Abstract
Dietary manipulation, including caloric restriction, has been shown to significantly impact host response capabilities, particularly associated with aging. This investigation compared systemic inflammatory and immune response molecules in rhesus monkeys (Macaca mulatta) on continuous long term calorie-restricted (CR) diets with a matched group of animals on a control diet, examining the effects of both gender and aging. The results demonstrated that haptoglobin and α1anti-glycoprotein were elevated in serum of male monkeys. Serum IgG antibody responses to C. rectus, A. actinomycetemcomitans, and P. gingivalis were significantly elevated in female monkeys. While only the antibody to F. nucleatum was significantly affected by the calorie-restricted diet in females, antibody levels to P. intermedia, C. rectus and T. denticola demonstrated a similar trend. In this investigation, only selected serum antibody levels were influenced by the age in male animals, seemingly related to increasing clinical disease in this gender. More generally, analytes were modulated by gender and/or diet in this oral model system of mucosal microbial challenge.
Keywords: Nonhuman primates, calorie-restriction, oral infections, host responses
Introduction
The inflammatory response involves humoral and cellular responses to a given challenge. There has been an increasing demand to assess the effects of aging on immune cell functions. It has been well documented that T cell and B cell numbers and functions decrease progressively during aging (1), although the impact of aging on innate immunity remains to be clarified. Numerous studies in rodent models have documented a decline in immune responsiveness with age (2–5). In particular, these studies have indicated that advancing age produces a general depression in the adaptive immune response (5), accompanied by an increase in the production and release of reactive oxygen species, reactive nitrogen species, and the activity of cyclooxygenase enzymes with an accompanying increase in prostaglandin production (1, 3, 4). In addition, there appears to be an up-regulation of inflammatory cytokine gene expression with aging including TNFα, IL-1, IL-6, INFγ, and TGFβ [(5–7). However, assessing the impact of aging on cellular functions in humans is complicated by the effects of chronic diseases frequently observed in elderly persons. Thus, in human systems it continues to be a challenge to delineate the effects of aging versus the effects of systemic or environmental conditions (8).
Caloric restriction (CR) of dietary intake has been shown to significantly alter a wide range of biological processes and, in particular, attenuate age-related disease in rodent models of aging (4, 8–11). This dietary manipulation has been demonstrated to attenuate the development of oxygen radical induced cell damage, to maintain more robust host responses protecting against deleterious extrinsic and intrinsic challenges to normal cell, tissue, and organ function, and to maintain general body-wide physiologic functions (12–25). Recent studies have interpreted these macro-observations at the molecular level by identifying that CR could stop aging-associated changes in the expression of numerous genes (12, 13), including altering insulin-like growth factor 1 (IGF-1) associated with age-related decreases in insulin sensitivity (20, 26, 27). Only recently have reports emerged regarding the potential for this dietary manipulation to also alter physiologic parameters in nonhuman primates, a species more closely related to humans (28–36). Since many of these findings are similar to those seen in rodent models, the nonhuman primates may provide a valuable link between rodent studies of reduced calorie diets and application of this approach to a human population.
Periodontal disease is a predominant chronic inflammatory disease of mankind (37–39) that is a consequence of oral infection, chronic inflammation, and destruction of collagen and bone, and can be documented to occur naturally with aging in humans and nonhuman primates (37, 40, 41). The extent and severity of tissue destruction is affected by the magnitude and characteristics of the host response and may be modulated by environmental, systemic or genetic factors (38, 39, 42). Periodontal destruction is cumulative and not naturally reversible, thus, it is unclear as to whether aging impacts the rate of disease progression or just reflects the accumulation of disease over time (41, 43). The importance of periodontal disease as a model of host-bacterial interactions, inflammation, and inflammatory disease lies in the ability to isolate and characterize bacterial and host factors from the oral cavity in a non-invasive manner and to correlate these changes with host tissue pathology. The nonhuman primate model has provided the essential bridge for understanding the interaction of the subgingival microbiota with the inflammatory/immune response targeted to selected members of this microbiota (44–48). Increasing evidence also suggests that these oral microorganisms can translocate to the systemic circulation and may routinely stimulate the reticuloendothelial and immune systems (49–51). Recent studies have provided clear evidence that the oral cavity can function as a nidus for a variety of potential medical problems (49, 51, 52). Several members of the periodontopathic microbiota have been found to be involved in other systemic infections, as well as in the induction of an acute phase response (APR) (53). Increased levels of acute phase proteins have been identified in adult patients with periodontitis, e.g. CRP, haptoglobin, and may reflect the infection and manifestations of acute and chronic inflammation that exist in the periodontium (53–56). Moreover, it was also evident that patients exhibiting the most severe disease had the greatest levels of each of the acute phase reactants. In addition, a serum antibody response is observed in these localized periodontal infections. It has been suggested that this serum response may reflect a local gingival inflammatory/immune response to the bacteria. Thus, the systemic antibody response observed in periodontitis patients appears to result from specific elicitation of antibody to an infection with the microorganism (50, 57, 58).
This study utilizes the accessibility and natural development of chronic inflammation and disease in the oral cavity to examine the effects of long term dietary calorie restriction on inflammatory/immune responses in a human-like model system, the rhesus monkey.
Materials and methods
Animals and diet
Eighty-three rhesus monkeys (Macaca mulatta) that are part of an ongoing study of caloric restriction and aging were used in these studies (Table 1). These animals have been housed at the National Institutes of Health Animal Center, Poolesville, MD. Details of the study have been described previously (Reynolds M, G. Branch-Mays, D. Dawson III, K.F. Novak, J. Mattison, J. Gunsolley, D. Ingram, M. Lane, G. Roth, and M.J. Novak. Effects of dietary calorie restriction on inflammatory disease in a nonhuman primate model. Submitted). At the time of the current study, animals had been assigned to continuous long term CR or control diets for periods of 13–17 years.
Table 1.
Age distribution of nonhuman primate cohort in study.
| Gender | Diet Group | N | Mean ± SD |
|---|---|---|---|
| Female | CON | 19 | 18.74 ± 1.29 |
| CR | 16 | 16.94 ± 1.22 | |
| Male | CON | 26 | 22.35 ± 1.21 |
| CR | 20 | 22.70 ± 1.53 |
Serum analyses
Blood was collected from all monkeys under ketamine or telazol anesthesia following an overnight fast, serum was separated and stored at −80°C until assay, when IgG antibody to six oral bacteria was evaluated using an ELISA as described previously (59, 60). Briefly, Campylobacter rectus, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans, Prevotella intermedia, Treponema denticola, and Porphyromonas gingivalis were grown in broth under anaerobic conditions, harvested by centrifugation, formalin-killed, washed, and stored at −20°C for use as antigens (61).
Selected acute phase reactants were quantified using ELISA procedures developed in our laboratory (53, 56, 62). Specifically we examined levels of C-reactive protein (CRP), haptoglobin (HG), fibrinogen (Fib), α1-antiproteinase (α1-AT), and α1-acid glycoprotein (α1-AG) in serum samples from all animals.
Statistical analyses
In the primary analysis, the effects of age and CR were analyzed separately by gender due to different age distributions (Table 1). Age was modeled as a linear variable. In secondary analyses, data were submitted to a linear regression analysis in which gender was included in the model. The purpose of the secondary analysis was to verify the robustness of the results. Statistical analysis was performed using JMP (SAS, Inc.). Statistical significance was set at an alpha level of 0.05.
Results
Systemic acute phase reactants
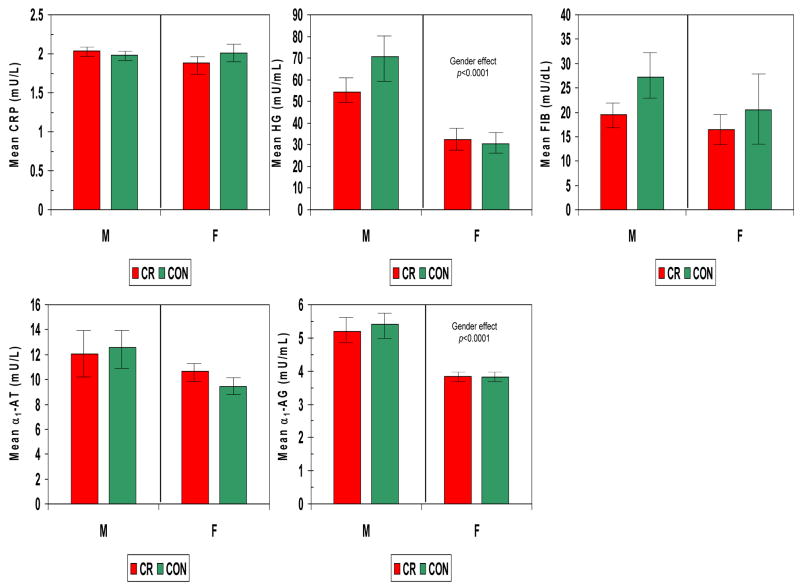
The levels of various acute phase reactants were determined in serum from each monkey and compared based upon gender and diet. Fig. 1 demonstrates that haptoglobin, and α1-antiglycoprotein were significantly greater in males compared to females and were not affected by CR diet.
Figure 1.
Acute phase reactants in serum from nonhuman primates categorized based upon gender (F – female; M – male) and diet (CR – calorie restricted; CON – control ad libitum). The bars denote the mean levels of each mediator (HG – haptoglobin; FIB - fibrinogen; CRP – C-reactive protein; α1-AT –α1-antiproteinase; α1-AG –α1-acid glycoprotein) and the vertical brackets denote 1 standard error.
Systemic antibody responses to oral bacteria
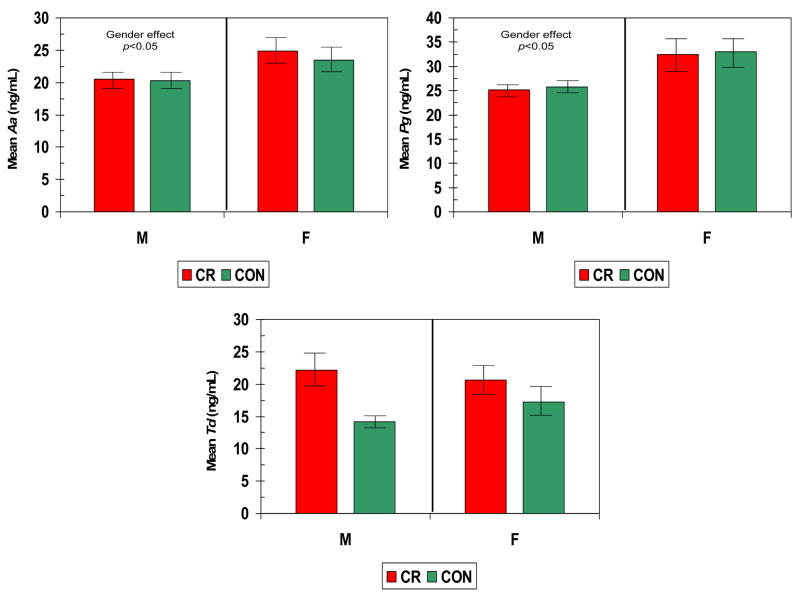
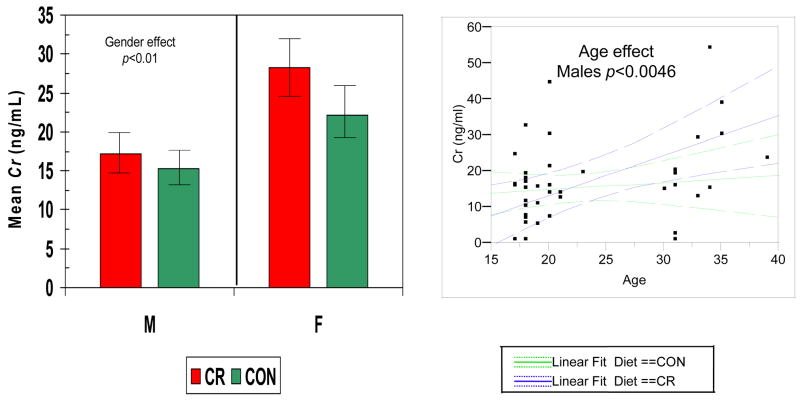
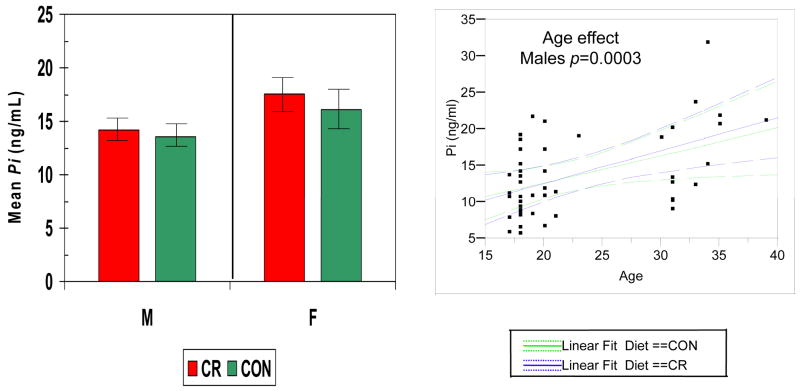
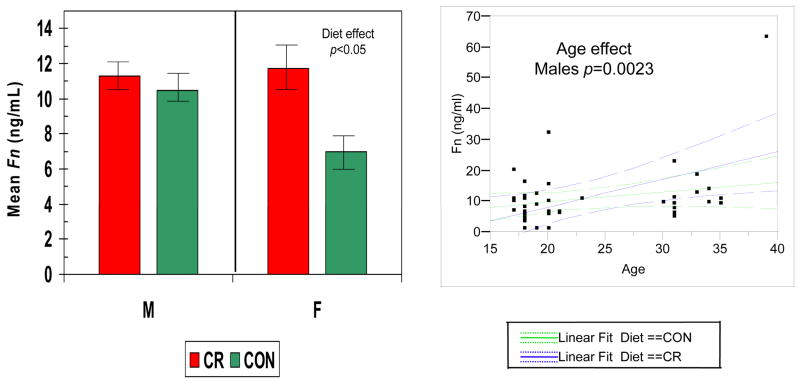
Figures 2–5 show the levels of serum IgG antibody to a group of oral bacteria commonly associated with periodontal disease (63, 64). In Fig. 2 antibody levels to A. actinomycetemcomitans and P. gingivalis were significantly elevated in the female monkeys compared to males with no effect of diet or age. In Fig. 3 the antibody to P. intermedia was significantly related to age in male animals, although the females did exhibit a trend toward higher levels of antibody, irrespective of diet. Fig. 4 illustrates that serum IgG antibody to F. nucleatum was significantly elevated by a CR diet in the females only, and the levels increased significantly with age in the males unrelated to diet. In Fig. 5 the serum antibody levels to C. rectus were also significantly elevated in females compared to males, and these levels increased significantly in males with age unrelated to diet.
Figure 2.
Serum IgG antibody levels to A. actinomycetemcomitans (Aa), P. gingivalis (Pg), and T. denticola(Td) in nonhuman primates categorized based upon gender and diet. The bars denote the mean levels of each mediator and the vertical brackets denote 1 standard error.
Figure 5.
Serum IgG antibody levels to C. rectus (Cr) in nonhuman primate categorized based upon gender and diet (LEFT). The bars denote the mean levels of each mediator and the vertical brackets denote 1 standard error. (RIGHT) Antibody levels related to age classified according to gender (green – female; blue – male). The dashed lines denote 95% confidence interval.
Figure 3.
Serum IgG antibody levels to P. intermedia (Pi) in nonhuman primates categorized based upon gender and diet (LEFT). The bars denote the mean levels of each mediator and the vertical brackets denote 1 standard error. (RIGHT) Antibody levels related to age classified according to diet (green –control; blue – calorie restricted). Dashed lines denote 95% confidence interval.
Figure 4.
Serum IgG antibody levels to F. nucleatum (Fn) in nonhuman primate categorized according to gender and diet (LEFT). The bars denote the mean levels of each mediator and the vertical brackets denote 1 standard error. (RIGHT) Antibody levels related to age classified according to gender (green – female; blue – male). The dashed lines denote 95% confidence interval.
Discussion
This investigation described the characteristics of systemic inflammatory and immune responses of a nonhuman primate cohort related to a calorie-restricted diet. This dietary manipulation has been demonstrated to contribute toward potential therapeutic outcomes related to biologic processes adversely affected by aging (10, 11, 15, 16). CR has been shown to minimize decline in specific immune functions (1, 5), as well as attenuate destructive inflammatory responses (4). Various physiologic parameters (4, 8, 14, 25, 35, 65) and hormonal changes (thyroid hormones, melatonin, and dehydroepiandrosterone sulfate) (19, 35, 66, 67) related to improved aging have been reported in nonhuman primates on a long-term CR diet.
In the current study, the nonhuman primate model was used to examine the effect of a calorie restricted diet on systemic inflammatory and antibody responses to oral commensal and opportunistic bacterial pathogens. Periodontal disease is a complex microbial infection with similarities between humans and nonhman primate (48, 54). This oral infection elicits a chronic immunoinflammatory lesion that destroys soft and hard tissues resulting in destruction of the periodontium (37, 68–70). While the extent and severity of periodontal disease is related to aging (41, 71), it is unclear if this finding represents a cumulative expression of years/decades of challenge to the tissues or an exacerbated disease process reflecting altered aging processes measured at a molecular level. Periodontal disease provides a model of host-bacterial interactions, inflammation, and adaptive immune responses that can be used to examine nutritional and aging changes in the oral cavity. In addition, ample evidence has demonstrated that these local oral infections also stimulate a systemic inflammatory and humoral immune response (50, 53, 58, 72–77).
We have previously reported an age-associated increase in periodontal disease in nonhuman primates (Reynolds M, G. Branch-Mays, D. Dawson III, K.F. Novak, J. Mattison, J. Gunsolley, D. Ingram, M. Lane, G. Roth, and M.J. Novak. Effects of dietary calorie restriction on inflammatory disease in a nonhuman primate model. Submitted). Periodontal disease was more prevalent in the males with a more dramatic effect related to aging. The current study suggested that characteristics of the systemic host responses were consistent with these disease findings. Systemic inflammatory mediators were significantly greater in male nonhuman primate compared to females. Human studies have shown that increased severity/extent of periodontitis results in higher serum levels of these host response molecules (53, 72, 78, 79). Thus, it was expected that the males would have elevated levels of these mediators. Although the male monkeys on a long term CR diet had generally lower levels across the profile of acute phase reactants, this difference was not statistically significant. These outcomes are consistent with cross-sectional and longitudinal observations of human populations demonstrating elevated acute phase reactants in periodontitis and a decrease in these mediators after mechanical and anti-inflammatory therapies (53, 72, 78, 79). Since these systemic inflammatory responses have been suggested to reflect and/or contribute to chronic inflammatory diseases, eg. cardiovascular and diabetes, the contribution of chronic periodontitis to these systemic biomolecules has been suggested to be a biologic link between oral and systemic diseases (80).
In humans, both the specificity and levels of serum antibody responses to oral pathogens are clearly related to periodontal disease (50, 58, 77, 81, 82). With increasing disease both antibody frequency and level also increase and various studies have demonstrated that these serum antibody levels will be elevated following mechanical therapy and will correlate with response to treatment (50, 83–86). Moreover, changes in serum antibody to selected oral pathogens appear to occur following emergence of the microorganisms in oral biofilm samples and prior to identification of progressing disease (50, 87). These findings suggest that the humoral immune response in local tissues, and reflected in the systemic circulation, is likely an important component of the host’s responses trying to re-establish homeostasis by controlling the challenge of these extracellular bacterial pathogens. Interestingly, we observed significantly elevated antibody to these oral pathogens in female monkeys who displayed less periodontal inflammation and disease than the males (Reynolds M, G. Branch-Mays, D. Dawson III, K.F. Novak, J. Mattison, J. Gunsolley, D. Ingram, M. Lane, G. Roth, and M.J. Novak. Effects of dietary calorie restriction on inflammatory disease in a nonhuman primate model. Submitted). The antibody responses also appeared to be generally elevated with CR, with the most substantive impact in females. These results suggested a gender specific differentiation of responses oriented towards a potentially destructive inflammatory response in males versus a protective adaptive immune response in females. This type of observation has a basis in existing data demonstrating inherent gender-based variations in levels of immunoglobulins (88–90) and other host response biomarkers (91, 92). Subsequent studies implementing a longitudinal, prospective design creating a ligature-induced periodontal challenge in these animals should help clarify the dynamics of the relationship of periodontal disease to these response profiles. Lastly, of the analytes measured, serum antibody levels demonstrated some positive correlations with aging, primarily in the males, consistent with increased clinical parameters of periodontal disease in this group.
These cross-sectional observations provide a snapshot of host serum acute phase and antibody responses in nonhuman primate. The response profiles supported an inherently different response pattern in monkeys that was gender determined, as well as differences in the genders with respect to the impact of CR on these systemic responses. Further analyses will determine the interaction of oral clinical presentation and these responses demonstrating the usefulness of the oral cavity as a model for aging studies of host-bacterial interactions.
Acknowledgments
This work was supported by USPHS grant U01 AG-021406 from the National Institute of Aging and by funds from the Intramural Research Program of the National Institute on Aging and the Veterinary Research Program of the Division of Research Resources of the National Institutes of Health. We extend our gratitude to the entire technical support group from the NIH Animal Research Center, especially April Hobbs, Edward Tilmont, Tommy Thompson, and Suzanne Pazzi for managing the sample collection and shipment for analyses, and to Rick Herbert, DVM, and Doug Powell, DVM, for their outstanding clinical assistance that assures the good health of the monkeys in this study The contributions of Drs. George Roth and Mark Lane in facilitating this research program are also greatly appreciated.
References
- 1.Hirokawa K, Utsuyama M. Animal models and possible human application of immunological restoration in the elderly. Mech Ageing Dev. 2002;123:1055–1063. doi: 10.1016/s0047-6374(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 2.Anisimov VN, Ukraintseva SV, Anikin IV, et al. Effects of phentermine and phenformin on biomarkers of aging in rats. Gerontology. 2005;51:19–28. doi: 10.1159/000081430. [DOI] [PubMed] [Google Scholar]
- 3.Merry BJ. Calorie restriction and age-related oxidative stress. Ann N Y Acad Sci. 2000;908:180–198. doi: 10.1111/j.1749-6632.2000.tb06646.x. [DOI] [PubMed] [Google Scholar]
- 4.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Pahlavani MA. Influence of caloric restriction on aging immune system. J Nutr Health Aging. 2004;8:38–47. [PubMed] [Google Scholar]
- 6.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascarucci P, Taub D, Saccani S, et al. Age-related changes in cytokine production by leukocytes in rhesus monkeys. Aging (Milano) 2001;13:85–94. doi: 10.1007/BF03351530. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 9.Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 10.Smith JV, Heilbronn LK, Ravussin E. Energy restriction and aging. Curr Opin Clin Nutr Metab Care. 2004;7:615–622. doi: 10.1097/00075197-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Van Remmen H, Guo Z, Richardson A. The anti-ageing action of dietary restriction. Novartis Found Symp. 2001;235:221–230. doi: 10.1002/0470868694.ch18. discussion 230–223. [DOI] [PubMed] [Google Scholar]
- 12.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 13.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Dietary restriction and life-span. Science. 2002;296:2141–2142. doi: 10.1126/science.296.5576.2141. author reply 2141–2142. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DK, Anson RM, de Cabo R, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 15.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 16.Roth GS, Lane MA, Ingram DK. Caloric restriction mimetics: the next phase. Ann N Y Acad Sci. 2005;1057:365–371. doi: 10.1196/annals.1356.027. [DOI] [PubMed] [Google Scholar]
- 17.Lane MA, Black A, Handy AM, et al. Energy restriction does not alter bone mineral metabolism or reproductive cycling and hormones in female rhesus monkeys. J Nutr. 2001;131:820–827. doi: 10.1093/jn/131.3.820. [DOI] [PubMed] [Google Scholar]
- 18.Lane MA, Reznick AZ, Tilmont EM, et al. Aging and food restriction alter some indices of bone metabolism in male rhesus monkeys (Macaca mulatta) J Nutr. 1995;125:1600–1610. doi: 10.1093/jn/125.6.1600. [DOI] [PubMed] [Google Scholar]
- 19.Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocrinol Metab. 2001;86:3292–3295. doi: 10.1210/jcem.86.7.7655. [DOI] [PubMed] [Google Scholar]
- 20.Cutler RG, Davis BJ, Ingram DK, Roth GS. Plasma concentrations of glucose, insulin, and percent glycosylated hemoglobin are unaltered by food restriction in rhesus and squirrel monkeys. J Gerontol. 1992;47:B9–12. doi: 10.1093/geronj/47.1.b9. [DOI] [PubMed] [Google Scholar]
- 21.DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA. Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci. 1999;54:B5–11. doi: 10.1093/gerona/54.1.b5. discussion B12–13. [DOI] [PubMed] [Google Scholar]
- 22.Lane MA, Baer DJ, Tilmont EM, et al. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995;50:B295–302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- 23.Lee IM, Blair SN, Allison DB, et al. Epidemiologic data on the relationships of caloric intake, energy balance, and weight gain over the life span with longevity and morbidity. J Gerontol A Biol Sci Med Sci. 2001;(56 Spec No 1):7–19. doi: 10.1093/gerona/56.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 24.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lane MA, Baer DJ, Rumpler WV, et al. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodkin NL, Ortmeyer HK, Hansen BC. Long-term dietary restriction in older-aged rhesus monkeys: effects on insulin resistance. J Gerontol A Biol Sci Med Sci. 1995;50:B142–147. doi: 10.1093/gerona/50a.3.b142. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi S, Kamino Y, Hiratsuka K, Kiyama-Kishikawa M, Abiko Y. Age-related changes in IGF-1 expression in submandibular glands of senescence-accelerated mice. J Oral Sci. 2004;46:119–125. doi: 10.2334/josnusd.46.119. [DOI] [PubMed] [Google Scholar]
- 28.Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 29.Edwards IJ, Rudel LL, Terry JG, et al. Caloric restriction lowers plasma lipoprotein (a) in male but not female rhesus monkeys. Exp Gerontol. 2001;36:1413–1418. doi: 10.1016/s0531-5565(01)00107-3. [DOI] [PubMed] [Google Scholar]
- 30.Ingram DK, Chefer S, Matochik J, et al. Aging and caloric restriction in nonhuman primates: behavioral and in vivo brain imaging studies. Ann N Y Acad Sci. 2001;928:316–326. doi: 10.1111/j.1749-6632.2001.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 31.Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 32.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates. Ann N Y Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 33.Lane MA, Mattison J, Ingram DK, Roth GS. Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc Res Tech. 2002;59:335–338. doi: 10.1002/jemt.10214. [DOI] [PubMed] [Google Scholar]
- 34.Roth GS, Ingram DK, Lane MA. Calorie restriction in primates: will it work and how will we know? J Am Geriatr Soc. 1999;47:896–903. doi: 10.1111/j.1532-5415.1999.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 35.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 36.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 37.Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49:491–516. v. doi: 10.1016/j.cden.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Preshaw PM, Seymour RA, Heasman PA. Current concepts in periodontal pathogenesis. Dent Update. 2004;31:570–572. 574–578. doi: 10.12968/denu.2004.31.10.570. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie CS, Kinane DF. Nutrition, inflammation, and periodontal disease. Nutrition. 2003;19:475–476. doi: 10.1016/s0899-9007(02)01043-2. [DOI] [PubMed] [Google Scholar]
- 40.Streckfus CF, Parsell DE, Streckfus JE, Pennington W, Johnson RB. Relationship between oral alveolar bone loss and aging among African-American and Caucasian individuals. Gerontology. 1999;45:110–114. doi: 10.1159/000022072. [DOI] [PubMed] [Google Scholar]
- 41.Willershausen-Zonnchen B, Gleissner C. Periodontal disease in elderly patients. Eur J Med Res. 1998;3:55–64. [PubMed] [Google Scholar]
- 42.Skaleric U, Kovac-Kavcic M. Some risk factors for the progression of periodontal disease. J Int Acad Periodontol. 2000;2:19–23. [PubMed] [Google Scholar]
- 43.van der Velden U, Abbas F, Hart AA. Experimental gingivitis in relation to susceptibility to periodontal disease. (I) Clinical observations. J Clin Periodontol. 1985;12:61–68. doi: 10.1111/j.1600-051x.1985.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 44.Ebersole JL, Cappelli D, Holt SC, Singer RE, Filloon T. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol Immunol. 2000;15:19–26. doi: 10.1034/j.1399-302x.2000.150104.x. [DOI] [PubMed] [Google Scholar]
- 45.Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect Immun. 2004;72:1166–1168. doi: 10.1128/IAI.72.2.1166-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 47.Persson GR, Engel LD, Moncla BJ, Page RC. Macaca nemestrina: a non-human primate model for studies of periodontal disease. J Periodontal Res. 1993;28:294–300. doi: 10.1111/j.1600-0765.1993.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 48.Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993;64:497–508. doi: 10.1902/jop.1993.64.6.497. [DOI] [PubMed] [Google Scholar]
- 49.Mombelli A. Periodontitis as an infectious disease: specific features and their implications. Oral Dis. 2003;9(Suppl 1):6–10. doi: 10.1034/j.1601-0825.9.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 50.Ebersole JL, Cappelli D, Holt SC. Periodontal diseases: to protect or not to protect is the question? Acta Odontol Scand. 2001;59:161–166. doi: 10.1080/000163501750266756. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page RC. The microbiological case for adjunctive therapy for periodontitis. J Int Acad Periodontol. 2004;6:143–149. [PubMed] [Google Scholar]
- 53.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 54.Ebersole JL, Cappelli D, Mathys EC, et al. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann Periodontol. 2002;7:102–111. doi: 10.1902/annals.2002.7.1.102. [DOI] [PubMed] [Google Scholar]
- 55.Adonogianaki E, Mooney J, Kinane DF. The ability of gingival crevicular fluid acute phase proteins to distinguish healthy, gingivitis and periodontitis sites. J Clin Periodontol. 1992;19:98–102. doi: 10.1111/j.1600-051x.1992.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 56.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schenkein HA, Gunsolley JC, Best AM, et al. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinane DF, Mooney J, Ebersole JL. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 59.Giardino A, Ebersole JL, Holt SC. Characteristics of systemic antibody responses of nonhuman primates following active immunization with Porphyromonas gingivalis, Prevotella intermedia and Bacteroides fragilis. Oral Microbiol Immunol. 1996;11:79–87. doi: 10.1111/j.1399-302x.1996.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 60.Ebersole JL, Kornman KS. Systemic antibody responses to oral microorganisms in the cynomolgus monkey: development of methodology and longitudinal responses during ligature-induced disease. Res Immunol. 1991;142:829–839. doi: 10.1016/0923-2494(91)90128-6. [DOI] [PubMed] [Google Scholar]
- 61.Ebersole JL, Taubman MA, Smith DJ, Frey DE, Haffajee AD, Socransky SS. Human serum antibody responses to oral microorganisms. IV Correlation with homologous infection. Oral Microbiol Immunol. 1987;2:53–59. doi: 10.1111/j.1399-302x.1987.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 62.Ebersole JL, Cappelli D, Mott G, Kesavalu L, Holt SC, Singer RE. Systemic manifestations of periodontitis in the non-human primate. J Periodontal Res. 1999;34:358–362. doi: 10.1111/j.1600-0765.1999.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 63.Socransky SS, Haffajee AD, Smith C, et al. Use of checkerboard DNA-DNA hybridization to study complex microbial ecosystems. Oral Microbiol Immunol. 2004;19:352–362. doi: 10.1111/j.1399-302x.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 64.Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J Clin Periodontol. 2004;31:996–1002. doi: 10.1111/j.1600-051X.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 65.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 66.Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- 67.Urbanski HF, Downs JL, Garyfallou VT, et al. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- 68.Kinane DF, Shiba H, Hart TC. The genetic basis of periodontitis. Periodontol 2000. 2005;39:91–117. doi: 10.1111/j.1600-0757.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 69.Kantarci A, Van Dyke TE. Resolution of inflammation in periodontitis. J Periodontol. 2005;76:2168–2174. doi: 10.1902/jop.2005.76.11-S.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bascones A, Noronha S, Gomez M, Mota P, Gonzalez Moles MA, Dorrego MV. Tissue destruction in periodontitis: bacteria or cytokines fault? Quintessence Int. 2005;36:299–306. [PubMed] [Google Scholar]
- 71.Garcia RI, Krall EA, Vokonas PS. Periodontal disease and mortality from all causes in the VA Dental Longitudinal Study. Ann Periodontol. 1998;3:339–349. doi: 10.1902/annals.1998.3.1.339. [DOI] [PubMed] [Google Scholar]
- 72.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 73.Scannapieco FA. Periodontal inflammation: from gingivitis to systemic disease? Compend Contin Educ Dent. 2004;25:16–25. [PubMed] [Google Scholar]
- 74.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–318. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 75.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–166. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 76.Kinane DF, Lappin DF. Immune processes in periodontal disease: a review. Ann Periodontol. 2002;7:62–71. doi: 10.1902/annals.2002.7.1.62. [DOI] [PubMed] [Google Scholar]
- 77.Podmore M, Ebersole JL, Kinane DF. Immunodominant antigens in periodontal disease: a real or illusive concept? Crit Rev Oral Biol Med. 2001;12:179–185. doi: 10.1177/10454411010120020701. [DOI] [PubMed] [Google Scholar]
- 78.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–340. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 79.Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007–1016. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 80.Williams RC, Offenbacher S. Periodontal medicine: the emergence of a new branch of periodontology. Periodontol 2000. 2000;23:9–12. doi: 10.1034/j.1600-0757.2000.2230101.x. [DOI] [PubMed] [Google Scholar]
- 81.Haffajee AD, Socransky SS, Dzink JL, Taubman MA, Ebersole JL, Smith DJ. Clinical, microbiological and immunological features of subjects with destructive periodontal diseases. J Clin Periodontol. 1988;15:240–246. doi: 10.1111/j.1600-051x.1988.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 82.Ebersole JL. Systemic humoral immune responses in periodontal disease. Crit Rev Oral Biol Med. 1990;1:283–331. doi: 10.1177/10454411900010040601. [DOI] [PubMed] [Google Scholar]
- 83.Darby IB, Mooney J, Kinane DF. Changes in subgingival microflora and humoral immune response following periodontal therapy. J Clin Periodontol. 2001;28:796–805. doi: 10.1034/j.1600-051x.2001.280812.x. [DOI] [PubMed] [Google Scholar]
- 84.Holbrook WP, Mooney J, Sigurdsson T, Kitsiou N, Kinane DF. Putative periodontal pathogens, antibody titres and avidities to them in a longitudinal study of patients with resistant periodontitis. Oral Dis. 1996;2:217–223. doi: 10.1111/j.1601-0825.1996.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 85.Ebersole JL, Cappelli D, Steffen MJ, Willmann DE, O’Dell DS. Host response assessment in recurring periodontitis. J Clin Periodontol. 1996;23:258–262. doi: 10.1111/j.1600-051x.1996.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 86.Ebersole JL, Taubman MA, Smith DJ, Haffajee AD. Effect of subgingival scaling on systemic antibody responses to oral microorganisms. Infect Immun. 1985;48:534–539. doi: 10.1128/iai.48.2.534-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ebersole JL, Cappelli D, Steffen MJ. Characteristics and utilization of antibody measurements in clinical studies of periodontal disease. J Periodontol. 1992;63:1110–1116. doi: 10.1902/jop.1992.63.12s.1110. [DOI] [PubMed] [Google Scholar]
- 88.Weber-Mzell D, Kotanko P, Hauer AC, et al. Gender, age and seasonal effects on IgA deficiency: a study of 7293 Caucasians. Eur J Clin Invest. 2004;34:224–228. doi: 10.1111/j.1365-2362.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- 89.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O. Reference distributions for immunoglobulins A, G, and M: a comparison of a large cohort to the world’s literature. J Clin Lab Anal. 1998;12:371–377. doi: 10.1002/(SICI)1098-2825(1998)12:6<371::AID-JCLA7>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jarvis D, Luczynska C, Chinn S, Burney P. The association of age, gender and smoking with total IgE and specific IgE. Clin Exp Allergy. 1995;25:1083–1091. doi: 10.1111/j.1365-2222.1995.tb03255.x. [DOI] [PubMed] [Google Scholar]
- 91.Scheingraber S, Dobbert D, Schmiedel P, Seliger E, Dralle H. Gender-specific differences in sex hormones and cytokines in patients undergoing major abdominal surgery. Surg Today. 2005;35:846–854. doi: 10.1007/s00595-005-3044-1. [DOI] [PubMed] [Google Scholar]
- 92.Mabley JG, Horvath EM, Murthy KG, et al. Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation. J Pharmacol Exp Ther. 2005;315:812–820. doi: 10.1124/jpet.105.090480. [DOI] [PubMed] [Google Scholar]