Abstract
Gabapentin is a γ-aminobutyric acid (GABA) analogue, with GABAmimetic pharmacological properties. Gabapentin is used for the treatment of seizures, anxiety and neuropathic pain. It has been proposed that gabapentin may be useful in the treatment of cocaine dependence. However, clinical trials with gabapentin have shown conflicting results, while preclinical studies are sparse. In the present study, we investigated the effects of gabapentin on intravenous cocaine self-administration and cocaine-triggered reinstatement of drug-seeking behavior, as well as on cocaine-enhanced dopamine (DA) in the nucleus accumbens (NAc). We found that gabapentin (25–200 mg/kg, i.p., 30 min or 2 h prior to cocaine) failed to inhibit intravenous cocaine (0.5 mg/kg/infusion) self-administration under a fixed-ratio reinforcement schedule or cocaine-triggered reinstatement of cocaine-seeking behavior. In vivo microdialysis showed that the same doses of gabapentin produced a modest increase (∼50%, p < 0.05) in extracellular NAc GABA levels, but failed to alter either basal or cocaine-enhanced NAc DA. These data suggest that gabapentin is a weak GABA-mimic drug. At the doses tested, it has no effect in the addiction-related animal behavioral models here tested. This is in striking contrast to positive findings in the same animal models shown by another GABAmimetic – γ-vinyl GABA (see companion piece to present article).
Keywords: Gabapentin, Cocaine, Dopamine, GABA, Self-administration, Relapse
1. Introduction
Cocaine addiction is a serious health problem, yet there are no effective medications for its treatment. The rewarding effects of cocaine have been thought to be mediated primarily by inhibition of dopamine (DA) re-uptake in the mesolimbic dopamine system, which originates from the ventral tegmental area (VTA) in the midbrain and projects to the nucleus accumbens (NAc) and other forebrain regions (Wise, 1996; Gardner, 2000). Given this, most current pharmacotherapeutic strategies for the treatment of cocaine addiction have focused on this DA system by either mimicking cocaine's inhibition of DA re-uptake (as a substitution type therapy) or by blocking DA transmission with various DA receptor antagonists (as a blocker type therapy to inhibit the enhanced DA produced by cocaine). However, these interventions have proven ineffective in many clinical trials to date (Nann-Vernotica et al., 2001; Haney et al., 2001; Grabowski et al., 2000; Gorelick et al., 2004).
In addition to modulating DA, cocaine also produces an inhibitory effect on GABAergic neurons in the VTA and NAc (Cameron and Williams, 1994; Henry and White, 1995; Kiyatkin and Rebec, 2000), which has been thought to play an important role in cocaine reward and relapse (Carlezon and Wise, 1996). Thus, it has been proposed that pharmacological elevation of brain GABA levels or augmentation of GABAergic neurotransmission would directly counteract cocaine's actions on GABAergic transmission, and thus on cocaine reward and relapse (Roberts et al., 1996; Dewey et al., 1997; Giorgetti et al., 1998). Several lines of evidence support this hypothesis. First, potentiation of GABAA receptor activity inhibits cocaine-induced seizures (Gasior et al., 1999) and cocaine-enhanced extracellular NAc DA (Giorgetti et al., 1998). Also, GABAB receptor agonists inhibit both cocaine self-administration (Roberts et al., 1996; Roberts, 2005) and cocaine-triggered reinstatement of drug-seeking behavior (Campbell et al., 1999; Di Ciano and Everitt, 2003). Second, elevation of extracellular GABA levels by inhibition of GABA degradation by γ-vinyl GABA (GVG) inhibits the rewarding effects of cocaine as assessed by intravenous self-administration, conditioned place preference and electrical brain stimulation reward (Kushner, 1997; Kushner et al., 1999; Dewey et al., 1998). GVG has also been reported to inhibit cocaine-enhanced NAc DA levels (Dewey et al., 1998; Schiffer et al., 2003). Furthermore, clinical trials with the GABAA receptor modulator topiramate, the GABAB receptor agonist baclofen, or the GABA enhancers GVG or tiagabine (a GABA-uptake inhibitor) have shown significant decreases in self-reported cocaine craving and urine-confirmed cocaine use (Kampman et al., 2004; Shoptaw et al., 2003; Johnson, 2005; Brodie et al., 2003; González et al., 2003).
Gabapentin is another GABAergic modulator which facilitates GABAergic neurotransmission, possibly by increasing the synthesis and nonvesicular release of GABA as well as by preventing GABA catabolism (reviewed in Taylor et al., 1998). Early studies and small-scale (9–30 patient) open outpatient trials resulted in reports of reduced cocaine cravings and use after initiation of treatment with gabapentin (Raby, 2000; Raby and Coomaraswamy, 2004; Myrick et al., 2001; Hart et al., 2004; Haney et al., 2005). However, these findings were later challenged by other larger-scale, double-blind, placebo-controlled clinical trials demonstrating that gabapentin, at doses up to 2400–3200 mg/day for 6–12 weeks, had no effect on abstinence rates, craving or subjective effects of cocaine (Bisaga et al., 2006; Berger et al., 2005; González et al., 2007; Hart et al., 2007). However, there is no evidence demonstrating whether pretreatment with gabapentin significantly alters cocaine's direct rewarding effects or cocaine-triggered reinstatement of drug-seeking behavior at preclinical levels in experimental animals. Therefore, in the present study, we first observed the effects of systemic administration of gabapentin (25–200 mg/kg) on intravenous cocaine self-administration and cocaine-induced reinstatement of cocaine-seeking behavior in laboratory rats, and then further observed the effects of gabapentin on NAc extracellular DA, GABA and cocaine-enhanced NAc DA levels.
2. Materials and methods
2.1. Animals
Male Long-Evans rats (Charles River Laboratories, Raleigh, NC) weighing 250–300 g were housed individually in a climate-controlled animal room on a reversed light–dark cycle with free access to food and water. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse.
2.2. Cocaine self-administration and reinstatement of drug-seeking behavior
Animals were surgically implanted with intravenous (i.v.) catheters under sodium pentobarbital anesthesia using aseptic surgical technique. After the right external jugular vein was separated from surrounding tissues and fascia by blunt dissection and catheterized, the free end of the catheter was passed subcutaneously to the top of the skull, where it exited into a connector (a modified 22 gauge cannula; Plastics One, Roanoke, VA) mounted to the skull with stainless steel skull screws (Small Parts Inc., Miami Lakes, FL) and dental acrylic. To help prevent clogging, the catheters were flushed daily with a gentamicin–heparin–saline solution (5 mg/kg gentamicin, 30 IU/ml heparin; ICN Biochemicals, Cleveland, OH). After full recovery from surgery, animals were placed into standard operant chambers for cocaine self-administration and reinstatement as described previously (Xi et al., 2005, 2006). Initially, animals pressed the active lever for cocaine (1 mg/kg/infusion) for 3 h per day under fixed-ratio 1 (FR1) reinforcement, and were then switched to 0.5 mg/kg/infusion under FR2 reinforcement. Cocaine infusions were associated with light and sound cues. Inactive lever-presses were counted, but had no consequence.
For those rats used to evaluate the effects of gabapentin on cocaine self-administration, stable cocaine-maintained responding (0.5 mg/kg/infusion) under a FR2 schedule was established before gabapentin testing was initiated. Less than 10% variability in inter-response interval and less than 10% variability in number of presses on the active lever for at least three consecutive days were required for inclusion in the study. Animals then randomly received vehicle (25% 2-hydroxypropyl-β-cyclodextrin) or one dose of gabapentin (25, 60, 100, 200 mg/kg, i.p). Thirty minutes or 2 h later, all rats were put into test chambers, and the numbers of cocaine infusions and active lever-presses were recorded for 3h.
For those rats used to evaluate the effects of gabapentin on cocaine-triggered relapse, stable self-administration (0.5 mg/kg/infusion) under a FR2 reinforcement schedule was established, and animals were then exposed to extinction conditions, during which cocaine was replaced by saline and the cocaine-associated cue-light and tone turned off. Daily extinction sessions continued until lever-pressing was less than 10 per 3 h session for three consecutive days. Then, animals were divided into experimental groups, and reinstatement testing was begun 24 h later. On the reinstatement test day, each group of rats received either vehicle or one dose of gabapentin (25, 60, 100, 200 mg/kg, i.p). Thirty minutes or 2 h later, all rats received a priming injection of cocaine (10 mg/kg i.p.) and active lever-presses were recorded for 3 h. Inactive lever-presses were also counted but had no consequence.
2.3. In vivo brain microdialysis
Four groups of rats were used to evaluate the effects of gabapentin (0, 25, 100, 200 mg/kg i.p.) on NAc GABA and DA levels. Additionally, two groups of rats were used to evaluate the effects of the same doses of gabapentin on cocaine-induced changes in NAc DA. Microdialysis procedures were as reported previously (Xi et al., 2006). Guide cannulae (20 gauge, Plastics One, Roanoke, VA) were surgically implanted into the NAc (AP +1.6 mm, ML ±1.8 mm, DV −4.3 mm, angled 6° from vertical) under sodium pentobarbital anesthesia. The guide cannulae were fixed to the skull with stainless steel skull screws (Small Parts Inc., Miami Lakes, FL) and dental acrylic. Animals were allowed to fully recover from surgery before experiments began. Microdialysis probes were inserted through the previously-implanted guide cannulae into the NAc 12 h before each experiment to minimize damage-induced neurotransmitter release. During each experiment, microdialysis buffer (5 mM glucose, 2.5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, 0.15% phosphate buffered saline, pH 7.4) was perfused through the probe (2.0 μl/min) for at least 2 h before sampling began. Samples were collected every 20 min into 10 μl of 0.5 M perchloric acid (to prevent neurotransmitter degradation). After sample collection, all samples were frozen at −80 °C until analyzed.
Microdialysate GABA was determined using high performance liquid chromatography (HPLC) with flourometric detection (Xi et al., 2006). Excitation (Exλ) and emission (Emλ) wavelengths were 336 nm and 420 nm, respectively. Concentrations of DA were measured by HPLC with an electrochemical (EC) detection system (ESA Associates, Chelmsford, MA) as described previously (Xi et al., 2006), upgraded with a Coulochem III EC detector (ESA Associates, Chelmsford, MA). Areas under the curve (AUC) for DA or GABA were measured.
2.4. Drugs
Cocaine HCl (Sigma-RBI., St. Louis, MO) was dissolved in physiological saline. Gabapentin was purchased from Tocris Bioscience (Ellisville, MO) or synthesized at the H.C. Brown Center for Borane Research, Department of Chemistry, Purdue University, West Lafayette, IN, and was dissolved in 25% 2-hydroxypropyl-β-cyclodextrin (Sigma-RBI, St. Louis, MO).
2.5. Histology
Following microdialysis, rats were euthanized by pentobarbital overdose (>100 mg/kg i.p.) and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed and placed in 10% formalin for 1 week. The tissue was blocked around the NAc and coronal sections (100 μm thick) were made using a vibratome. The sections were stained with cresyl violet and examined by light microscopy.
2.6. Data analyses
All data are presented as means (±S.E.M.). AUC data were used for quantifying gabapentin's effects on basal extracellular NAc GABA, and basal or cocaine-enhanced extracellular NAc DA levels. The AUC (%) was calculated for each animal by subtracting 100 from the percent of baseline value for each data point, and subsequently summing all data points collected after drug administration. One-way analysis of variance (ANOVA) was used to analyze the effects of gabapentin on intravenous cocaine self-administration (Fig. 1), cocaine-induced reinstatement (Fig. 2), and basal or cocaine-induced neurochemical events (Figs. 3B, 3D, 4B, 4D). Two-way ANOVA with repeated measures over time was used to analyze the effects of gabapentin alone (Figs. 3A, C) or of gabapentin pretreatment on cocaine-induced changes in NAc neurotransmitter levels (Figs. 4A, 4C). Post-ANOVA pre-planned individual group comparisons were conducted using the Bonferroni procedure.
Fig. 1.
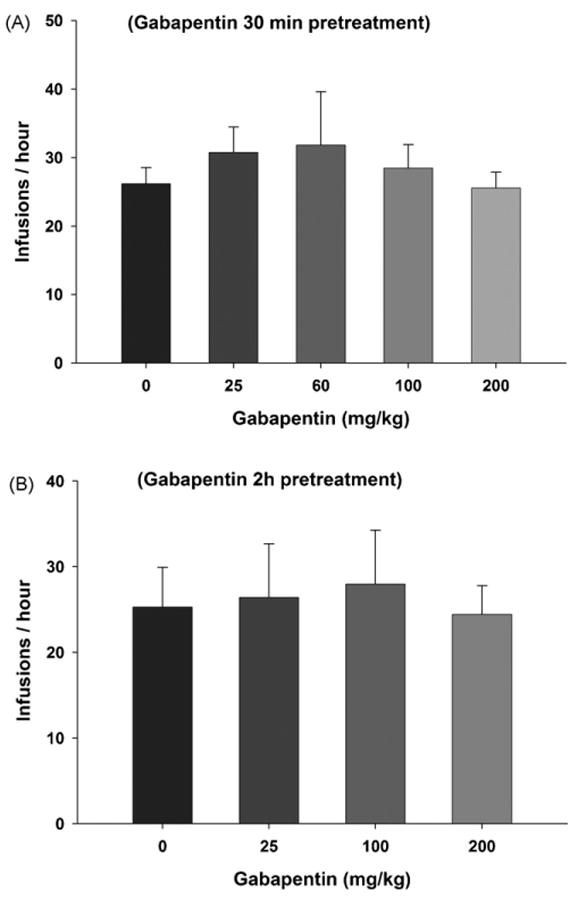
Effects of gabapentin (25–200 mg/kg) on intravenous cocaine (0.5 mg/kg/infusion) self-administration under a fixed-ratio 2 (FR2) reinforcement schedule, when gabapentin was administered either 30 min (Panel A) or 2 h (Panel B) prior to cocaine self-administration tests.
Fig. 2.
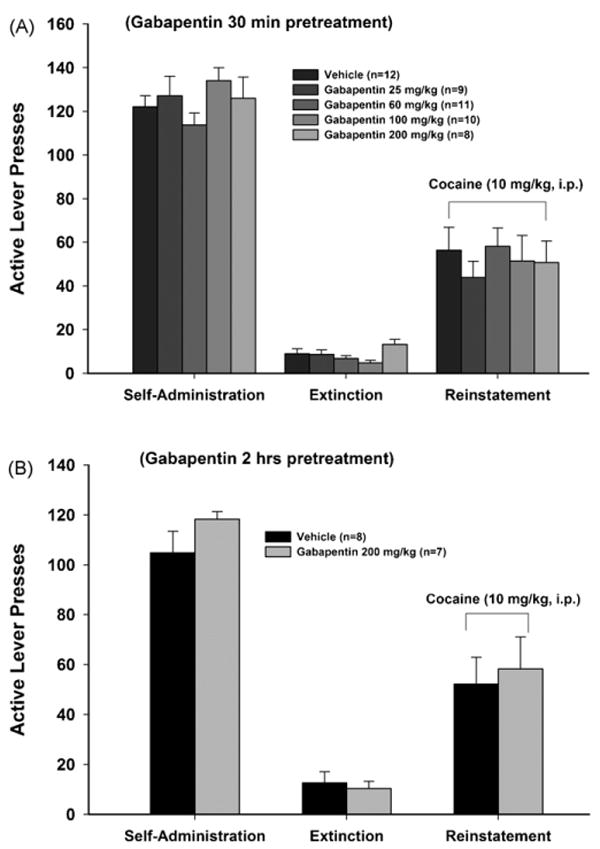
Effects of gabapentin (25–200 mg/kg) on cocaine (10 mg/kg)-induced reinstatement of drug-seeking behavior in rats, when gabapentin was administered either 30 min (Panel A) or 2 h (Panel B) prior to reinstatement tests.
Fig. 3.
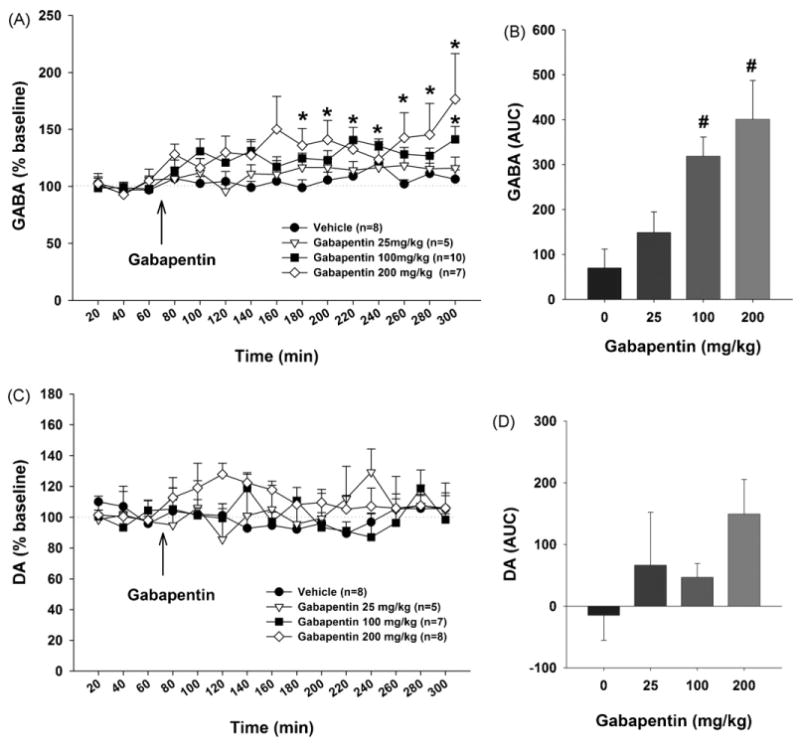
Effects of gabapentin on basal extracellular GABA and DA levels in the NAc. Systemic administration of gabapentin (25–200 mg/kg, i.p.) modestly elevated extracellular GABA levels in the NAc (Panels A and B), but failed to alter extracellular DA levels in the NAc (Panels C and D). *p < 0.05, compared with the baseline in each group; #p < 0.05, compared with the vehicle treatment group.
Fig. 4.
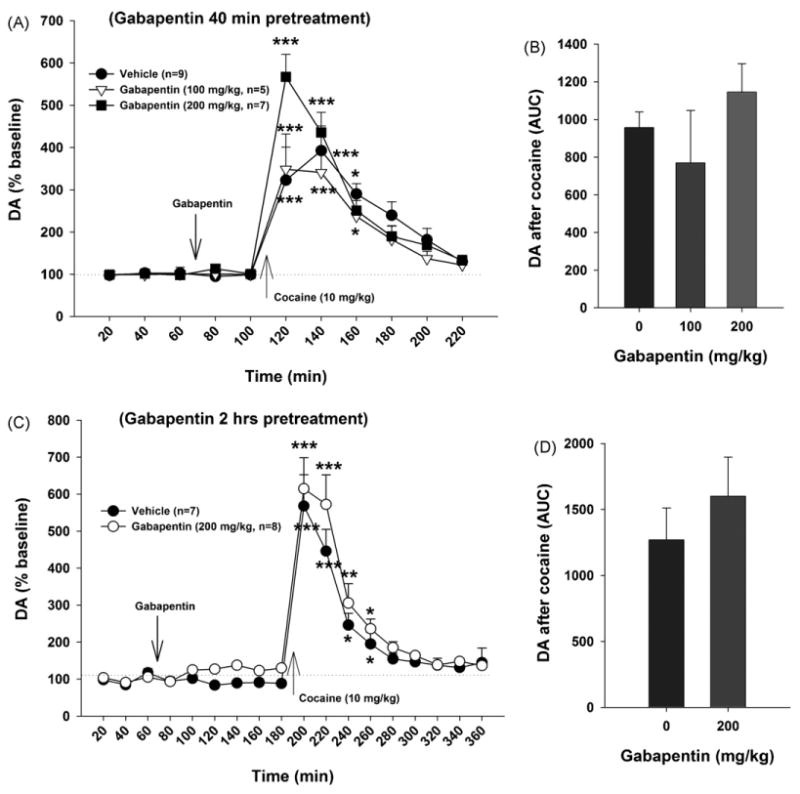
Effects of gabapentin on cocaine-induced increases in extracellular DA in the NAc. Systemic administration of cocaine (10 mg/kg, i.p.) significantly elevated extracellular DA in the NAc, while pretreatment with gabapentin (100–200 mg/kg, i.p.) failed to inhibit cocaine-enhanced DA in the NAc, when gabapentin was administered either 40 min (Panels A and B) or 2 h (Panels C and D) prior to cocaine priming. *p < 0.05, **p < 0.01, ***p < 0.001, compared with the baseline in each group.
3. Results
3.1. Gabapentin had no effect on cocaine self-administration behavior
Fig. 1 shows the effects of gabapentin (25, 60, 100, 200 mg/kg) on cocaine self-administration rate under FR2 reinforcement conditions at a unit cocaine reinforcement dose of 0.5 mg/kg/infusion, when compared to vehicle treatment animals. One-way ANOVA for repeated measures over the gabapentin doses showed no statistically significant effect of gabapentin on cocaine self-administration, when gabapentin was administered either 30 min (F4,33 = 0.67, p > 0.05) or 2 h (F3,15 = 0.21, p > 0.05) prior to cocaine self-administration testing.
3.2. Gabapentin had no effect on cocaine-triggered reinstatement of drug-seeking behavior
Five additional groups of rats were used to evaluate the effects of gabapentin on cocaine-induced reinstatement of drug-seeking behavior. Rats in all five groups exhibited stable responses on the active lever during the last 5–7 days of cocaine self-administration. There was no statistically significant difference in mean numbers of cocaine infusions, active lever presses, or inactive lever presses between the different groups of rats during the last three sessions of cocaine self-administration or extinction. A single, noncontingent cocaine injection (10 mg/kg i.p.) produced robust reinstatement of lever-pressing behavior within 30–40 min after cocaine administration. Pretreatment with gabapentin (25, 60, 100, 200 mg/kg, i.p) had no significant effect on cocaine-induced reinstatement of drug-seeking behavior, when gabapentin was administered 30 min prior to cocaine priming (Fig. 2A: F4,45 = 1.47, p > 0.05). Similarly, gabapentin (200 mg/kg) also failed to alter cocaine-induced reinstatement, when it was administered 2 h prior to cocaine priming in an additional experiment (Fig. 2B: F1,13 = 0.15, p > 0.05).
3.3. Gabapentin modestly elevated extracellular GABA levels, but did not alter extracellular DA levels in the NAc
Fig. 3 shows the effects of gabapentin (25, 100, 200 mg/kg, i.p.) on NAc extracellular GABA and DA levels, demonstrating that gabapentin dose-dependently elevated extracellular NAc GABA levels, but had no effect on NAc DA levels. Two-way ANOVA for repeated measures over time for the data shown in Fig. 3A revealed a statistically significant treatment (gabapentin vs. vehicle) main effect (F3,22 = 4.75, p < 0.05), and a statistically significant time main effect (F14,308 = 2.08, p < 0.05), but no significant treatment × time interaction (F42, 308 = 0.75, p > 0.05). One-way ANOVA for the AUC data shown in Fig. 3B revealed a significant dose-dependent increase in NAc GABA after gabapentin administration (F3,21 = 3.5, p < 0.05). Individual comparisons with the Bonferroni t-test showed a significant increase in NAc GABA after 100 mg/kg (t = 2.54, p < 0.05) or 200 mg/kg (t = 2.96, p < 0.05), but not after 25 mg/kg (t = 2.45, p > 0.05), gabapentin. Two-way ANOVA for repeated measurements over time for the data shown in Fig. 3C did not reveal a statistically significant treatment main effect (F3,21 = 2.69, p > 0.05), time main effect (F14,294 = 1.03, p > 0.05), or treatment × time interaction (F42, 294 = 1.13, p > 0.05). Further, one-way ANOVA for the AUC data shown in Fig. 3D revealed no significant treatment main effect (F3,44 = 1.22, p > 0.05).
3.4. Gabapentin had no effect on cocaine-enhanced NAc DA
Fig. 4 shows that cocaine alone (10 mg/kg, i.p.) produced a significant increase (300–400% of baseline, p < 0.05) in extracellular DA in the NAc. Gabapentin pretreatment (100–200 mg/kg, i.p., 40 min prior to cocaine priming) did not significantly alter such cocaine-induced increases in DA. Two-way ANOVA for repeated measures over time for the data shown in Fig. 4A revealed a statistically significant time main effect (F10,230 = 43.49, p < 0.001) and a statistically significant treatment × time interaction (F20,230 = 1.99, p < 0.01), but no significant treatment main effect (F2,23 = 1.24, p > 0.05). One-way ANOVA for the AUC data shown in Fig. 4B revealed no significant effect of gabapentin on cocaine-enhanced NAc DA (F2,23 = 1.19, p > 0.05). Similarly, gabapentin (200 mg/kg), when administered 2 h prior to cocaine injection, also failed to inhibit cocaine-induced increases in NAc extracellular DA in an additional experiment (Fig. 4C, D). Two-way ANOVA for repeated measures over time for the data shown in Fig. 4C revealed a statistically significant time main effect (F11,176 = 14.23, p < 0.001) and a statistically significant treatment × time interaction (F11,176 = 17.85, p < 0.001), but no significant treatment main effect (F1,13 = 0.008, p > 0.05). One-way ANOVA for the AUC data shown in Fig. 4D revealed no significant effect of gabapentin on cocaine-enhanced NAc DA (F1,13 = 1.43, p > 0.05).
Finally, histological examination indicated that the active membranes of the microdialysis probes were located within the NAc core and shell, but more within the core compartment (data not shown).
4. Discussion
The present study demonstrates that gabapentin, within the dose range 25–200 mg/kg, inhibited neither intravenous cocaine self-administration nor cocaine-induced reinstatement of cocaine-seeking behavior in experimental animals. Furthermore, the same doses of gabapentin only modestly (∼50%) elevated NAc extracellular GABA levels, but had no effect on either basal levels of extracellular DA or cocaine-induced increases in extracellular DA in the NAc. These results suggest that gabapentin is a weak GABA-mimic drug, which, at the doses tested, has no effect on cocaine's reinforcing ability or on cocaine-triggered relapse to drug-seeking behavior.
Gabapentin is structurally analogous to GABA, but, unlike GABA, crosses the blood–brain barrier when administered systemically (Wang and Welty, 1996; Goa and Sorkin, 1993). Gabapentin has little affinity at ionotropic GABAA receptors or metabotropic GABAB receptors (Suman-Chauhan et al., 1993), but significantly increases GABA synthesis (Löscher et al., 1991) and release (Götz et al., 1993), most likely by reversal of the GABA transporter (Honmou et al., 1995). In addition, high concentrations of gabapentin also inhibit GABA uptake by blocking GABA binding to GABA transporters (Eckstein-Ludwig et al., 1999). Consistent with these findings observed in vitro, the present in vivo brain microdialysis study demonstrated that gabapentin (100–200 mg/kg) produced a modest (∼50%), delayed (∼2 h) increase in extracellular GABA levels, which lasted for at least 4 h. In contrast to this present finding, another in vivo microdialysis study reported that 100 mg/kg gabapentin alters neither basal levels of extracellular GABA, nor K+-, glutamate- or nipecotic acid-induced increases in extracellular GABA in the substantia nigra (SN) (Timmerman et al., 2000). Such a difference in gabapentin's action on extracellular GABA could be related to the different doses (100 mg/kg vs. 200 mg/kg), the different brain regions sampled (NAc vs. SN) and/or the different sample sizes (n = 10 vs. n = 4). We also found that gabapentin neither lowers basal levels of extracellular NAc DA, nor inhibits cocaine-enhanced NAc DA, when gabapentin was administered either 40 min or 2 h prior to cocaine administration. Such pretreatment times were chosen based upon the finding that gabapentin-induced increases in NAc GABA are slow-onset and long-acting with significant increases occurring at around 2 h after gabapentin. These data suggest that gabapentin-induced increases in NAc GABA levels are not robust, and therefore likely not strong enough to inhibit NAc DA release.
Another important finding in the present study is that pretreatment with gabapentin (25–200 mg/kg) inhibits neither cocaine self-administration nor cocaine-triggered relapse to drug-seeking behavior, when administered either 30 min or 2 h prior to cocaine self-administration test. Intravenous cocaine self-administration and reinstatement of drug-seeking behavior are commonly used animal models to predict rewarding effects and relapse-provoking potential of addictive drugs in humans (Shaham et al., 2003; Xi et al., 2005, 2006). Congruent with the present findings, Filip et al. (2007) recently reported that lower doses of gabapentin (10–30 mg/kg, 1 h prior to testing) failed to alter cocaine self-administration and cocaine-induced relapse in rats (Filip et al., 2007). Such low doses of gabapentin (1–30 mg/kg) also failed to alter acute cocaine-induced hyperactivity or repeated cocaine-induced behavioral sensitization in rats (Itzhak and Martin, 2000; but see Filip et al., 2006). In contrast, one study reported that gabapentin (10–100 mg/kg) dose-dependently protected against cocaine (75 mg/kg)-induced seizures in mice (Gasior et al., 1999), which may result from a pharmacologically distinct (i.e., non-GABAergic) underlying mechanism (Bossert and Franklin, 2003).
The present findings that gabapentin (25–200 mg/kg) failed to inhibit cocaine self-administration, cocaine-triggered relapse and cocaine-enhanced NAc DA suggest that gabapentin, at the doses tested, has little potential for altering cocaine's rewarding effects or cocaine craving and relapse at the human level. This is consistent with findings from recent large-scale clinical trials demonstrating that gabapentin (1600–3200 mg/day, 6–12 weeks) had no effect on cocaine craving or urine-confirmed cocaine use (Berger et al., 2005; Bisaga et al., 2006; Hart et al., 2007; González et al., 2007). Since large-scale, double-blind clinical trials have obvious advantages over the case reports described in the Introduction, we interpret these negative findings as being more reliable and conclusive. In addition to the above-noted negative or positive reports in human subjects, there are two more small-scale clinical reports (Hart et al., 2004; Haney et al., 2005) of mixed results with gabapentin (600–1200 mg/day, in 7–8 cocaine-dependent patients, for 7 weeks), in which gabapentin appeared to inhibit cocaine craving and subjective effects of smoked cocaine, but had no effect on either cocaine self-administration or on cognitive or cardiovascular effects of smoked cocaine. In addition, there are two case reports indicating that gabapentin itself appears to have abuse potential in cocaine addicts (Markowitz et al., 1997; Reccoppa et al., 2004).
The failure of gabapentin to inhibit acute cocaine's behavioral and neurochemical effects is unlikely due to too low doses being used in the present study. First, 100–200 mg/kg gabapentin did produce an increase (∼50%, p < 0.05) in NAc extracellular GABA levels in the present study; Second, the doses used in the present experiments are equivalent to or higher than doses found to be effective in other behavioral tests in experimental animals. For example, it was reported that 10–30 mg/kg gabapentin significantly inhibits spontaneous activity and cocaine-induced behavioral sensitization (Filip et al., 2006), as well as nociceptive responses to noxious stimuli (Tanabe et al., 2006). In addition, 10–200 mg/kg gabapentin was reported to significantly inhibit cocaine- or pilocarpin-induced seizures (Gasior et al., 1999; Pereira et al., 2007). Third, by conversion of animal doses to human equivalent doses (i.e., rat dose in mg/kg × 0.16, as recommended by the U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research) (U.S. Department of Health and Human Services, 2005), the doses (25–200 mg/kg) of gabapentin we used in the present study in rats are equivalent to 4–30 mg/kg in humans. This is equivalent to the daily effective doses used for relieving pain (300–1200 mg/day, ≈5–20 mg/kg based on average body weight of 60 kg) (Mathiesen et al., 2007) or for controlling partial seizures (300–1800 mg/day, ≈5–30 mg/kg/day) (Morris, 1999) or for cocaine use (600–2400 mg/day, ≈10–40 mg/kg/day) in other clinical trials (Hart et al., 2004; Myrick et al., 2001; Raby, 2000; Raby and Coomaraswamy, 2004). It may be argued that such direct comparisons between a single animal dose and multiple human doses may be inaccurate or inappropriate. However, we point out that although the doses used in the present study are higher than the majority of doses reported in the literature for controlling pain and seizures (see review by Mathiesen et al., 2007), such high doses of gabapentin still had no effect on cocaine self-administration and cocaine-triggered reinstatement in experimental animals, and thus may be taken as an a forteriori demonstration of lack of effect.
Taken together, all these data suggest that gabapentin is a weak GABA enhancer. The therapeutic effects of gabapentin in the treatment of seizures or other psychiatric diseases could be related to its actions on other neurotransmitters or ion channels, in addition to mild augmentation of GABAergic transmission (Timmerman et al., 2000; Goa and Sorkin, 1993).
In conclusion, the present data – and their preliminary presentations in abstract form (Peng et al., 2004; Li et al., 2006) – show, for the first time, that gabapentin neither alters cocaine self-administration nor cocaine-triggered reinstatement of drug-seeking behavior in laboratory animals, suggesting a limited potential in treating cocaine dependence in humans. This could be related to its weak GABA-mimic potency, which does not appear strong enough to effectively inhibit cocaine-induced increases in extracellular DA in the NAc. The present findings with gabapentin are thus in contrast to our findings with γ-vinyl GABA (GVG), another GABAmimetic compound that does show effectiveness in some of the same addiction-related preclinical animal models used in the present study (see companion piece to present article). We therefore conclude that there are significant differences among putative GABAmimetic compounds with respect to their potential utility as anti-addiction therapeutic agents at the human level.
Acknowledgments
Role of funding source: Funding for this study was provided by funds from the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), U.S. Public Health Service (PHS), U.S. Department of Health and Human Services (DHHS), and by funds from the Herbert C. Brown Center for Borane Research, Department of Chemistry, Purdue University. Assistance in preparing the first draft of the manuscript was received from the NIH Fellows Editorial Board.
Footnotes
Preliminary reports on some of the present findings, in abbreviated abstract form, were presented at the 34th annual meeting of the Society for Neuroscience in October 2004, San Diego, CA and the 68th annual scientific meeting of the College on Problems of Drug Dependence in June 2006, Scottsdale, AZ.
Conflict of interest: All authors declare no financial activities or financial holdings that could be perceived as constituting a conflict of interest.
Contributors: Authors Peng, Xia Li, Gardner, and Xi designed the study. Authors Peng, Xia Li, Ashby, Gardner, and Xi carried out the literature searches and summaries of previous work. Authors Peng, Xia Li, and Jie Li carried out surgery on the experimental animals. Authors Ramachandran, Gagare, and Pratihar designed and developed novel and more efficient chemical syntheses for gabapentin for the in vivo animal studies. Authors Peng, Xia Li, and Jie Li ran animals in the behavioral paradigms, carried out the in vivo microdialysis experiments, and collected the data. Author Xi carried out the statistical analyses of the collected data, with additional statistical advice from author Gardner. Author Xia Li wrote the first draft of the manuscript, and authors Gardner and Xi contributed to correcting, revising, and rewriting the manuscript. Author Xi prepared the graphs and figures. All authors contributed to and have approved the final manuscript.
References
- Berger SP, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, Montgomery MA, Goldsmith RJ, Bloch DA, Singal BM, Elkashef A. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Franklin KBJ. Reinforcing versus anticonvulsant drugs: effects on intracranial self-stimulation rate-frequency M50 indices. Behav Brain Res. 2003;144:243–247. doi: 10.1016/s0166-4328(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with γ-vinyl GABA. Synapse. 2003;50:261–265. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology. 1999;143:209–214. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alexoff DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Morgan AE, Ashby CR, Jr, Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–129. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. The GABAB receptor agonist baclofen attenuates cocaine- and heroin-seeking behavior by rats. Neuropsychopharmacology. 2003;28:510–518. doi: 10.1038/sj.npp.1300088. [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Fei J, Schwarz W. Inhibition of uptake, steady-state currents, and transient charge movements generated by the neuronal GABA transporter by various anticonvulsant drugs. Br J Pharmacol. 1999;128:92–102. doi: 10.1038/sj.bjp.0702794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Gotda A, Zaniewska M, Vetulani J, Przegaliski E. Various GABA-mimetic drugs differently affect cocaine-evoked hyperlocomotion and sensitization. Eur J Pharmacol. 2006;541:163–170. doi: 10.1016/j.ejphar.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Gotda A, Przegaliski E, Vetulani J. Diverse effects of GABA-mimetic drugs on cocaine-evoked self-administration and discriminative stimulus effects in rats. Psychopharmacology. 2007;192:17–26. doi: 10.1007/s00213-006-0694-7. [DOI] [PubMed] [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Witkin JM. Preclinical evaluation of newly approved and potential antiepileptic drugs against cocaine-induced seizures. J Pharmacol Exp Ther. 1999;290:1148–1156. [PubMed] [Google Scholar]
- Giorgetti M, Javaid JI, Davis JM, Costa E, Guidotti A, Appel SB, Brodie MS. Imidazenil, a positive allosteric GABAA receptor modulator, inhibits the effects of cocaine on locomotor activity and extracellular dopamine in the nucleus accumbens shell without tolerance liability. J Pharmacol Exp Ther. 1998;287:58–66. [PubMed] [Google Scholar]
- Goa KL, Sorkin EM. Gabapentin: a review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409–427. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- González G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, Kosten TR. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- González G, Sevarino K, Sofuoglu M, Polng J, Oliveto A, Gonsai K, George TP, Kosten TR. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–1632. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Götz E, Feuerstein TJ, Lais A, Meyer DK. Effects of gabapentin on release of gamma-aminobutyric acid from slices of rat neostriatum. Arzneimittelforschung. 1993;43:636–638. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart C, Collins ED, Foltin RW. Smoked cocaine discrimination in humans: effects of gabapentin. Drug Alcohol Depend. 2005;80:53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86:274–277. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73:279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Oyelese AA, Kocsis JD. The anticonvulsant gabapentin enhances promoted release of GABA in hippocampus: a field potential analysis. Brain Res. 1995;692:273–277. doi: 10.1016/0006-8993(95)00634-3. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology. 2000;151:226–233. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O'Brien CP. Apilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Dopamine-independent action of cocaine on striatal and accumbal neurons. Eur J Neurosci. 2000;12:1789–1800. doi: 10.1046/j.1460-9568.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- Kushner SA, Dewey SL, Kornetsky C. Gamma-vinyl GABA attenuates cocaine-induced lowering of brain stimulation reward thresholds. Psychopharmacology. 1997;133:383–388. doi: 10.1007/s002130050418. [DOI] [PubMed] [Google Scholar]
- Kushner SA, Dewey SL, Kornetsky C. The irreversible γ-aminobutyric acid (GABA) transaminase inhibitor γ-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. [PubMed] [Google Scholar]
- Li X, Peng XQ, Gilbert J, Xi ZX, Gardner E. Gabapentin has no effect on cocaine-primed relapse and cocaine-induced increases in dopamine in the nucleus accumbens. Abstracts of the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence, 2006; Phoenix, Arizona. 2006. abstract # 460. [Google Scholar]
- Löscher W, Hönack D, Taylor CP. Gabapentin increases aminooxyacetic acid-induced GABA accumulation in several regions of rat brain. Neurosci Lett. 1991;128:150–154. doi: 10.1016/0304-3940(91)90249-s. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Finkenbine R, Myrick H, King L, Carson WH. Gabapentin abuse in a cocaine user: implications for treatment. J Clin Psychopharmacol. 1997;17:423–424. doi: 10.1097/00004714-199710000-00012. [DOI] [PubMed] [Google Scholar]
- Mathiesen O, Moiniche S, Dahl JB. Gabapentin and postoperative pain; a qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiol. 2007;7(6) doi: 10.1186/1471-2253-7-6. published online 07 July 07 at http://www.biomedcentral.com/1471-2253/7/6) [DOI] [PMC free article] [PubMed]
- Morris GL. Gabapentin. Epilepsia. 1999;40(Suppl 5):S63–S70. doi: 10.1111/j.1528-1157.1999.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Henderson S, Brady KT, Malcolm R. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62:19–23. doi: 10.4088/jcp.v62n0105. [DOI] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Peng XQ, Xi ZX, Gilbert J, Campos A, Dewey SL, Schiffer WK, Brodie JD, Ashby CR, Gardner EL. Gamma-vinyl GABA, but not gabapentin, inhibits cocaine-triggered reinstatement of drug-seeking behavior in the rat. Abstracts of the 34th Annual Meeting of the Society for Neuroscience, 2004; San Diego, CA. 2004. abstract # 691.8. [Google Scholar]
- Pereira MB, Freitas RL, Assis MA, Silva RF, Fonteles MM, Freitas RM, Takahashi RN. Study pharmacologic of the GABAergic and glutamatergic drugs on seizures and status epilepticus induced by pilocarpine in adult Wistar rats. Neurosci Lett. 2007;419:253–257. doi: 10.1016/j.neulet.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Raby WN. Gabapentin therapy for cocaine cravings. Am J Psychiatry. 2000;157:2058–2059. doi: 10.1176/appi.ajp.157.12.2058-a. [DOI] [PubMed] [Google Scholar]
- Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65:84–86. doi: 10.4088/jcp.v65n0114. [DOI] [PubMed] [Google Scholar]
- Reccoppa L, Malcolm R, Ware M. Gabapentin abuse in inmates with prior history of cocaine dependence. Am J Addict. 2004;13:321–323. doi: 10.1080/10550490490460300. [DOI] [PubMed] [Google Scholar]
- Roberts DCS. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Marsteller D, Dewey SL. Sub-chronic low dose γ-vinyl GABA (vigabatrin) inhibits cocaine-induced increases in nucleus accumbens dopamine. Psychopharmacology. 2003;168:339–343. doi: 10.1007/s00213-003-1446-6. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–1448. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Suman-Chauhan N, Webdale L, Hill DR, Woodruff GN. Characterisation of [3H]gabapentin binding to a novel site in rat brain: homogenate binding studies. Eur J Pharmacol. 1993;244:293–301. doi: 10.1016/0922-4106(93)90155-3. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Murakami H, Honda M, Ono H. Gabapentin depresses C-fiber-evoked field potentials in rat spinal dorsal horn only after induction of long-term potentiation. Exp Neurol. 2006;202:280–286. doi: 10.1016/j.expneurol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L. Summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Bouma M, De Vries JB, Davis M, Westerink BHC. A microdialysis study on the mechanism of action of gabapentin. Eur J Pharmacol. 2000;398:53–57. doi: 10.1016/s0014-2999(00)00309-5. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. [October 19, 2007];2005 http://www.fda.gov/cder/guidance/index.htm.
- Wang Y, Welty DF. The simultaneous estimation of the influx and efflux blood-brain barrier permeabilities of gabapentin using microdialysis-pharmacokinetic approach. Pharm Res. 1996;13:398–403. doi: 10.1023/a:1016092525901. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost – variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8636. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


