Abstract
During spore formation in Bacillus subtilis, σE-directed gene expression in the mother-cell compartment of the sporangium triggers the activation of σG in the forespore by a pathway of intercellular signaling that is composed of multiple proteins of unknown function. Here, we confirm that the vegetative protein SpoIIIJ, the forespore protein SpoIIQ, and eight membrane proteins (SpoIIIAA through SpoIIIAH) produced in the mother cell under the control of σE are ordinarily required for intercellular signaling. In contrast, an anti-σG factor previously implicated in the pathway is shown to be dispensable. We also present evidence suggesting that SpoIIIJ is a membrane protein translocase that facilitates the insertion of SpoIIIAE into the membrane. In addition, we report the isolation of a mutation that partially bypasses the requirement for SpoIIIJ and for SpoIIIAA through SpoIIIAG, but not for SpoIIIAH or SpoIIQ, in the activation of σG. We therefore propose that under certain genetic conditions, SpoIIIAH and SpoIIQ can constitute a minimal pathway for the activation of σG. Finally, based on the similarity of SpoIIIAH to a component of type III secretion systems, we speculate that signaling is mediated by a channel that links the mother cell to the forespore.
Keywords: signal transduction, cell-cell communication, sigma factor, sporulation
Introduction
Long thought to exist only as solitary cells, bacteria are now known to communicate with one another during growth, pathogenesis, and development (reviewed in Bassler & Losick, 2006). A powerful system in which to study bacterial communication is spore formation by the gram-positive soil bacterium Bacillus subtilis, which involves three close-range intercellular signaling events (see below and Figure 1A). Early in sporulation an asymmetrically positioned septum is formed and divides the rod-shaped cell into two compartments of unequal size. The larger cell is called the mother cell and the smaller cell, which ultimately becomes the spore, is called the forespore. The forespore is subsequently engulfed by the mother cell in a phagocytic-like process in which the forespore is pinched off as a free protoplast within the mother cell. As a consequence of engulfment, the forespore is surrounded by two membranes: an inner forespore membrane and an outer forespore membrane that derives from the mother cell. Later in development a thick layer of peptidoglycan known as the cortex is produced in the space between the inner and outer forespore membranes and a thick, proteinaceous coat is produced in the mother cell and deposited around the outside of the forespore. Eventually, when morphogenesis is complete, the mature spore is released by lysis of the mother cell.
Figure 1. σG activation is tightly coupled to σE–directed gene expression independently of sigG transcriptional regulation and the antisigma factors CsfB and SpoIIAB.
A. Cartoon depicting the morphological stages of B. subtilis sporulation and the cell-cell signaling pathways controlling σ factor activation in each compartment. Immediately following asymmetric septation, σF becomes active in the smaller forespore compartment and initiates a signal transduction pathway that leads to σE activation in the larger mother cell. Upon the completion of engulfment, σE directs the activation of σG in the forespore by a poorly understood mechanism. Finally, active σG initiates a signal transduction pathway that activates σK in the mother cell.
B. σG activity commences during the third hour of sporulation. σG–dependent expression of a translational PsspB-lacZ reporter gene was monitored during sporulation of cells expressing sigG from its normal promoter and at its normal position (solid squares, strain AHB41).
C. The timing of σG activation and its dependence on intercellular signaling are maintained in cells expressing PspoIIQ-sigG in place of the endogenous sigG gene. σG–dependent PsspB-lacZ expression during sporulation was monitored in wild type (solid squares), sigE (open circles), Q (open squares), AA-AH (open diamonds), or J (open triangles) mutant cells, all of which expressed PspoIIQ-sigG in place of the endogenous sigG gene (strains AHB350, AHB415, AHB363, AHB411, and AHB365, respectively).
D. The timing of σG activation and its dependence on mother cell gene expression are unaffected by the absence of CsfB. The σG–dependent accumulation of β-galactosidase from the PsspB-lacZ reporter gene in wild type (closed squares), csfB (open squares), sigE (closed triangles), and sigE csfB double mutant cells (open triangles) was monitored through sporulation (strains AHB563, AHB580, AHB608, and AHB611, respectively). All strains expressed PspoIIQ-sigG in place of the endogenous sigG gene. A slight increase in the basal levels of σG activity in csfB mutant cells can be observed when the y-axis scale is changed (inset).
E. The timing of σG activation and its dependence on mother cell gene expression remains intact in the absence of regulation by the antisigma factors SpoIIAB and CsfB. σG–dependent PsspB-lacZ expression during sporulation was monitored in wild type (closed squares), csfB (open squares), sigE (closed triangles), and sigE csfB double mutant cells (open triangles), all of which expressed PspoIIQ-sigGE156K in place of the endogenous sigG gene (strains AHB565, AHB582, AHB610, and AHB613, respectively). A slight increase in the basal levels of σG activity in csfB mutant cells can be observed when the y-axis scale is changed (inset).
The morphological events of sporulation are orchestrated by the sequential and compartmentalized appearance of four alternative sigma (σ) factors that coordinate distinct programs of gene expression in the two cells of the developing sporangium. The first compartment-specific σ factor, σF, becomes active in the forespore soon after asymmetric division. An intercellular signaling pathway emanating from the forespore under σF control, in turn, directs activation of σE in the mother cell. Once engulfment is complete, σG is activated in the forespore by a second intercellular signaling pathway initiated by σE–directed gene activity in the mother cell. Finally, σG triggers a third and final signaling pathway that activates the last compartment-specific transcription factor, σK, in the mother cell. In all, these three tandem signal tranduction pathways ensure that gene expression is kept in register between the two developing cells throughout morphogenesis (Figure 1A).
The intercompartmental signaling pathways that govern the activation of σE and σK in the mother cell are relatively well understood. Both involve the proteolytic conversion of inactive pro-protein precursor proteins, pro-σE and pro-σK, to the mature and active form of the transcription factors by a chain of events triggered by the forespore transcription factors σF and σG, respectively (reviewed in Rudner & Losick, 2001). In contrast, the molecular mechanisms that link σE-directed gene expression in the mother cell to the activation of σG in the forespore have remained elusive. One potential explanation for the difficulty in deciphering this pathway is that more than ten genes are known to be required for the activation of σG: the gene for σE sigE, the eight members of the spoIIIA operon, which is under the control of σE, the spoIIQ gene, which is under the control of σF, and the vegetatively expressed gene spoIIIJ (Errington et al., 1992, Partridge & Errington, 1993, Londono-Vallejo et al., 1997, Sun et al., 2000, Serrano et al., 2003). Activation of σG is also coupled to the completion of engulfment, as evidenced by the finding that σG remains inactive in cells mutant for engulfment (for example, Margolis et al., 1993, Smith et al., 1993, Frandsen & Stragier, 1995). As yet an additional complication, the gene encoding σG, sigG (also known as spoIIIG), is subject to complex and poorly-understood transcriptional regulation. The sigG gene is initially transcribed by σF and later by σG itself, thereby confining its expression to the forespore and ultimately creating a positive feedback loop (Karmazyn-Campelli et al., 1989, Sun et al., 1991b). Furthermore, sigG transcription has been reported to be delayed relative to other σF target genes and to require σE–directed gene activity in the mother cell as well as the transcription of spoIIQ in the forespore (Partridge & Errington, 1993, Sun et al., 2000). Finally, several proteins have been identified as inhibitors of σG, including the anti-σF factor SpoIIAB and, recently, the anti-σG factor CsfB (also known as Gin) (Kirchman et al., 1993, Kellner et al., 1996, Chary et al., 2007, Karmazyn-Campelli et al., 2008).
Here we have used genetic approaches in an effort to simplify the problem and hence increase our understanding of the intercellular signaling pathway that controls σG. First, we confirm the importance of σE, the eight SpoIIIA proteins (SpoIIIAA-AH), SpoIIQ, and SpoIIIJ in σG activation, even when the expression of sigG is uncoupled from its normal transcriptional control. Second, we show that the antisigma factors SpoIIAB and CsfB are dispensable for σG regulation via mother cell signaling. Third, through the isolation of bypass mutants, we present evidence indicating that under certain genetic conditions, SpoIIIAH and SpoIIQ can constitute the minimal components of the pathway for activating σG. Finally, based on the similarity of SpoIIIAH to a family of multimerizing proteins from pathogenic, gram-negative bacteria, we propose that intercellular signaling is mediated by a channel across the inner and outer forespore membranes.
Results
Activation of σG is tightly coupled to σE–directed gene expression
As starting point for this investigation, we sought to confirm that the activation of σG in the forespore is strongly dependent upon σE–directed gene expression in the mother cell. A complication in doing so, however, was that the normal promoter (PsigG) that governs the transcription of the gene (sigG) for σG is controlled both by σF and, autogenously, by σG itself (Karmazyn-Campelli et al., 1989, Sun et al., 1991b). Additionally, PsigG activity has been reported to require σE-directed gene expression in the mother cell and expression of the spoIIQ gene in the forespore (Partridge & Errington, 1993, Sun et al., 2000). To circumvent these complications, we utilized a previously described sigG construct in which PsigG was replaced by a promoter that is under the exclusive control of σF, PspoIIQ (Stragier & Losick, 1996, Londono-Vallejo et al., 1997, Karmazyn-Campelli et al., 2008). This PspoIIQ-sigG construct was integrated into an exogenous locus (amyE) on the chromosome of a strain deleted for the native sigG gene. Consistent with previous reports, the sigG amyE::PspoIIQ-sigG strain (henceforth referred to as the PspoIIQ-sigG strain for simplicity) sporulated at wild-type levels (data not shown) and displayed normal timing of σG activation as measured with a translational fusion of lacZ to a promoter (PsspB) that is under the exclusive control of σG (Sun et al., 1991a, Stragier & Losick, 1996, Serrano et al., 2004, Karmazyn-Campelli et al., 2008). More specifically, σG–dependent PsspB-lacZ activation commenced three hours after the onset of sporulation in cells expressing PspoIIQ-sigG (Figure 1C) as well as in control cells expressing sigG from its normal promoter and at its normal location (Figure 1B). The level of σG activity in cells harboring PspoIIQ-sigG was, however, modestly (∼2-fold) lower than in cells containing PsigG-sigG.
We then tested the dependence of σG activity upon σE and upon the σE–controlled spoIIIA operon in the PspoIIQ-sigG strain background. The spoIIIA operon consists of eight genes spoIIIAA-spoIIIAH, which for simplicity we henceforth refer to as AA, AB, AC,…, AH. We introduced deletions of the gene (sigE) encoding σE or the entire spoIIIAA operon (AA-AH) into cells harboring the PsspB-lacZ fusion. In both cases, σG-directed synthesis of β-galactosidase was almost completely abolished (Figure 1C), consistent with previously published results obtained with these mutants in otherwise wild-type backgrounds (Partridge & Errington, 1993, Kellner et al., 1996).
Activation of σG is also reported to be dependent upon the vegetatively expressed gene spoIIIJ (henceforth J) and the σF–controlled gene spoIIQ (henceforth Q) (Errington et al., 1992, Londono-Vallejo et al., 1997, Serrano et al., 2003). Indeed, σG activity was largely abolished by deletion of either J or Q in the PspoIIQ-sigG strain, although a low level of σG activity was detected in the absence of J (Figure 1C).
The absence of antisigma factors does not uncouple σG activation from σE
Recent work has revealed the existence of a previously unrecognized inhibitor of σG activity called CsfB. Evidence presented by Chary et al. (2007) and by Karmazyn-Campelli et al. (2008) indicates that CsfB (referred to as Gin in the latter study) helps to suppress low levels of σG activity that escape the control mechanisms that normally restrict its activity to the forespore after engulfment. In addition, Karmazyn-Campelli et al. (2008) concluded that the absence of CsfB uncoupled σG activation from σE–directed gene expression during sporulation. We noticed, however, that these workers used as a reporter for σG activity lacZ fused to PsspE, a promoter that has been shown to be recognized by both σG and σF in vivo and in vitro (Sun et al., 1991a). Indeed, PsspE harbors one member of the pair of guanines (Gs) at positions -15 and -16 that are features of efficient promoter recognition by σF–RNA polymerase. Mutational analysis has confirmed that the G at -16 contributes to PsspE recognition by σF in vivo and in vitro (Sun et al., 1991a). In contrast, the sspB promoter has no Gs at position -15 and -16 and is highly specific for σG (Sun et al., 1991a).
The weak selectivity of PsspE likely explains the puzzling finding by Karmazyn-Campelli et al. (2008) that PsspE-lacZ expression was only partially impaired in cells lacking σE. In contrast, we observed that expression of PsspB-lacZ was almost completely blocked in cells mutant for the mother-cell transcription factor. These contrasting findings can be seen both in Figure 4B from the report of Karmazyn-Campelli et al. (2008) and in Figure 1C from the current investigation. Also, in other unpublished work, we have seen that PsspE-lacZ expression is only incompletely impaired in cells expressing PspoIIQ-sigG and mutant for sigE (data not shown).
Figure 4. AH and Q are required for σG activation in pbpGΔR147-K148 cells.
σG–dependent PsspB-lacZ expression during sporulation was monitored in cells lacking (A) J, (B) sigE, (C) AA-AH, (D) AA, (E) AE, (F) AA-AG, (G) AH, or (H) Q either in the presence of wild type pbpG (closed circles) or the pbpGΔR147-K148 mutant (open circles). σG–dependent PsspB-lacZ activity in an isogenic wild type strain is also shown in each panel (closed squares). All strains expressed PspoIIQ-sigG in place of endogenous sigG and were deleted for csfB. In all cases, β-galactosidase activity was normalized to the maximal activity observed for the wild type control strain in each experiment (% WT maximum).
A. PsspB-lacZ activity in cells harboring wild type J (closed squares), deleted for J (closed circles), or deleted for J and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB639, and AHB905, respectively).
B. PsspB-lacZ activity in cells harboring wild type sigE (closed squares), deleted for sigE (closed circles), or deleted for sigE and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB863, and AHB909, respectively).
C. PsspB-lacZ activity in cells harboring wild type AA-AH (closed squares), deleted for AA-AH (closed circles), or deleted for AA-AH and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB992, and AHB1006, respectively).
D. PsspB-lacZ activity in cells harboring wild type AA (closed squares), deleted for AA (closed circles), or deleted for AA and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB1034, and AHB1048, respectively).
E. PsspB-lacZ activity in cells harboring wild type AE (closed squares), deleted for AE (closed circles), or deleted for AE and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB1040, and AHB1054, respectively).
F. PsspB-lacZ activity in cells harboring wild type AA-AG (closed squares), deleted for AA-AG (closed circles), or deleted for AA-AG and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB1164, and AHB1166, respectively).
G. PsspB-lacZ activity in cells harboring wild type AH (closed squares), deleted for AH (closed circles), or deleted for AH and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB1046, and AHB1060, respectively).
H. PsspB-lacZ activity in cells harboring wild type Q (closed squares), deleted for Q (closed circles), or deleted for Q and harboring pbpGΔR147-K148 (open circles) (strains AHB640, AHB949, and AHB964, respectively).
Accordingly, we decided to reinvestigate the role of CsfB in σG activation using the σG–specific reporter PsspB-lacZ. As shown in Figure 1D, deletion of csfB did not alter the timing of σG activation during sporulation, nor did it permit σG activation in a sigE mutant. Furthermore, σG activation remained almost wholly dependent on AA-AH, Q, and J in cells deleted for csfB (data not shown). We did, however, detect a small increase in the basal level of σG activity prior to its normal time of induction in the absence of CsfB (Figure 1D inset). In agreement with Chary et al. (2007), we therefore conclude that CsfB suppresses low levels of inappropriate σG activity. However, because the overall profile of σG activation was largely unaffected in a csfB mutant, relief from CsfB is evidently not the mechanism by which σE-directed gene expression triggers the activation of σG.
The antisigma factor SpoIIAB is responsible for inhibiting σF at early times during sporulation and has also been shown to bind to, and inhibit, σG (Kirchman et al., 1993, Kellner et al., 1996). But analogous to our findings with CsfB, more recent studies have indicated that SpoIIAB is not responsible for σG inhibition in the forespore prior to the completion of engulfment or in the absence of AA-AH or J (Evans et al., 2003, Serrano et al., 2004, Chary et al., 2005). Conceivably, however, CsfB and SpoIIAB function redundantly and both must be inactivated in order to uncouple σG activation from σE-directed gene expression. To test this idea, we introduced a point mutation into the PspoIIQ-sigG construct, such that glutamate 156 of the resulting σG protein was switched to lysine. This E156K substitution has been shown to render σG immune to SpoIIAB inhibition, thereby eliminating the influence of SpoIIAB on σG without affecting SpoIIAB–mediated σF regulation (Serrano et al., 2004). We then monitored σG–directed expression of the PsspB-lacZ reporter both in a PspoIIQ-sigGE156K mutant and in a PspoIIQ-sigGE156K csfB double mutant. In both cases, we observed no significant change in the timing or extent of σG activation (Figure 1E). We did, however, see a slight increase in the basal level of σG activity in the double mutant (Figure 1E inset). Evidently, SpoIIAB cooperates with CsfB to suppress low levels of basal σG activity that escape the control mechanisms that normally delay its activation until after engulfment.
Finally, we examined the dependence of σG activation on σE by introducing a sigE deletion into the PspoIIQ-sigGE156K csfB double mutant. The results show that σG activation remained almost completely dependent on the mother-cell transcription factor (Figure 1E). We conclude that escape from SpoIIAB and CsfB, individually or together, is not the basis for activation of σG.
Isolation of suppressor mutants that partially bypass the dependence of σG activation on J
In an effort to elucidate the pathway by which σG is activated, we turned our attention to the ten proteins that are required for activation of the forespore transcription factor but are not needed for the process of engulfment. These are the eight proteins (AA-AH) encoded by the spoIIIA operon, the forespore protein Q, and the vegetative protein J. Our strategy was to attempt to eliminate some of these proteins by seeking bypass mutants and thereby identify those remaining proteins that are minimally required for the activation of σG. We reasoned that as a vegetative protein, J might be the most peripheral, and we therefore initially focused on seeking bypass mutants of it.
We therefore executed a genetic selection to identify spontaneous mutations that permitted spore formation and σG activation in the absence of J. To avoid the complications associated with the PsigG promoter (discussed above), we performed the selection in a strain expressing PspoIIQ-sigG. Furthermore, we included a deletion of csfB to eliminate the possibility that CsfB–mediated σG inhibition might mask the effect of relevant mutations. [Unless otherwise noted, all strains utilized for the remainder of this study expressed PspoIIQ-sigG and were deleted for csfB.] The resulting PspoIIQ-sigG csfB J mutant strain (referred to henceforth as the J mutant for simplicity) was subjected to repeated rounds of sporulation, heat-treatment (to kill cells that had not successfully sporulated), germination, and growth to enrich for spontaneously-arising mutants with increased sporulation efficiency. After selection in this manner, several mutants were isolated with increased spore forming ability as compared to the parental J mutant. As shown in Figure 2A, the isolated mutations restored sporulation efficiency to the J mutant by 1-4 orders of magnitude. For comparison, the presence of the wild-type J gene increased spore formation by 7 orders of magnitude, to a level nearly identical to the sporulation efficiency of a fully wild type strain.
Figure 2. Isolation of J suppressor mutants partially restored for spore formation and σG activation.
A. Spore formation is partially restored to J suppressor mutants a-e. Cells were induced to sporulate for 24 hours in DSM medium, and spores were measured as heat resistant colony forming units. With the exception of the parental wild-type strain (WT; PY79), all strains expressed PspoIIQ-sigG in place of endogenous sigG and were deleted for csfB. Cells harbored a wild-type copy of the J gene (J+; AHB480), lacked J (ΔJ; AHB434), or lacked J and harbored one of the five indicated suppressors (ΔJ suppressors a-e; AHB442, AHB547, AHB543, AHB438, AHB551, respectively). Error bars indicate standard deviation.
B. σG activation is partially restored to J mutant cells harboring suppressors a-e. σG–dependent PsspB-lacZ expression was monitored during sporulation of cells harboring a wild-type copy of the J gene (J+; AHB352), lacking J (ΔJ; AHB366), or lacking J and harboring one of the five indicated suppressors (ΔJ suppressors a-e; AHB419, AHB422, AHB420, AHB418, AHB423, respectively). All strains expressed PspoIIQ-sigG in place of endogenous sigG and were deleted for csfB. β-galactosidase activity was normalized to the maximal activity observed for the J+ wild type control strain (% WT maximum).
C. Cartoon of proteins encoded by J suppressor mutant genes yqjG, spoIIIAE, and pbpG. The predicted membrane topology of YqjG is based on that presented by Tjalsma et al. (2003). The N-terminal signal peptide and membrane topology of SpoIIIAE (referred to in the main text as AE) was predicted by the PolyPhobius algorithm (Kall et al., 2005, Kall et al., 2007). The SignalP algorithm (Bendtsen et al., 2004) indicated a high likelihood of signal peptidase (SP) cleavage at the site indicated. The membrane topology of PbpG is based on results from the PolyPhobius algorithm (Kall et al., 2005, Kall et al., 2007) and is consistent with the fact that high molecular weight PBPs are typically anchored in the cytoplasmic membrane by a non-cleavable N-terminal signal peptide (Ghuysen, 1994). Amino acid alterations isolated as J suppressors in each of the three proteins are indicated with asterisks.
We then monitored expression of the PsspB-lacZ reporter in the J suppressors and observed that σG activation was also partially restored in the isolated mutants, ranging from 10-40% of the levels displayed by an isogenic wild type (J+) strain (Figure 2B). In contrast, the J mutant displayed <5% of the σG activity displayed by the wild type. Thus, the suppressor mutations had bypassed the dependence of σG activation and spore formation on the J gene to a substantial extent. It is worth noting, however, that the J suppressors restored σG activity to J mutant cells much more potently (10-40% of wild type levels) than spore formation (0.001-0.1% of wild type levels). One possible explanation is that J plays an additional, as yet unknown role in sporulation, independent of σG activation.
The J suppressor mutations map to three genes
Standard mapping analysis and genetic characterization revealed five, non-identical suppressor mutations (designated a-e) located in one of three genes: yqjG, AE, and pbpG (Figure 2C). Suppressor mutations a and b fell within the yqjG coding sequence and resulted in an alanine to serine substitution at codon 238 (yqjGA238S) and a glycine to valine substitution at codon 247 (yqjGG247V), respectively. [Details regarding the nucleotide changes in the isolated mutants are given in Experimental Procedures.] The c and d mutations were in the AE gene, with c resulting in an alanine to glycine substitution at codon 254 (AEA245G) and d changing serine codon 265 to a proline codon (AES265P). Finally, mutation e was a six nucleotide deletion in the pbpG gene, resulting in deletion of arginine and lysine codons 147 and 148 (pbpGΔR147-K148). Inspection of the sequence near the deletion in pbpG revealed the presence of a direct repeat of 5 nucleotides spaced 1 nucleotide apart, suggesting that slippage during DNA replication produced the isolated deletion. [During the course of this analysis we detected two discrepancies in the AE sequence and one in the pbpG sequence as compared to published sequences; see Experimental Procedures.]
To determine whether the J suppressor mutations caused a sporulation phenotype on their own, we integrated a wild-type copy of the J gene at the thrC locus of each suppressor mutant. The resulting strains displayed approximately the same level of sporulation as a corresponding wild type and, as a control, the parental J mutant that harbored thrC::J (data not shown). These findings indicated that the J suppressor mutants do not significantly affect sporulation in the presence of J. Next we wondered whether the absence of the σG-inhibitor CsfB influenced the ability of the suppressor mutants to partially restore spore formation to cells lacking J. (As discussed above, the suppressor selection was performed in a strain deleted for csfB.) To test this possibility, we integrated a functional, wild-type copy of the csfB gene at the thrC locus and measured the sporulation efficiency of the resulting strains. Strikingly, the thrC::csfB complementation construct had no measurable effect on the sporulation efficiency of any of the suppressor mutants (data not shown).
The absence of J can be alleviated by alterations to its paralog YqjG or its likely substrate AE
As reported above, spore formation and σG activation can be restored to a J mutant by mutations in the yqjG or AE genes. (The suppression of J by pbpG mutation will be addressed separately below.) Interestingly, yqjG encodes a paralog of the J protein (displaying 37% identity), and both are members of a family of membrane protein translocases present in the inner mitochondrial membrane (Oxa1), the chloroplast thylakoid membrane (Alb3), and the inner membrane of Escherichia coli (YidC) (Yen et al., 2001). Evidence indicates that proteins in this Oxa1/Alb3/YidC family play critical roles in the insertion, translocation, folding, and assembly of membrane proteins and protein complexes into their respective membranes (reviewed in Yi & Dalbey, 2005, Xie & Dalbey, 2008). Consistent with this important cellular function, J and YqjG have been shown to be redundantly required for viability in B. subtilis (Murakami et al., 2002). However, J is also specifically required for sporulation, performing a function for which YqjG cannot substitute (Errington et al., 1992, Murakami et al., 2002). It appears likely, then, that the yqjGA238S and yqjGG247V alleles that we have isolated as J suppressors encode variants of the YqjG protein that have acquired some of the sporulation-specific functionality of its paralog protein J.
We have also found that spore formation and σG activation can be partially restored to cells lacking J by mutation of the AE gene. Fittingly, the AE gene is a member of the spoIIIA operon, which we have shown in Figure 1C to be required, like J, for σG activation. Inspection of the AE gene indicated that it encodes a highly hydrophobic protein. As such, we submitted the AE protein sequence to a signal peptide and transmembrane domain prediction program (PolyPhobius), which calculated the presence of an N-terminal signal peptide and seven additional membrane-spanning domains (Kall et al., 2005, Kall et al., 2007). Furthermore, a signal peptide prediction program (SignalP) indicated that the predicted AE signal peptide is likely to be cleaved by signal peptidase after position 24 (Bendtsen et al., 2004). The resulting predicted membrane topology of AE is shown in Figure 2C. In all, these predicted features suggest that AE is a polytopic membrane protein that utilizes the Sec system for assembly into the membrane.
Given that members of the Oxa1/Alb3/YidC protein family, which includes J, are known to aid in the insertion and folding of polytopic membrane proteins, at times in cooperation with the Sec machinery, we suggest that AE is a likely substrate of J. According to this model, AE does not assemble properly into the membrane in the absence of J, thereby blocking σG activation. The ability of AE mutations to suppress the σG activation defect of J mutant cells strongly supports this contention: it is likely that the isolated variants of AE (AEA245G and AES265P) are more capable of assembling into the membrane on their own and/or have acquired the ability to be recognized as a substrate by the J paralog YqjG.
If AE represents a major sporulation-specific substrate of J, we reasoned that the isolated YqjG variants described above might have acquired the ability to recognize AE as a substrate. If this were true, we would not expect the isolated yqjG suppressors of J to suppress the σG–activation defect of cells lacking AE. Indeed, we observed that σG–dependent activity of the PsspB-lacZ reporter was almost fully blocked in an AE single mutant as well as in an AE yqjGG247V double mutant (Figure 3B). While these findings do not establish that AE is the target of YqjGG247V, they are consistent with this idea. Next, we wondered whether the isolated AE and yqjG mutations might display a genetic interaction if combined in cells lacking J. Strikingly, we found that a triple J yqjGG247V AES265P mutant strain activated σG to a significant extent (nearly 50% of the activity displayed by the corresponding wild type strain) (Figure 3A). In contrast, the two corresponding double mutants J yqjGG247V and J AES265P displayed σG activity that reached 16 and 20% of the wild type levels, respectively, while the J single mutant activated σG to a level <5% of wild type.
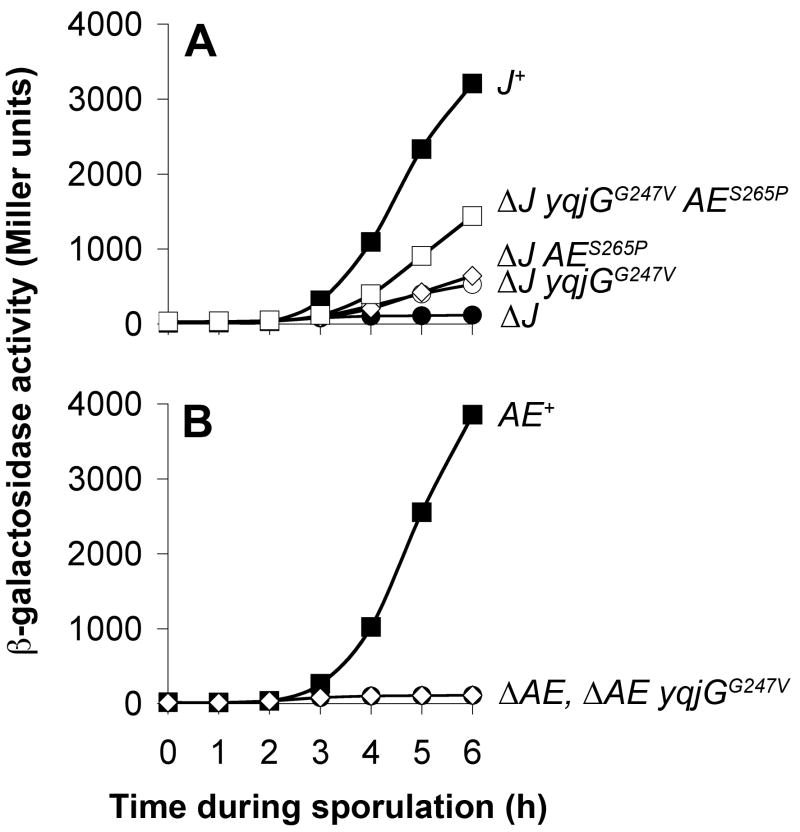
Figure 3. yqjGG247V acts synergistically with AES265P to restore σG activity to J mutant cells and cannot restore σG activity to cells lacking AE.
A. yqjGG247V cannot restore σG activity to cells lacking AE. σG–dependent PsspB-lacZ expression was monitored during sporulation of cells harboring a wild-type copy of the J gene (closed squares), deleted for J (closed circles), or deleted for J and harboring yqjGG247V (open circles), AES265P (open diamonds), or both yqjGG247V and AES265P (open squares) (strains AHB640, AHB655, AHB657, AHB659, and AHB661, respectively). All strains expressed PspoIIQ-sigG in place of endogenous sigG and were deleted for csfB.
B. yqjGG247V acts synergistically with AES265P to restore σG activity to J mutant cells. The σG–dependent accumulation of β-galactosidase from the PsspB-lacZ reporter gene in cells containing a wild-type copy of the AE gene (closed squares), deleted for AE (closed circles), or deleted for AE and harboring yqjGG247V (open diamonds) was monitored through sporulation (strains AHB640, AHB936, and AHB937, respectively). All strains expressed PspoIIQ-sigG in place of endogenous sigG and were deleted for csfB.
Alteration of a peptidoglycan biosynthetic enzyme alleviates the σG–activation defect of cells lacking J
The strongest J suppressor mutant (e) was determined to harbor a six-nucleotide deletion in the pbpG gene, which encodes a Class A high-molecular-weight penicillin-binding protein (PBP). PBPs are enzymes that direct the assembly of peptidoglycan, a structural component of the bacterial cell wall composed of strands of repeating N-acetylglucosamine and N-acetyl muramic acid sugar residues crosslinked by peptide side chains (reviewed in Ghuysen, 1991). Class A PBPs display two enzymatic activities: the first is a transglycosylase activity required for the addition of disaccharide pentapeptide subunits onto growing glycan strands, and second, a transpeptidase activity that promotes the covalent linkage (crosslinking) of peptides across glycan strands. The pbpG gene encodes one of four Class A PBPs in B. subtilis, and interestingly, this gene is expressed specifically in the forespore under the control of σF and σG (Pedersen et al., 2000). Cells lacking pbpG do not display a sporulation phenotype; however, when pbpG and pbpF (which encodes a second forespore-expressed Class A PBP) (Popham & Setlow, 1993) are simultaneously deleted, the resulting cells display severe defects in the layer of peptidoglycan that surrounds the developing spore (known as the cortex) and are ultimately unable to form stable, mature spores (McPherson et al., 2001).
Close inspection of the amino acid sequences of Class A PBP family members revealed that the isolated PbpGΔR147-K148 variant lacks an arginine and lysine that are part of a highly conserved motif (RKxxE) within the transglycosylase domain (Goffin & Ghuysen, 1998). We therefore predicted that this altered PbpG protein was non-functional. To test this idea, we determined the ability of pbpGΔR147-K148 to complement the sporulation defect of a pbpG pbpF double mutant. Wild type pbpG integrated at an exogenous locus (thrC) fully restored spore formation to the pbpG pbpF double mutant. In contrast, thrC::pbpGΔR147-K148 was unable to correct the sporulation defect of the pbpG pbpF cells, confirming that the PbpGΔR147-K148 protein is non-functional (data not shown).
Given that pbpGΔR147-K148 appears to encode a non-functional version of PbpG, we wondered whether a deletion of pbpG could restore σG activation to J mutant cells in the same manner as pbpGΔR147-K148. Surprisingly, we found that the J pbpG double mutant was blocked for σG activation to the same extent as the J mutant alone (data not shown). Furthermore, we obtained the same results with a J pbpF double mutant and J pbpG pbpF triple mutant (data not shown). These findings indicate that loss of PbpG function (or PbpF function, or both) is not sufficient to suppress the σG–activation defect of J mutant cells. To further investigate this unexpected finding, we performed complementation analysis to determine whether pbpGΔR147-K148 behaved in a recessive or dominant manner regarding J suppression. Importantly, if pbpGΔR147-K148–mediated suppression of J were due to a loss of function, it would be expected to behave recessively. In contrast, we observed that pbpGΔR147-K148 behaved in a semi-dominant manner: J mutant strains harboring both the pbpGΔR147-K148 and wild type pbpG alleles continued to display increased spore formation, albeit not to the same levels as a J mutant harboring pbpGΔR147-K148 alone (data not shown). Altogether, the simplest explanation for the complex genetic behavior of the pbpGΔR147-K148 allele is that the produced protein behaves as a dominant-negative, possibly compromising the overall activity of the peptidoglycan biosynthetic machinery in the space between the forespore and mother cell. Alternatively, the PbpGΔR147-K148 variant protein may cause an imbalance in the rates of peptidoglycan transglycosylation versus transpeptidation that cannot be rescued by the presence of wild type PbpG. In either case, it appears likely that an impairment and/or delay in cortex synthesis in the intercompartmental space between the inner and outer membranes that surround the forespore can partially restore σG activation and spore formation to J mutant cells.
PbpGΔR147-K148 reveals a minimal pathway for σG activation
Based on our characterization of PbpGΔR147-K148–mediated suppression of the J mutant phenotype (above), we hypothesized that cortex synthesis in the intercompartmental space between the mother cell and forespore might normally serve to sterically dampen and/or ultimately shut down the signaling pathway that links σE activity in the mother cell to σG activation in the forespore. A disruption or delay in cortex synthesis might therefore be expected to potentiate the σE–dependent, intercellular pathway for σG activation, as long as the most critical components of that pathway were not compromised. Based on this logic, we concluded that J is not a core component of the σG–activation pathway, given that a J mutant was suppressed by pbpGΔR147-K148. This contention is further supported by our earlier finding that J is likely to influence σG activation indirectly through its potential substrate protein AE.
We reasoned that the pbpGΔR147-K148 mutation might serve as a powerful tool to identify the most critical components of the post-transcriptional σG–activation pathway. More specifically, if cells lack a core factor of the activation pathway, we expected that pbpGΔR147-K148 would not be able to restore σG activity (given that a nonexistent signal cannot be amplified). Conversely, if cells lack an accessory component of the activation pathway, such as J, pbpGΔR147-K148 would (partially) re-establish σG activity. We therefore tested the ability of the pbpGΔR147-K148 mutation to restore σG activation to cells lacking each of the genes required for activation of the forespore transcription factor. First, as a control, we introduced the pbpGΔR147-K148 allele into J mutant cells and confirmed that σG activity (as measured by PsspB-lacZ reporter gene expression) was significantly rescued in the J pbpGΔR147-K148 double mutant as compared to the J mutant alone (Figure 4A). Next, we tested σG activation in sigE pbpGΔR147-K148 double mutant cells. The results clearly indicated that pbpGΔR147-K148 was unable to suppress the σG–activation defect of cells lacking σE (Figure 4B). This finding was satisfying, as it indicated that pbpGΔR147-K148 was unable to re-establish σG activity altogether independently of the σE–coupled activation pathway.
Next, we tested the ability of pbpGΔR147-K148 to suppress the σG–activation defect of cells mutant for the spoIIIA operon. To begin, we tested cells deleted for the entire operon, and found that AA-AH pbpGΔR147-K148 mutant cells were nearly completely defective for σG-dependent PsspB-lacZ reporter gene activation (Figure 4C). This result indicated that at least one of the eight genes in the spoIIIA operon is required for σG activation in cells harboring the pbpGΔR147-K148 suppressor mutation. To more specifically identify the critical protein(s), we tested the ability of pbpGΔR147-K148 to suppress the σG–activation defect of cells harboring in-frame, non-polar deletions of AA, AB, AC-AD, AE, AF, AG, and AH. Strikingly, cells individually lacking AA, AB, AC-AD, AE, AF, or AG displayed significant σG-dependent β-galactosidase production (∼20% wild type levels) when combined with pbpGΔR147-K148. The data showing pbpGΔR147-K148–mediated restoration of σG activity in cells individually deleted for AA or AE are shown in Figure 4D-E, and are representative of the data obtained for other individual deletions of the spoIIIA genes AA-AG. (Data for AB, AC-AD, AF and AG deleted cells are given in Supplemental Figure S1.) Even more remarkably, we observed that cells simultaneously lacking AA-AG were similarly restored for σG activity in the presence of pbpGΔR147-K148 (Figure 4F). In contrast, deletion of AH alone blocked significant σG activation regardless of the presence of pbpGΔR147-K148 (Figure 4G). We did, however, note that AH mutant cells were not quite as severely defective in σG activity when compared to cells lacking other members of the spoIIIA operon. Although only a very slight difference, we reproducibly observed that cells lacking AA-AG reached only ∼2-3% of wild type σG activity levels by hour 6, whereas cells lacking AH displayed ∼5-6% wild type σG activity levels at the same time. Nevertheless, neither AH single nor AH pbpGΔR147-K148 double mutant cells achieved substantial levels of σG activity.
Our genetic data indicate that AH is the only protein encoded by the spoIIIA operon required for significant σG activation in pbpGΔR147-K148 cells. One potentially trivial explanation for this finding is that cells deleted for AH (but not AA-AG) and simultaneously mutant for pbpG might fail to complete forespore engulfment. AH has been observed to become required for engulfment in certain genetic backgrounds (Broder & Pogliano, 2006), and furthermore, σG is well-established to remain inactive in mutants blocked for engulfment (for example, Margolis et al., 1993, Smith et al., 1993, Frandsen & Stragier, 1995). To address this possibility, we monitored the completion of engulfment in the AH pbpGΔR147-K148 double mutant and control strains using a previously described microscopic assay (Sharp & Pogliano, 1999). Importantly, we found that AH pbpGΔR147-K148 cells completed forespore engulfment to the same extent as AH single mutant and isogenic wild type cells (data not shown).
To confirm that the pbpGΔR147-K148 suppressor restored σG activity to AA-AG mutant cells specifically in the forespore compartment of the developing sporangium, we introduced a reporter gene encoding cyan fluorescent protein (cfp) under the control of PsspB into wild type, AA-AG, and AA-AG pbpGΔR147-K148 cells. (As with all strains in this study, unless otherwise noted, these cells expressed PspoIIQ-sigG and were deleted for csfB.) As expected, significant σG–dependent PsspB-cfp expression was observed by florescence microscopy in sporulating wild type cells, specifically in the forespore compartment of sporangia that had completed forespore engulfment (a representative image from hour 5 is shown in Figure 5A). In contrast, only low levels of CFP florescence were detected in the engulfed forespores of AA-AG mutant sporangia (Figure 5B). Strikingly, we observed intermediate levels of PsspB-cfp expression in AA-AG pbpGΔR147-K148 double mutant forespores, consistent with significant compartment-appropriate restoration of σG activity (Figure 5C).
Figure 5. The pbpGΔR147-K148 suppressor mutation restores σG activity to AA-AG mutant cells in a compartment-specific manner.

σG–dependent expression of a PsspB-cfp reporter gene was monitored by fluorescence microscopy in cells collected at hour 5 of sporulation with the following relevant genotypes: (A) wild type, (B) AA-AG, and (C) AA-AG pbpGΔR147-K148 (strains AHB1331, AHB1352, and AHB1353, respectively). All strains expressed PspoIIQ-sigG and were deleted for csfB. CFP fluorescence is shown in grayscale (“PsspB-CFP”-labeled column) or false-colored green (“Merge”-labeled column). CFP images were acquired and processed with identical parameters to permit direct comparison of fluorescence intensity among samples. Membrane fluorescence from FM 4-64 staining is shown in grayscale (“Membrane”-labeled column) or in false-colored red (“Merge”-labeled column). Note that the membranes surrounding engulfed forespores are not detectable due to membrane-impermeability of the FM 4-64 stain.
In toto, these results suggest that of the eight proteins encoded by the spoIIIA operon, only one (AH) is indispensible for σG activation in pbpGΔR147-K148 cells; the remaining seven proteins (AA-AG) appear to be unneccesary, at least under certain genetic conditions, for significant σG activity.
Finally, we tested whether pbpGΔR147-K148 was able to restore σG activity to cells lacking Q. As shown in Figure 4H, Q pbpGΔR147-K148 double mutant cells remained nearly fully blocked for activation of the forespore transcription factor. As described above for AH pbpGΔR147-K148 cells, we confirmed that Q pbpGΔR147-K148 double mutant cells completed forespore engulfment to the same extent as Q single mutant and isogenic wild type cells (data not shown). Altogether, these results indicate that of the ten proteins required for σG activation (AA-AH, Q, and J), just two (AH and Q) appear to constitute a minimal pathway that is sufficient for significant σG activation in pbpGΔR147-K148 cells.
AH is homologous to a family of multimeric pore-forming proteins
Next, we turned our attention to AH and Q. AH is expressed in the mother cell under the control of σE, while Q is expressed under the control of σF in the forespore. Both AH and Q harbor N-terminal transmembrane segments and large extracellular C-terminal domains; importantly, two recent reports demonstrated that the AH and Q extracellular domains directly interact across the intermembrane space between the mother cell and forespore (Blaylock et al., 2004, Doan et al., 2005). A simple BLAST search against a non-redundant protein database with the AH protein sequence revealed homology to its orthologs in other spore forming bacteria but little else. In the hopes of finding more remote homologs of AH, we subjected the sequence to the HHpred search algorithm, which performs a PSI-BLAST on a query protein sequence to generate a hidden Markov model (HMM) that is in turn used to perform a pair-wise comparison with a database of HMMs (Soding et al., 2005). Importantly, the HHpred algorithm can consider both amino acid conservation (based on the alignment of close homologs) and secondary structure conservation (based on known or predicted secondary structures of close homologs) in its search criteria. Strikingly, the extracellular domain of AH displayed similarity (92% probability score) to a family of proteins associated with type III secretion systems (T3SSs) in pathogenic gram negative bacteria and the flagellum in gram negative and gram positive bacteria (Pfam “YscJ_FliF” family, Finn et al., 2008). Representative proteins from this family include the T3SS proteins EscJ from enteropathogenic E. coli, PrgK from Salmonella typhimurium, and YscJ from Yersinia pestis, as well as the flagellar protein FliF. (For simplicity, these proteins will be referred to henceforth as the EscJ family.) The recently solved EscJ crystal structure indicates that it is composed of two domains, both of which are capable of intermolecular contacts (Crepin et al., 2005, Yip et al., 2005). Remarkably, further molecular modeling indicated that these intermolecular contacts could cause the EscJ protein to multimerize to form a 24-subunit hollow ring (Figure 6A) (Yip et al., 2005). As shown in Figure 6B, many residues that are highly conserved in Domain 2 of EscJ family members are also conserved among AH family members. Furthermore, the predicted secondary structure of AH family members aligns remarkably well with the known secondary structure of EscJ family members. In all, the apparent similarity of AH to this family of proteins raises the intriguing possibility that AH may be capable of multimerization in the mother-cell membrane (Figure 6A).
Figure 6. Similarity of AH to a family of multimeric pore-forming proteins suggests a model for the signal transduction pathway governing the activation of σG.
A. Cartoon depicting the domain structures of the EscJ and AH families of proteins and their assembly into a multimeric pore structure (known [Yip et al., 2005] or speculative, respectively). As indicated, the C-terminal extracellular region of AH proteins displays similarity to Domain 2 of the EscJ family of proteins.
B. Detailed depiction of the homology and secondary structure similarity detected between EscJ and AH proteins by the HHPred algorithm (92% probability score) (Soding et al., 2005). Multiple sequence alignments of proteins from the EscJ family (Domain 2 region) and AH family (C-terminal region) were generated with ClustalW program (Thompson et al., 1994) and juxtaposed to indicate amino acid conservation. Connector lines indicate amino acids that show high conservation among and between the family members. The known secondary structure of EscJ (Yip et al., 2005) is shown above the multiple sequence alignment of EscJ family members (alpha helices 3 and 4, beta strands 4, 5, and 6). Likewise, the predicted secondary structure of AH (predicted with the PSIPRED algorithm) (McGuffin et al., 2000), which shows striking similarity to the experimentally determined secondary structure of EscJ family members, is indicated below the multiple sequence alignment of AH family members. Fragments from the following proteins from the EscJ family were included in the multiple sequence alignment: Salmonella typhimurium PrgK, Shigella flexneri MxiJ, enteropathogenic E. coli EscJ, Yersinia pestis YscJ, Pseudomonas aeruginosa PscJ, and E. coli FliF. The AH proteins from the following spore forming bacteria were included in the multiple sequence alignment: B. subtilis (Bsu_AH), B. licheniformis (Bli_AH), B. halodurans (Bha_AH), B. clausii (Bcl_AH), Oceanobacillus iheyensis (Oih_AH), B. anthracis (Ban_AH), and Clostridium difficile (Cdi_AH).
C. Model for the signal transduction pathway governing the activation of σG. We propose that the interaction between AH and Q across the intermembrane space between the mother cell and forespore, coupled with AH multimerization, leads to the formation of a channel between the two compartments of the developing sporangium. In our model, the AH-Q channel permits the transport of an unknown substrate required for σG activation into the forespore. According to this model, σE is required for σG activation in part because it is required for expression of proteins that comprise the channel. Further details are provided in the Discussion.
Discussion
The intercellular signaling pathway controlling σG activity during B. subtilis sporulation is poorly understood, due in part to the fact that at least ten proteins, few of known or predicted structure or function, are required. Here we have identified genetic conditions under which only two of these proteins, AH and Q, are required for significant σG activity while the remaining proteins, J and AA-AG, are dispensable. This suggests that AH and Q are capable of constituting a minimal pathway for the activation of σG. The similarity of AH to type III secretion proteins, together with the fact that AH interacts with Q (Blaylock et al., 2004, Doan et al., 2005), raises the intriguing possibility that these two proteins direct the formation of a multimeric channel or pore that spans the mother cell and forespore membranes (Figure 6C). AH and Q are known to co-localize as several punctuate foci around the engulfed forespore (Blaylock et al., 2004), and it is conceivable that these foci correspond to channels across the intercompartmental space between the two cells.
We speculate that this putative AH-Q channel transports an effector molecule from the mother cell to the forespore that triggers the activation σG. If so, what is the nature of this effector molecule and how does it act? One formal possibility is that the effector is an anti-antisigma factor for an as-yet-to-be-discovered antisigma factor that holds σG inactive in the forespore. (As we have demonstrated in this study, the antisigma factor cannot be SpoIIAB or CsfB.) In such a model, a mutant of the anti-antisigma factor would be expected to be blocked in the activation of σG. If so, a candidate for the gene encoding the anti-antisigma factor has eluded many years of intensive genetic analysis. Similarly, other kinds of models in which the AH-Q channel pumps a protein effector for σG activation into the forespore seem unlikely given the absence of candidate sporulation genes whose mutant phenotypes indicate a role in the control of σG.
It nonetheless remains possible that the hypothetical effector protein was missed in genetic analysis because it is essential for viability or because it plays an additional role early in sporulation. Nevertheless, and in light of these considerations, it seems simplest to suppose that if the proposed AH-Q channel pumps an effector into the forespore, the molecule in question is not a protein. Specifically, we are entertaining the idea that the mother cell nurtures or feeds the developing spore through the putative AH-Q channel, providing substrates for macromolecular synthesis. This idea is especially appealing because at the time of σG activation, the engulfed forespore is isolated from the external environment and thus unable to take up resources on its own. Furthermore, it may be necessary for the forespore, which is destined to become a dormant spore, to halt certain metabolic activities at an earlier time during sporulation than was previously appreciated. Regardless, in this model, σG inactivity in sigE, AA-AH, J, or Q mutants is not due to an antagonist of σG, but rather to the absence of basic components required for transcription or translation, such as nucleotides or amino acids. A specific version of this model holds that the AH-Q channel provides ATP and other nucleotides that become limiting for transcription at intermediate to late stages of development in the forespore. In this view, these nucleotides are needed to sustain all transcription in the forespore. This model predicts that in cells engineered to produce in the forespore a sigma factor unrelated to sporulation, the activity of this alternative sigma factor would also exhibit dependence on the channel.
Another question raised by our model is the role of J and the other proteins encoded by the spoIIIA operon, AA-AG, in σG activation. Our genetic suppressor data suggest that each of these proteins can be dispensable for σG activity, indicating that they may only play accessory, or indirect roles in the intercellular signaling pathway. Indeed, we propose that J influences σG activity indirectly by facilitating the folding and assembly of AE into the membrane. This idea is supported by our isolation of AE mutations that restore significant σG activity and spore formation to J mutant cells, as well as by the predicted function of J (membrane protein insertase related to E. coli YidC) and attributes of AE (predicted to be a polytopic membrane protein). One apparent contradiction to our speculation that the mother-cell protein AE is a substrate of J during sporulation comes from a study demonstrating that expression of J by a forespore-specific promoter (PspoIIQ), but not a mother-cell-specific promoter (PspoIID), was sufficient to promote wild-type levels of sporulation (Serrano et al., 2003). (The endogenous J promoter is expressed vegetatively, thereby ordinarily allowing J to accumulate in both compartments during sporulation.) The authors of this study did note, however, that expression of PspoIID-J permitted significant levels of sporulation (∼5% wild type). One possible explanation for these apparently contradictory findings is that J may act on AE in the mother-cell membrane and on an unknown substrate(s) in the forespore membrane, both of which are required for efficient sporulation. In this case, it is possible that the PspoIIQ-J construct utilized by Serrano et al. (2003) permitted a small amount of J expression during vegetative growth and/or in the mother cell to satisfy the requirement for J in AE assembly into the mother-cell membrane.
What are the roles of AA-AG in σG activation? Our genetic suppressor data indicate that AA-AG may be less important than AH for σG activation, given that AA-AG pbpGΔR147-K148 cells, but not AH pbpGΔR147-K148 cells, display significant σG activity. In this light, it is tempting to speculate that AA-AG may play accessory roles in σG activation, such as regulating the assembly, activity, or specificity of a channel formed by AH and Q. However, this view is inconsistent with a second line of evidence suggesting that AA-AG are ordinarily more critically required for σG activity and sporulation than AH. More specifically, we observed in this work that cells mutant for AH are not quite as severely defective for σG activation as AA-AG mutants (although all mutants were significantly defective when compared to the corresponding wild type strain). Additionally, we and others (D. Rudner, personal communication) have observed that cells individually mutant for AA-AG are more severely impaired in sporulation (by a factor of ∼105) than a strain lacking AH (impaired by a factor of ∼102-103). Altogether, these data suggest that AA-AG, more so than AH, are critically important for σG activation and spore formation under normal conditions. How are we to reconcile these apparently contradictory findings? An appealing possibility is that AH and Q serve as a platform for assembly of the proposed channel, which is composed of the AA-AG proteins. In this scenerio, AA-AG would, under normal circumstances, be most critical for function of the channel. However, in the presence of the pbpGΔR147-K148 suppressor, AH and Q alone might serve as a functioning channel to a significant extent. An independent line of investigation has led J. Meisner, X. Wang, and C. Moran (personal communication) to also infer that the AA-AH proteins constitute a channel that links the mother cell to the forespore.
Finally, we return to the role of CsfB in σG activation. As presented earlier, CsfB is not sufficient to explain the inhibition of σG prior to engulfment or in cells mutant for sigE (or AA-AH, J, or Q). Nonetheless, CsfB is produced in the forespore under σF control and is capable of inhibiting σG (Decatur & Losick, 1996, Chary et al., 2007, Karmazyn-Campelli et al., 2008). This means that as a precondition to the activation of σG in wild type cells, CsfB must be inactivated at the appropriate time during sporulation (i.e. after forespore engulfment). In their recent study, Karmazyn-Campelli et al. (2008) point out that the CsfB protein resembles zinc metalloproteins, whose activities are often regulated by zinc ion binding. They suggest, therefore, that engulfment of the forespore (resulting in its isolation from the external environment) could, in principle, create physiological conditions that alter the ability of CsfB to bind zinc, and thereby block the ability of CsfB to interact with and inhibit σG. An especially appealing aspect of this model, consistent with the data we present here, is that CsfB deactivation need not rely on AA-AH, J, or Q, but instead depends solely on the completion of the morphological event of engulfment. Nonetheless, CsfB inactivation evidently does not serve as the signal for the completion of engulfment, as the absence of CsfB did not permit σG activation in a mutant (of the spoIIM gene) that fails to complete engulfment (data not shown).
In conclusion, we have executed a genetic strategy that identified conditions under which AH and Q are the two most critical components of the pathway linking σE–directed gene expression in the mother cell to the activation of σG in the forespore. In contrast, the remaining proteins required for σG activation through this pathway (AA-AG and J) appear to be partially dispensable under certain genetic conditions. Finally, in silico analysis of the AH protein sequence leads to a model for signal transduction in which σG activation relies on the transport of a substrate molecule from the mother cell to the forespore through a multimeric channed formed by AH and Q. One intriguing possibility is that the transported molecules do not specifically regulate σG but instead contribute more generally to the ability of the forespore to carry out macromolecular synthesis.
Experimental Procedures
General methods
For general propagation, bacterial strains were grown in Luria-Bertani medium (LB). When appropriate, antibiotics were included in the growth medium as follows: chloramphenicol (5 μg/ml), erythromycin plus lincomycin (1 μg/ml and 25 μg/ml respectively), spectinomycin (100 μg/ml), kanamycin (5 μg/ml), tetracycline (10 μg/ml), phleomycin (0.4 μg/ml), and ampicillin (100 μg/ml). To measure spore formation, cells were induced to sporulate by nutrient exhaustion for approximately 24 hours at 37°C in Difco sporulation medium (DSM) (Schaeffer et al., 1965, Nicholson & Setlow, 1990), and the number of colony forming units that survived heat treatment (80°C for 20 minutes) were calculated. For measurements of gene activity with lacZ reporter genes, cells were induced to sporulate at 37°C by the resuspension method (Sterlini & Mandelstam, 1969, Nicholson & Setlow, 1990). Aliquots of cells were collected at intervals, stored at -80°C, and processed for β-galactosidase activity as previously described (Nicholson & Setlow, 1990).
Strain and plasmid construction
All experiments were performed with strains derived by transformation of the prototrophic laboratory strain PY79 (Youngman et al., 1984). Competent B. subtilis cells were prepared as previously described (Wilson & Bott, 1968). Details regarding strain and plasmid sources and construction are provided in Supplemental Materials. Additionally, see Supplemental Table S1 for the full genotypes of strains, Supplemental Table S2 for a description of plasmids, and Supplemental Table S3 for a list of primers utilized in this study.
Isolation of spoIIIJ suppressor mutants
Strain AHB366, harboring a deletion of the spoIIIJ gene, was grown for 24 hours in 100 ml DSM supplemented with 5 μg/ml chloramphenicol at 37°C to accumulate spontaneous mutations and sporulate. The culture was subjected to heat treatment at 80°C for 20 minutes to kill any cells that had not sporulated. To perform a second round of selection, 11 mL of the heat treated cells were inoculated into 100 ml fresh DSM/chloroamphenicol, grown at 37°C for 24 hours, and heat treated as above. Finally, a third round of enrichment for sporulation competent mutants was performed in the same manner. Eight mutants that displayed increased spore-forming ability after two or three rounds of selection were chosen for further analysis.
Standard B. subtilis mapping techniques or direct tests with candidate genes revealed five non-identical spoIIIJ suppressor mutants among the eight mutants chosen for further analysis. The yqjGA238S mutation (designated suppressor a) was recovered once and was due to a G to T transversion mutation at yqjG nucleotide 712. The yqjGG247V mutation (designated suppressor b) was recovered twice and was due to G to T transversion mutation at yqjG nucleotide 740. Suppressor mutation c (spoIIIAEA245G) was isolated twice and was caused by C to G transversion mutation at spoIIIA nucleotide 761. Suppressor mutation d (spoIIIAES265P) was also isolated twice and was caused by a T to C transition mutation at nucleotide 793. Finally, pbpGΔR147-K148 (designated suppressor e) was isolated once and was due to the precise deletion of pbpG nucleotides 438-443.
In the process of sequencing the mutant alleles of spoIIIAE and pbpG, several discrepancies were found between the published sequence of the wild-type B. subtilis 168 strain (http://genolist.pasteur.fr/SubtiList/) and our wild-type PY79 strain (which is derived from 168). These differences are as follows: (1) spoIIIAE nucleotide 86 was determined to be an A, not G as annotated, resulting in a glutamic acid residue at SpoIIIAE amino acid position 29 in place of the annotated glycine; (2) an annotated G at spoIIIAE position 1100 was absent in PY79-derived sequence, thereby altering the downstream reading frame; (3) spoIIIAG nucleotide 41 was determined to be a T, not C as annotated, resulting in a phenylalanine residue at SpoIIIAG amino acid position 14 in place of the annotated serine; (4) spoIIIAH nucleotide 464 was determined to be an A, not G as annotated, resulting in a glutamic acid residue at SpoIIIAH amino acid position 155 in place of the annotated glycine; (5) an annotated C in the intergenic space between spoIIIAH and accB (position 69 downstream of the spoIIIAH stop codon) was determined to be an A; and (6) an extra C was found immediately after pbpG nucleotide 1910 in PY79 when compared to the published B. subtilis 168 sequence, thereby altering the downstream reading frame. Importantly, the changes we documented for the predicted amino acid sequences of SpoIIIAE, SpoIIIAG, and SpoIIIAH are consistent with those submitted to the NCBI database by P. Stragier (Accession #AAA76724, AAA76726, and AAA76727, respectively). We did not investigate whether these differences are due to errors in the published sequence or represent bona fide variations between B. subtilis 168 and PY79. The extra C residue observed in the pbpG ORF was previously noted by Pedersen et al. (2000) and appears to be a variation between B. subtilis 168 and other 168-derived laboratory strains such as PY79.
Microscopy
Cells were induced to sporulate at 37°C by the resuspension method (Sterlini & Mandelstam, 1969, Nicholson & Setlow, 1990). At hour 5 of sporulation, cells were harvested and resuspended in 1× PBS containing 1 μg/ml of the membrane stain FM 4-64 (Invitrogen). Cells were then spotted onto a thin 1% agarose pad prepared on a glass slide and immobilized with a poly L-lysine-treated coverslip. Fluorescence microscopy was performed with an Olympus BX60 microscope fitted with a UplanFI 100× phase contrast objective. CFP was visualized using Chroma filter set 31044v2 (excitation filter 426-446 nm, dichroic mirror 455 nm, emission filter 460-500 nm), while FM 4-64 fluorescence was visualized using Olympus filter set U-MWG (excitation filter 510-550 nm, dichroic mirror 570 nm, barrier filter >590 nm). Images were captured using the Simple PCI imaging software version 6.0 (Hamamatsu Corporation). Exposure times for CFP fluorescence were set to 2000 ms without variation, while FM 4-64 images were acquired with exposure times ranging from 500-2000 ms. Images were adjusted for brightness and contrast, false colored, and overlayed using the Metamorph software version 4.6r5 (Universal Imaging). Finally, images were exported to Adobe Photoshop CS3 software. Importantly, CFP images were adjusted identically to permit comparisons of fluorescence intensity among samples.
Protein prediction and search tools
Transmembrane, signal peptides, and signal peptidase cleavage sites were predicted using the PolyPhobius (Kall et al., 2005, Kall et al., 2007) and SignalP (Bendtsen et al., 2004) algorithms. Secondary structure prediction was performed using the PSIPRED algorithm (McGuffin et al., 2000). Finally, homology searches were peformed with the HHpred program (Soding et al., 2005).
Supplementary Material
Acknowledgments
We thank P. Piggot, K. Pogliano, and D. Rudner for critical comments on the manuscript and members of the laboratory for helpful discussions. We would also like to thank C. Moran for communicating results prior to publication. This work was supported by a Helen Hay Whitney Foundation postdoctoral fellowship to A.H.C. and NIH grant GM18568 to R.L.
References
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Blaylock B, Jiang X, Rubio A, Moran CP, Jr, Pogliano K. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev. 2004;18:2916–2928. doi: 10.1101/gad.1252704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder DH, Pogliano K. Forespore engulfment mediated by a ratchet-like mechanism. Cell. 2006;126:917–928. doi: 10.1016/j.cell.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary VK, Meloni M, Hilbert DW, Piggot PJ. Control of the expression and compartmentalization of (sigma)G activity during sporulation of Bacillus subtilis by regulators of (sigma)F and (sigma)E. J Bacteriol. 2005;187:6832–6840. doi: 10.1128/JB.187.19.6832-6840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chary VK, Xenopoulos P, Piggot PJ. Expression of the sigma(F)-directed csfB locus prevents premature appearance of sigma(G) activity during sporulation of Bacillus subtilis. J Bacteriol. 2007;189:8754–8757. doi: 10.1128/JB.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepin VF, Prasannan S, Shaw RK, Wilson RK, Creasey E, Abe CM, Knutton S, Frankel G, Matthews S. Structural and functional studies of the enteropathogenic Escherichia coli type III needle complex protein EscJ. Mol Microbiol. 2005;55:1658–1670. doi: 10.1111/j.1365-2958.2005.04508.x. [DOI] [PubMed] [Google Scholar]
- Decatur A, Losick R. Identification of additional genes under the control of the transcription factor sigma(F) of Bacillus subtilis. J Bacteriol. 1996;178:5039–5041. doi: 10.1128/jb.178.16.5039-5041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T, Marquis KA, Rudner DZ. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol Microbiol. 2005;55:1767–1781. doi: 10.1111/j.1365-2958.2005.04501.x. [DOI] [PubMed] [Google Scholar]
- Errington J, Appleby L, Daniel RA, Goodfellow H, Partridge SR, Yudkin MD. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for sigma(G) activity at an intermediate stage of sporulation. J Gen Microbiol. 1992;138:2609–2618. doi: 10.1099/00221287-138-12-2609. [DOI] [PubMed] [Google Scholar]
- Evans L, Clarkson J, Yudkin MD, Errington J, Feucht A. Analysis of the interaction between the transcription factor sigma(G) and the anti-sigma factor SpoIIAB of Bacillus subtilis. J Bacteriol. 2003;185:4615–4619. doi: 10.1128/JB.185.15.4615-4619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen N, Stragier P. Identification and characterization of the Bacillus subtilis spoIIP locus. J Bacteriol. 1995;177:716–722. doi: 10.1128/jb.177.3.716-722.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen JM. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- Ghuysen JM. Molecular structures of penicillin-binding proteins and beta-lactamases. Trends Microbiol. 1994;2:372–380. doi: 10.1016/0966-842x(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Goffin C, Ghuysen JM. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev. 1998;62:1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL. An HMM posterior decoder for sequence feature prediction that includes homology information. Bioinformatics. 2005;21 1:i251–257. doi: 10.1093/bioinformatics/bti1014. [DOI] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007;35:W429–432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn-Campelli C, Bonamy C, Savelli B, Stragier P. Tandem genes encoding sigma-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989;3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- Karmazyn-Campelli C, Rhayat L, Carballido-Lopez R, Duperrier S, Frandsen N, Stragier P. How the early sporulation sigma factor sigma(F) delays the switch to late development in Bacillus subtilis. Mol Microbiol. 2008;67:1169–1180. doi: 10.1111/j.1365-2958.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Kellner EM, Decatur A, Moran CP., Jr Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol Microbiol. 1996;21:913–924. doi: 10.1046/j.1365-2958.1996.461408.x. [DOI] [PubMed] [Google Scholar]
- Kirchman PA, DeGrazia H, Kellner EM, Moran CP., Jr Forespore-specific disappearance of the sigma-factor antagonist SpoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol Microbiol. 1993;8:663–671. doi: 10.1111/j.1365-2958.1993.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Londono-Vallejo JA, Frehel C, Stragier P. SpoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol Microbiol. 1997;24:29–39. doi: 10.1046/j.1365-2958.1997.3181680.x. [DOI] [PubMed] [Google Scholar]
- Margolis PS, Driks A, Losick R. Sporulation gene spoIIB from Bacillus subtilis. J Bacteriol. 1993;175:528–540. doi: 10.1128/jb.175.2.528-540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- McPherson DC, Driks A, Popham DL. Two class A high-molecular-weight penicillin-binding proteins of Bacillus subtilis play redundant roles in sporulation. J Bacteriol. 2001;183:6046–6053. doi: 10.1128/JB.183.20.6046-6053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Haga K, Takeuchi M, Sato T. Analysis of the Bacillus subtilis spoIIIJ gene and its paralogue gene, yqjG. J Bacteriol. 2002;184:1998–2004. doi: 10.1128/JB.184.7.1998-2004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson WL, Setlow P. Sporulation, germination, and outgrowth. In: Harwood CR, Cutting SM, editors. Molecular Biological Methods for Bacillus. New York: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- Partridge SR, Errington J. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol Microbiol. 1993;8:945–955. doi: 10.1111/j.1365-2958.1993.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Ragkousi K, Cammett TJ, Melly E, Sekowska A, Schopick E, Murray T, Setlow P. Characterization of ywhE, which encodes a putative high-molecular-weight class A penicillin-binding protein in Bacillus subtilis. Gene. 2000;246:187–196. doi: 10.1016/s0378-1119(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Popham DL, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpF gene, which codes for a putative class A high-molecular-weight penicillin-binding protein. J Bacteriol. 1993;175:4870–4876. doi: 10.1128/jb.175.15.4870-4876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner DZ, Losick R. Morphological coupling in development: lessons from prokaryotes. Dev Cell. 2001;1:733–742. doi: 10.1016/s1534-5807(01)00094-6. [DOI] [PubMed] [Google Scholar]
- Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Corte L, Opdyke J, Moran CP, Jr, Henriques AO. Expression of spoIIIJ in the prespore is sufficient for activation of sigma(G) and for sporulation in Bacillus subtilis. J Bacteriol. 2003;185:3905–3917. doi: 10.1128/JB.185.13.3905-3917.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Neves A, Soares CM, Moran CP, Jr, Henriques AO. Role of the anti-sigma factor SpoIIAB in regulation of sigma(G) during Bacillus subtilis sporulation. J Bacteriol. 2004;186:4000–4013. doi: 10.1128/JB.186.12.4000-4013.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp MD, Pogliano K. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc Natl Acad Sci U S A. 1999;96:14553–14558. doi: 10.1073/pnas.96.25.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Bayer ME, Youngman P. Physical and functional characterization of the Bacillus subtilis spoIIM gene. J Bacteriol. 1993;175:3607–3617. doi: 10.1128/jb.175.11.3607-3617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini JM, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–241. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- Sun D, Fajardo-Cavazos P, Sussman MD, Tovar-Rojo F, Cabrera-Martinez RM, Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by sigma(F): identification of features of good sigma(F)-dependent promoters. J Bacteriol. 1991a;173:7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DX, Cabrera-Martinez RM, Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma(G) J Bacteriol. 1991b;173:2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YL, Sharp MD, Pogliano K. A dispensable role for forespore-specific gene expression in engulfment of the forespore during sporulation of Bacillus subtilis. J Bacteriol. 2000;182:2919–2927. doi: 10.1128/jb.182.10.2919-2927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalsma H, Bron S, van Dijl JM. Complementary impact of paralogous Oxa1-like proteins of Bacillus subtilis on post-translocational stages in protein secretion. J Biol Chem. 2003;278:15622–15632. doi: 10.1074/jbc.M301205200. [DOI] [PubMed] [Google Scholar]
- Wilson GA, Bott KF. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968;95:1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Dalbey RE. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat Rev Microbiol. 2008;6:234–244. doi: 10.1038/nrmicro3595. [DOI] [PubMed] [Google Scholar]
- Yen MR, Harley KT, Tseng YH, Saier MH., Jr Phylogenetic and structural analyses of the Oxa1 family of protein translocases. FEMS Microbiol Lett. 2001;204:223–231. doi: 10.1111/j.1574-6968.2001.tb10889.x. [DOI] [PubMed] [Google Scholar]
- Yi L, Dalbey RE. Oxa1/Alb3/YidC system for insertion of membrane proteins in mitochondria, chloroplasts and bacteria. Molecular Membr Biol. 2005;22:101–111. doi: 10.1080/09687860500041718. [DOI] [PubMed] [Google Scholar]
- Yip CK, Kimbrough TG, Felise HB, Vuckovic M, Thomas NA, Pfuetzner RA, Frey EA, Finlay BB, Miller SI, Strynadka NC. Structural characterization of the molecular platform for type III secretion system assembly. Nature. 2005;435:702–707. doi: 10.1038/nature03554. [DOI] [PubMed] [Google Scholar]
- Youngman P, Perkins JB, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.