Abstract
The lactate dehydrogenase (LDH) protein family members characteristically are distributed in tissue- and cell-type specific patterns and serve as the terminal enzyme of glycolysis, catalyzing reversible oxidation-reduction between pyruvate and lactate. They are present as tetramers and one family member, LDHC, is abundant in spermatocytes, spermatids, and sperm, but also is found in modest amounts in oocytes. We disrupted the Ldhc gene to determine whether LDHC is required for spermatogenesis, oogenesis, and/or sperm and egg function. The targeted disruption of Ldhc severely impaired fertility in male Ldhc-/- mice, but not in female Ldhc-/- mice. Testis and sperm morphology and sperm production appeared to be normal. However, total LDH enzymatic activity was considerably lower in Ldhc-/- sperm than in wild type sperm, indicating that the LDHC homotetramer (LDH-C4) is responsible for most of the LDH activity in sperm. Although initially motile when isolated, there was a more rapid reduction in the level of ATP and in motility in Ldhc-/- sperm than in wild type sperm. Moreover, Ldhc-/- sperm did not acquire hyperactivated motility, were unable to penetrate the zona pellucida in vitro, and failed to undergo the phosphorylation events characteristic of capacitation. These studies showed that LDHC plays an essential role in maintenance of the processes of glycolysis and ATP production in the flagellum that are required for male fertility and sperm function.
Keywords: sperm, glycolysis, gene targeting, capacitation, fertilization, testis, mouse
INTRODUCTION
Lactate dehydrogenase (LDH) catalyzes the reduction of pyruvate to lactate with the concomitant oxidation of NADH to NAD+ [1]. Catalytically active LDH consists of A and B subunits that assemble into homo- or heterotetramers and are distributed in the body in combinations reflecting the metabolic requirements of different tissues. For example, LDHA is most active in skeletal muscle where oxygen deficiency requires glycolysis to satisfy metabolic needs, while LDHB is abundantly expressed in cardiac muscle that is dependent upon aerobic metabolic pathways. Exquisite tissue specificity is exemplified by the Ldhc gene, the third member of the family; it is expressed only in male [2, 3] and female [4] germ cells, and its protein product forms the enzymatically active homotetramer, classically referred to as LDH-C4.
Originally, LDH-C4 was considered specific to male germ cells [5], while LDHB was described as the predominant LDH isozyme in oocytes [6]. However, Coonrod et al. [4] showed recently that Ldhc transcripts are present in germinal vesicle-stage oocytes and not in fertilized eggs, whereas LDHC protein is present in germinal-vesicle stage oocytes, fertilized eggs, and persist to preimplantation blastocyst development. Because of these observations, the function of LDH-C4 during oogenesis, oocyte maturation, or early development is unclear.
However, Ldhc transcript levels are substantially lower in oocytes than in male germ cells. The NCBI UniGene expression profiles indicate that 102 per million transcripts present in oocytes are for Ldhc, whereas 9340 per million transcripts present in pachytene spermatocytes and round spermatids are for Ldhc (UniGene build #168, UniGene accession number for Ldhc: Mm.16563). In addition, Ldhc transcripts are more abundant in whole testis (2844 per million) than Ldha transcripts (584 per million; Mm.29324) and Ldhb transcripts (160 per million; Mm.9745). Several studies have shown that isolated meiotic and post-meiotic male germ cells preferentially use lactate and pyruvate over glucose as an energy substrate ([7-9]; reviewed in [10]), suggesting that lactate oxidation by LDH isozymes might be significant in energy metabolism during the middle and later stages of spermatogenesis. In male germ cells, Ldhc expression is activated with the onset of meiosis. The LDHC protein can be detected first in preleptotene spermatocytes and is abundant in spermatids and spermatozoa [11, 12]. Heterotetramers containing LDHA and LDHB also were present in spermatogonia, spermatocytes and spermatids [13]. However, LDH-C4 was the major LDH isozyme in male germ cells, and heterotetramers containing LDHC and either LDHA or LDHB were not detected in murine or human testes [13].
It was thought that LDH-C4 is the only member of the LDH family active in spermatozoa [5, 13]. However, recent studies have shown that LDHA also is present in spermatozoa [14, 15]. LDHA was localized to the principal piece of the sperm flagellum where LDHC [16, 17] and other glycolytic enzymes also are concentrated [14]. However, LDHA but not LDHC was bound tightly to the fibrous sheath [14]. In contrast to spermatogenic cells, sperm exhibit high levels of glycolysis. Several in vitro studies provided evidence that glucose metabolism has an important role in production of the ATP required for sperm motility, hyperactivation, and capacitation [18-21]. This was proven when inactivation of the gene for the sperm-specific glycolysis pathway enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDHS) [22] dramatically reduced the level of ATP in sperm, caused severe defects in progressive sperm motility, and resulted in male infertility. Why Ldhc gene expression has been conserved in mammalian germ cells where Ldha and Ldhb genes also are transcribed remains an enigma. Kinetics of catalysis between the isozymes differ but not by the orders of magnitude sufficient to confer advantage to one of the three relative to germ cell metabolism [23]. However, LDH-C4 has high thermostability [23] and is able to metabolize α-hydroxyvalerate [24, 25], indicating that it has unique structural and functional properties [26]. In this study, we have disrupted the Ldhc gene to address the role of LDH-C4 in male and female germ cell development, gamete function and fertility.
MATERIALS AND METHODS
Materials and Reagents
All materials and reagents were of the highest quality available and purchased from Sigma-Aldrich (St Louis, MO; http://sigmaaldrich.com) or Mallinckrodt Baker (Phillipsburg, NJ; http://www.mallbaker.com) unless designated otherwise.
Targeting construct and targeted disruption of the Ldhc gene
Mice with exon 3 of the Ldhc gene deleted were generated using 129SvEv embryonic stem cells and C57BL/6N blastocysts to produce chimeras), Lhdctm1Erg (Supplemental Fig. 1 available online at www.biolreprod.org). All wild type animals were purchased from Charles Rivers (Raleigh, NC; http://www.criver.com). This study was performed with animals from the third and fifth backcrossed generations with wild type C57BL/6N mice.
Phenotype determination: body and reproductive organ weights, sperm number, and daily sperm production
All animal procedures were performed in accordance with NIH guidelines and approved by the National Institute of Environmental Health Sciences (NIEHS) and/or Northwestern University Animal Care and Use Committee. Mice from 12 to 32 wk of age were euthanized by CO2 asphyxiation followed by cervical dislocation. Total body, testis, epididymis, and seminal vesicle weights were determined. Sperm numbers were determined using a hemocytometer. Daily sperm production (DSP) was estimated as described previously [27, 28]. Briefly, testes were weighed and homogenized in an extraction buffer (0.15 M NaCl, 0.1 mM NaN3, 0.05% Triton X-100) to yield homogenization-resistant condensed spermatid heads. The number of spermatid heads was determined using a hemocytometer [27, 28].
Sperm preparation
Cauda epididymides collected in 1X PBS (Ca2+/Mg2+-free) were carefully dissected to remove blood vessels and fat, several small cuts were made with iridectomy scissors, and sperm were allowed to swim out into the medium. Capacitating medium was a modified Krebs-Ringer bicarbonate solution (119.37 mM NaCl, 4.78 mM KCl, 1.71 mM CaCl2 •2H2O, 1.19 mM MgSO4 •7H2O, 1.19 mM KH2PO4, 25.07 mM NaHCO3, 5.6mM glucose) with 4 mg/ml bovine serum albumin (BSA) [17, 29], or human tubular fluid medium (HTF) supplemented with 4 mg/ml BSA (Millipore, Bedford, MA; http://www.millipore.com). Sperm were incubated for selected lengths of time at 37°C in 5% CO2 in humidified air.
For some experiments, sperm isolated from cauda epididymides as described above were purified with the PureSperm 40/80 kit (NIDACON Int.; http://www.nidacon.com) following the manufacturer’s instructions. Briefly, the sperm suspension was layered onto the PureSperm gradient and centrifuged at 300 × g for 20 min. The sperm pellet was washed in PureSperm Wash buffer. Sperm purity was approximately 99-100%, with negligible contamination by somatic cells.
Histology and immunodetection of LDHC
Testes were fixed in Bouins solution (Polysciences, Inc., Warrington, PA; http://www.polysciences.com), dehydrated, and embedded in paraffin using standard methods. Five μm thick sections were stained with hematoxylin and eosin for histological analysis. The antibody to LDHC was diluted 1:10,000 in 1X PBS and 1% BSA for immunohistochemistry (IHC) and 1:4,000 for indirect immunofluorescence (IIF) [16, 17]. IHC on paraffin sections was performed using a Vectastain ABC kit (Vector Laboratory, Burlingame, CA; http://www.vectorlabs.com) following the manufacturer’s instructions. For IIF, sperm suspensions were pipetted onto slides (Superfrost/Plus, Fisher Scientific; http://www.fishersci.com), allowed to settle and attach at 4°C for 10 min, and permeabilized with 0.5% Triton X-100 in PBS for 2 min followed by 1 min in cold methanol 100% (-20°C). After incubation in antibody to LDHC for 2 h at room temperature (RT), sperm were incubated with Alexa Fluor 488 donkey anti-rabbit IgG (dilution 1:200; Invitrogen, Carlsbad, CA; http://www.invitrogen.com) for 1 h and mounted with Vectashield (Vector Laboratory). Samples were prepared for transmission and scanning electron microscopy as described previously [19].
RNA extraction, RT-PCR, and real time RT-PCR
RNA was extracted with TRIzol reagent (Invitrogen). Specific primers were designed using the Primer3 processor (http://frodo.wi.mit.edu) [30], and the optimal temperature of annealing was defined for each primer pair (Supplemental Table 1 available online at www.biolreprod.org). Primers for Ldhc RT-PCR were chosen in different exons and PCR products were sequenced using Big Dye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA; http://www.appliedbiosystems.com). Real-time RT-PCR was performed using the SybrGreen kit (Applied Biosystems). All reactions were repeated three times and 5S rRNA was used as an internal control to ensure equal amplification efficiency.
LDH activity
Homogenized testis fragment or purified sperm were suspended in buffer (0.1 M Tris-HCl, pH 7) and subjected to three 10-sec ultrasonic pulses. The suspension was centrifuged at 14,000 × g for 10 min at 4°C. The supernatant was collected and protein levels measured using the BCA protein assay kit (Pierce Biotechnology, Indianapolis, IN; http://www.piercenet.com). LDH activity was determined as previously described [31]. Briefly, proteins were loaded on a 10% native polyacrylamide gel and separated at 100 V for 5 h at 4°C. Lactate dehydrogenase isozyme activity was visualized by incubating the gel for 15 min in the dark in a staining mix [0.05 M Tris-HCl pH 8.4, 2 mM NAD, 0.8 mM NBT, 62.5 mM DL-lactate, and a few crystals of phenazine methosulfate (PMS)]. Global LDH activity was assayed in solution by spectrophotometry [17]. Ten μg of protein extract was added to 1ml of reaction buffer (0.05 M Na2HPO4 pH 7, 0.1 mg/ml NADH, and 27.5 μg/ml pyruvate). LDH activity was calculated as the change in absorbance at 340 nm over a period of 1 min and expressed as units/min/μg of protein.
Lactate level measurements
Purified sperm were washed in capacitating medium without lactate and pyruvate (present in the PureSperm buffer) and then incubated in capacitating medium at a concentration of 4×106 spermatozoa/ml at 37°C in 5% CO2 in humidified air. An aliquot was taken at time 0 to measure the residual levels of lactate and then after 0.5, 1, 2, and 4 h of incubation. After centrifugation for 5 min at 10,000 × g, duplicate 5 μl aliquots of medium were collected. Lactate levels were determined using a commercial kit (BioVision, Mountain View, CA; http://www.biovision.com) based on an enzymatic reaction by lactate oxidase and interaction of the product with a probe to produce fluorescence (at excitation/emission = 535/587nm). The concentration of each sample was calculated using a standard curve. The detailed protocol for this assay is available on the manufacturer’s website.
Western blot assays
Proteins were either extracted as previously described (see LDH activity section) for LDHC immunodetection, or in a denaturating extraction buffer [125 mM Tris-HCl pH 7.5, 4% SDS, complete cocktail inhibitors 1X (Roche Applied Science, Indianapolis, IN; https://www.roche-applied-science.com)] for immunodetection of phosphotyrosine-containing proteins. Proteins were separated on a 10% polyacrylamide gel and transferred onto Immobilon membrane (Millipore). The membrane was incubated in blocking buffer [PBS with 0.1% Tween-20 (PBS-T) plus 5% nonfat dry milk for anti-LDHC or 1% BSA for anti-phosphotyrosine] for 1 h at RT with shaking. After three washes in 1X PBS-T, the membrane was incubated with the primary antibody against either LDHC (diluted 1:10,000; incubated overnight at 4°C) or phosphotyrosine (clone PT-66, Sigma-Aldrich; diluted 1:1,000; incubated for 1 h at RT). After several stringency washes, the membrane was incubated for 1 h at RT with the appropriate HRP-conjugated secondary antibody. The Amersham ECL+ kit (GE Healthcare Bio-Sciences Corp., Piscataway, NJ; http://www1.gelifesciences.com) was used to detect the signal.
Assessment of fertility by natural mating
To assess male fertility, individual Ldhc-/- males were mated continuously for two or more 4 wk periods with two C57BL/6N wild type females. Individual Ldhc-/- females were mated with one wild type C57BL/6N male for 8 wk. All females were maintained for 4 wk after the mating period to assess possible pregnancy.
Individual Ldhc-/- (n=10) and wild type males (n=6) were mated with one superovulated wild type female overnight to assess male mating ability and to determine the percentage of eggs fertilized in vivo. Superovulated females that were not mated were used to determine the number of unfertilized two-cell eggs resulting from either parthenogenetic activation or oocyte fragmentation. In the morning following mating, females were examined for copulatory plugs and euthanized (experiment repeated 4 times). Zygote-cumulus complexes were isolated from the swollen ampullae. Zygotes were denuded by a brief exposure to hyaluronidase (200 IU) and then washed thoroughly in M2 medium (Millipore). Zygotes were cultured in groups in 10 in 20 μl drops of KSOM+AA medium (Millipore) in 60 mm dishes covered with light mineral oil in an atmosphere of 5% CO2 in humidified air at 37°C. They were examined at 4 h for the presence of pronuclei, and two-cell and four-cell embryos were counted one and two days later, respectively. Blastocysts were collected from superovulated females sacrificed on day 4.5 post-insemination (experiment repeated 3 times). Uteri were flushed with M2 medium, and expanded blastocysts morula, or degenerated eggs were washed in M2 medium and counted.
In vitro fertilization
Cauda epididymal sperm were capacitated in HTF medium supplemented with 4 mg/ml BSA for 1.5 h at 37°C in 5% CO2 in humidified air. Oocytes from superovulated CD-1 mice were treated with hyaluronidase to remove cumulus cells. In some cases, zonae pellucidae were removed by chymotrypsin treatment (10 μg/ml, less than 5 min). Cumulus- and zona pellucida-free eggs were incubated in groups of 20 in 100 μl droplets for 4 and 1 h, respectively, with Ldhc+/- and Ldhc-/- sperm at a concentration of 0.2 × 103 sperm/μ1 in humidified air at in 5% CO2 37°C. The eggs were washed (to remove cellular debris and surplus sperm) and incubated overnight in KSOM+AA medium. The following morning, two-cell embryos were counted and transferred to fresh KSOM+AA medium. The eggs were considered fertilized if they developed to blastocysts after 4 days in culture.
ATP level measurements
Sperm were incubated in capacitating medium (described above) at 37°C in 5% CO2 in humidified air and assayed after 10 min, 90 min and 4 h. Sperm ATP levels were measured as previously described [22]. After centrifugation at 1,000 × g for 3 min, the pellet was resuspended in 100°C buffer (100 mM Tris-HCl, 4 mM EDTA pH 7.8) and incubated for another 2 min at 100°C. Samples were centrifuged at 10,000 × g for 5 min and aliquots of the supernatant were analyzed in duplicate. ATP was measured using a luciferase bioluminescence assay according to the manufacturer’s protocol (ATP Bioluminescence Assay kit CLS II; Roche Applied Science).
Sperm motility assessment
Quantitative parameters of sperm motility were determined as described by computer-assisted sperm analysis (CASA) instrument (Hamilton Thorne Research, Beverly, MA, software version 12; http://www.hamiltonthorne.com) after incubation for 0.5, 1.5, and 4 h in capacitating medium. Median values of each of the kinematic parameters were obtained for each sample (for definitions see [32]). Kinematic parameters measured included curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), amplitude of the lateral displacement of sperm head (ALH), beat-cross frequency (BCF), linearity (LIN = 100% × VSL/VCL), and straightness (STR = 100% × VSL/VAP) [32-34]. Sperm were counted as motile with any type of movement and progressively motile when VAP > 50 μm/sec and STR > 50%. Hyperactive sperm were identified with the “sort fraction” function of the Hamilton Thorne analyzer by using these criteria: VCL > 240μm/sec, ALH > 18 μm and BCF < 40Hz.
Data analysis
All results are represented as the mean values of each group ± SEM. The significance of the results was determined using one-way ANOVA followed by the Mann-Whitney U-test. Differences were considered significant at p < 0.05.
RESULTS
Generation and fertility of LDHC-deficient mice
The Ldhc locus was inactivated by gene targeting to determine if LDH-C4 activity is required for gametogenesis and/or gamete function in male and female mice. Exon 3, which encodes both the coenzyme-binding domain and part of the substrate-binding domain, was flanked by loxP sites (Supplemental Fig. 1 available online at www.biolreprod.org). The targeted allele was transmitted through the germline. Offspring heterozygous for the targeted floxed allele were mated with transgenic mice expressing Cre to produce heterozygous Ldhc+/- mice and these were mated to produce homozygous Ldhc-/- male and female mice. Both Ldhc-/- male and female mice were generated in expected Mendelian ratios (data not shown).
The Ldhc-/- female mice exhibited normal fertility, producing approximately one litter of 8 ± 3 pups per month, which was not significantly different from control C57BL/6N wild type mice (7 ± 3 per month). The fertility of Ldhc+/- males also was comparable to that of control wild type males (8 ± 4 pups per month). In their fertility trials, each of the eleven 60-days old Ldhc-/- males (third backcross generation, n=8; fifth backcross generation, n=3) were mated with 4 different C57BL/6N females over 2 to 4 month periods. Two of the Ldhc-/- males (one third and one fifth backcross generation) sired single litters of one and three pups, respectively, during the first trial. To extend this analysis, these two males were kept for 2 additional trials (total of 6 different females over a 4 months period), but they did not sire litters in these subsequent matings. No other Ldhc-/- males have produced litters. Genotyping confirmed that these two males were Ldhc-/- and that their offspring were Ldhc+/-.
Morphology of testes and sperm from Ldhc-/- mice appears normal
There were no significant differences in body weights of wild type, Ldhc+/-, and Ldhc-/- males. The same was true for testis, epididymis, and seminal vesicle weights (Table 1). Sperm production was unaffected, with no differences seen in daily sperm production or epididymal sperm counts (Table 1). Sperm from Ldhc-/- mice examined by light and scanning electron microscopy also exhibited normal morphology (data not shown).
Table 1.
Phenotypic characteristics of wild type (WT), Ldhc+/- (HET), and Ldhc-/- (KO) mice.
| Genotype | Body weight (g) | Seminal vesicle weight (mg) | Epididymis weight (mg) | Testis weight (mg) | Sperm count, (106/cauda epididymis) | Daily sperm count # (105 DSP) |
|---|---|---|---|---|---|---|
| WT (n=11, #8) | 33.05 ± 6.98 | 399.45 ± 58 | 41.27 ± 7.38 | 88.00 ± 12.68 | 17.33 ±2.94 | 3.97 ±1.21 |
|
| ||||||
| HET (n=10, #7) | 36.49 ± 5.36 | 421.80 ± 78.11 | 44.65 ± 8.68 | 91.55 ± 14.74 | 17.77 ±2.94 | 4.51 ±0.89 |
|
| ||||||
| KO (n=12, #10) | 34.51 ± 4.59 | 380.75 ± 66.93 | 45.71 ± 9.27 | 97.16 ± 18.40 | 18.37 ±3.71 | 4.68 ±1.93 |
Values are means ±SEM for each genotype. Seminal vesicle weight was the combined weight of both glands; testis and epididymis weight was the average weight between right and left organs. The sperm count was the number collected from one cauda epididymis in one ml. The number of samples (n) is indicated below, # correspond to the number of samples for the DSP.
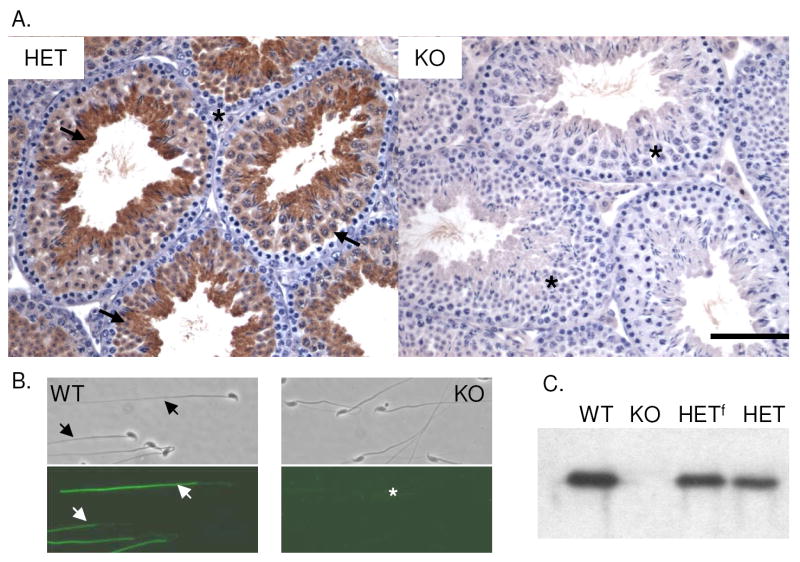
Testis morphology as assessed by histological analysis and transmission electron microscopy was comparable in wild type, Ldhc+/- and Ldhc-/- animals (data not shown). Using both IHC and IIF, we confirmed that LDHC is present in the cytoplasm of meiotic and post-meiotic germ cells and in the sperm flagellum of wild type and Ldhc+/- mice (Fig. 1, A and B). As expected, no LDHC was detectable in Ldhc-/- testes and sperm (Fig. 1, A and B) or oocytes (Supplemental Fig. 2 available online at www.biolreprod.org). The absence of LDHC in the testes of Ldhc-/- mice also was confirmed by western blotting (Fig. 2C).
Fig.1.
(A) Immunohistochemical localization of LDHC in testis from Ldhc+/- (HET) and Ldhc-/- (KO) mice. Arrows indicate positive areas and the asterisk indicates the absence of LDHC. Bar = 100 μm. (B) Immunofluorescence of LDHC on sperm from wild type (WT) and Ldhc-/- (KO) mice. (C) Western blot analysis of testis extracts from wild type (WT), Ldhc-/- (KO), heterozygote type 1 (HETf; floxed), and Ldhc+/- (HET) mice, probed with anti-LDHC.
Fig.2.
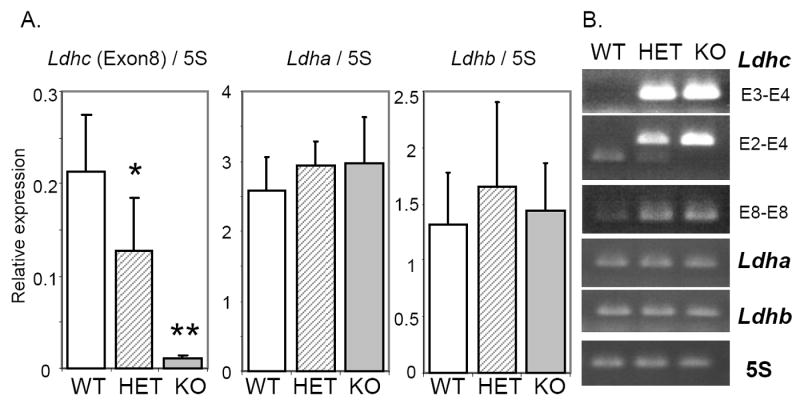
RT-PCR analysis of Ldha, Ldhb and Ldhc transcript levels. Total RNAs were extracted from whole testis from wild type (WT; n=8), Ldhc+/- (HET; n=7), and Ldhc-/- (KO; n=9) mice. (A) Real-time RT-PCR analysis of Ldha, Ldhb and Ldhc (E8-E8 primers). Each reaction was done in triplicate for each gene and the amount of cDNA was determined by using a standard curve. Data are means ± SEM and are expressed in arbitrary units after normalization with 5S rRNA. *p < 0.05; **p < 0.01 compared to wild type. (B) RT-PCR products were separated on 2% agarose gels and their sizes were confirmed with a DNA ladder (not shown). Primer pair E3-E4 for Ldhc identifies the native transcript and will not produce a band when Exon 3 is deleted; primer pair E2-E4 amplifies transcripts containing exon 3 with a 254 bp product, and the truncated transcript with a 127 bp product; primer pair E8-E8 amplifies both Ldhc transcripts without size distinction.
Ldha and Ldhb transcript levels are not changed in Ldhc-/- testes
We used real-time RT-PCR and gene-specific primers (Supplemental Table 1 available online at www.biolreprod.org) to measure the Ldha, Ldhb and Ldhc transcript levels in the testes from wild type, Ldhc+/- and Ldhc-/- mice. No significant differences were observed in Ldha and Ldhb transcript levels in Ldhc+/- or Ldhc-/- mice compared to wild type mice (Fig. 2A). However, with PCR primers specific for exon 8, Ldhc transcript levels were 5.2% and 60% in testes from Ldhc-/- and Ldhc+/- mice respectively, of the levels in testes from wild type mice (Fig. 2A), rather than 0% and 50% as expected, indicating that transcription of the mutant allele was not inhibited completely. Sequencing of the PCR product confirmed that it was amplified from transcripts containing exon 8 of Ldhc (data not shown). To determine if these results were due to the presence of variant transcripts lacking exon 3, PCR assays were performed with primer in exons 3 and 4 (E3-E4) and primers in exons 2 and 4 (E2-E4). With RNA from wild type testes, we observed a unique PCR product with both primer pairs. While the E3-E4 primer pair did not yield a PCR product from Ldhc-/- testis RNA (row E3-E4, Fig. 2B), the E2-E4 primer pair generated a second PCR products from Ldhc-/- and Ldhc+/- testis RNA (row E2-E4, Fig. 2B). Sequence analysis of the E2-E4 primer pair PCR product from Ldhc-/- testis RNA determined that it lacked nucleotides 161-288 (data not shown), which corresponds to exon 3. However, no full-length transcripts were found in Ldhc-/- testis. The transcripts detected at low levels were always missing exon 3. Because exon 3 encodes both the coenzyme-binding domain and part of the substrate-binding domain, it is not likely that the variant transcript produces a functional LDH-C4 enzyme.
Total LDH activity is lower in Ldhc-/- testes and sperm
LDH activity differed significantly in testis and sperm extracts from wild type, Ldhc+/-, and Ldhc-/- mice (Fig. 3, A and B). Total LDH activity was reduced by one-half in Ldhc-/- testis extracts as compared to wild type. While total LDH activity from Ldhc+/- testis extracts appeared to be reduced by approximately one-quarter as compared to wild type, this was not statistically significant. A similar pattern was observed in spermatozoa, with significant reductions of 24.7% and 82.2% in LDH activity respectively, in Ldhc+/- and Ldhc-/- sperm as compared to wild type sperm (Fig. 3A). However, the total LDH activities in extracts of Ldhc-/- testis and sperm were 50% and 17.8% of the total LDH activity in testis and sperm from wild type mice. This is to be expected since both LDHA and LDHB are present in testes [13] and LDHA has been found in sperm by proteomic screens [14, 15].
Fig.3.
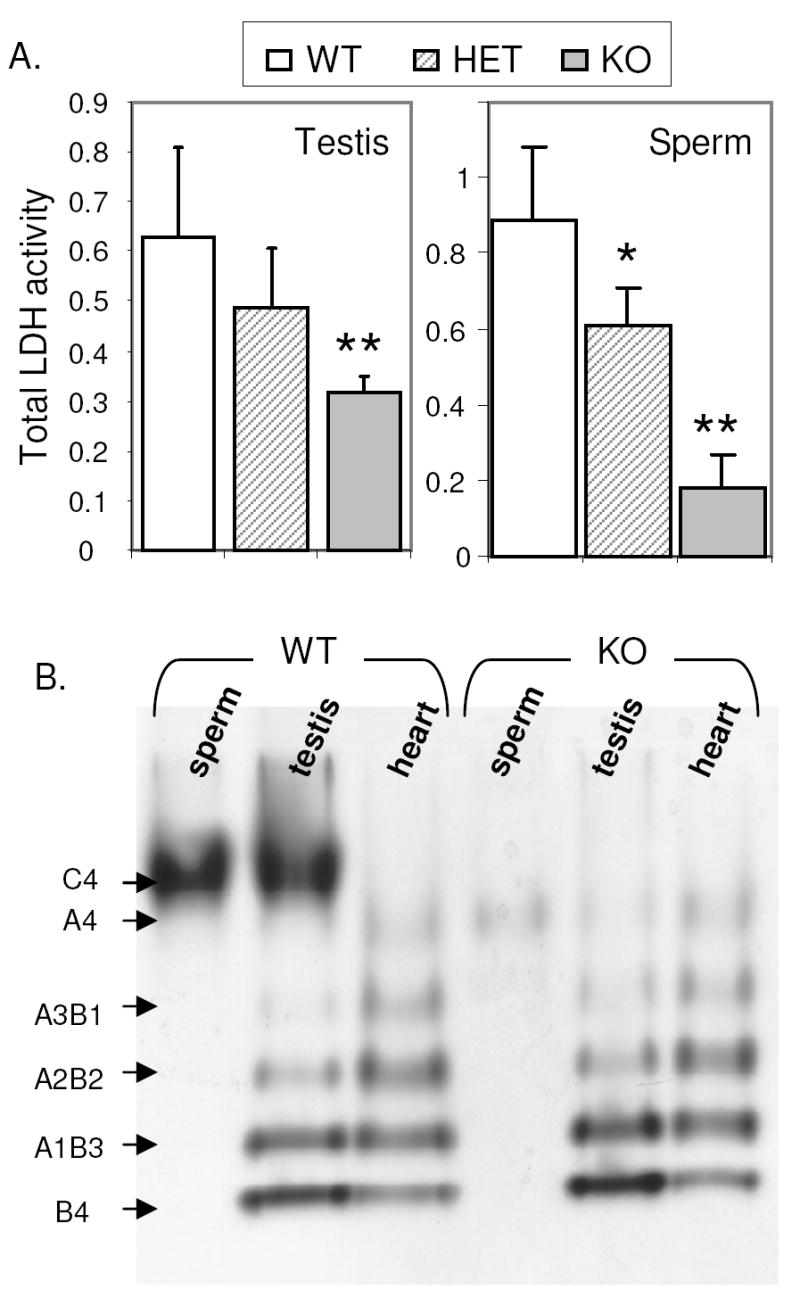
LDH activity analysis in sperm from wild type (WT), Ldhc+/- (HET) and Ldhc-/- (KO) mice. (A) Global LDH activity was measured by spectrophotometry as the decrease in absorbance at 340 nm over 1 min (ODt0-ODt1min). Values are mean ± SEM (n=6). * p<0.05, ** p < 0.01, compared to wild type mice. (B) LDH activity visualized on an activity staining gel. A representative gel is shown (n=3). Subunit composition of LDH isozymes are indicated on the left.
The activity of the different LDH isozymes was visualized after tissue extracts were separated by electrophoresis on a non-denaturing polyacrylamide gel. Proteins extracted from heart were used as a control to detect isozymes containing LDHA and LDHB. As previously reported [5, 13], bands corresponding to homotetramers and heterotetramers of LDHA and LDHB activity, and a strong band corresponding to LDH-C4 homotetramer activity were visualized in proteins extracted from wild type testis. In spermatozoa, only the LDH-C4 activity band was observed (Fig. 3B). As expected, no LDH-C4 activity was detected in testis and sperm of Ldhc-/- mice, whereas the other LDH isozymes activities in testis extract appeared comparable to those in testis of wild type mice (Fig. 3B). However, consistent with our previous observation on total LDH activity, a minor band on an LDH activity gel corresponding to LDH-A4 was observed in extracts of sperm and testis from Ldhc-/- mice. This band was not visualized in wild type sperm, presumably because the strong band of LDH-C4 obscured that of LDH-A4.
Lactate production by Ldhc-/- sperm is compromised
We measured lactate production by wild type, Ldhc+/-, and Ldhc-/- sperm. Pyruvate is the end product of glycolysis and LDH-C4 converts the pyruvate into lactate. Lactate can diffuse across the sperm plasma membrane and thus the accumulation of lactate in the medium indicates LDH activity. Lactate was found to accumulate over time in media containing wild type and Ldhc+/- sperm. After incubation of wild type sperm for 30 min, 61.4 nmol/ml of lactate was present. In contrast, after incubation of Ldhc-/- sperm for 30 min, there was little if any lactate production (Fig. 4). However, Ldhc-/- sperm were capable of lactate production and after 4 h of incubation, 46.6 nmol/ml of lactate were present. Nevertheless, this was less than 10% of the amount of lactate in media containing wild type sperm after the same time period (576.6 nmol/ml).
Fig.4.
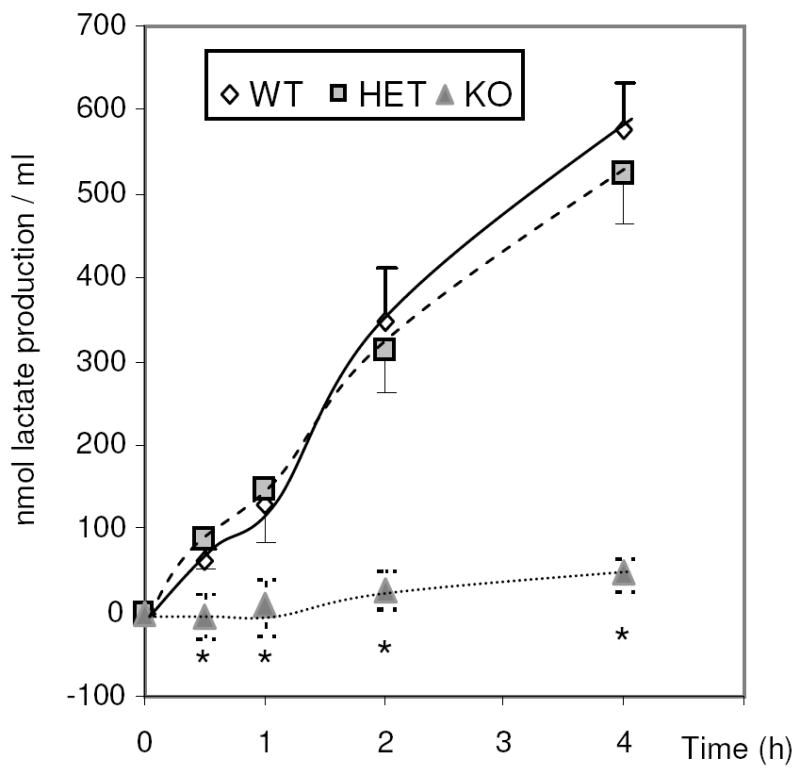
Lactate level in medium containing wild type (WT), Ldhc+/- (HET) and Ldhc-/- (KO) sperm (4 ×106 /ml) after incubation in capacitating medium at different times. Values are the mean lactate levels ± SEM (n=3). * p < 0.001 compared to wild type.
In vivo and in vitro fertilization by Ldhc-/- sperm is defective
Copulatory plugs were produced by Ldhc-/- males mated overnight with superovulated wild type female mice. We found that 62.8% of the eggs recovered from matings with wild type males were fertilized (presence of pronuclei) but fewer than 0.5% from Ldhc-/- males appeared to be fertilized (Table 2). The number of one cell eggs developing to the two-cell stage was similar for eggs recovered from females bred to Ldhc-/- male and eggs recovered from females not bred, and none of these divided to the four-cell stage in culture (Control; Table 2, A). A similar result was found when female reproductive tracts were flushed 4.5 days after mating. No morula or blastocysts were recovered from females mated with Ldhc-/- males, whereas 75.3% of the embryos recovered from females mated with wild type males had developed to the morula stage (16.9%), or the blastocyst-stage (58.4%) (Table 2, B).
Table 2.
Percentage of in vivo fertilized eggs after natural mating with wild type (WT) or Ldhc-/- (KO) males.
| A.
|
B.
|
||||
|---|---|---|---|---|---|
| genotype | Fertilized | 2-cell embryos | 4-cell embryos | Morula + blastocyst | Degenerated cells |
| WT (A.179/B.229) | 62.77 % ±17.03 | 52.18 % ±23.65 | 46.93 % ±22.29 | 75.33 % ±12.38 | 24.67 % ±11.67 |
|
| |||||
| KO (A.263/ B.259) | 0.30% ±0.81 | 3.41 % ±5.30 | 0 % | 0 % | 100 % |
|
| |||||
| Control (195) | 0 % | 6.38 % ±6.08 | 0% | ||
Eggs were collected: (A) the day following mating; fertilization was assessed by the presence of pronuclei and development to two-cell and four-cell embryos; (B) 4.5 days after mating; number of in vivo-derived blastocysts were counted. Results are expressed in percentage of fertilized, two-cell, four-cell embryos or blastocysts from total eggs collected. Controls are eggs from superovulated non-mated females, and represent spontaneous divisions. The total numbers of eggs analyzed for (A) and (B) are indicated in parentheses. At least 3 independent experiments were performed. Values are means ±SEM.
The in vitro fertilization assay results were consistent with the results of the mating studies. Only 3% of cumulus-free eggs incubated with sperm from Ldhc-/- males became two-cell embryos, none of which developed into blastocysts. In contrast, 51% of eggs fertilized by Ldhc+/- sperm became two-cell embryos and 39% became blastocysts (Table 3, A). However, 29% of eggs with the zona pellucida removed and incubated with sperm from Ldhc-/- males became two-cell embryos and 20% developed into blastocysts (Table 3, B). Even so, the number of zona pellucida-free eggs fertilized was only one-third of the 87% observed with Ldhc+/- sperm. These IVF assays were performed with a small number of sperm (20,000 sperm per 100 μl droplet per 20 eggs) to avoid polyspermy. However, some tests were performed with a larger number of sperm (2 and 10 times more) and the same results were observed (data not shown).
Table 3.
In vitro fertilization with Ldhc+/- (HET) or Ldhc-/- (KO) sperm.
| A. Cumulus-free eggs
|
B. Zona pellucida-free eggs
|
|||
|---|---|---|---|---|
| genotype | 2-cell embryos | Blastocysts | 2-cell embryos | Blastocysts |
| HET (A.179/B.168) | 51.29 % ±14.67 | 33.87 % ±12.27 | 87.73 % ±2.30 | 69.41 % ±14.7 |
|
| ||||
| KO (A.263/B.265) | 3.33% ±0.81 | 0 % | 29.41% ±10.26 | 20.15% ±3.32 |
Fertilization was assessed by recording the number of two-cell embryos 24 h after fertilization, and fertilization was confirmed after 4 days in culture by development to blastocysts. Results are expressed in percentage of two-cell or blastocysts from total eggs collected; total numbers of cumulus-free eggs (A) and zona pellucida-free eggs (B) are indicated in parenthesis. At least 4 independent experiments were performed, and data are reported as mean ± SEM.
Lack of LDH-C4 alters sperm motility
Maintenance of progressive motility and acquisition of hyperactivated motility by sperm are required for fertility. Motility parameters of Ldhc-/- sperm were analyzed by CASA after 30 min, 90 min, and 4 h of incubation in capacitating medium (Fig. 5). Initially (t0.5), the motility of Ldhc-/- sperm was comparable to wild type sperm (Fig.6 and Supplemental Movies 1 and 2 available online at www.biolreprod.org). After 4 h of incubation, the percentage of Ldhc-/- sperm that were motile was reduced and there was an even greater decrease in the percentage with progressive motility (Fig. 5; Fig. 6 and Supplemental Movies 3 and 4 available online at www.biolreprod.org). Moreover, Ldhc-/- sperm failed to develop the hyperactivity pattern characterized by vigorous flagellar movements. This was identified by CASA as showing a high VCL and ALH and lower BCF [33]. Only 1.93% of Ldhc-/- sperm were hyperactive, while 46.9% of Ldhc+/- sperm were hyperactive (t1.5 h) (Fig. 5).
Fig.5.
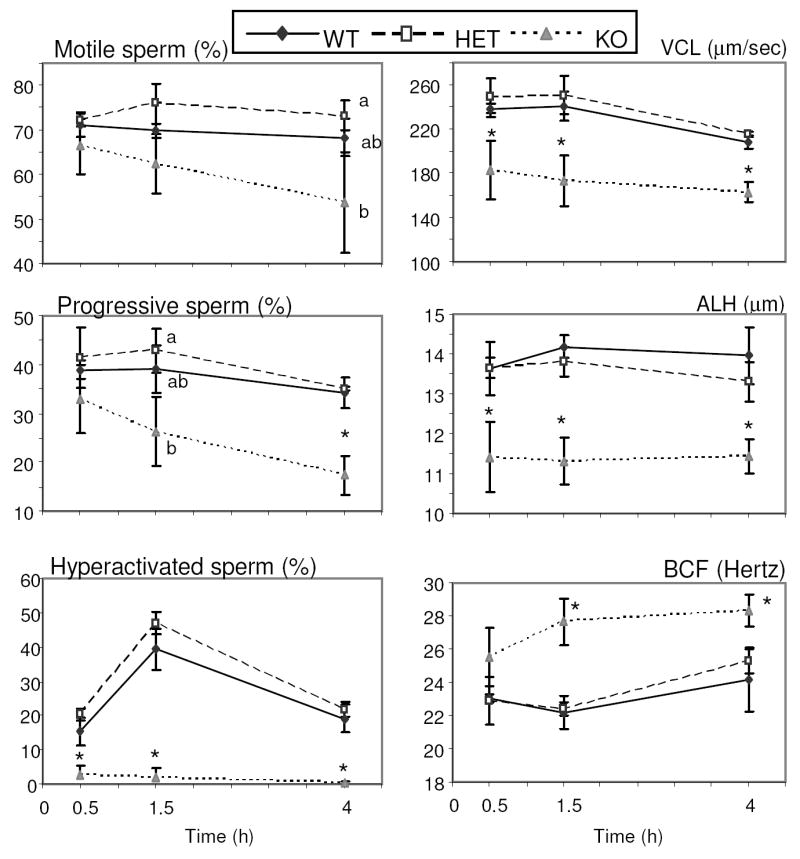
Sperm motility assessed by CASA. Sperm were collected in capacitating medium and incubated at 37°C in 5% CO2 in humidified air. Motile sperm, progressively motile sperm, and hyperactived sperm are expressed in percentage of sperm presenting indicated characteristic (see Materials and Methods) of total sperm counted. VCL = curvilinear velocity, ALH = amplitude of the lateral displacement of sperm head, BCF = beat-cross frequency. Median values ± SEM (minimum 200 sperm recorded) of each of the kinematic parameters were obtained for each sample (n=3). Letters (a, b) above the means denote no significant difference if identical or significant difference if not identical. * p< 0.05 compared to wild type.
Fig.6.
Still images taken from the supplemental movies (available online at www.biolreprod.org). Sperm from Ldhc+/- (HET) and Ldhc-/- (KO) mice were collected in capacitation medium and incubated 1 h or 4 h at 37°C in 5% CO2 in humidified air. Movies were taken with a Leica DFC420 digital camera mounted on a Leica DM IRB inverted phase microscope with a 20X lens and captured using Leica LAS software version 2.8.1.
Loss of LDH-C4 alters protein tyrosine phosphorylation in sperm
A hallmark of sperm capacitation is an increased level of protein tyrosine phosphorylation [35-37]. The level of tyrosine phosphorylation in sperm from wild type, Ldhc+/-, and Ldhc-/- mice was evaluated by Western blotting to determine if LDH-C4 is required for this process. The extent of tyrosine phosphorylation was substantial in wild type and Ldhc+/- sperm incubated in capacitating medium for 90 min, but negligible in Ldhc-/- sperm (Fig. 7). The constitutively phosphorylated hexokinase (molecular mass 116kDa) served as an internal positive control for the antibody and demonstrated that the samples contained equal amounts of protein.
Fig.7.
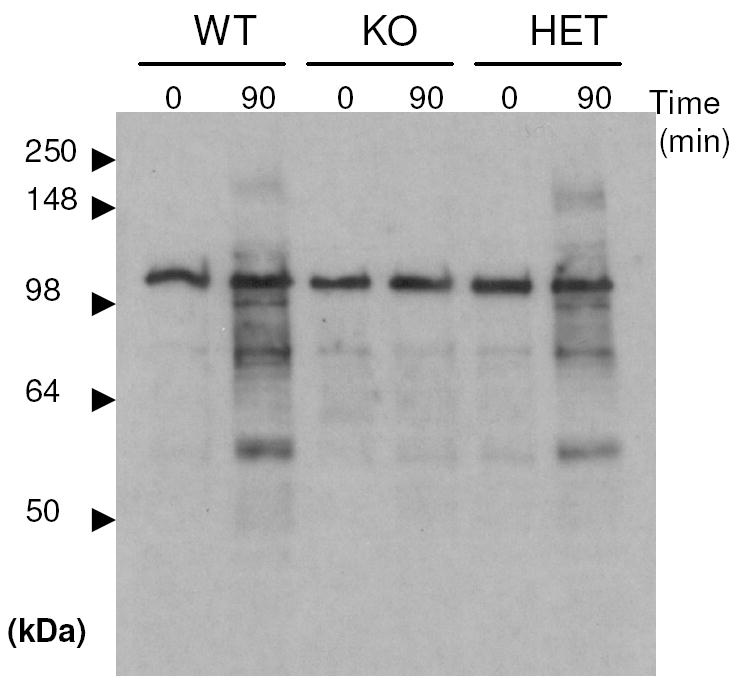
Protein tyrosine phosphorylation occurring in wild type (WT), Ldhc+/- (HET) and Ldhc-/- (KO) sperm assayed by western blotting with an antibody against phosphotyrosine. Proteins were extracted immediately after sperm collection or after 90 min incubation in capacitating medium. The band at 116kDa corresponds to hexokinase. The example shown is representative of three experiments.
LDH-C4 is required to maintain sperm ATP levels
High ATP levels are required for sperm motility, hyperactivity and capacitation [19, 21]. There were no differences in the ATP levels in sperm from wild type, Ldhc+/-, and Ldhc-/- mice after incubation for 10 min in capacitating medium (Fig. 8). However, the ATP level was 52.3% lower at 90 min in Ldhc-/- sperm than in wild type sperm and even lower (81.8%) after 4 h. There was not a significant difference in ATP levels between Ldhc+/- and wild type sperm at any of the time points (Fig. 8).
Fig.8.
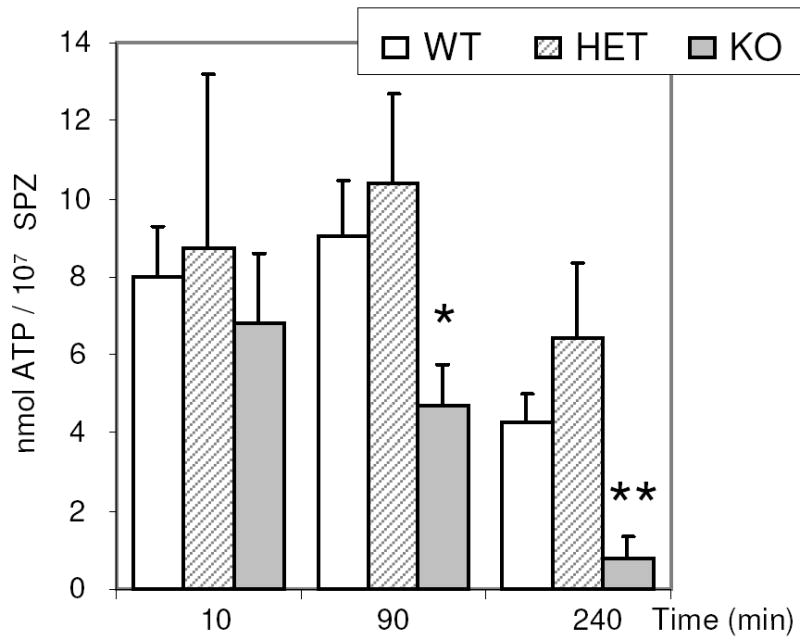
Time-dependent decrease in ATP levels in Ldhc-/- sperm (A). Values are the mean ATP levels ± SEM from wild type (WT; n=7), Ldhc+/- (HET; n=8), and Ldhc-/- (KO; n=10) mice. * p < 0.05, **p < 0.001 compared to wild type.
DISCUSSION
This study confirms reports that LDHC is present in both spermatogenic cells and oocytes [4, 5] (Fig.1 and Supplemental Fig. 2 available online at www.biolreprod.org). Disruption of the Ldhc gene caused male but not female infertility and established that LDHC is essential for sperm function, but unlikely to have a significant role in oocyte function. As expected, native transcripts, LDHC protein and LDH-C4 enzymatic activity were not detected in Ldhc-/- mice. However, a variant transcript lacking the exon 3 coding region was present at low levels in Ldhc-/- and Ldhc+/- testes. The low transcript level might suggest that sequences in exon 3 and/or the intronic regions flanking the exon that are deleted during the Cre-mediated LoxP recombination event might contain regulatory region(s) that have effects on transcription or Ldhc mRNA stability.
The level of Ldhc transcript levels in testis from Ldhc+/- mice was reduced by 40%. We also found a decrease of global LDH activity of 19.1% in testis and 24.7% in sperm from Ldhc+/- mice. However, the fertility of Ldhc+/- mice was comparable to the fertility of wild type mice. Moreover, no differences were observed between wild type and Ldhc+/- sperm in ATP levels, motility, hyperactivation or capacitation-related changes in phosphorylation. These observations indicate that sperm contain substantially more LDH-C4 than is needed to maintain normal fertility.
The total LDH activity was reduced to 50% in the testis and 17.8% in sperm from Ldhc-/- mice, compared to the total LDH activity in testis and sperm from wild type mice. Although LDHA and LDHB heterotetramers are present in testis, and LDHA homotetramers are present in sperm [16, 17], these reductions indicate that a substantial amount of the LDH activity in testis and most of the LDH activity in sperm is due to LDH-C4.
The substantial level of LDH-C4 activity in pachytene spermatocytes and spermatids might indicate that this isozyme has a significant role in spermatogenesis. However, based on histology and sperm production numbers, spermatogenesis appears to progress normally in the Ldhc-/- testis. This seems not to be due to functional compensation by the other LDH isozymes because Ldha and Ldhb transcript levels were comparable in testes of Ldhc-/- and wild type mice. Although these results suggest that LDH-C4 does not play an essential role in the maintenance of spermatogenesis, it does not rule out that the absence of LDH-C4 has a subtle effect on spermatogenesis that subsequently compromises sperm function.
The fertility of Ldhc-/- males was severely compromised, even though the sperm morphology, numbers and motility observed soon after isolation appeared normal. The assessment of male fertility by natural mating showed that the majority of Ldhc-/- males were infertile, but 2 males were subfertile, each producing one small litter. However, no morula or blastocysts were recovered from the reproductive tracts of superovulated females mated with Ldhc-/- males, and no blastocyst embryos were produced by in vitro fertilization using sperm from Ldhc-/- males and eggs with an intact zona pellucida. Furthermore, the absence of LDH-C4 in sperm resulted in a decline in progressive motility compared to wild type sperm and a failure to achieve hyperactivated motility. These findings strongly suggest that the qualitative and quantitative effects that the absence of LDH-C4 has on sperm motility contribute to the severely compromised fertility of Ldhc-/- males. Nevertheless, the mating studies indicated that there is a small probability that Ldhc-/- sperm can reach and fertilize the oocyte. This presumably occurs quite soon after mating, before sperm motility declines, and suggests that the in vivo conditions are more conducive to fertilization by these sperm than the in vitro conditions.
Our results parallel clinical observations that have been made on sperm from patients. It was reported that LDHC could not be detected in spermatozoa from some men presenting at a fertility clinic [38]. These men were infertile and produced normal numbers of sperm which initially were motile and soon became poorly motile or non-motile. Thus a more complete understanding of the role of LDH-C4 will be important for diagnosing some causes of infertility and for advancing the development of new approaches to controlling fertility in men.
LDH is the terminal enzyme of glycolysis with the usual role of reducing pyruvate to lactate when oxygen is limiting. However, aerobic glycolysis is the main source of ATP in sperm of mice [22] and presumably of many other mammalian species to provide energy required for the flagellar activity that produces sperm motility. The failure of Ldhc-/- sperm to acquire hyperactivated motility and to fertilize eggs in vitro with an intact zona pellucida is quite likely a consequence of ATP deficit. However, higher levels of ATP and sperm motility were observed in Ldhc-/- sperm than in Gapdhs-/- sperm [22]. This suggests that the LDHA activity present in Ldhc-/- sperm might generate enough ATP to support motility initially, but cannot compensate sufficiently for the lack of LDH-C4 sufficiently to allow later processes required for normal fertility to occur, such as hyperactivation and capacitation.
However, when the zona pellucida was removed, Ldhc-/- sperm fertilized 29% of eggs. Because this is a lower percentage of fertilization than occurred with Ldhc+/- sperm, it implies a defect in sperm-egg fusion by Ldhc-/- sperm. These results are consistent with previous studies indicating that glucose is necessary for sperm capacitation, protein tyrosine phosphorylation and sperm-oocyte fusion [18, 39]. In addition, the Ldhc-/- sperm did not undergo the protein tyrosine phosphorylation changes characteristic of capacitation, suggesting that defects in phosphorylation also contributed to the failure to fertilize zona pellucida-intact eggs.
Our results suggest that the fertility defect in Ldhc-/- males has more than one cause. These appear to include a more rapid decline in motility than in sperm from wild type males, a defect in capacitation, as indicated by a failure in phosphorylation events that are hallmarks of this process, and a fertilization defect. All of these probably are due to compromised ATP production caused by the disruption of glycolysis. However, the underlying defects that lead to these changes remain to be determined. Because LDH catalyzes the reduction of pyruvate to lactate with a concomitant oxidation of NADH to NAD+, accumulation of pyruvate or other upstream metabolites that inhibit glycolysis and/or the effects of disruption of NADH oxidation may be involved. The effects of a lack of LDH-C4 on these parameters currently are being studied. In addition, we cannot exclude that LDH-C4 may have another essential function which remains to be determined.
Supplementary Material
Acknowledgments
We thank Dr. Philip Leder for providing TC1 embryonic stem cells, Linwood Koonce from NIEHS for excellent technical assistance, John Otstot from NIEHS for sequencing, Drs. Kiyoshi Miki and Masuo Goto for helpful suggestions, and Drs. Christopher Geyer, Michael G. O’Rand and Brigitte Le Magueresse for critically reading this manuscript.
Footnotes
This research was supported in part by NIH HD05863 (EG) and in part by the Intramural Research Program of the NIH, National Institutes of Environmental Health Sciences (EME).
Summary Sentence: Targeted disruption of the Ldhc gene results in compromised sperm function and male but not female infertility.
References
- 1.Everse J, Kaplan NO. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg E. Reproductive implications of LDH-C4 and other testis-specific isozymes. Exp Clin Immunogenet. 1985;2:120–124. [PubMed] [Google Scholar]
- 3.Wheat TE, Goldberg E. An allelic variant of the sperm-specific lactate dehydrogenase C4 (LDH-X) isozyme in humans. J Exp Zool. 1977;202:425–430. doi: 10.1002/jez.1402020312. [DOI] [PubMed] [Google Scholar]
- 4.Coonrod S, Vitale A, Duan C, Bristol-Gould S, Herr J, Goldberg E. Testis-specific lactate dehydrogenase (LDH-C4; Ldh3) in murine oocytes and preimplantation embryos. J Androl. 2006;27:502–509. doi: 10.2164/jandrol.05185. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg E. Lactate dehydrogenase-X from mouse testes and spermatozoa. Methods Enzymol. 1975;41:318–323. doi: 10.1016/s0076-6879(75)41072-2. [DOI] [PubMed] [Google Scholar]
- 6.Roller RJ, Kinloch RA, Hiraoka BY, Li SS, Wassarman PM. Gene expression during mammalian oogenesis and early embryogenesis: quantification of three messenger RNAs abundant in fully grown mouse oocytes. Development. 1989;106:251–261. doi: 10.1242/dev.106.2.251. [DOI] [PubMed] [Google Scholar]
- 7.Mita M, Hall PF. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol Reprod. 1982;26:445–455. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- 8.Grootegoed JA, Jansen R, Van der Molen HJ. The role of glucose, pyruvate and lactate in ATP production by rat spermatocytes and spermatids. Biochim Biophys Acta. 1984;767:248–256. doi: 10.1016/0005-2728(84)90194-4. [DOI] [PubMed] [Google Scholar]
- 9.Jutte NH, Jansen R, Grootegoed JA, Rommerts FF, Clausen OP, van der Molen HJ. Regulation of survival of rat pachytene spermatocytes by lactate supply from Sertoli cells. J Reprod Fertil. 1982;65:431–438. doi: 10.1530/jrf.0.0650431. [DOI] [PubMed] [Google Scholar]
- 10.Boussouar F, Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol Metab. 2004;15:345–350. doi: 10.1016/j.tem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg E, Hawtrey C. The ontogeny of sperm specific lactate dehydrogenase in mice. J Exp Zool. 1967;164:309–316. doi: 10.1002/jez.1401640302. [DOI] [PubMed] [Google Scholar]
- 12.Alcivar AA, Trasler JM, Hake LE, Salehi-Ashtiani K, Goldberg E, Hecht NB. DNA methylation and expression of the genes coding for lactate dehydrogenases A and C during rodent spermatogenesis. Biol Reprod. 1991;44:527–535. doi: 10.1095/biolreprod44.3.527. [DOI] [PubMed] [Google Scholar]
- 13.Li SS, O’Brien DA, Hou EW, Versola J, Rockett DL, Eddy EM. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol Reprod. 1989;40:173–180. doi: 10.1095/biolreprod40.1.173. [DOI] [PubMed] [Google Scholar]
- 14.Krisfalusi M, Miki K, Magyar PL, O’Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- 15.Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, Scrable H, Herr JC, Visconti PE. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73:721–729. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- 16.Beyler SA, Wheat TE, Goldberg E. Binding of antibodies against antigenic domains of murine lactate dehydrogenase-C4 to human and mouse spermatozoa. Biol Reprod. 1985;32:1201–1210. doi: 10.1095/biolreprod32.5.1201. [DOI] [PubMed] [Google Scholar]
- 17.Duan C, Goldberg E. Inhibition of lactate dehydrogenase C4 (LDH-C4) blocks capacitation of mouse sperm in vitro. Cytogenet Genome Res. 2003;103:352–359. doi: 10.1159/000076824. [DOI] [PubMed] [Google Scholar]
- 18.Travis AJ, Tutuncu L, Jorgez CJ, Ord TS, Jones BH, Kopf GS, Williams CJ. Requirements for glucose beyond sperm capacitation during in vitro fertilization in the mouse. Biol Reprod. 2004;71:139–145. doi: 10.1095/biolreprod.103.025809. [DOI] [PubMed] [Google Scholar]
- 19.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 20.Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–695. [PubMed] [Google Scholar]
- 21.Miki K. Energy metabolism and sperm function. Soc Reprod Fertil Suppl. 2007;65:309–325. [PubMed] [Google Scholar]
- 22.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O’Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg E. Amino acid composition and properties of crystalline lactate dehydrogenase X from mouse testes. J Biol Chem. 1972;247:2044–2048. [PubMed] [Google Scholar]
- 24.Allen JM. Multiple forms of lactic dehydrogenase in tissues of the mouse: their specificity, cellular localization, and response to altered physiological conditions. Ann N Y Acad Sci. 1961;94:937–951. doi: 10.1111/j.1749-6632.1961.tb35586.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg E. Lactate dehydrogenases in spermatozoa: subunit interactions in vitro. Arch Biochem Biophys. 1965;109:134–141. doi: 10.1016/0003-9861(65)90298-5. [DOI] [PubMed] [Google Scholar]
- 26.Li SS, Feldmann RJ, Okabe M, Pan YC. Molecular features and immunological properties of lactate dehydrogenase C4 isozymes from mouse and rat testes. J Biol Chem. 1983;258:7017–7028. [PubMed] [Google Scholar]
- 27.Cotton L, Gibbs GM, Sanchez-Partida LG, Morrison JR, de Kretser DM, O’Bryan MK. FGFR-1 [corrected] signaling is involved in spermiogenesis and sperm capacitation. J Cell Sci. 2006;119:75–84. doi: 10.1242/jcs.02704. [DOI] [PubMed] [Google Scholar]
- 28.Robb GW, Amann RP, Killian GJ. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil. 1978;54:103–107. doi: 10.1530/jrf.0.0540103. [DOI] [PubMed] [Google Scholar]
- 29.Lee MA, Storey BT. Bicarbonate is essential for fertilization of mouse eggs: mouse sperm require it to undergo the acrosome reaction. Biol Reprod. 1986;34:349–356. doi: 10.1095/biolreprod34.2.349. [DOI] [PubMed] [Google Scholar]
- 30.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg E. Lactate Dehydrogenases and Malate Dehydrogenases in Sperm: Studied by Polyacrylamide Gel Electrophoresis. Ann N Y Acad Sci. 1964;121:560–570. doi: 10.1111/j.1749-6632.1964.tb14226.x. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer ST. CASA--practical aspects. J Androl. 2000;21:515–524. [PubMed] [Google Scholar]
- 33.Neill JM, Olds-Clarke P. A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res. 1987;18:121–140. doi: 10.1002/mrd.1120180204. [DOI] [PubMed] [Google Scholar]
- 34.Cancel AM, Lobdell D, Mendola P, Perreault SD. Objective evaluation of hyperactivated motility in rat spermatozoa using computer-assisted sperm analysis. Hum Reprod. 2000;15:1322–1328. doi: 10.1093/humrep/15.6.1322. [DOI] [PubMed] [Google Scholar]
- 35.Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- 36.Urner F, Sakkas D. Protein phosphorylation in mammalian spermatozoa. Reproduction. 2003;125:17–26. doi: 10.1530/rep.0.1250017. [DOI] [PubMed] [Google Scholar]
- 37.Emiliozzi C, Fenichel P. Protein tyrosine phosphorylation is associated with capacitation of human sperm in vitro but is not sufficient for its completion. Biol Reprod. 1997;56:674–679. doi: 10.1095/biolreprod56.3.674. [DOI] [PubMed] [Google Scholar]
- 38.Gavella M, Cvitkovic P. Semen LDH-X deficiency and male infertility. Arch Androl. 1985;15:173–176. doi: 10.3109/01485018508986907. [DOI] [PubMed] [Google Scholar]
- 39.Urner F, Sakkas D. Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod. 1996;55:917–922. doi: 10.1095/biolreprod55.4.917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.