Abstract
The lack of a safe and reliable alternative to hormone therapy (HT) for treating menopausal symptoms underscores the need for alternative therapies. OBJECTIVE: The purpose of this study was to assess the in vivo estrogenic effects of the botanical dietary supplements Trifolium pratense (red clover) and Humulus lupulus (hops), and two compounds obtained from H. lupulus, isoxanthohumol and 8-prenylnaringenin (8-PN) using the ovariectomized uterotrophic adult rat model. A H. lupulus extract and a 30% isoflavone extract of T. pratense were tested at three escalating doses as was one dose of isoxanthohumol for 21 days. 8-Prenylnaringenin, the major estrogen in H. lupulus, was also tested at three relevant escalating doses. In order to determine the in vivo metabolism of 8-PN, the major Phase I and Phase II metabolites were also identified. The primary outcome measure, uterus weight gain, indicated that H. lupulus and T. pratense did not have an estrogenic effect on the uterus, and none of the secondary outcome measures were positive. In contrast, there was a clear dose response when 8-PN was evaluated where the middle and high doses of 8-PN were active. 8-Prenylnaringenin in rat plasma, liver, and mammary gland was measured and the major Phase I and Phase II 8-PN metabolites were detected. Our findings suggest that while both the H. lupulus and T. pratense extracts do not have an effect on the rat uterus, 8-PN at equivalent doses to those previously used in humans did have an effect, and may therefore have a deleterious effect in women.
Keywords: 8-prenylnaringenin, estrogen, Humulus lupulus, isoxanthohumol, menopause, Trifolium pratense
1 Introduction
Symptoms associated with menopause such as insomnia, loss of libido, vaginal atrophy, depression, and hot flashes can greatly affect the quality of life for women. Many women have used hormone therapy (HT) to alleviate menopausal symptoms; however, with the publication of the Women's Health Initiative in 2002 [1], the number of women using HT has decreased dramatically [2]. Even before the publication of several large studies of HT, many women have been turning to herbal remedies for the relief of menopausal symptoms [3-5], perhaps because they are viewed as safe [6]. Unfortunately, few botanicals are chemically and biologically standardized to relevant active compounds using an appropriate mechanism of action [7]. Among the herbal remedies currently being used, many contain phytochemicals that mimic the effects of human estrogen in vitro or in vivo. Such phytochemicals are commonly referred to as phytoestrogens.
Trifolium pratense L. (red clover) contains the estrogenic isoflavones, daidzein, formononetin, biochanin A, and genistein [8, 9]. While currently being marketed for use in alleviating menopausal symptoms, there is an absence of an ethnomedical history and clinical data to support the use of T. pratense for these purposes. It has historically been used as an anti-spasmodic and an anti-cancer treatment. Metabolism and clinical pharmacokinetic studies [10] have shown that formononetin and biochanin A can be converted by cytochrome P450 to their more active estrogenic metabolites, daidzein and genistein, respectively, which are the same isoflavones found in Glycine max Merrill (soy) [11]. Trifolium pratense has been tested in clinical trials as a menopause therapy [12] with formulations based on the active metabolites and their metabolic precursors [13]. These studies have shown mixed results. A single year long trial evaluated the isoflavones, but used a dose of 40 mg/day total isoflavone content, which may not have been high enough to be efficacious [13].
The strobiles of Humulus lupulus L. (Cannabaceae) (hops) are primarily used to flavor beer, and have been studied since 1953 for a potential estrogenic mechanism of action [13-15]. In the past, the flavonoids 8-PN and 6-PN, and the chalcone, isoxanthohumol (IX), have been isolated and reported to be estrogenic in vitro and/or in vivo [16, 17]. The most potent estrogen in H. lupulus is 8-PN, which is in actuality an artifact formed through isomerization of the precursor chalcone, desmethylxanthohumol [18, 19]. 8-Prenylnaringenin has been shown in a variety of in vitro assays to be estrogenic in the nanomolar range [16, 17, 20, 21]. It can also be formed from precursors such as IX, and xanthohumol (XH), through metabolism [22].
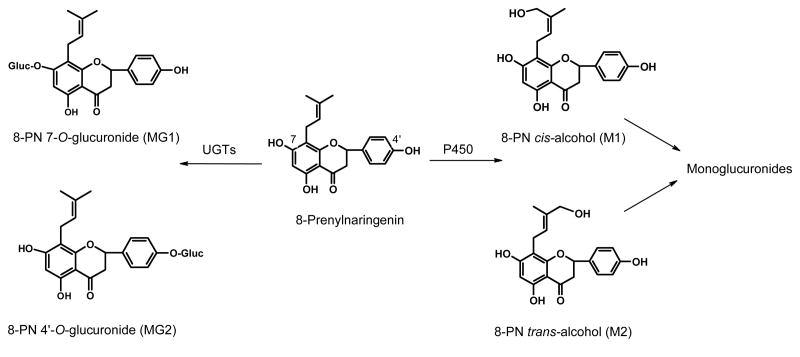
In previous metabolic studies, it has been shown that human liver microsomes convert 8-PN to 12 metabolites [22]. Among them, 8-PN cis- and trans-alcohols (8-PN-M1 and 8-PN-M2) are the most abundant monooxygenated metabolites (Figure 1). The estrogenicities of the 8-PN alcohols have been previously determined [23] and were found to be ∼10 fold less estrogenic than 8-PN. Phase II metabolites of 8-PN, including glucuronides and sulfate conjugates, have been identified in incubations with human Caco-2 cells and human hepatocytes [24] (see structures in Figure 1). Conjugates of 8-PN were also reported in urine of men after consuming beer or beer spiked with 8-PN [25], but the radioimmunoassay used in this study did not allow for the identification of specific conjugates. However, little information is available concerning the formation of metabolites of 8-PN in vivo.
Figure 1.
8-Prenylnaringenin and its major phase I and phase II metabolites.
Recently, 8-PN was tested in animals [26, 27] and in a human phase I clinical trial [28]. While H. lupulus extracts have also been tested in two phase I clinical trials in menopausal women [29], extracts have not been rigorously tested in vivo following the identification of 8-PN as the compound primarily responsible for estrogenic activity. The authors of the in vivo animal study found that a high dose of 8-PN (68.4 mg/kg) had an estrogenic effect by suppressing serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) and body weight gain, and stimulated serum prolactin levels, uterus weight, and the mRNA transcription levels of the progesterone receptor, insulin-like growth factor I, and complement protein C3 [27], Additionally, the high dose of 8-PN was reported separately to have histological features similar to the estrogen control, and induced epithelial polyploidy formation. 8-PN also caused secretions in the mammary gland, proliferation, and progesterone receptor expression [26]. The authors of both papers recognized that the estrogenic effects of 8-PN may possibly be deleterious to humans and called attention to the doses used in humans and the possible need to include a progestin in future formulations [26, 27].
The purpose of the present study was to compare two botanicals, T. pratense and H. lupulus and the two compounds from H. lupulus that were either estrogenic (8-PN) or that could be metabolized to 8-PN (IX). Based on the dosages used in previous studies in humans and in animals, we tested both the extracts and the pure compounds in order to compare our data both within the experiment and with previously published data. To our knowledge, this represents the first report comparing 8-PN with a H. lupulus botanical extract in the adult rat uterotrophic model.
2 Materials and Methods
2.1 Materials
For the preparation of the hops extract, spent cones of the Hallertauer Herzbrugger cultivar of H. lupulus (harvest 2002, HHE02) were bulk extracted with EtOH by SS Steiner (Mainburg, Germany). The hops extract was standardized to a minimum of 0.50 % isoxanthohumol, 2.00 % xanthohumol, 0.09 % 8-prenylnaringenin, and contained very small amounts (0.9 ppm) of desmethylxanthohumol (DMX). A T. pratense extract standardized to 30% isoflavones was provided by Naturex (South Hackensack, NJ). The red clover extract was standardized to a minimum of 30 % isoflavone content by weight (30 g of total isoflavones per 100 g of total extract) of four isoflavones: genistein, 0.41 %; daidzein, 0.23 %; biochanin A, 14.47 %; and formononetin, 14.26 %, present as hydrolyzed aglycones.
Isoxanthohumol (IX) was isolated as reported previously [19]. Synthetic 8-Prenylnaringenin (8-PN) was prepared using literature procedures with minor modifications to achieve higher chemoselectivity and regioselectivity of the product during the scale-up of the synthesis. Briefly, racemic 8-PN was prepared by the tandem Claisen-Cope rearrangement of 5-prenyl-7,4′-diacetylnaringenin [30] by combining 5-prenyl, 7,4′-diacetylnaringenin (1.3 g, 3.06 mmol) in toluene (25 mL) with Eu(fod)3 (264 mg, 0.25 mmol, 0.083 equivalents). The solution was heated in a CEM Discoverer microwave apparatus at 120 °C for 11 min. After cool ing, the solution was then treated with pyrrolidine (0.60 mL) and stirred at room temperature for 15 min. The reaction was then worked up by dilution with EtOAc (25 mL) and washing with 2M H2SO4. The organic phase was dried (anhydrous Na2SO4) and evaporated, and the residue was purified by gravity column chromatography on silica gel (125 g) using CHCl3-EtOH 99:1 as eluant to provide crude 8-PN. Washing with ether-chloroform 2:1 afforded 853 mg (82%) of purified 8-PN as a pale-yellow powder having physical (mp, optical rotation) and spectroscopic (MS, 1H-and 13C NMR) properties identical to those of the compound isolated from H. lupulus [16]. The purity of 8-PN was determined using quantitative 1H NMR (qHNMR) [31, 32] and the quantitative results were as follows: 95.0 % 8-PN, 1.5 % of 6-prenylnaringenin, 2.4 % of related prenylflavanones including 8-iso-PN and 6-iso-PN, and 1.0 % unrelated impurities.
2.2 Animals
Guidelines established by our institutional Animal Care and Use Committee and state and federal regulations were followed for all procedures. The protocol complied with the Guide for the Care and Use of Laboratory Animals, and the facilities are Association for the Assessment and Accreditation of Laboratory Animals Care approved. Female ovariectomized Sprague-Dawley rats weighing ∼200 g were received at eight weeks of age from Harlan (Indianapolis, IN). Animals were allowed one week to acclimate.
2.3 Diets and Treatment
All rats consumed Harland/Teklad purified diet AIN-93M that was certified isoflavone-free (Indianapolis, IN). Since soybean oil was a component of the diet, LC-MS analysis was used to confirm that common isoflavones found in soybean were not present in the diet. An isoflavone-free diet was used to minimize the potential for confounding experimental results with the phytoestrogens found in T. pratense and H. lupulus. Access to food and water was unrestricted. The T. pratense and H. lupulus extracts and the negative control, carboxymethylcellulose, at 1% (purchased from Sigma-Aldrich) were administered by gavage. 17β-Estradiol or IX were suspended in corn oil and were administered subcutaneously as was the negative control oil vehicle. In the second experiment evaluating a dose-dependant effect of 8-PN, synthesized 8-PN was administered by intraperitoneal (ip) injection as were the positive and negative controls.
2.4 Treatment, Preparation, and Dosing
The T. pratense and H. lupulus powdered extracts were ground using a mortar and pestle before being suspended in a 10 g/L CMC solution to yield concentrations of 1.7, 17, and 60 mg/mL in 1% CMC. 17β-Estradiol was suspended in corn oil at a concentration of 33.3 μg/mL (10 μg/rat•d). Each rat was treated for 21 d. The doses of T. pratense were based on a clinical dose of 120 mg/(woman•d) isoflavones contained in 400 mg/(woman•d) of T. pratense extract. An allometric scaling factor of 6.0 was used to convert the human dose to a rat dose based on body surface area [33]. For example, if a woman weighing 64 kg received a dose of 400 mg (6.25 mg/kg•d), then the equivalent rat dose would be approximately 40 mg/kg•d. Concentrations 10-fold lower and higher (4 mg/kg•d and 400 mg/kg•d) were used to determine if there were estrogenic effects in this range. Additionally, the dose of 400 mg/(kg•d) of a 30% isoflavone T. pratense extract was in the same order of magnitude as a 15% isoflavone T. pratense extract previously tested at 750 mg/(kg•d). The H. lupulus extract was tested at the same doses as T. pratense and prepared in the same manner. The IX dose was based on half of the amount of IX in the highest dose of H. lupulus. The IX concentration was 0.2%, therefore in the high dose of H. lupulus there was 0.8 mg/kg of IX in a 400 mg/kg dose of hop extract. Half of that is 0.4 mg/kg of IX. The 8-PN dose was based on the amount of 8-PN given in the highest dose of (400 mg/kg•d). Since approximately 0.1% of the H. lupulus extract is 8-PN, this equates to a 0.4 mg/kg•d dose. We also tested at 10 and 100 times this concentration (4 mg/kg•d and 40 mg/kg•d, respectively) to determine if there was a dose response. Estrogenic effects were evaluated primarily on uterine weight and body weight. Rats were weighed to monitor body weight maintenance, toxicity, and to adjust the dose accordingly as rats increased in bodyweight.
2.5 Tissue Collection
The animals in the extract study were anesthetized with ketamine/xylazine (ip) 24 h after the last dose. The uterus was collected, trimmed of fat and connective tissue, blotted with filter paper, weighed, and stored for histological processing. A sample of blood (1 mL) and urine (1 mL) from the bladder was collected using syringe needles and placed on ice before being stored at -80 °C. The animal was then transcardially perfused with phosphate buffer, (50 mL, 0.1 M, pH 7.4) and 4% paraformaldehyde in phosphate buffer (200 mL). In the second animal study, the animals receiving 8-PN or controls were sacrificed using bottled gas CO2. The blood and uterus were collected and processed as described above.
2.6 Vaginal Cell Cornification
Animals in the T. pratense and H. lupulus study were evaluated using the Allen-Doisy test for estrogenicity as describe previously [34] with minor modifications. Smears were stained using 1% crystal violet stain and observed under a light microscope using a 10X eyepiece and a 40X objective. Smears were read at the end of the study and cells were identified as leukocytes, nucleated, or cornified epithelial cells. An estimated percentage was assigned to each cell population such that the total cell population was 100%.
2.7 Histology
Each uterus from animals in the 8-PN study was processed for paraffin embedding. The uteri were arranged horizontally and sectioned in 4 μm increments. One slide per animal was stained using hemotoxylin and eosin. Slides were coverslipped using paramount and representative pictures of the luminal epithelium were acquired (Nikon E600 microscope with a Nikon DXM 1200 camera; 400 ×). Representative epithelial cell height was measured in pixels using MetaVue (Universal Imaging Corp, Madison WI) software (n = 30 per picture) and averaged. Data were combined using GraphPad Prism and average height in pixels for each group was calculated.
2.8 Rat Plasma Preparation for Metabolite Analysis
Ice-cold methanol (125 μL) containing 0.5 μM naringenin as internal standard was added to rat plasma (25 μL) to precipitate protein. After centrifugation at 10,000 × g for 10 min, the supernatant was removed and analyzed using HPLC-MS-MS. Blank rat plasma, spiked with concentrations of 8-PN from 1-1,000 ng/mL, was used for calibration curves and analytical validation.
2.9 Rat Liver Preparation for Metabolite Analysis
Each rat liver (approximately 300 mg) was homogenized in phosphate buffer (three volumes of deionized water, 0.05 M, pH 7.4). Then naringenin (2 μL, 2.5 μM) was added as internal standard. Each homogenate (300 μL) was extracted three times with ethyl acetate saturated with water (1 mL). The organic layer was removed, and evaporated to dryness under a stream of dry nitrogen, and then reconstituted with 50% aqueous methanol (100 μL). After centrifugation, the samples were analyzed using LC-MS-MS as described below.
2.10 Rat Mammary Gland Preparation for Metabolite Analysis
Rat mammary gland (approximately 100 mg) was homogenized in phosphate buffer (three volumes of deionized water, 0.05 M, pH 7.4). Each sample was spiked with an aliquot of naringenin (0.5 μM) as internal standard and vortexed for 30 s. Proteins in the mammary homogenates were precipitated by the addition of ice-cold methanol/acetonitrile (1.5 mL, 1:1, v/v) and then centrifuged at 4 °C (10,000 × g for 10 min). The supernatant was evaporated to dryness under a stream of nitrogen. The residues were reconstituted in 70% methanol (150 μL) prior to LC-MS-MS analysis.
2.11 Metabolism of IX by Rat Liver Microsomes
Rat liver microsomes from female Sprague-Dawley rats were purchased from In Vitro Technologies (Baltimore, MD, USA) and were used as reported previously for human microsomes [22]. Briefly, a typical incubation mixture (0.4 mL) contained 1 mg mL-1microsomal protein, 10 μM IX and 1 mM NADPH in 50 mM phosphate buffer, pH 7.4. After a 60 min incubation at 37 °C, the reactio n was stopped by chilling the mixture on ice and by addition of 1.6 ml of a cold mixture of acetonitrile–ethanol (1 : 1, v/v) to precipitate proteins. Samples were centrifuged, the supernatant was evaporated to dryness under nitrogen and the residue was dissolved in the mobile phase prior to LC-MS analysis. Control incubations were carried out either without microsomal protein or without NADPH.
2.12 LC-MS-MS Tissue Analysis
All quantitative data were obtained using LC-MS-MS with negative ion electrospray and multiple reaction monitoring (MRM) with an Applied Biosystems (Framingham, CT) API 4000 triple quadruple mass spectrometer. The HPLC system consisted of Shimadzu (Columbia, MD, USA) LC-10ADvp pumps and a LC PAL (CTC Analytics AG, Switzerland) autosampler. Separation of 8-PN and its metabolites was carried out using a ZORBAX SB 2.1 × 100 mm C18 column (3.5 μm particle size) with a 15 min gradient from 40-75% methanol in 0.1% aqueous formic acid followed by 90% methanol for 5 min. The flow rate was 0.3 mL/min, and the injection volume was 10 μL. The column and the autosampler were maintained at room temperature (19-22 °C). Electrospray ionization was carried out at -4.2kV, the declustering potential was -100 eV, the entrance potential was -10 eV, and the capillary temperature was 450 °C. Nitrogen was used for collision-induced dissociation at a collision energy (CE) of -28 eV and collision cell exit potential of -13 eV. The MRM transitions of m/z 339-219 and m/z 271-151 were measured for 8-PN and naringenin (internal standard), respectively. For the hydroxylated 8-PN, metabolites 8-PN-M1 and 8-PN-M2, the MRM transition of m/z 355-235 was monitored. Monoglucuronidated 8-PN metabolites were measured using the MRM transition of m/z 515-339. For the quantitative analysis of 8-PN, a calibration curve (using spiked rat plasma) was linear (R2 = 0.9971) over the range 58.8 fmol – 14.7 pmol. The limits of detection and quantitation were 14.7 and 58.8 fmol, respectively. The reproducibility of this LC-MS-MS method was measured by triplicate analysis of the three quality controls (low, medium, and high concentrations). The accuracy and precision was within ± 15 %, respectively.
2.13 Statistics
All statistical tests were performed using GraphPad prism (San Diego, California). Data were analyzed using one-way ANOVA with the Dunnett post test, and the confidence interval was set at 95%.
3 Results
3.1 Uterus Weight
Extracts of H. lupulus and T. pratense, as well as the pure compounds IX and 8-PN, were tested in the rat uterotrophic model. Animals were dosed as milligrams of extract or compound per kg bodyweight or per animal. The dose of each tested compound was based on the concentration of the compounds found in the highest dose of H. lupulus. Two animals in each of the high doses for T. pratense and H. lupulus had to be euthanized due to morbidity caused by gavage (Table 1). While a trend toward increasing uterus weight with increasing dose appeared, there were no statistically significant differences between the uterus weights of the vehicle control, 1% CMC (w/v; ig) and oil (s.c.), and either T. pratense or H. lupulus at any of the doses (Figure 2). However, in the 8-PN study (Table 1) there was a clear dose response where 0.4 mg/kg•d was not statistically significant, but 4 and 40 mg/kg•d were significant (p-value < 0.05) (Figure 2). The positive control, 17β-estradiol, was statistically significant compared to the vehicle (corn oil).
Table 1. Animal Groups.
| Treatment | N |
|---|---|
| 1% CMC and corn oil | 7 |
| 17β-estradiol, 10 μg/(rat•d) | 6 |
| T. pratense, 4 mg/(kg•d) | 7 |
| T. pratense, 40 mg/(kg•d) | 7 |
| T. pratense, 400 mg/(kg•d) | 5 (7)1 |
| H. lupulus, 4 mg/(kg•d) | 7 |
| H. lupulus, 40 mg/(kg•d) | 7 |
| H. lupulus, 400 mg/(kg•d) | 5 (7)1 |
| Isoxanthohumol 0.4 mg/(rat•d) | 6 |
| Oil | 6 |
| 17β-estadiol, 10μg/(rat•d) | 6 |
| 8-PN 0.4 mg/(kg•d) | 6 |
| 8-PN 4.0 mg/(kg•d) | 6 |
| 8-PN 40 mg/(kg•d) | 6 |
Number in parenthesis is the starting number of animals.
Figure 2.
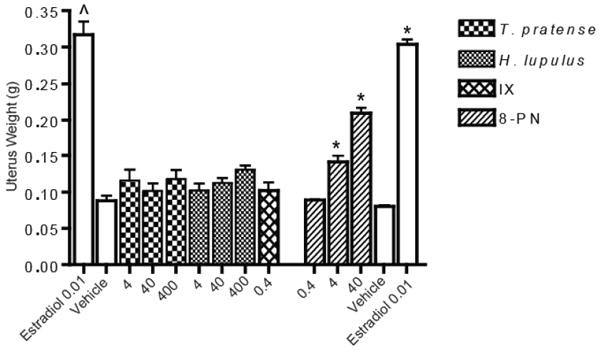
Uterus weight of animals treated with T. pratense, H. lupulus, isoxanthohumol (IX), 8-prenylnaringenin (8-PN), or controls. ˆ and * indicate p-value < 0.05 compared to respective control group using ANOVA. N = 5, 6, or 7.
3.2 Body Weights
The rats receiving T. pratense or H. lupulus extracts or purified IX did not at any of the tested doses maintain body weight comparable with those that received the positive control, 17β-estradiol (Figure 3). In the 8-PN study, the highest dose, 40 mg/kg•d, clearly maintained bodyweight to the same extent as the positive control (Figure 3). The middle dose appears to also have had an effect on body weight since the animals did not gain as much weight as quickly as the control animals. The low dose, 0.4 mg/kg•d did not have any effect on body weight maintenance compared with the positive control.
Figure 3.
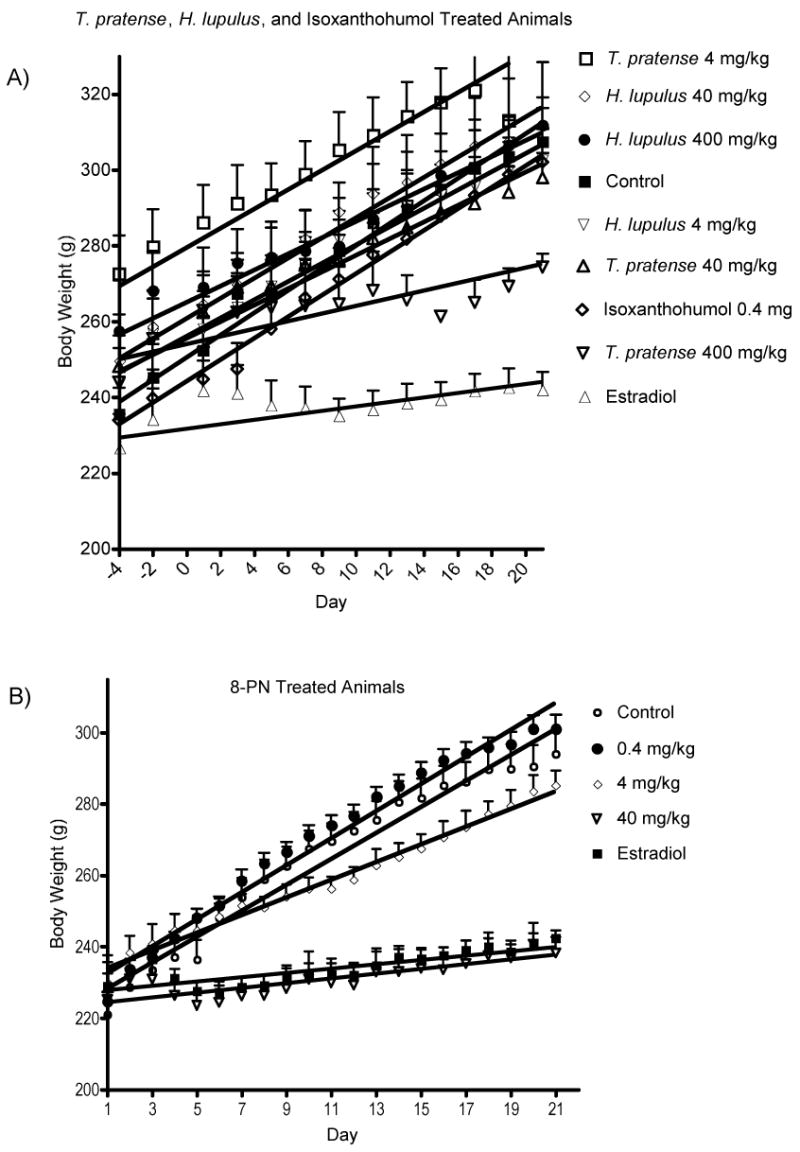
Body weights. A) H. lupulus and T. pratense. B) 8-prenylnaringenin. Body weight was measured every other day.
3.3 Vaginal Cell Cornification
None of the animals in the treatment groups had vaginal epithelial cell cornification similar to the positive control, estradiol (data not shown). In fact, none of the treatments appeared to have more than 50% cornified cells at any given time point, and in general during the last five days of the study remained lower than 25% cornified cells.
3.4 Histology
Uterus luminal epithelial cell height is an outcome measure of estrogenicity in ovariectomized adult rats. When treated with an estrogen, these epithelial cells will grow vertically at a faster rate than in untreated animals. In this study, after measuring the height of the epithelial cells, there was a statistical difference in height between each groups and the control group (Figure 4). However, visually there did not appear to be any significant morphological change in epithelial cell height, with the exception of the estradiol treated animals (Figure 5).
Figure 4.
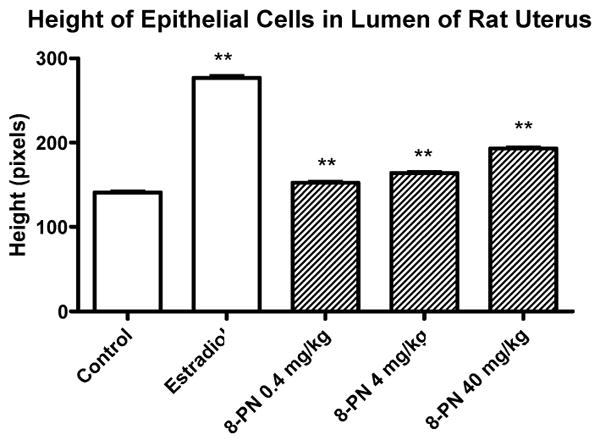
Height of luminal epithelial cells in the lumen of rat uterus treated with 8-prenylnaringenin. Uterus sections stained with hemotoxylin and eosin were evaluated for luminal epithelial height by measuring in pixels the average height of 30 cells per slide, one slide per animal. ** p-Value < 0.01 based on ANOVA, N = 6.
Figure 5.
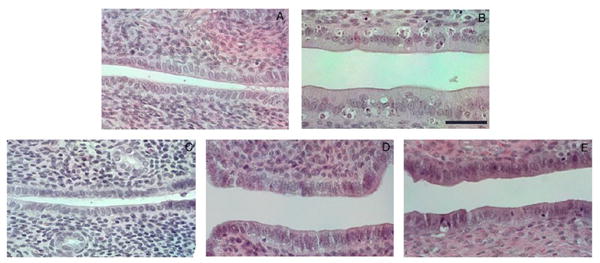
Hemotoxylin and eosin staining of rat uterus treated with 8-prenylnaringenin. Uterus tissue was embedded in parafin and sectioned (4 μm) longitudinally. Slides were stained with hemotoxylin and eosin and coverslipped using paramount. Magnification = 400 ×. Scale bar = 40 μm.
3.5 8-Prenylnaringenin Detection in Tissues
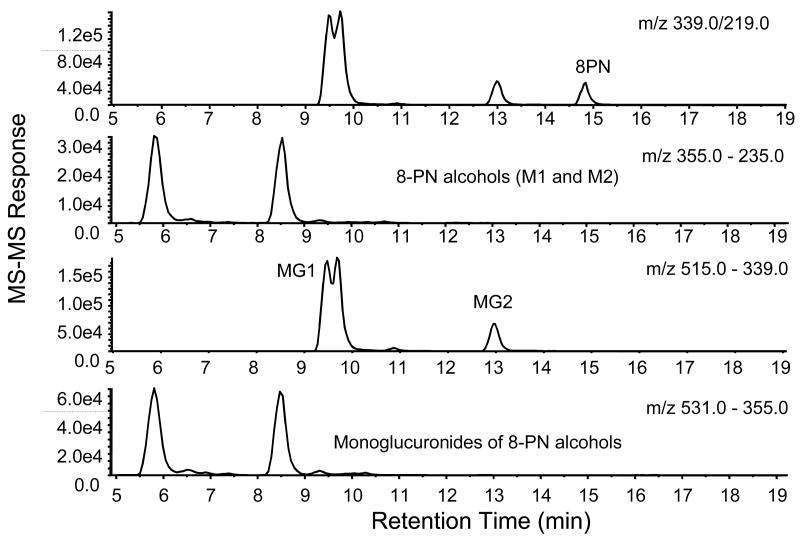
Using LC-MS-MS, 8-PN was detected in the plasma of rats that had been dosed with 40 mg/kg•d of 8-PN; however, none was detected in plasma from rats dosed with 4.0 or 0.4 mg/kg•d of 8-PN (Table 2) or those dosed with IX. The concentration of 8-PN measured in rat plasma at a dosage of 40 mg/kg•d was 3.7 ± 1.6 ng/mL. LC-MS-MS chromatograms for these analyses are shown in Figure 6. Two 8-PN monoglucuronides (MG1 and MG2; Figure 1) were detected in the plasma of the rats receiving 40 mg/kg•d of 8-PN (Figure 6). The major monoglucuronide metabolite of 8-PN, MG1, was also detected in plasma from rats dosed with 4.0 and 0.4 mg/kg•d of 8-PN (data not shown). Peaks for MG1 and MG2 were detected in the MRM chromatogram (Figure 6) eluting at 9.5 and 13.0 min, respectively. Signals for these glucuronides were also detected in the MRM chromatogram for 8-PN, which was caused by some fragmentation in the ion source resulting in the formation of the aglycone at m/z 339. As indicated in the MRM chromatogram in Figure 6, the amount of MG1 that fragmented in the ion source to form 8-PN exceeded the level of free 8-PN in rat plasma by more than 4-fold. Not only did this indicate extensive glucuronidation of 8-PN in vivo, but it showed that formation of MG1 exceeded formation of MG2 by more that 3-fold. In addition, two forms of MG1 were detected as indicated by the two partially resolved peaks eluting ∼ 9.5 min in the LC-MS-MS chromatogram in Figure 6, which were the result of diastereomer formation following glucuronidation of racemic 8-PN. The assignment of the structures to MG1 and MG2 was based on previous studies in our laboratory [24]. Although no peaks were detected for free 8-PN-M1 or 8-PN-M2, monoglucuronides of these metabolites were observed eluting at ∼ 5.8 and 8.6 min (Figure 6). Due to fragmentation in the ion source in which glucuronic acid was eliminated, peaks for the corresponding aglycones were also detected at the same retention times in the MRM chromatogram of 8-PN-M1 and 8-PN-M2. Since standards were not available, the specific sites of glucuronidation of 8-PN-M1 and 8-PN-M2 were not determined. Finally, no sulfate conjugates of 8-PN, 8-PN-M1 or 8-PN-M2 were detected in the plasma for rats following administration of any dose of 8-PN.
Table 2. 8-Prenylnaringenin measured in rat plasma, liver, and mammary glands using LC-MS-MS.
| Treatment group |
1Plasma
(N=6) |
1Liver
(N=6) |
1Mammary gland
(N=6) |
|---|---|---|---|
| (ng/mL) | (μg/g tissue) | (μg/g tissue) | |
| Control | <0.5002 | <0.0022 | 0.003 ± 0.0022 |
| Estradiol | <0.5002 | <0.0022 | 0.004 ± 0.0032 |
| 0.4 mg/kg of 8-PN | <0.5002 | 0.10 ± 0.05 | 0.014 ± 0.014 |
| 4.0 mg/kg of 8-PN | <0.5002 | 0.4 ± 0.3 | 0.047 ± 0.046 |
| 40 mg/kg of 8-PN | 3.7 ± 1.63 | 4.4 ± 4.13 | 0.6 ± 0.43 |
Values are expressed as mean ± S.D.
Below the limit of quantitation
The difference from control was determine using one-way ANOVA with Dunnett posthoc test (p< 0.01)
Figure 6.
LC-MS-MS chromatograms of 8-PN (dosed at 40 mg/kg/rat) and its metabolites in rat plasma. MRM was carried out using negative ion electrospray and collision-induced dissociation. The precursor and product ion transition for each measurement are indicated above each chromatogram.
Unlike plasma, 8-PN was detected in liver from animals treated with all dosages of 8-PN (Table 2) but not after treatment with IX only. The levels of 8-PN in liver varied according to dosage with the highest level, 4.4 ± 4.1 μg/g liver tissue, corresponding to an 8-PN dosage of 40 mg/kg•d and the lowest levels of 0.03 ± 0.03 μg/g liver tissue corresponding to an 8-PN dosage of 0.4 mg/kg•d. Although the concentrations were not determined, the most abundant metabolite, MG1, was detected in liver tissues from all of the three dosages of 8-PN. In addition, the mono-hydroxylated metabolite 8-PN-M1 was detected in liver tissue for the rats receiving only the highest dosage of 8-PN (40 mg/kg•d) (data not shown).
8-PN, 8-PN-M1, and MG1 were detected in rat liver samples. The amount of 8-PN detected in liver samples from rat dosed with 40 mg/kg•d of 8-PN was significantly higher (p-value < 0.01) than in controls (Table 2). 8-PN was also found in liver samples from rats treated with lower dosages. Both 8-PN and MG1 were found in liver samples from rats dosed at 4.0 and 0.4 mg/kg•d. Small amounts of the phase I metabolite 8-PN-M1 were also found in liver from rats dosed with 40 mg/kg•d of 8-PN (data not shown).
8-PN was detected in mammary gland tissue from all animals administered 8-PN, but not from those given IX. The highest level of 8-PN in mammary gland tissue was from rats dosed with 40 mg/kg•d of 8-PN and was 0.6 ± 0.4 μg/g tissue (Table 2). Overall, the mammary gland level of 8-PN was less than the corresponding levels in liver tissue. In addition, 8-PN levels in mammary tissues were dose-dependent, and levels in rats dosed with 40 mg/kg•d 8-PN were significantly higher (p-value < 0.01) than in control animals that did not receive 8-PN (Table 2). Finally, the monoglucuronide of 8-PN (MG1) was also detected in mammary gland tissue using LC-MS-MS (data not shown).
4 Discussion
Historically, in the search for estrogenic activity en route to the treatment of symptoms associated with menopause, an estrogenic outcome in the rat uterotrophic model has been considered a positive outcome both for estrogenicity as well as for further pursuit in humans. However, in light of the several large-scale clinical trials of HT that showed an increased risk in hormone-dependent cancers [1, 35], it is pertinent to question whether an estrogenic outcome in the uterotrophic animal model really is desirable.
In this study we chose to use T. pratense as a “positive botanical control” since it had previously been demonstrated that an extract standardized to the same compounds was weakly estrogenic in vivo [34], and a H. lupulus cultivar was selected based on its reported chemical/biological properties and availability. Furthermore, 8-PN and IX were also evaluated since 8-PN is the major active phytoestrogen in H. lupulus [16, 17, 20, 21], and IX can be metabolized to 8-PN [22]. The major phytoestrogens in T. pratense, daidzein and genistein, and their metabolic precursors, formononetin and biochanin A, were not evaluated since there is already a large body of data on these compounds [36, 37], and the primary focus was on H. lupulus. Uterus weight was used as the primary outcome measure and body weight was one of the secondary outcome measures. Uterus weight has been validated as a method of evaluating estrogens and antiestrogens in rodents [37]. In ovariectomized adult rats the uterus weight is significantly decreased, and when supplemented with estrogens the uterus weight increases to sham control weight. Estrogens have also been know to maintain body weight in ovariectomized rats, while animals treated with vehicle have an increased body weight [38]. Recently, ERα has been implicated in this process [39].
Initially the results of the current study were questioned since the positive botanical control did not appear to be positive in the same assays. It is possible that the differences in activity may be attributed to the different chemical composition of the extracts and the dosing concentrations. However, upon more careful inspection the differences in the conclusions between the current study and the previous study are likely attributed to the previous study using flawed methodology and a change in perspective regarding a uterotrophic outcome. The original paper concluded that the T. pratense extract was estrogenic based on data from the vaginal cell cornification assay, and did not find a statistically significant increase in uterus weight for T. pratense treated animals [34]. It was further concluded that if the extract was estrogenic, then it was a viable candidate for clinical trial. In the current study, we confirmed that T. pratense, as in the original study, had no significant effect on the uterus weight or body weight maintenance. However, we were unable to reproduce a statistical difference for any of the treatments in the Allen-Doisy assay. In fact, we were unable to see more than 50% cornified cells, and at the end of the study, the percentage dropped to 25%. Unfortunately, in the previous study the methodology and conclusions based primarily on this assay may be flawed. The vaginal cell cornification assay is semi-quantitative at best, and highly subjective, particularly if an unblinded researcher is interpreting then data. Therefore, based on uterus weight, we conclude that neither H. lupulus nor T. pratense extracts had an estrogen effect on the uterus. While the conclusion that a uterotrophic outcome indicates estrogenic activity has not changed, the interpretation and desirability of this outcome measure has changed. Currently, uterotrophic action in vivo is cause for concern since the same action in women would be deleterious. In light of this concern and the semi-quantitative assay that was used in the original interpretation of the data, we conclude that data from the two studies do not conflict regarding the lack of T. pratense uterotrophic activity. Therefore, since there was a lack of uterotrophic effect in rats, these extracts are therefore candidates for further phase I/II clinical trials.
While the present work was the second report testing the uterotrophic activity of T. pratense, it is the first report testing in this model a H. lupulus extract that has been chemically standardized to estrogenic compounds and potential metabolic precursor compounds. Previous reports published prior to the identification of 8-PN have ranged from no detectable estrogenic activity in Spanish H. lupulus [40], to the presence of estrogenic activity in a juvenile model [41], to antigonodal activity [42]. One study, which is the most similar in nature to the current study, was reported where a dietary supplement used for breast enhancement was tested [43]. After five days of treatment either in feed or by subcutaneous injection, they reported that H. lupulus exhibited weakly estrogenic activity, but only at an 8-PN concentration up to 250-times higher than that recommended for women. Coldham and Sauer used a Student's t-test and found that in prepubertal mice, the middle two doses of the dietary supplement containing 0.24 and 0.84 μg 8-PN, respectively, were weakly active (P < 0.05). In the ovariectomized mouse model, only the lowest dose (13.6 ng dietary supplement/ g bodyweight /day) was weakly active (P < 0.05). The analysis might have been more conclusive were more rigorous statistical analysis, such as ANOVA, carried out.
Following the identification of 8-PN, it is noteworthy that while there were no methodologically rigorous reports of a H. lupulus extract being tested for estrogenic activity in the adult rat ovariectomized model, there is a growing body of research that has tested purified 8-PN both in animals and humans. There were two reports of a H. lupulus extract having been tested in menopausal women. The first [44] was not randomized, and the control group (N = 5) received a low dose of H. lupulus, instead of a placebo. Furthermore there were no statistical tests reported, and some subjects received a second herb in addition to H. lupulus. The second report tested two H. lupulus extracts with different concentrations of 8-PN using a standardized extract [29]. They found that the lower dose of 8-PN (100 μg 8-PN/dose) had a significant reduction of the Kuiper Index at six weeks, but not at 12 weeks, and the higher dose (250 μg 8-PN/dose) was not significant at either time point [29].
Species differences regarding the metabolism of IX were also observed. Although previous in vitro and in vivo studies have indicated that IX can be converted to the more estrogenic agent 8-PN in humans [22, 45], we did not observe this O-demethylation reaction when IX was incubated with rat liver microsomes (data not shown). Therefore, species differences in the metabolism of IX might explain why 8-PN was not detected in the plasma from rats treated with pure IX. This is also consistent with the factor that IX did not exhibit significant estrogenic activity in rats in this study.
8-Prenylnaringenin, its two most abundant monoglucuronides MG1 and MG2, and two monoglucuronides of 8-PN-M1 and 8-PN-M2 were found in plasma of rats dosed with 40 mg/kg of 8-PN. These metabolites were predicted by our previous in vitro studies on the metabolism of 8-PN [22, 24]. In addition, 8-PN and some of its metabolites were detected in liver and mammary gland tissue. The relative levels of 8-PN glucuronides were considerably higher than those of free 8-PN, which indicated that 8-PN was rapidly conjugated with glucuronic acid following absorption in vivo. Rapid conjugation of 8-PN with glucuronic acid might explain its relatively low estrogenicity in vivo compared with in vitro studies. However, it is important to note that although extensive conjugation with glucuronic acid was predicted by our previous in vitro studies [24], no sulfate conjugates were observed. This difference between in vitro and in vivo conjugation are due at least in part to species differences, as our in vitro models utilized human hepatocytes and the human intestinal epithelial cell line Caco-2. It is also possible that the sulfate metabolites were excreted in the feces.
In summary, while the 15% isoflavone T. pratense extract has been reported to be weakly estrogenic [34], this effect was based mainly on the semi-quantitative results of the Allen-Doisy vaginal cell cornification assay. The 30% isoflavone T. pratense extract tested in the present study did not have a uterotrophic effect in rats and, therefore, is unlikely to have a uterotrophic effect nor increase the risk for endometrial cancer in menopausal women. Similarly the H. lupulus extract did not have a statistically significant effect on the weight of the uterus. Based on the lack of uterotrophic activity in the rat, both extracts are candidates for phase I safety and phase II efficacy clinical trials in menopausal women. However, the escalating doses of pure 8-PN did have a clear effect on uterus weight and body weight, and care should be taken when considering using the human equivalent dose as an unopposed estrogen in menopausal women.
Acknowledgments
The authors thank SS Steiner for providing various cultivars of H. lupulus and NATUREX for providing the 30% isoflavone T. pratense extract.
Funding: This work was supported, in part, by Grant P50 AT00155 provided jointly by the Office of Dietary Supplements (ODS), National Center for Complementary and Alternative Medicine (NCCAM), the Office for Research on Women's Health (ORWH), and the National Institute of General Medicine (NIGMS) of the National Institutes of Health (NIH). C.R.O. is grateful for a Ruth L. Kirschstein NRSA NCCAM Predoctoral fellowship F31 AT24232. None of the authors declare a conflict of interest that would prejudice their impartiality. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Sources of financial support: Grant P50 AT00155 provided jointly ODS, NCCAM, ORWH, and NIGMS. C.R.O. is grateful for a Ruth L. Kirschstein NRSA NCCAM Predoctoral fellowship F31 AT24232.
Cited Literature
- 1.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 5.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. What are bioidentical hormones. Harvard Women's Health Watch. 2006;13:1–3. [PubMed] [Google Scholar]
- 7.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 9.Liggins J, Bluck LJ, Coward WA, Bingham SA. Extraction and quantification of daidzein and genistein in food. Anal Biochem. 1998;264:1–7. doi: 10.1006/abio.1998.2825. [DOI] [PubMed] [Google Scholar]
- 10.Piersen CE, Booth NL, Sun Y, Liang W, Burdette JE, van Breemen RB, Geller SE, Gu C, Banuvar S, Shulman LP, Bolton JL, Farnsworth NR. Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Curr Med Chem. 2004;11:1361–1374. doi: 10.2174/0929867043365134. [DOI] [PubMed] [Google Scholar]
- 11.Hu M, Krausz K, Chen J, Ge X, Li J, Gelboin HL, Gonzalez FJ. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab Dispos. 2003;31:924–931. doi: 10.1124/dmd.31.7.924. [DOI] [PubMed] [Google Scholar]
- 12.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 13.Piersen CE. Phytoestrogens in botanical dietary supplements: implications for cancer. Integr Cancer Ther. 2003;2:120–138. doi: 10.1177/1534735403002002004. [DOI] [PubMed] [Google Scholar]
- 14.Stevens JF, Miranda CL, Buhler DR, Deinzer ML. Chemistry and biology of hop flavonoids. J Am Soc Brew Chem. 1998;56:136–145. [Google Scholar]
- 15.Koch W, Heim G. Estrogens in hops and beer. Munch Med Wochenschr. 1953;95:845. [PubMed] [Google Scholar]
- 16.Milligan SR, Kalita JC, Heyerick A, Rong H, De Cooman L, De Keukeleire D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J Clin Endocrinol Metab. 1999;84:2249–2252. doi: 10.1210/jcem.84.6.5887. [DOI] [PubMed] [Google Scholar]
- 17.Milligan SR, Kalita JC, Pocock V, Van De Kauter V, Stevens JF, Deinzer ML, Rong H, De Keukeleire D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J Clin Endocrinol Metab. 2000;85:4912–4915. doi: 10.1210/jcem.85.12.7168. [DOI] [PubMed] [Google Scholar]
- 18.Hänsel RV, Schulz J. Desmethylxanthohumol: Isolierung aus Hopfen und Cyclisierung zu Flavanonen. Arch Pharm Weinheim. 1988;321:37–40. [Google Scholar]
- 19.Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Froehlich R, Fong HH, Farnsworth NR, Pauli GF. Estrogens and Congeners from Spent Hops (Humulus lupulus L.) J Nat Prod. 2004;67:2024–2032. doi: 10.1021/np049783i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, De Keukeleire D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–242. [PubMed] [Google Scholar]
- 21.Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J Agric Food Chem. 2005;53:6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- 23.Zierau O, Hauswald S, Schwab P, Metz P, Vollmer G. Two major metabolites of 8-prenylnaringenin are estrogenic in vitro. J Steroid Biochem Mol Biol. 2004;92:107–110. doi: 10.1016/j.jsbmb.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic D, Li Y, Chadwick LR, van Breemen RB. In Vitro Studies of Intestinal Permeability and Hepatic and Intestinal metabolism of 8-Prenylnaringenin, a Po ten Phytoestrogen from Hops (Humulus lupulus L.) Pharm Res. 2006;23:864–872. doi: 10.1007/s11095-006-9902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer O, Bohlmann R, Schleuning WD, Schulze-Forster K, H M. Development of a radioimmunoassay for the quantitative determination of 8-prenylnaringenin in biological matrices. J Agric Food Chem. 2005;53:2881–2889. doi: 10.1021/jf047897u. [DOI] [PubMed] [Google Scholar]
- 26.Rimoldi G, Christoffel J, Wuttke W. Morphologic changes induced by oral long-term treatment with 8-prenylnaringenin in the uterus, vagina, and mammary gland of castrated rats. Menopause. 2006;13:669–677. doi: 10.1097/01.gme.0000196596.90076.d0. [DOI] [PubMed] [Google Scholar]
- 27.Christoffel J, Rimoldi G, Wuttke W. Effects of 8-prenylnaringenin on the hypothalamo-pituitary-uterine axis in rats after 3-month treatment. J Endocrinol. 2006;188:397–405. doi: 10.1677/joe.1.06384. [DOI] [PubMed] [Google Scholar]
- 28.Rad M, Humpel M, Schaefer O, Schoemaker RC, Schleuning WD, Cohen AF, Burggraaf J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br J Clin Pharmacol. 2006;62:288–296. doi: 10.1111/j.1365-2125.2006.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heyerick A, Vervarcke S, Depypere H, Bracke M, De Keukeleire D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54:164–175. doi: 10.1016/j.maturitas.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Gester S, Metz P, Zierau O, Vollmer G. An efficient synthesis of the potent phytoestrogens 8-prenylnaringenin and 6-(1,1-dimethylallyl)naringenin by europium(III)-catalyzed Claisen rearrangement. Tetrahedron. 2001;57:1015–1018. [Google Scholar]
- 31.Pauli GF, Jaki BU, Lankin DC. A Routine Experimental Protocol for qHNMR Illustrated with Taxol. J Nat Prod. 2007;70:589–595. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: Development of and Potential of a Method for Natural Products Analysis. J Nat Prod. 2005;68:113–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- 33.USFDA. 2007 http://www.fda.gov/cber/gdlns/dose.htm#iii.
- 34.Burdette JE, Liu J, Lantvit D, Lim E, Booth N, Bhat KP, Hedayat S, Van Breemen RB, Constantinou AI, Pezzuto JM, Farnsworth NR, Bolton JL. Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr. 2002;132:27–30. doi: 10.1093/jn/132.1.27. [DOI] [PubMed] [Google Scholar]
- 35.Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114:448–454. doi: 10.1002/ijc.20710. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:179–189. doi: 10.1016/s1570-0232(02)00493-2. [DOI] [PubMed] [Google Scholar]
- 37.Owens W, Koeter HB. The OECD program to validate the rat uterotrophic bioassay: an overview. Environ Health Perspect. 2003;111:1527–1529. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–780. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 39.Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Bravo L, Cabo J, Fraile A, Jiminez J, Villar A. Pharmacodynamic study of hops (Humulus lupulus). II. Estrogenic action. Ars Pharmaceutica. 1971;12:421–425. [Google Scholar]
- 41.Kumani A, Okamoto R. Extraction of the hormonal substance from hop. Toxicol Lett. 1984;21:203–207. doi: 10.1016/0378-4274(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 42.Okamoto R, Kumani A. Anitgonadotropic activity of hop extract. Acta Endocrinologica. 1992;127:371–377. doi: 10.1530/acta.0.1270371. [DOI] [PubMed] [Google Scholar]
- 43.Coldham NG, Sauer MJ. Identification, quantitation and biological activity of phytoestrogens in a dietary supplement for breast enhancement. Food Chem Toxicol. 2001;39:1211–1224. doi: 10.1016/s0278-6915(01)00081-3. [DOI] [PubMed] [Google Scholar]
- 44.Goetz P. Treatment of hot flashes due to ovarian insufficiency using a hops extract (Humulus lupus) Phytotherapie Pratique. 1990;4:13–15. [Google Scholar]
- 45.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De Keukeleire D, Bracke M, De Henauw S, Verstraete W, Van de Wiele T. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–2246. doi: 10.1093/jn/137.10.2242. [DOI] [PubMed] [Google Scholar]