Abstract
Anti-apoptotic pathways play a central role in the survival of multiple myeloma cells. The contribution of PI3-kinase and Akt kinase in mediating myeloma cell survival is well established although the role of glycogen synthase kinase-3 (GSK3) is less defined. In this study we determined the contribution of GSK3 in growth regulation of myeloma cells. We treated six different multiple myeloma cell lines with a Thiadiazolidinone (TDZD), a non-competitive inhibitor of GSK3 and determined its effects on proliferation and apoptosis. In addition we determined the activation of forkhead transcription factors (FOXO) in response to TDZD. TDZD inhibited proliferation and induced apoptosis of all myeloma cell lines. TDZD was also effective in inducing apoptosis of primary myeloma cells whereas CD34 positive normal hematopoietic cells were protected from apoptosis. Furthermore, TDZD-mediated inhibition of GSK3 resulted in dephosphorylation and activation of FOXO3a. In primary myeloma cells FOXO transcription factors were highly phosphorylated where as the levels of GSK3 phosphorylation was quite low. The levels of the pro-apoptotic proteins Fas lignad (FasL) and IκBα increased after treatment with TDZD in myeloma cell lines. These studies provide the basis for further testing of GSK3 inhibitors in the clinical setting.
Keywords: myeloma, apoptosis, glycogen synthase kinase-3
Introduction
Multiple myeloma (MM) is a post-germinal center B cell malignancy characterized by the accumulation of terminal but incompletely differentiated, antibody-producing plasma cells in the bone marrow [1-3]. MM cells have low capacity for proliferation but are resistant to apoptosis. The interaction of the clonal plasma cells with the bone marrow micro-environment is crucial for the progression of MM [1]. In addition to being dependent on bone marrow stroma, plasma cells secrete high levels of cytokines including interleukin-6 (IL-6) and insulin-like growth factor-1 (IGF-1) that provide anti-apoptotic and the growth promoting signals to sustain the malignant clone [2-5]. Disruption of signaling pathways activated by these cytokines may provide a means to affect the growth and induce apoptosis of myeloma plasma cells. One of the key anti-apoptotic pathways in myeloma is the PI3-kinase/Akt signaling pathway [6-8]. In vitro inhibition of this pathway by PI3-kinase and Akt kinase inhibitors induce apoptosis. Recent studies have suggested that GSK3, one of the down-stream molecules of Akt kinase is potentially a good target for pharmacologic inhibition in cancer due to its role in cell cycle regulation/apoptosis and cell adhesion [9-11].
GSK3 is a serine/threonine kinase expressed in all eukaryotes, which was originally discovered as part of the glycogen biosynthesis pathway. Subsequently it was demonstrated that this enzyme is involved in multiple biological processes including early embryonic development, cell cycle regulation and cell death [12,13]. Unlike most kinases phosphorylation of GSK3 leads to its inactivation. Akt kinase, mitogen-activated protein kinase-1 (MAPK) and p70 ribosomal S6 kinase-1 (S6K1) target GSK3 for phosphorylation at several serine and threonine sites [14]. Multiple growth factors exert their effects on GSK3 inactivation through different signaling cascades. For example insulin phosphorylates GSK3 via the Akt kinase pathway whereas epidermal growth factor targets GSK3 through the classical MAPK and as well as Akt pathway [15]. GSK3 phosphorylation by S6K1 is initiated by amino acids in human myocytes [16]. We have previously shown that addition of lithium chloride to multiple myeloma cells, an inhibitor of GSK3, resulted in dephosphorylation and activation of two members of the forkhead transcription factor family [17].
FOXO family of transcription factors is characterized by their conserved DNA-binding domain including three α-helices and two large loops. FOXO1a, FOXO3a, and FOXO4 are the three members of the FOXO family of transcription factors that are expressed in mammalian cells [17-20]. They play important roles in the regulation of cell cycle, apoptosis, and response to oxidative stress [19]. FOXO proteins regulate transcription of several pro-apoptotic genes including FasL and Bim [21]. They also regulate p27kip gene transcription resulting in cell cycle arrest [18]. The activities of FOXO proteins are modulated by phosphorylation at several serine and/or threonine residues which blocks their translocation to the nucleus [21]. On the other hand non-phosphorylated form of FOXO proteins are localized to the nucleus and are transcriptionally active. In the context of understanding the growth regulation of myeloma, we examined the effect of GSK3 inhibition on FOXO transcription factors. In our current study we have demonstrated that inhibition of GSK3 by the non-competitive inhibitor, TDZD [22] suppresses growth and induces apoptosis in multiple myeloma cell lines through activation of forkhead transcription factors. We also demonstrate that primary plasma cells isolated from multiple myeloma patients show high level of phosphorylation/inactivation of forkhead transcription factors whereas phosphorylation of GSK3 is quite minimal. In addition our data also show that one of the FOXO transcription factor targets, Fas-L is activated in myeloma cell lines in response to treatment with TDZD.
Material and Methods
Cell Lines
Multiple Myeloma cell lines U266, NCI-H929, and RPMI-8266 were purchased from ATCC. MM.1S and MM.1R were kindly provided by Drs. Steven T. Rosen and Nancy Krett (Northwestern University, Chicago, IL)). Dox10V was kindly provided by Dr. S.H. Lim (Texas Tech University Health Sciences Center, Amarillo, TX). All cell lines are maintained in RPMI-1640 containing 10% FBS, except MM.1S and MM.1R, which were maintained in RPMI-1640 containing 10% FBS, supplemented with antibiotics (100 units/ml penicillin, 100μg/ml streptomycin), fungizone (2.5 μg/ml) (Invitrogen, Carlsbad, CA, USA), and plasmocin (5 μg/ml) (InvivoGen San Diego, CA, USA).
Reagents
Anti- phospho (Ser256) FOXO1a, anti- phospho FOXO1a (Thr24)/FOXO3a (Thr32), anti-phospho GSK3, and antibody against FasL and IκBα, GSK3β were purchased from Cell Signaling Technologies (Beverly, MA, USA). Antibodies against phospho GSK3β (Ser9) and non-phospho GSK3α/β were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-tubulin antibody was purchased from NeoMarkers (Fremont, CA). Human CD138 micro beads were purchased from Miltenyi Biotec, Inc. (Auburn, CA, USA). Antibody against CD138 and CD38 conjugated with fluorescent PE and FITC respectively were purchased from Beckman Coulter (Fullerton, CA, USA). GSK3β inhibitor TDZD was from Santa Cruz Biotechnology or Bio Synthesis Inc. (Lewisville, TX, USA). MTT was purchased from Sigma-Aldrich (St. Louis, MO, USA). Protein G Sepharose 4 Fast Flow was purchased from Amersham Biosciences (Uppsala, Sweden).
Annexin V staining
Apoptosis of myeloma cells was assessed by annexin V-FITC apoptosis detection kit (BD Biosciences). Cells were treated with TDZD at the indicated concentrations, after 24 hrs cells were collected and washed with phosphate-buffered saline (PBS) and were stained with annexin V-FITC and PI according to manufacture's instructions. Samples were acquired on a flow cytometer (DakoCytomation, Glostrup, Denmark) and analyzed with Flowjo software (Tree Star, Inc. Ashland, OR, USA).
Cell Proliferation Assay (MTT)
Myeloma cell proliferation in response to TDZD was assessed by MTT assay, based on the method from Roche, Switzerland. Cells were seeded in 96-well flat bottom microtiter plate (3-8×105/ml) with varying concentrations of TDZD. After appropriate incubation period, MTT labeling reagent (5mg/ml in PBS) was added at 10μl/well, and incubation was continued in a cell culture incubator for 4-5 hrs. After solubilization buffer (10% SDS in 0.01M HCl) was added at 100μl/well, plates were incubated overnight to allow complete solubilization of the purple formazan crystals. The absorbance was measured at A570nm with AD340 Absorbance Detector from Beckman Coulter (Fullerton, CA, USA).
Immunoblotting and immunoprecipitation
Immunoprecipitation and Immunoblotting were performed as previously described [23].
In vitro kinase assay
In vitro kinase assay was performed following protocols provided by Upstate Biotechnology (Lake Placid, NY). Briefly, Glutathione-S-transferase (GST) tagged, full-length fusion FOXO1a protein (2μg) purchased from Upstate Biotechnology, Inc. was incubated with 10ng/μl of active recombinant GSK3β enzyme (Upstate Biotechnology, Inc.) in kinase buffer (5X buffer; 500μM ATP, 75 mM MgCl, 40 mM MOPS, pH 7.2, 25 mM β-glycerophosphate, 5 mM EGTA and 1 mM DTT) for 30 minutes at 30°C. Kinase reaction was terminated by adding SDS-sample buffer and boiling for 5 minutes. Samples were separated on SDS-PAGE and immunobloted using anti-phospho FOXO antibody that recognize phosphorylated forms of FOXO3a and FOXO1a (Cell Signaling Technologies, Beverly MA).
Isolation of plasma cells from multiple myeloma patient samples
Bone marrow aspirates were obtained from multiple myeloma patients after IRB approval and informed consent. These samples were separated over ficoll-hypaque (density=1.077g/ml) to obtain mononuclear cells. CD138 positive cells were isolated from the mononuclear cells using the antibody-coated paramagnetic micro beads in the VarioMACS™ cell isolation devise (Milteny Biotec, Inc.). The purity of the isolated CD138 positive cells was assessed by flow cytometry.
Results
Growth arrest of myeloma cells by GSK3 inhibition
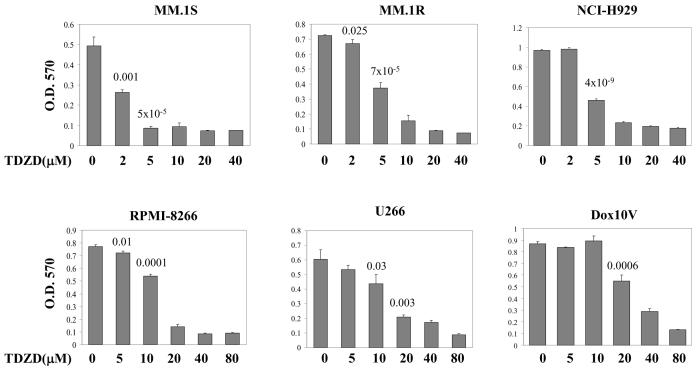
We studied six different multiple myeloma cell lines to determine whether inhibition of GSK3 by TDZD inhibits growth and induces apoptosis. TDZD is a non-competitive inhibitor and therefore more selective than compounds that bind the ATP binding pocket. The six cell lines studied have various chromosomal abnormalities and secrete different immunoglobulins consistent with different myeloma subtypes. In addition all six cell lines expressed syndecan-1 (CD138), a marker protein common to plasma cells and none of the lines are infected with EBV (Table 1). Myeloma cell lines were treated with varying concentrations of TDZD for 24 hrs and cell proliferation was determined by MTT cell proliferation assay. As shown in figure 1, proliferation of all of the myeloma cell lines was inhibited to a variable degree by TDZD treatment. MM.1S (IC50 = 2.6μM), MM.1R (IC50 = 5.7 μM), and NCI-H929 (IC50 = 5.6 μM) cells were highly sensitive to TDZD treatment whereas U266 (IC50 = 16.6 μM), RPMI-8266 (IC50 = 13.2 μM) and Dox10V (IC50 = 30 μM) required higher concentrations for inhibition. The range of sensitivity to the drug among various myeloma lines reflected the diverse biochemical characteristics exhibited by these cells as shown in Table 1. The IC50 values listed in table 1 reflect the compound concentration that inhibited 50% of cell growth after treatment for 24 hrs.
Table 1.
Characteristics of Multiple Myeloma Cell Lines
| Cell line | +Ig Type |
++EBV status |
Characteristics | TDZD (IC50) μM |
Reference |
|---|---|---|---|---|---|
| MM.1S | λ | no | CD38+, CD138+, glucocorticoid sensitive | 2.6 | [37] |
| MM.1R | λ | no | Derived from MM.1S, glucocorticoid resistant | 5.7 | [38] |
| NCI-H929 | IgA | no | PCA-1+, CD38+, CD138+ | 5.6 | [39] |
| RPMI 8266 | λ | no | CD38±, CD138+, chemo sensitive | 13.2 | [40] |
| U266 | IgE | no | Produces IL-6+, CD38−, CD138+ | 16.6 | [41,42] |
| Dox 10V | λ | no | Derived from PRMI 8266, chemo resistant | 30.0 | [43,44] |
Ig: Immunoglobulin
EBV: Epstein Barr Virus
All lines negative for B-cell markers CD19 & CD20
Figure 1.
The effect of inhibition of GSK3 activity on the proliferation of myeloma cells. Myeloma cell lines MM.1S, MM.1R, NCI-H929, U266, RPMI-8266, and Dox10V were cultured in the absence or presence of various concentrations of TDZD as indicated for 24 hrs. MTT cell proliferation assay was performed on each sample. The data are mean of triplicate determinations. P values are indicated for data that showed statistical significance.
Induction of apoptosis by TDZD
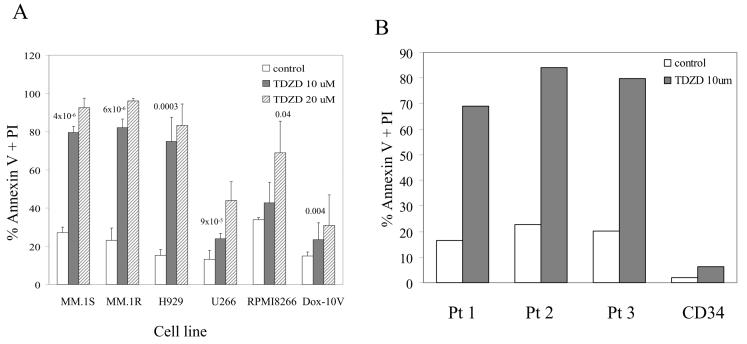
We then determined whether treatment of myeloma cell lines with TDZD induces apoptosis of myeloma cells. Cells were treated with two concentrations of TDZD for 24 hrs and analyzed by annexin-V and PI staining. As shown in figure 2, after 24 hours of TDZD treatment, all six cell lines showed apoptosis. MM.1S, MM.1R, and H929 myeloma cell lines were very sensitive to apoptosis, but U266, RPMI required higher doses to achieve a similar level of apoptosis. Dox10V cell line was most resistant to TDZD exhibiting very little apoptosis even at the higher dose (Fig. 2A). Overall these results were consistent with the data for proliferation where we observed MM.1S and MM.1R to be very sensitive to the drug where as RPMI-8266 and Dox10V were less responsive to growth suppression. We then tested whether myeloma cells isolated and cultured in the presence of TDZD will undergo apoptosis. We isolated CD138 positive plasma cells from the bone marrow of three myeloma patients and cultured for 24 hrs in the presence and absence of TDZD. In addition we also cultured CD34 positive normal hematopoietic early progenitors with and without TDZD. Our results showed that primary myeloma plasma cells treated with TDZD were very sensitive to apoptosis compared to untreated cells (Fig 2B). Furthermore CD34 early hematopoietic cells were not affected by the treatment of TDZD during the 24 hrs treatment (Fig. 2 B).
Figure 2.
Induction of apoptosis of myeloma cells by GSK3 inhibition. (A) Myeloma cell lines MM.1S, MM.1R, NCI-H929, U266, RPMI-8266, and Dox10V were cultured in the absence or presence of TDZD at indicated concentrations for 24 hrs. Cells were analyzed for apoptosis by flow cytometry after staining for Annexin V /PI. The data shown are mean of triplicate experiments. (B) Primary myeloma cells (CD138+) isolated from 3 patients (Pt) and early hematopoietic progenitors (CD34+) from a normal donor were cultured in the absence of presence of TDZD for 24 hrs prior to evaluating for apoptosis.
Inhibition of GSK3 by TDZD affects FOXO transcription factors
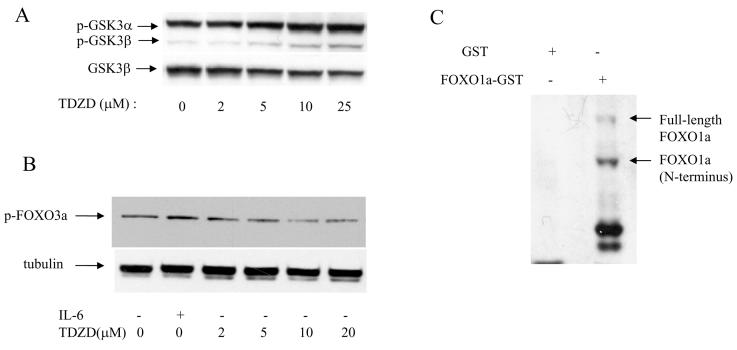
In order to determine the effect of TDZD on phosphorylation (inactivation) of GSK3, we performed immunoblot analysis using the U266 myeloma cell line after treatment with increasing concentrations of TDZD. Addition of TDZD to U266 cells increased phosphorylation/inactivation of GSK3 in a dose dependent fashion (Fig. 3A). Both GSK3α and GSK3β isoforms were affected by TDZD although the GSK3α isoform was the prominent protein that was phosphorylated in myeloma cells (Fig. 3A). We then determined whether FOXO3a, one of the members of the FOXO family of proteins is also attenuated in response to TDZD treatment. Addition of increasing concentrations of TDZD dephosphorylated FOXO3a in a dose dependent manner suggesting that inhibition of GSK3 activated FOXO transcription factors (Fig. 3B). To further investigate whether FOXO proteins acts as substrates for GSK3 enzyme, we performed in vitro kinase assays. We incubated recombinant FOXO1a-GST fusion protein in the presence of active GSK3 enzyme, kinase buffer containing ATP and analyzed the reaction products by immunoblotting against an anti-phospho FOXO antibody that recognize phosphorylated form of FOXO1a. Our results showed that both full-length and truncated versions of FOXO1a were phosphorylated by GSK3 whereas GST protein, which was used as a control, was not phosphorylated (Fig. 3C).
Figure 3.
Inhibition of GSK3 activity leads to activation of FOXO3a transcription factor in myeloma cells. (A) U266 cells were cultured in low serum for 20 hrs in the presence or absence of increasing concentrations of TDZD prior to analysis by SDS-PAGE, and immunoblotting against an anti-phospho GSK3 antibody. The same blot was subsequently immunoblotted against a non phospho GSK3 antibody for protein loading. (B) U266 cells were cultured in low serum for 20 hrs in the presence or absence of TDZD prior to analysis by SDS-PAGE and immunoblotting against phospho-FOXO3a antibody. One sample was also analyzed after stimulation with 50ng/ml of IL-6 for 30 minutes. The same blot was subsequently immunoblotted against a tubulin antibody for protein loading.
(C) Inactivation/phosphorylation of FOXO3a transcription factors by GSK3β. GST-FOXO1a fusion protein was incubated with recombinant GSK3β in the presence of ATP in kinase buffer. The reactions were resolved by SDS-PAGE, and blotted with anti-phospho-FOXO antibody that recognizes phosphorylated forms of FOXO3a and FOXO1a.
Phosphorylation of FOXO transcription factors in primary myeloma plasma cells
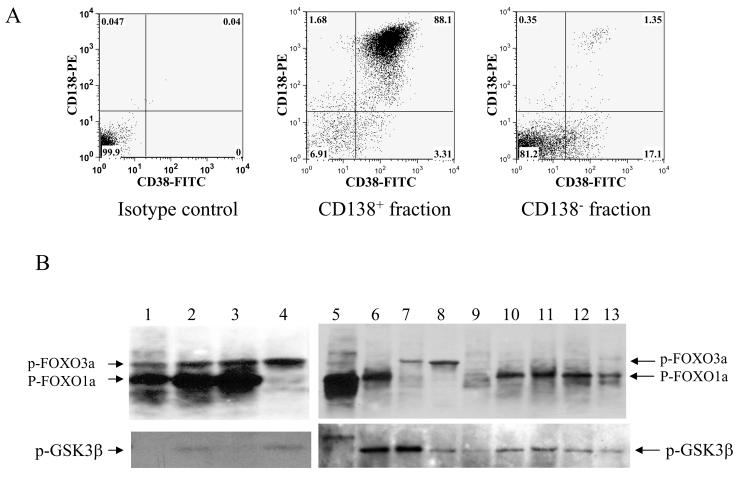
In order to determine whether plasma cells isolated from multiple myeloma patients exhibit highly phosphorylated (inactive) forms of FOXO proteins, we first analyzed bone marrow samples obtained from patients for the level of CD138 and CD38 surface protein expression. Most myeloma patients have large number of plasma cells in their bone marrow, which is part of the malignant clone. These malignant cells were purified by selecting for CD138 positive cells. Flow cytometry analysis for CD138 and CD38 expression levels from a patient bone marrow sample is shown in Figure 4A. We used thirteen bone marrow samples from myeloma patients to purify malignant plasma cells using CD138 immunomagnetic beads prior to determining the levels of phosphorylation of FOXO family members by immunoblot analysis. The results of immunoblot analysis using phospho-specific FOXO antibody are shown in Figure 4B. This experiment showed that a majority of patient samples exhibited high level of phosphorylation of FOXO proteins (Fig. 4B). We then reprobed the same blot to determine the phosphorylation status of GSK3 and found that a majority of the samples had low level of GSK3 phosphorylation suggesting that high-level of GSK3 activity co-relates with inactivation/phosphorylation of FOXO transcription factors (Fig. 4B).
Figure 4.
Phosphorylation of FOXO transcription factors and GSK3 in primary myeloma patient samples. (A) Bone marrow mononuclear cells from a bone marrow aspirate of a myeloma patient was selected for CD138+ cells and analyzed by flow cytometry to confirm enrichment of plasma cells. (B) Total cell lysates from CD138 positive plasma cells were analyzed by SDS-PAGE and probed with anti-phospho-FOXO3a/FOXO1a and anti-phosphoGSK3β antibodies.
Activation of apoptotic cascades by TDZD-mediated GSK3 inhibition
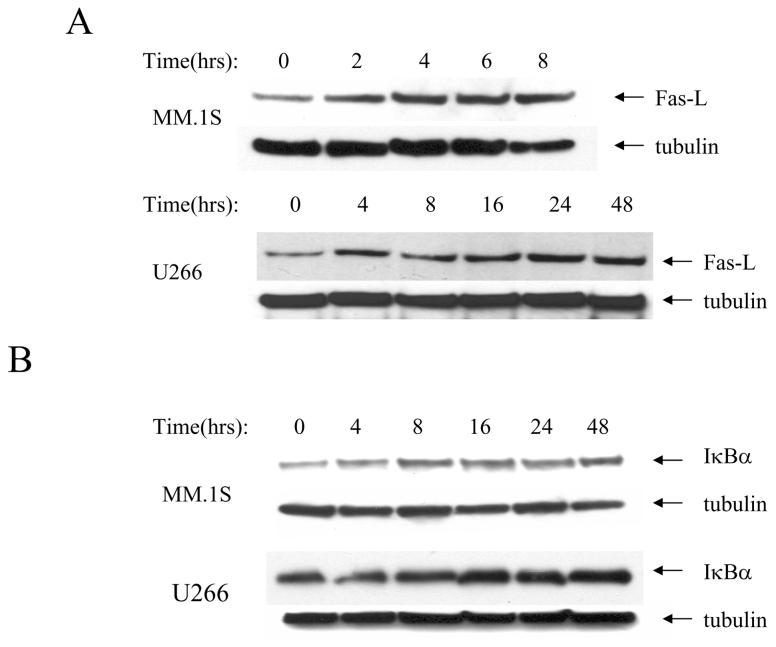
To further understand the mechanism of action of GSK3 inhibition by TDZD we examined the expression of FasL and one of the targets of IκB kinase (IKK) complex, IκBα. FasL is a positive effecter of apoptosis and is one of the targets of FOXO transcription factors in multiple cell types. On the other hand the pro or anti-apoptotic outcome of IκBα upregulation is context and cell type specific [24]. We performed a time course experiment where U266 and MM.1S myeloma cells were incubated with TDZD over various period of time and examined the level of FasL by immunoblot analysis. Our data showed that levels of FasL increased over the time period the cells were exposed to TDZD (Fig. 5A) whereas tubulin, which was used as a protein loading control remained constant. In a parallel experiment we also examined the level of IκBα protein levels in both MM.1S and U266 cell lines and found that TDZD up regulated IκBα although the effect was greater in U266 cell line suggesting that inhibition of GSK3 by TDZD affects several downstream proteins that are associated with apoptosis (Fig. 5B).
Figure 5.
Expression of Fas-L and IκBα in response to TDZD treatment (A) MM.1S and U266 cells were treated with 10 μM TDZD for various time periods as indicated. Cells were collected and total cell lysates were analyzed by SDA-PAGE and immunoblotted with anti-Fas-L antibody. Subsequently the same blots were probed with anti-tubulin antibody as a protein loading control. (B) MM.1S and U266 cells were treated with 10 μM TDZD for various time periods as indicated. Cells were collected and total lysates were analyzed by SDA-PAGE and immunoblotted with anti-IkBα antibody. Subsequently the same blots were probed with anti-tubulin antibody for loading control.
Discussion
The role of PI3-kinase and protein kinase B pathway in providing anti-apoptotic signals in multiple myeloma cells is well documented [6,25,26]. However, the impact of GSK3 inhibition and its influence on FOXO transcription factors in regulating growth and survival of myeloma cells have not been studied. Furthermore, almost all small molecule drugs that are being developed to regulate growth and induction of apoptosis are ATP competitive inhibitors, which are likely to affect other kinases as well. In the current study we examined the use of a non-competitive ATP inhibitor of GSK3 to determine the impact on growth/apoptosis of myeloma cells and the effect of GSK3 inhibition on several important downstream regulators. In a previous study we had observed that relatively high concentrations of lithium and SB216763, two agents that are known to inhibit GSK3 affected the growth of the myeloma cell line RPMI8226 [26]. Therefore, we investigated other inhibitors that have been developed to regulate GSK3 inhibition in neuronal cells and found that the ATP non-competitive inhibitor, TDZD, was very effective in suppression of myeloma cell proliferation and induction of apoptosis. We showed that this inhibitor induced apoptosis in multiple myeloma cell lines. Moreover, we showed that GSK3 inhibition by TDZD induces apoptosis of primary myeloma plasma cells but spares CD34-positive early hematopoietic cells suggesting that TDZD may be potentially useful to purge hematopoietic stem cell autografts of malignant plasma cells.
We also provide several lines of evidence for regulation of FOXO transcription factors by GSK3. Although PKB is well-established as the primary kinase responsible for phosphorylation and inactivation of FOXO transcription factors, it is likely that other kinases such as GSK3 also directly influence the activity of FOXO transcription factors. Our current data provide several lines of evidence for regulation of FOXO transcription factors by GSK3. First we are able to show that a highly specific inhibitor of GSK3 dephosphorylates FOXO3a, one of the members of the FOXO family. Secondly, we demonstrate that FOXO proteins are substrates for GSK3 enzyme. Finally, we were able to demonstrate that FOXO transcription factors are inactive/phosphorylated in myeloma patient samples contributing to increased survival of myeloma cells as a result of suppression of FasL and the cell cycle inhibitor p27kip, which are transcriptional targets of FOXO transcription factors [21,27]. Therefore the high-level of inactivation/phosphorylation of FOXO proteins seen in myeloma patient samples could potentially be reversed by GSK3 inhibition leading to apoptosis of myeloma cells. In addition to examining downstream targets of PKB such as GSK3 and FOXO proteins we also examined the effect of TDZD on the NF-kappa B pathway, a key signaling pathways affected in myeloma [7,28]. Specifically we examined the level of IκBα in response to TDZD treatment in MM cell lines and found that protein level of IκBα steadily increased with time after inhibition of GSK3. These results were in agreement with several studies at least in other cell types where it has been demonstrated that regulation of the NF kappa B signaling cascade through modulation of one or more components of the IκB kinase complex [28-30].
We also provide evidence for selective nature of TDZD especially in protecting early CD34 positive hematopoietic cells from apoptosis. One of the standard therapies used for the treatment of multiple myeloma is transplantation of peripheral blood-collected CD34 positive hematopoietic cells although often residual malignant cells are present in these grafts [31-34]. Therefore by devising means to in vitro purge these grafts we will be able to reduce or eliminate the possibility of relapse of the disease. Moreover, two studies have demonstrated that inhibition of GSK3 by small-molecule drugs have the potential of expanding the early hematopoietic stem cells, which are needed for long-term engraftment of the hematopoietic compartment [35,36]. Therefore, utilization of ATP non-competitive inhibitors such as TDZD and its derivatives will potentially accomplish two objectives in the context of treating multiple myeloma. Future studies will focus on testing GSK3 inhibitors in myeloma mouse models and determining whether paracrine affects of the bone marrow microenvironment can be overcome to induce apoptosis of myeloma cells.
Acknowledgements
This work was supported in part by National Cancer Institute grant CA98550 (AW) and the Multiple Myeloma Research Foundation.
References
- 1.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 2.Xu FH, Sharma S, Gardner A, Tu Y, Raitano A, Sawyers C, Lichtenstein A. Interleukin-6-induced inhibition of multiple myeloma cell apoptosis: support for the hypothesis that protection is mediated via inhibition of the JNK/SAPK pathway. Blood. 1998;92:241–51. [PubMed] [Google Scholar]
- 3.Puthier D, Bataille R, Amiot M. IL-6 up-regulates mcl-1 in human myeloma cells through JAK / STAT rather than ras / MAP kinase pathway. Eur J Immunol. 1999;29:3945–50. doi: 10.1002/(SICI)1521-4141(199912)29:12<3945::AID-IMMU3945>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 4.Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth. Blood. 2000;96:2856–61. [PubMed] [Google Scholar]
- 5.Stromberg T, Ekman S, Girnita L, Dimberg LY, Larsson O, Axelson M, Lennartsson J, Hellman U, Carlson K, Osterborg A. IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood. 2006;107:669–78. doi: 10.1182/blood-2005-01-0306. others. [DOI] [PubMed] [Google Scholar]
- 6.Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses. Cancer Res. 2000;60:6763–70. [PubMed] [Google Scholar]
- 7.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–83. doi: 10.1038/sj.onc.1205664. others. [DOI] [PubMed] [Google Scholar]
- 8.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–27. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh JC, Altieri DC. Activation of p53-dependent apoptosis by acute ablation of glycogen synthase kinase-3beta in colorectal cancer cells. Clin Cancer Res. 2005;11:4580–8. doi: 10.1158/1078-0432.CCR-04-2624. [DOI] [PubMed] [Google Scholar]
- 10.Ougolkov AV, Billadeau DD. Targeting GSK-3: a promising approach for cancer therapy? Future Oncol. 2006;2:91–100. doi: 10.2217/14796694.2.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya K, Nakamura T, Okamoto R, Kanai T, Watanabe M. Reciprocal targeting of Hath1 and beta-catenin by Wnt glycogen synthase kinase 3beta in human colon cancer. Gastroenterology. 2007;132:208–20. doi: 10.1053/j.gastro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 13.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–86. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 1999;461:120–4. doi: 10.1016/s0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong JL, Bonavaud SM, Toole BJ, Yeaman SJ. Regulation of glycogen synthesis by amino acids in cultured human muscle cells. J Biol Chem. 2001;276:952–6. doi: 10.1074/jbc.M004812200. [DOI] [PubMed] [Google Scholar]
- 17.Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–7. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 18.Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol Cell Biol. 2000;20:9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025–36. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez A, Alonso M, Castro A, Perez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J Med Chem. 2002;45:1292–9. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- 23.Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, Sawyer ST, Krystal G, Yi T, Nishada K. Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol Chem. 1999;274:24469–74. doi: 10.1074/jbc.274.35.24469. others. [DOI] [PubMed] [Google Scholar]
- 24.Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–75. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991–6000. doi: 10.1038/sj.onc.1204833. [DOI] [PubMed] [Google Scholar]
- 26.M GA, Uddin S, Mahmud D, Damacela I, Lavelle D, Ahmed M, van Besien K, Wickrema A. Regulation of myeloma cell growth through Akt/Gsk3/forkhead signaling pathway. Biochem Biophys Res Commun. 2002;297:760–4. doi: 10.1016/s0006-291x(02)02278-7. [DOI] [PubMed] [Google Scholar]
- 27.Reagan-Shaw S, Ahmad N. The role of Forkhead-box Class O (FoxO) transcription factors in cancer: a target for the management of cancer. Toxicol Appl Pharmacol. 2007;224:360–8. doi: 10.1016/j.taap.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, Neri P, Tassone P, Yasui H, Ishitsuka K, Raje N, Chauhan D, Podar K, Mitsiades C, Dang L. MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res. 2006;12:5887–94. doi: 10.1158/1078-0432.CCR-05-2501. others. [DOI] [PubMed] [Google Scholar]
- 29.Frelin C, Imbert V, Griessinger E, Loubat A, Dreano M, Peyron JF. AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-alpha in Jurkat leukemic cells. Oncogene. 2003;22:8187–94. doi: 10.1038/sj.onc.1206963. [DOI] [PubMed] [Google Scholar]
- 30.Ni H, Ergin M, Huang Q, Qin JZ, Amin HM, Martinez RL, Saeed S, Barton K, Alkan S. Analysis of expression of nuclear factor kappa B (NF-kappa B) in multiple myeloma: downregulation of NF-kappa B induces apoptosis. Br J Haematol. 2001;115:279–86. doi: 10.1046/j.1365-2141.2001.03102.x. [DOI] [PubMed] [Google Scholar]
- 31.Bjorkstrand B, Gahrton G. High-dose treatment with autologous stem cell transplantation in multiple myeloma: past, present, and future. Semin Hematol. 2007;44:227–33. doi: 10.1053/j.seminhematol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Vescio RA, Han EJ, Schiller GJ, Lee JC, Wu CH, Cao J, Shin J, Kim A, Lichtenstein AK, Berenson JR. Quantitative comparison of multiple myeloma tumor contamination in bone marrow harvest and leukapheresis autografts. Bone Marrow Transplant. 1996;18:103–10. [PubMed] [Google Scholar]
- 33.Vogel W, Kopp HG, Kanz L, Einsele H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J Cancer Res Clin Oncol. 2005;131:214–8. doi: 10.1007/s00432-004-0635-y. [DOI] [PubMed] [Google Scholar]
- 34.Gazitt Y, Reading CC, Hoffman R, Wickrema A, Vesole DH, Jagannath S, Condino J, Lee B, Barlogie B, Tricot G. Purified CD34+ Lin− Thy+ stem cells do not contain clonal myeloma cells. Blood. 1995;86:381–9. [PubMed] [Google Scholar]
- 35.Trowbridge JJ, Xenocostas A, Moon RT, Bhatia M. Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med. 2006;12:89–98. doi: 10.1038/nm1339. [DOI] [PubMed] [Google Scholar]
- 36.Bug G, Gul H, Schwarz K, Pfeifer H, Kampfmann M, Zheng X, Beissert T, Boehrer S, Hoelzer D, Ottmann OG. Valproic acid stimulates proliferation and self-renewal of hematopoietic stem cells. Cancer Res. 2005;65:2537–41. doi: 10.1158/0008-5472.CAN-04-3011. others. [DOI] [PubMed] [Google Scholar]
- 37.Goldman-Leikin RE, Salwen HR, Herst CV, Variakojis D, Bian ML, Le Beau MM, Selvanayagan P, Marder R, Anderson R, Weitzman S. Characterization of a novel myeloma cell line, MM.1. J Lab Clin Med. 1989;113:335–45. others. [PubMed] [Google Scholar]
- 38.Moalli PA, Pillay S, Weiner D, Leikin R, Rosen ST. A mechanism of resistance to glucocorticoids in multiple myeloma: transient expression of a truncated glucocorticoid receptor mRNA. Blood. 1992;79:213–22. [PubMed] [Google Scholar]
- 39.Gazdar AF, Oie HK, Kirsch IR, Hollis GF. Establishment and characterization of a human plasma cell myeloma culture having a rearranged cellular myc proto-oncogene. Blood. 1986;67:1542–9. [PubMed] [Google Scholar]
- 40.Matsuoka Y, Moore GE, Yagi Y, Pressman D. Production of free light chains of immunoglobulin by a hematopoietic cell line derived from a patient with multiple myeloma. Proc Soc Exp Biol Med. 1967;125:1246–50. doi: 10.3181/00379727-125-32327. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson K. Synthesis and secretion of IgE by an established human myeloma cell line. Clin Exp Immunol. 1971;9:785–93. [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsson K, Bennich H, Johansson SG, Ponten J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970;7:477–89. [PMC free article] [PubMed] [Google Scholar]
- 43.Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Res. 1986;46:5125–30. [PubMed] [Google Scholar]
- 44.Dalton WS, Grogan TM, Meltzer PS, Scheper RJ, Durie BG, Taylor CW, Miller TP, Salmon SE. Drug-resistance in multiple myeloma and non-Hodgkin's lymphoma: detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J Clin Oncol. 1989;7:415–24. doi: 10.1200/JCO.1989.7.4.415. [DOI] [PubMed] [Google Scholar]