Abstract
Objective
As we previously reported, ADAMTS-7 and ADAMTS-12, two members of ADAMTS (adisintegrin and metalloprotease with thrombospondin motifs) family, degrade COMP in vitro and are significantly induced in the cartilage and synovium of arthritic patients. The purpose of this study was to determine 1) whether cleavage activity of ADAMTS-7 and -12 of COMP are associated with COMP degradation in osteoarthritis; 2) whether a2M is a novel substrate for ADAMTS-7 and -12; and 3) whether a2M inhibits ADAMTS-7 or -12 cleavage of COMP.
Methods
An in vitro digestion assay was used to examine the degradation of COMP by ADAMTS-7 and ADAMTS-12 in the cartilage of OA patients; in cartilage explants incubated with TNF-α or IL-1β with or without blocking antibodies; and in human chondrocytes treated with specific siRNA to knock down ADAMTS-7 or/and-12. Digestion of alpha-2-macroglobulin (a2M) by ADAMTS-7 and -12 in vitro and the inhibition of ADAMTS-7 or -12-mediated digestion of COMP by α2M were also analyzed.
Results
The molecular mass of the COMP fragments produced by either ADAMTS-7 or ADAMTS-12 were similar to those observed in OA patients. Specific blocking antibodies against ADAMTS-7 and ADAMTS-12 dramatically inhibited TNF-α- or IL-1β-induced COMP degradation in the cultured cartilage explants. The suppression of ADAMTS-7 or ADAMTS-12 expression by siRNA silencing in the human chondrocytes also prevented TNF-α- or IL-1β-induced COMP degradation. Both ADAMTS-7 and ADAMTS-12 were able to cleave α2M, giving rise to 180 and 105 kDa cleavage products, respectively. Furthermore, α2M inhibited both ADAMTS-7- and ADAMTS-12-mediated COMP degradation in a concentration (or dose)-dependent manner.
Conclusion
Our observations demonstrate the importance of COMP degradation by ADAMTS-7 and ADAMTS-12 in vivo. Furthermore, α2M is a novel substrate for ADAMTS-7 and ADAMTS-12. More significantly, α2M represents the first endogenous inhibitor of ADAMTS-7 and ADAMTS-12.
Introduction
Cartilage consists mainly of extracellular matrix (ECM) with very few cells, mostly chondrocytes. Arthritis is characterized by the breakdown of the ECM and subsequent loss of articular cartilage typically mediated by an excessive amount of active proteolytic activity[1]. The ECM is a network of proteins and macromolecules that provides both strength and nutrients for the cells. Articular cartilage is composed of 60-85% water, 15-22% type II collagen, 4-7% aggrecan and less than 5% other matrix proteins such as cartilage oligomeric matrix protein (COMP), decorin and collagens I, V, VI, IX, and XI among others. COMP, a prominent noncollagenous component of cartilage, accounts for approximately 1% of the wet weight of articular tissue [2, 3]. COMP is a 524-kDa pentameric, disulfide-bonded, multidomain glycoprotein composed of approximately equal subunits (∼110 kDa each) [4, 5]. COMP fragments have been detected in the cartilage, synovial fluid, and serum of patients with knee injuries, osteoarthritis and rheumatoid arthritis[6-8]. In previous studies to identify the physiological enzymes responsible for COMP degradation, we performed a functional genetic screen, which led to the isolation of ADAMTS-7 and -12 as COMP-binding partners [9, 10]. Subsequent studies showed that both ADAMTS-7 and ADAMTS-12 were able to digest COMP in vitro and that their levels were significantly upregulated in arthritic cartilage and synovium compared to a normal controls [6-10].
ADAMTS-7 and -12 belong to the metalloproteinase ADAMTS (adisintegrin and metalloprotease with thrombospondin motifs) family. The ADAMTS family consists of secreted zinc metalloproteinases with a precisely ordered modular organization that includes at least one thrombospondin type I repeat [11]. So far, nineteen members have been cloned in this family and some of them have known functions that have been implicated in specific diseases [12]. For instance, ADAMTS-13 mutants have a role in thrombotic thrombocytopenic purpura, a disease characterized by a decrease in the amount of circulating platelets[13]. Mutations in the ADAMTS-2 gene (procollagen I N-proteinase) cause Ehlers-Danlos syndrome Type VII C, a genetic condition characterized by defects in collagen synthesis, as well as bovine dermatopraxis[14]. A number of ADAMTS members have been implicated in the breakdown of cartilage in osteoarthritis and rheumatoid arthritis, including ADAMTS4 (aggrecanase 1), ADAMTS-5 (aggrecanase 2)[15-18], ADAMTS-7[9] and ADAMTS-12[10].
α2-Macroglobulin (α2M) is a member of the α-macroglobulin family of proteins found in the circulation of a broad range of species [19]. Human α2M is found at relatively high levels (2-4 mg/ml) in plasma and is a tetramer of four identical 185-kDa subunits, each of which has an exposed 39-amino acid “bait region” that contains cleavage sites for a variety of proteinases [20, 21]. The function of the bait region is to trap the proteinase, potentially accounting for its capacity to bind and inhibit ADAMTS-4, ADAMTS-5 and ADAMTS-10[22, 23]. ADAMTS-1 forms a stable complex with α2M that is dependent on the zinc binding catalytic domain of ADAMTS-1[24].
Inhibition of degradative enzymes can slow or block disease progression. The isolation of physiological inhibitors for the cartilage degradative enzymes is, therefore, of great interest from both a pathophysiological and a therapeutic standpoint. α2M is an inhibitor of several metalloproteases, including collagenase, stromelysis [25], ADAMTS-4 and ADAMTS-5 [23]. In addition, a2M also associates with ADAMTS-7 [26]. The purpose of the study was to examine the potential association of the ADAMTS-7 and -12-mediated cleavage of COMP with osteoarthritis damage, and the possible role of α2M as a substrate for and inhibitor of ADAMTS-7 or -12 enzyme activity against COMP.
Materials and methods
Sources of tissues
Normal adult articular cartilage were obtained from the knees of patients (mean age 56.7 years, range 43-64 years) who had died of diseases unrelated to arthritis (specimens obtained en bloc from the Musculoskeletal Transplant Foundation). The grade of osteoarthritis was determined using the Kellgren-Lawrence Grading System [27]. Normal cartilage samples were without radiographic or intra-articular evidence of arthritic disease (Kellgren-Lawrence Grade 0). Arthritic cartilages were obtained (with IRB#: 12758) from patients undergoing elective total knee arthroplasty for end-stage OA with Kellgren-Lawrence Grade of 3 or 4 from the distal femora of 8 patients (mean age 58.4 years, range 49-66 years). The samples were then stored at -80°C until analysis.
In vitro digestion assay of COMP by ADAMTS-7 and ADAMTS-12
An in vitro digestion protocol described previously [9, 10] was followed to determine whether the fragments resulted from COMP digestion by ADAMTS-7 and ADAMTS12 are the same as those seen in the cartilage of OA patients. Briefly, purified COMP was incubated with either recombinant ADAMTS-7 or ADAMTS-12 in a digestion buffer (50 mM Tris-HCl, 100mM NaCl, 5mM CaCl2, 2mM ZnCl2, pH 7.5) for 8 hours at 37°. The digested products were resolved by 8% SDS-PAGE, under reduced condition, and the gel was stained with Coomassie brilliant blue G-colloidal solution.
Cartilage explant cultures
Human cartilage was cultured as described previously[28, 29] with modifications. Briefly, human knee cartilage was dissected into pieces of diameter of approximately 4 mm by punches of 1- to 2-mm thickness. The cartilage was dispensed into tissue-culture flasks (0.7 g/flask) and incubated overnight in control, serum-free medium Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) containing 25 mM HEPES, 2 mM glutamine, 100 μg/ml streptomycin, 100 IU/ml penicillin, 2.5 μg/ml gentamicin, and 40 units/ml nystatin. Fresh control medium (10 ml) with TNF-α (5 ng/ml) or IL-1β (5 ng/ml) (in triplicate for statistical analysis) was then added (day 0). At day 2, the supernatants were harvested for COMP degradation analysis by Western blotting and the cartilage samples were extracted for RNA, as described below. In some cultures, antibodies against ADAMTS-7 or/and ADAMTS-12 (5μg/ml of anti-ADAMTS-7 or/and ADAMTS-12 rabbit polyclonal antibodies was added [9, 10]. At day 7, culture supernatants were harvested and COMP degradative fragments in the culture supernatants were determined using Western blotting assay.
COMP degradation analyses in the cartilage of OA patients
Extracts of normal and OA cartilages and supernatants from cultured cartilage explants were analyzed by Western Blotting as previously described[9, 10]. Briefly, the samples were loaded on 8% gels and separated by SDS-PAGE under reducing conditions. Separated proteins were transferred to polyvinylidene difluoride membranes and probed with a 1:2500 dilution of rabbit polyclonal anti-COMP antiserum [6, 9, 30, 31]. Subsequently, membranes were incubated with a 1:20000 dilution of goat anti-rabbit IgG horseradish peroxidase conjugate as the secondary antibody, and the signal detected using the ECL chemiluminescent system (Amersham Pharmacia Biotech, Upsala, Sweden).
Analysis of ADAMTS-7 and ADAMTS-12, mRNA in cultured cartilage explants
Total RNA was extracted as described previously[9, 10] and real-time PCR was performed using a sequence-specific probe and primers for ADAMTS-7 (fluorescence-labeled oligonucleotide probes [using 6-carboxy-fluorescein (FAM)] probe: 5′-AAGCGCTTCCGCCTCTGCAACC-3′ ; primers: 5′-CAGCCTACGCCCAAATACAAA-3′ and 5′-CCCTTGTAGAGCATAGCGTCAAA-3′) and ADAMTS-12 (fluorescence-labeled oligonucleotide probes [using 6-carboxy-fluorescein (FAM)] probe: 5′-AGGACATCTGTGCTGGTTTCAATCGCC-3′; primers: 5′-CACGACGTGGCTGTCCTTCT-3′ and 5′-CCGAATCTTCATTGATGTTACAACTG-3′). The PCR products obtained was confirmed by direct sequencing of the amplicons. A standard curve with copy numbers ranging from 103 to 109 was produced using human cartilage cDNA as the template. An XY scatter plot was produced using Microsoft Excel software, and the equation y = mx + b (where m = the slope of the standard curve and b = the y intercept of that line) was calculated and R2 values obtained. As an internal control, 18s rRNA was analyzed in parallel by using the Endogenous Control Human rRNA kit (Applied Biosystems, Foster City, CA).
PCR reactions for all samples were performed in duplicate in 96-well optical plates with 5 ng of cDNA (1 ng of cDNA for the 18S rRNA), 100 nM probe, 200 nM each primer, and 10.0 μl of TaqMan Universal 2× PCR Master Mix (PE-Applied Biosystems, St. Louis, MO) in a 20-μl reaction volume. The amplification reaction was carried out over 40 cycles (an initial holding stage of 2 min at 50°C and then 10 min at 95°C, followed by a two-step cycling program of 15 s at 95°C and 1 min at 60°C).
Knock-down of ADAMTS-7 and -12 by specific siRNA
The human chondrocyte cell line, C-28/12, was used as a model for analyzing the efficiency of knockdown by the siRNAs and for determining the consequences of knockdown of ADAMTS-7 and 12 on COMP degradation. The cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). Two regions of human ADAMTS-7 or/and ADAMTS-12 were targeted for small interfering RNA (siRNA) using mammalian expression pSUPER vector (OligoEngine, Seattle, WA) according to the manufacturer’s instructions. To generate each siRNA, equimolar amounts of complementary sense and antisense strands were mixed and annealed slowly by cooling to 10°C in a 50-μL reaction buffer (100 mM NaCl and 50 mM HEPES pH 7.4). The annealed oligos were inserted into the BglII/HindIII sites of pSUPER vector. The resulting plasmids and control vector pSUPER were co-transfected with the corresponding expression plasmid into C28I2 cells using LipofectAMINE 2000 reagent (Invitrogen, Rockville, MD) and the levels of ADAMTS-7 or/and ADAMTS-12 was monitored using immumofluorescence cell staining as deccribed below. The data demonstrated that the siRNA 5′-ACCTAAAGATCACGCACCA-3′ and the siRNA 5′-ACACATCACACACACCCAA-3′ were able to efficiently reduce the expression of human ADAMTS-7 and ADAMTS-12, respectively. The C-28/12 cells were then transfected with the siRNA described above (i.e. ADAMTS-7 siRNA (siTS7), ADAMTS-12 siRNA (siTS7), both (siTS7 +siTS12) or pSUPER control (CTR)) and cultured in the presence of TNF-α (5 ng/ml) or IL-1β (5 ng/ml). After incubation for 7 days, the media were collected and assayed by Western blotting with anti-COMP antibody.
Immumofluorescence cell staining
Briefly, cultures plated on chamber slides (Nalge Nunc International, Naperville, IL) were fixed with cold 100% methanol and air-dried. After rehydration in PBS and blocking with 30% goat serum for 30 min, the cells were incubated with primary antibodies against ADAMTS-7 (Santa Cruz; diluted 1:100) or ADAMTS-12 (diluted 1:100) for 1 hr. Secondary antibodies against rabbit IgG conjugated with FITC (Santa Cruz; diluted 1:100) were applied for 45 min, followed by an incubation with 0.5 mg of 49,69-diamidino-2-phenylindole dihydrochloride (DAPI) for 5 min. The specimens were observed under a fluorescence microscope with appropriate optical filters. Microscopic images were captured using the Image program (Media Cybernetics, Silver Spring, MD) and an Olympus microscope.
Digestion assay of α2M by ADAMTS-7 and -12 in vitro
To determine whether ADAMTS7 and ADAMTS12 cleave α2M, increasing amounts of recombinant ADAMTS-7 and ADAMTS-12 [9, 10] were incubated overnight at 37 °C with 140 nM or200 nM human α 2M (Sigma-Aldrich, St. Louis) in 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 10 mM CaCl2. Subsequently, reaction products were analyzed by 8% SDS-PAGE under non-reducing conditions and the gel was stained with Coomassie Brilliant Blue R-250.
Inhibition assays of α2M on ADAMTS-7 or -12 digestion of COMP
To test the ability of α2M to inhibit ADAMTS-7 and -12 cleavage of COMP, recombinant ADAMTS-7 or ADAMTS-12 was pre-incubated with various concentrations of α2M for 2 hours at 37°C. Then purified COMP was added into the above mixture in the digestion buffer (50 mM Tris-HCl, 100mM NaCl, 5mM CaCl2, 2mM ZnCl2, pH 7.5) for 2 more hours at 37°C. The digested products were resolved by 8% nonreduced SDS-PAGE gel, and the gel was either stained with Coomassie brilliant blue G-colloidal solution or detected using Western blotting with anti-COMP antibody [9, 10, 32].
Statistical test
Two-sample Student’s t-test was used to determine significant differences (p < 0.05) of the levels of ADAMTS-7 and ADAMTS-12 between control and TNF-α- or IL-1β-treated cartilage explants.
RESULTS
The sizes of the COMP fragments produced by ADAMTS-7 or -12 enzyme activity are similar to those in cartilage from OA patients
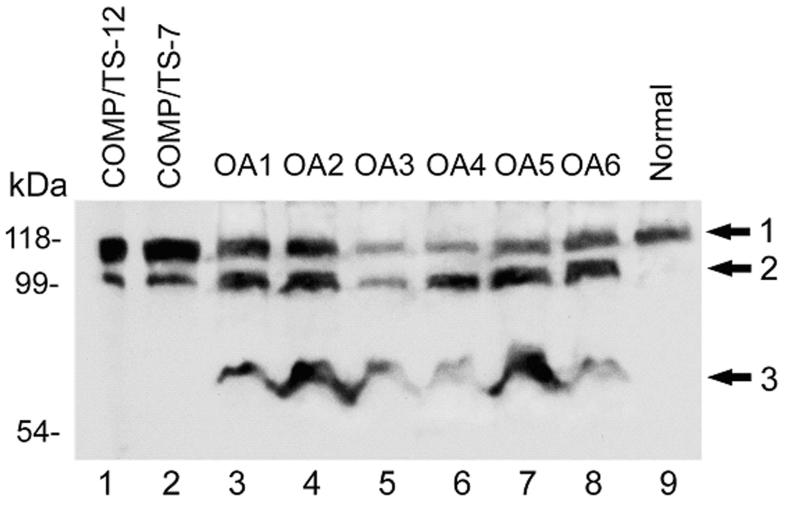
To elucidate the importance of ADAMTS-7- or ADAMTS-12-mediated COMP degradation in vivo, we determined whether OA cartilage contained the same fragment as we saw in ADAMTS-7- or ADAMTS-12-mediated COMP digestion in vitro. For this purpose, we analyzed the cartilage from 6 OA patients and COMP fragments produced by in vitro COMP digestion with the recombinant ADAMTS-7 or ADAMTS-12 using Western blotting with anti-COMP antibodies (Fig. 1). An approximately 110-kDa fragment (arrow 2) that was produced by digestion with ADAMTS-12 (lane 1) and ADAMTS-7 (lane 2) was an abundant component of all OA cartilage samples (lane 3 to 8); intact COMP monomer was also detected (arrow 1). Interestingly, an additional fragment (arrow 3) was observed in OA samples that was absent in the in vitro COMP digestion assay with ADAMTS-12- and ADAMTS-7,-, suggesting that additional enzyme(s) may contribute to COMP degradation in OA patients. Note that only intact COMP was detected in the normal cartilage (lane 9).
Fig. 1. Western blotting analysis of human OA cartilage samples and ADAMTS-7 (TS-7)- and ADAMTS-12 (TS-12)-mediated COMP digestion.
Samples were resolved on 8% SDS-PAGE gels, under reducing conditions, and COMP was detected using an anti-COMP antiserum. Intact COMP monomer, 110-kDa fragment and additional fragment in OA cartilage are indicated with arrow 1, 2, 3, respectively.
Induced expression of ADAMTS-7 and ADAMTS-12 by TNF-α and IL-1β
We next investigated whether TNF-α and IL-1β, two major inflammatory cytokines that induce the expression of a number of metalloproteinases involved in the development and progression of arthritic diseases [33-35], could regulate the expression of ADAMTS-7 and ADAMTS-12. Human cartilage explants were cultured in the absence or presence of either 5 ng/ml of TNF-α or 5ng/ml of IL-1β for 1 day in serum-free medium and real-time PCR was performed (see Fig. 2A). Both TNF-α and IL-1β induced the mRNA expression of ADAMTS-7 and ADAMTS-12 compared to untreated cartilage explants.
Fig. 2. ADAMTS-12 and ADAMTS-7 blocking antibodies inhibit TNF-α- or IL-1β-induced COMP degradation.
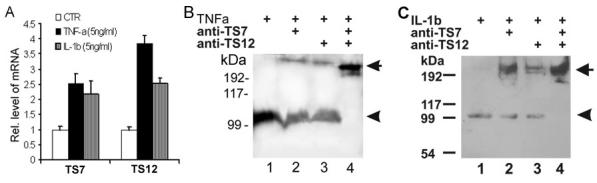
(A) Upregulation of ADAMTS-7 and ADAMTS-12 by TNF-α and IL-1β. The units are arbitrary and the leftmost bar in each group indicates a relative level of 1. * p < .05 vs. untreated controls. (B) & (C) Antibody blocking assays. OA cartilage explants were cultured in the presence of 5 ng/ml of TNF-α (B) or 5 ng/ml of IL-1β (C) with blocking antibodies, as indicated, for 7 days. The media were separated on non-reduced SDS-PAGE gels and COMP was detected using an anti-COMP antibody. Intact COMP and its degradative fragment are indicated with arrow and arrowhead respectively.
Antibodies against ADAMTS-7 and ADAMTS-12 antibody dramatically inhibits TNF-α- or IL-1β-induced COMP degradation
Since both TNF-α and IL-1β upregulate ADAMTS-7 and ADAMTS-12, two enzymes known to degrade COMP, we next determined whether these enzymes could account specifically for the COMP degradation induced by TNF-α or IL-1β in the cartilage organ culture system. Since TNF-α and IL-1β are known to induce the expression of various metalloproteinases, including ADAMTS-4 [34, 36], COMP degradation might be due to other enzymes alone or in combination with ADAMTS-7/-12 rather than to ADAMTS-7/-12 alone. To determine whether ADAMTS-7 or/and ADAMTS-12 is directly involved in the COMP degradation induced by these two proinflammatory cytokines, we compared COMP degradation in the absence or presence of ADAMTS-12 and/or ADAMTS-7 blocking antibody [9] (Figs. 2B and 2C). TNF-α and IL-1β treatments resulted in a 110-kDa COMP fragment (lane 1, indicated by the arrowhead). This fragment was reduced in the presence of either ADAMTS-12 (lane 2) or ADAMTS-7 (lane 3) blocking antibody; in addition, COMP-degradation was totally blocked by a combination of these two antibodies and intact COMP was observed (lane 4, indicated by the arrow), clearly indicating that ADAMTS-12 and ADAMTS-7 are important in TNF-α- and IL-1β-induced COMP degradation. Note that control antibody did not show any blocking activity (not shown).
Inhibition of ADAMTS-7 or/and ADAMTS-12 expression via siRNA-mediated silencing prevents COMP degradation in human chondrocytes
To further verify the importance of ADAMTS-7 and ADAMTS-12 in degrading COMP in vivo, we first suppressed ADAMTS-7 or/and ADAMTS-12 gene expression in human chondrocytes using the siRNA approach. We identified 19-nucleotide gene-specific sequences for ADAMTS-7 and ADAMTS-12, respectively, and then generated pSUPER-siTS-7 and pSUPER-siTS-12 constructs encoding siRNAs targeting the specific gene sequences. Immunofluorescent cell staining with human C-28/I2 chondrocytes transfected with either pSUPER-siTS-7, pSUPER-siTS-12 or pSUPER vector demonstrated that expression of the specific siRNAs efficiently reduced the levels of the corresponding proteins (Fig. 3A). Next we examined whether the siRNA knockdown of ADAMTS-7 or/and ADAMTS-12 would affect COMP degradation. The C-28/I2 chondrocytes were transfected with either pSUPER-siTS-7, pSUPER-siTS-12 or both and cultured with serum-free medium containing TNF-α or IL-1β for one week. Western blotting with anti-COMP antibody (Figs. 3B and 3C) showed a robust COMP degradative fragment in the medium from TNF-α- or IL-1β-treated cultures (lane 1). However, the intensity of the COMP fragment was reduced in the media collected from the cells transfected with pSUPER-siTS-7 or pSUPER-siTS-12 (lanes 2 and 3). Especially, the degradative fragment was barely detectable when the cells were co-transfected with pSUPER-siTS-7 and pSUPER-siTS-12 (lane 4). Collectively, these results further indicated that both ADAMTS-7 and ADAMTS-12 were critical for the TNF-a- or IL-1β-induced COMP degradation.
Fig. 3. Reduced expression of ADAMTS7 or/and ADAMTS-12 by siRNA silencing inhibits the degradation of COMP in human chondrocytes.
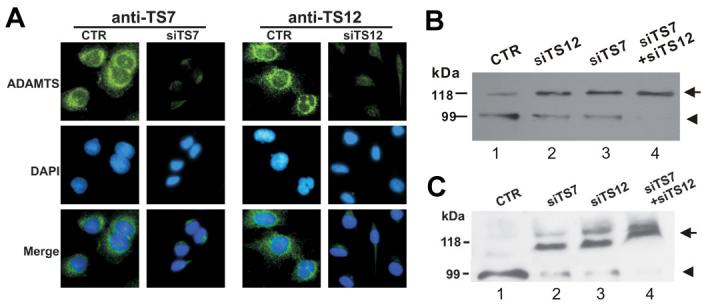
(A) siRNAs against ADAMTS-7 and ADAMTS-12 efficiently suppress the expression of their target molecules, assayed by immumofluorescence cell staining. Immortalized human chondrocytes, C-28/I2, transfected with either pSUPER plasmid (CTR), pSUPER-ADAMTS-7 siRNA (siTS7) or pSUPER-ADAMTS-12 siRNA (siTS12) were stained with either anti-ADAMTS-7 (left panel) or anti-ADAMTS-12 (right panel). The nuclei were stained with DAPI and the overlapping of these two signals is shown as “merge”. (B) & (C) Knockdown of either ADAMTS-7 or/and ADAMTS-12 dramatically inhibits COMP degradation. The C-28/12 cells were transfected with either ADAMTS-7 siRNA (siTS7), ADAMTS-12 siRNA (siTS7), both (siTS7 +siTS12) or pSUPER control (CTR), were cultured in the presence of 5 ng/ml of TNF- α (B) or 5 ng/ml of Il-1β (C) for 7 days. The media were separated on reduced SDS-PAGE gels and COMP was detected using an anti-COMP antibody. Intact COMP and its degradative fragment are indicated with arrow and arrowhead, respectively.
α 2M is a novel substrate for ADAMTS-7 and ADAMTS-12
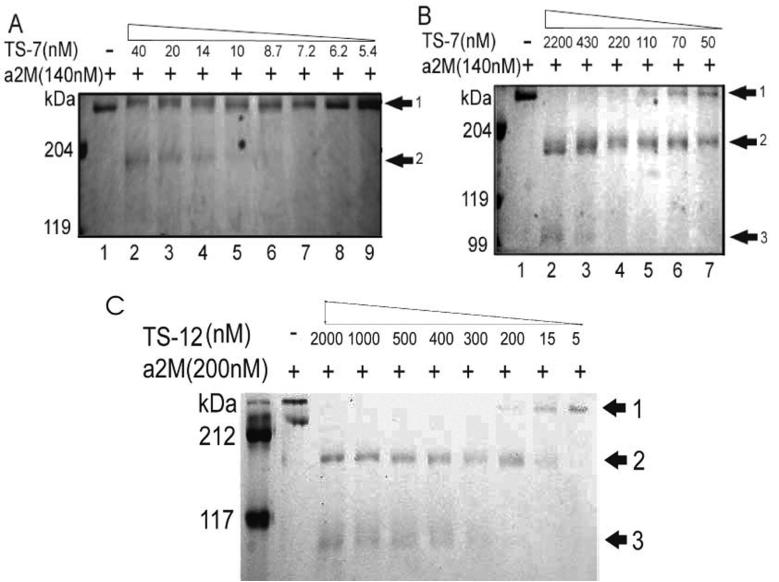
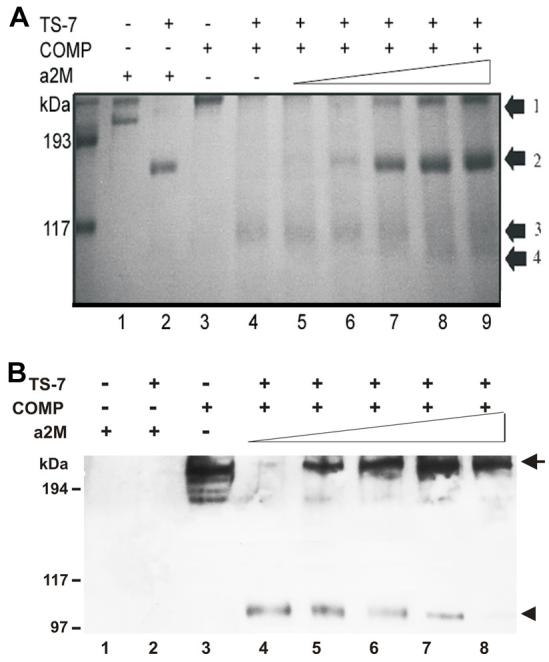
Since a2M associates with ADAMTS-7 [26], and α 2M inhibits ADAMTS-4 and ADAMTS-5 by competitive inhibition upon cleavage activity by the bait region of ADAMTS-4/-5 [23], we investigated whether α 2M is a also a substrate for ADAMTS-7- and ADAMTS-12. We first examined the digestion of α2M by ADAMTS-7 by incubation of 140nM of α2M with various concentrations of purified recombinant ADAMTS-7 and resolved the digests on Coomassie blue-stained SDS-PAGE. Intact α 2M in its tetramer form was detected at a molecular mass of ∼700 kDa (Fig. 4A, lane 1). One major α 2M cleavage product with the apparent molecular weight of approximately 180 kDa was observed when 10nM of ADAMTS-7 was applied and the intensity of this band strengthened gradually with increasing concentrations of ADAMTS-7 (Fig. 4A); A faint degradative fragment with the molecular weight of 105 kDa was observed using ADAMTS-7 at 430 nM or higher (Fig. 4B). A similar cleavage pattern of α2M was observed using recombinant ADAMTS-12(Fig. 4C).
Fig. 4. Cleavage of α 2M by ADAMTS-7 and ADAMTS-12.
In vitro digestion assay of α 2M by lower (A) or higher (B) amount of ADAMTS-7. 0.14 uM a2M was incubated in the absence or presence of various amount of ADAMTS-7, as indicated, for 2 h at 37 °C. The products were then separated by 8% non-reduced SDS-PAGE and visualized by Coomassie blue staining. (C) Digestion of α 2M by ADAMTS-12. 0.10 μM α 2M was incubated in the absence or presence of various amount of ADAMTS-12, as indicated, for 2 h at 37 °C. The products were then separated by 6% non-reduced SDS-PAGE and visualized by Coomassie blue staining. Arrow 1, 2, 3 indicate the intact α 2M, ∼180 kDa and 105 kDa resulted fragments, respectively.
α 2M inhibits ADAMTS-7- and ADAMTS-12-mediated COMP degradation
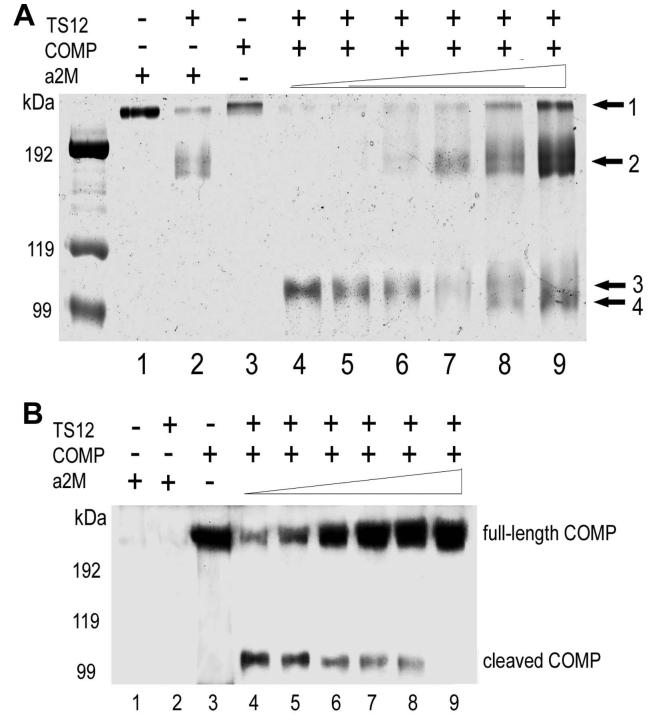
Since α2M can be digested by ADAMTS-4 and ADAMTS-5 and inhibits the cleavage of aggrecan by these enzymes [23], we next examined whether α 2M, as a substrate of ADAMTS-7 and ADAMTS-12, also acts as an competitive inhibitor of the degradation of COMP. ADAMTS-7 or ADAMTS-12 at a concentration of 330 nM were preincubated with various amounts of α 2M for 2 h at 37 °C. After the preincubation COMP was added to a final concentration of 170 nM, and then the reactions were carried out for another 2h at 37 °C. The products were first analyzed on a non-reduced SDS-PAGE gel and visualized by Coomassie blue staining (Fig. 5A and Fig. 6A). Accompanying the increase of α 2M, the intensities of the180 kDa (arrow 2) and 105 kDa (arrow 4) fragments of α 2M became stronger, whereas the 110 kDa COMP degradative fragment became weaker and finally not visible (arrow 3) and the intact COMP (arrow 1) band appeared (Fig. 6A and Fig. 7A). Since both intact α2M (700 kDa) and COMP (550 kDa) were retained at the very top of the gel, we next performed Western blotting with anti-COMP antibodies to determine whether the top band (arrow 1) was COMP rather than α 2M (Fig. 5B and Fig. 6B). Western blotting with anti-COMP antibodies (these do not cross-react with α 2M and its digested products (lane 1 and 2 of Fig. 5B and Fig. 6B)) clearly demonstrated that α2M efficiently protects COMP from degradation by either ADAMTS-7 or ADAMTS-12.
Fig. 5. α2M inhibits ADAMTS-7-mediated COMP degradation in a dose-dependent manner, assayed by Coomassie blue staining (A) and Western Blotting (B).
0.33 μM ADAMTS-7 was first incubated with increasing concentrations of α 2M, as indicated, for 2 h at 37 °C, then 0.17 μM COMP was added and allowed to incubate for additional 2 h at 37 °C. The resulting products were analyzed by 8% non-reduced SDS-PAGE and visualized by either Coomassie blue staining (A) or Western blotting with anti-COMP antibody (B). Arrows 1, 2, 3 and 4 in (A) indicate the intact COMP, the 180-kDa fragment of α 2M, the 110-kDa fragment of COMP and the 105-kDa fragment of α2M, respectively. In panel (B), arrow and arrowhead indicate intact COMP and its degradative fragment, respectively. Lanes 1 and 2 indicate that anti-COMP antibody does not cross-recognize either intact a2M nor its 180 kD degradative fragment. Lanes 4-8 indicate that intact COMP was protected from ADAMTS-7 cleavage by α 2M in a dose-dependent manner.
Fig. 6. α2M inhibits ADAMTS-12-mediated COMP degradation in a does-dependent manner, assayed by Coomassie blue staining (A) and Western Blotting (B).
0.20 uM ADAMTS-12 was first incubated with increasing concentrations of α 2M, as indicated, for 2 h at 37 °C, then 0.10 uM COMP was added and allowed to incubate for an additional 2 h at 37 °C. The resultant products were analyzed by 8% non-reduced SDS-PAGE and visualized by either Coomassie blue staining (A) or Western blotting with anti-COMP antibody (B). Arrows 1, 2, 3 and 4 in panel A indicate the intact COMP, the 180-kDa fragment of α 2M, the 110-kDa fragment of COMP and the 105-kDa fragment of α2M, respectively. In panel B, the intact COMP and its 110-kDa degradative fragment are indicated. Lanes 1 and 2 indicate that anti-COMP antibody does not cross-react with either intact α2M or its 180 kD degradative fragment. Lanes 4-9 indicate that intact COMP was protected from ADAMTS-7 cleavage by α2M in a dose-dependent manner.
DISCUSSION
ADAMTS family proteins have been implicated in the pathogenesis of different diseases, including arthritis [37-43]. We previously reported that ADAMTS-7 and ADAMTS-12, two members in this family sharing similar domain organization and structure, associated with and cleaved COMP in the in vitro digestion system, and their levels were significantly elevated in the cartilage and synovium of patients with arthritis [9, 10]. The present study provides insight into the importance of ADAMTS-7 and ADAMTS-12 in the degradation of COMP in the course of arthritis, since the size of the COMP fragment produced by ADAMTS-7 or -12 is similar to that of COMP-degradative fragments seen in OA patients (Fig. 1). Antibody blocking assays with cartilage explants have been widely used [44-46]. Using this model we found that anti-ADAMTS-7 and/or anti-ADAMTS-12 antibody dramatically inhibited COMP degradation induced by TNF-α and IL-1β, two key cytokines in the progression of arthritis that induce the expression of ADAMTS-7 and ADAMTS-12 (Figs. 2, 3). In addition, both anti-ADAMTS-7 or anti-ADAMTS-12 antibody did not inhibit ADAMTS-4-mediated COMP digestion in an in vitro assay (not shown). These data indicated that ADAMTS-7 and ADAMTS-12 are important for the cytokine-induced COMP degradation. The present study also presents evidence that α2M is a novel substrate for ADAMTS-7 and ADAMTS-12 (Figs. 4, 5) and acts as an inhibitor of COMP degradation mediated by these two enzymes (Figs. 6, 7).
Nineteen distinct human ADAMTS gene products have been cloned and can be divided into five subgroups based on their known functions [12]. The first of the divisions, consisting of ADAMTS-1, -4, -5, -8, -9, -15 and -20, cleave aggrecan [12]. ADAMTS-1 also cleaves the related hyalectan versican at analogous sites and ADAMTS-4 has been demonstrated to cleave brevican [47]. Among them, ADAMTS-4 and -5 have been best characterized and implicated in aggrecan degradation in osteoarthritis [16]. ADAMTS-5 is probably the major aggrecanase responsible for aggrecan degradation in vivo [41, 48]. ADAMTS-4 has been also shown to cleave COMP as well as fibromodulin and decorin [49-51]. A second subgroup contains ADAMTS-2, -3 and -14. ADAMTS-2 cleaves the amino peptides of type I, type II and type III procollagens [52, 53], ADAMTS-3 has since been identified as a type II procollagen N-propeptidase, whose expression is about 5-fold that of ADAMTS-2 in cartilage [54]. ADAMTS-14 has been identified as a homologue of ADAMTS-2, functioning as the major type I procollagen N-propeptidase activity in tendon [55]. ADAMTS-13, a von Willebrand factor-cleaving protease, stands as one subgroup [56, 57]. ADAMTS-7 and ADAMTS-12 that specifically associate with and degrade COMP represent another subgroup [9, 10, 26, 58]. And the remaining ADAMTS members form a loosely defined subgroup with unknown functions[12].
In addition to ADAMTS-7 and -12, ADAMTS-4 and several members of the family of matrix metalloproteinases (MMPs), including interstitial collagenase (MMP-1), collagenase-3 (MMP-13), stromelysin-1 (MMP-3), gelatinase-B (MMP-9), MMP-19, and enamelysin (MMP-20) also digest purified COMP in vitro. Here we also show that, an additional fragment (Fig. 1, small arrowhead) was observed in OA samples that was absent in ADAMTS-12- and ADAMTS-7-mediated COMP digestion, suggesting that additional enzyme(s) may contribute to COMP degradation in OA patients. In addition to COMP, a recent report revealed that aggrecan was also a substrate of ADAMTS-12[59], suggesting that competitive substrate might lead to the enzyme inhibition. In addition, several reports suggest that COMP may function to stabilize the articular cartilage extracellular matrix by specific cation-dependent interactions with matrix components, including collagen types II and IX, fibronectin, aggrecan, and matrilin-1, -3, and -4 [32, 60-63]. Thus, it is conceivable that the inhibition of COMP degradation may stabilize the cartilage matrix and in turn affect the degradation of the main macromolecules, including collagens and aggrecan.
Full length α2M is approximately 185 kD and forms a tetramer. It may be cleaved by ADAMTS-4 and ADAMTS-5, two major aggrecanases, and can inhibit their activities [23]. In this study we demonstrated that α2M is also a substrate for ADAMTS-7 and ADAMTS-12, two known COMP-associating and -degrading metalloproteinases (Figs. 4 and 5) and prevents the degradation of COMP by these enzymes in a does-dependent manner (Figs. 6 and 7). Degradation of a2M by ADAMTS-7 and ADAMTS-12 gives rise to two fragments, one major 180 kD and one minor 105 kDa fragment, suggesting that a2M may contain two cleavage sites of ADAMTS-7 and ADAMTS-12 (Figs. 4 and 5). Tortorella et al. have shown that ADAMTS-4 and ADAMTS-5 digested a2M at one major sensitive site and at additional insensitive sites with much less efficiency [23].
Evidence showing the importance of ADAMTS-7 and ADAMTS-12 for the in vivo degradation of COMP, identification of α2M as a novel substrate for ADAMTS-7 and ADAMTS-12, and subsequent characterization of its inhibitory activities on the degradation of COMP by ADAMTS-7 and ADAMTS-12 significantly extends our understanding of the degradative events that occur in joint disorders. These findings will also contribute to our ability to monitor the biological and physical properties of cartilage extracellular matrix, and provide us with promising therapeutic targets, including α2M or its derivatives, for treating or preventing extracellular matrix degeneration.
Acknowledgments
The authors thank the Musculoskeletal Transplant Foundation for providing human tissues. This work was aided by research grants NIH AR052022 (C. Liu), NIH AG029388 (C. Liu), NIH AR053210 (C. Liu) and NIH AR050620 (C. Liu), and New York Chapter of the Arthritis Foundation Dorothy W. Goldstein Young Scholar Award (C. Liu).
Abbreviations used
- COMP
cartilage oligomeric matrix protein
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- MMP
matrix metalloproteinases
- PCR
polymerase chain reaction
- TSP
thrombospondin
- TNF-α
tumor necrosis factor-alpha
- IL-1β
interleukin-1-beta
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- OA
osteoarthritis
- RA
rheumatoid arthritis
- α2M
α2-Macroglobulin
REFERENCES
- 1.Salzet M. Leech thrombin inhibitors. Curr Pharm Des. 2002;8(7):493–503. doi: 10.2174/1381612023395664. [DOI] [PubMed] [Google Scholar]
- 2.DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354(2):237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 3.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6136. [PubMed] [Google Scholar]
- 4.Morgelin M, Engel J, Heinegard D, Paulsson M. Proteoglycans from the swarm rat chondrosarcoma. Structure of the aggregates extracted with associative and dissociative solvents as revealed by electron microscopy. J Biol Chem. 1992;267(20):14275–14284. [PubMed] [Google Scholar]
- 5.Oldberg A, Antonsson P, Lindblom K, Heinegard D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267(31):22346–22350. [PubMed] [Google Scholar]
- 6.Di Cesare PE, Carlson CS, Stolerman ES, Hauser N, Tulli H, et al. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J Orthop Res. 1996;14:946–955. doi: 10.1002/jor.1100140615. H A-T. [DOI] [PubMed] [Google Scholar]
- 7.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 8.Saxne T, Heinegard D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 9.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20(7):988–990. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CJ, Kong W, Xu K, Luan Y, Ilalov K, Sehgal B, et al. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J Biol Chem. 2006;281(23):15800–15808. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36(6):981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 14.Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, et al. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65(2):308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274(33):23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- 16.Malfait AM, Liu RQ, Ijiri K, Komiya S, Tortorella MD. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J Biol Chem. 2002;277(25):22201–22208. doi: 10.1074/jbc.M200431200. [DOI] [PubMed] [Google Scholar]
- 17.Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, et al. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276(16):13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- 18.Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, et al. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284(5420):1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- 19.Sottrup-Jensen L. Alpha-macroglobulins: structure, shape, and mechanism of proteinase complex formation. J Biol Chem. 1989;264(20):11539–11542. [PubMed] [Google Scholar]
- 20.Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. Faseb J. 1992;6(15):3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 21.Feinman RD. The proteinase-binding reaction of alpha 2M. Ann N Y Acad Sci. 1994;737:245–266. doi: 10.1111/j.1749-6632.1994.tb44316.x. [DOI] [PubMed] [Google Scholar]
- 22.Barrett AJ, Starkey PM. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, et al. Alpha2-macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279(17):17554–17561. doi: 10.1074/jbc.M313041200. [DOI] [PubMed] [Google Scholar]
- 24.Kuno K, Terashima Y, Matsushima K. ADAMTS-1 is an active metalloproteinase associated with the extracellular matrix. J Biol Chem. 1999;274(26):18821–18826. doi: 10.1074/jbc.274.26.18821. [DOI] [PubMed] [Google Scholar]
- 25.Enghild JJ, Salvesen G, Brew K, Nagase H. Interaction of human rheumatoid synovial collagenase (matrix metalloproteinase 1) and stromelysin (matrix metalloproteinase 3) with human alpha 2-macroglobulin and chicken ovostatin. Binding kinetics and identification of matrix metalloproteinase cleavage sites. J Biol Chem. 1989;264(15):8779–8785. [PubMed] [Google Scholar]
- 26.Somerville RP, Longpre JM, Apel ED, Lewis RM, Wang LW, Sanes JR, et al. ADAMTS7B, the full-length product of the ADAMTS7 gene, is a chondroitin sulfate proteoglycan containing a mucin domain. J Biol Chem. 2004;279(34):35159–35175. doi: 10.1074/jbc.M402380200. [DOI] [PubMed] [Google Scholar]
- 27.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont J, Ionescu M, Reiner A, Poole AR, Tran-Khanh N, Hoemann CD, et al. Mature full-thickness articular cartilage explants attached to bone are physiologically stable over long-term culture in serum-free media. Connect Tissue Res. 1999;40(4):259–272. doi: 10.3109/03008209909000704. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276(44):41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 30.Di Cesare PE, Fang C, Leslie MP, Della Valle CJ, Gold JM, Tulli H, et al. Localization and expression of cartilage oligomeric matrix protein by human rheumatoid and osteoarthritic synovium and cartilage. J Orthop Res. 1999;17:437–445. doi: 10.1002/jor.1100170321. [DOI] [PubMed] [Google Scholar]
- 31.Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. Expression of cartilage oligomeric matrix protein (COMP) by embryonic and adult osteoblasts. J Orthop Res. 2000;18(5):713–720. doi: 10.1002/jor.1100180506. [DOI] [PubMed] [Google Scholar]
- 32.Di Cesare PE, Chen FS, Moergelin M, Carlson CS, Leslie MP, Perris R, et al. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002;21(5):461–470. doi: 10.1016/s0945-053x(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 33.Bevitt DJ, Mohamed J, Catterall JB, Li Z, Arris CE, Hiscott P, et al. Expression of ADAMTS metalloproteinases in the retinal pigment epithelium derived cell line ARPE-19: transcriptional regulation by TNFalpha. Biochim Biophys Acta. 2003;1626(13):83–91. doi: 10.1016/s0167-4781(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 34.Cross AK, Haddock G, Stock CJ, Allan S, Surr J, Bunning RA, et al. ADAMTS-1 and -4 are up-regulated following transient middle cerebral artery occlusion in the rat and their expression is modulated by TNF in cultured astrocytes. Brain Res. 2006;1088(1):19–30. doi: 10.1016/j.brainres.2006.02.136. [DOI] [PubMed] [Google Scholar]
- 35.Voros G, Maquoi E, Collen D, Lijnen HR. Differential expression of plasminogen activator inhibitor-1, tumor necrosis factor-alpha, TNF-alpha converting enzyme and ADAMTS family members in murine fat territories. Biochim Biophys Acta. 2003;1625(1):36–42. doi: 10.1016/s0167-4781(02)00589-4. [DOI] [PubMed] [Google Scholar]
- 36.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21(2):256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 37.Bayliss MT, Hutton S, Hayward J, Maciewicz RA. Distribution of aggrecanase (ADAMts 4/5) cleavage products in normal and osteoarthritic human articular cartilage: the influence of age, topography and zone of tissue. Osteoarthritis Cartilage. 2001;9(6):553–560. doi: 10.1053/joca.2001.0425. [DOI] [PubMed] [Google Scholar]
- 38.Behera AK, Hildebrand E, Szafranski J, Hung HH, Grodzinsky AJ, Lafyatis R, et al. Role of aggrecanase 1 in Lyme arthritis. Arthritis Rheum. 2006;54(10):3319–3329. doi: 10.1002/art.22128. [DOI] [PubMed] [Google Scholar]
- 39.Collins-Racie LA, Flannery CR, Zeng W, Corcoran C, Annis-Freeman B, Agostino MJ, Arai M, et al. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23(4):219–230. doi: 10.1016/j.matbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Demircan K, Hirohata S, Nishida K, Hatipoglu OF, Oohashi T, Yonezawa T, et al. ADAMTS-9 is synergistically induced by interleukin-1beta and tumor necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum. 2005;52(5):1451–1460. doi: 10.1002/art.21010. [DOI] [PubMed] [Google Scholar]
- 41.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 43.Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, et al. Expression and activity of ADAMTS-5 in synovium. Eur J Biochem. 2001;268(5):1259–1268. doi: 10.1046/j.1432-1327.2001.01990.x. [DOI] [PubMed] [Google Scholar]
- 44.Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004;50(2):516–525. doi: 10.1002/art.20004. [DOI] [PubMed] [Google Scholar]
- 45.Kakinuma T, Yasuda T, Nakagawa T, Hiramitsu T, Akiyoshi M, Akagi M, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates matrix metalloproteinase 3 synthesis enhanced by oxidized low-density lipoprotein in rheumatoid arthritis cartilage. Arthritis Rheum. 2004;50(11):3495–3503. doi: 10.1002/art.20581. [DOI] [PubMed] [Google Scholar]
- 46.van de Lest CH, van den Hoogen BM, van Weeren PR. Loading-induced changes in synovial fluid affect cartilage metabolism. Biorheology. 2000;37(12):45–55. [PubMed] [Google Scholar]
- 47.Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, et al. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275(49):38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- 48.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 49.Dickinson SC, Vankemmelbeke MN, Buttle DJ, Rosenberg K, Heinegard D, Hollander AP. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003;22(3):267–278. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 50.Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, et al. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279(11):10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 51.Melching LI, Fisher WD, Lee ER, Mort JS, Roughley PJ. The cleavage of biglycan by aggrecanases. Osteoarthritis Cartilage. 2006;14(11):1147–1154. doi: 10.1016/j.joca.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Colige A, Li SW, Sieron AL, Nusgens BV, Prockop DJ, Lapiere CM. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc Natl Acad Sci U S A. 1997;94(6):2374–2379. doi: 10.1073/pnas.94.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, et al. Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem. 2003;278(21):19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- 54.Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276(34):31502–31509. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- 55.Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, et al. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277(8):5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- 56.Hovinga JA, Studt JD, Alberio L, Lammle B. von Willebrand factor-cleaving protease (ADAMTS-13) activity determination in the diagnosis of thrombotic microangiopathies: the Swiss experience. Semin Hematol. 2004;41(1):75–82. doi: 10.1053/j.seminhematol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Soejima K, Matsumoto M, Kokame K, Yagi H, Ishizashi H, Maeda H, et al. ADAMTS-13 cysteine-rich/spacer domains are functionally essential for von Willebrand factor cleavage. Blood. 2003;102(9):3232–3237. doi: 10.1182/blood-2003-03-0908. [DOI] [PubMed] [Google Scholar]
- 58.Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274(36):25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- 59.Llamazares M, Obaya AJ, Moncada-Pazos A, Heljasvaara R, Espada J, Lopez-Otin C, et al. The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J Cell Sci. 2007;120(Pt 20):3544–3552. doi: 10.1242/jcs.005751. [DOI] [PubMed] [Google Scholar]
- 60.Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282(34):24591–24598. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansson B, Carey D, Alini M, Ionescu M, Rosenberg LC, Poole AR, et al. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J Clin Invest. 1995;95:1071–1077. doi: 10.1172/JCI117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 63.Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279(24):25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]