Abstract
Objective
To determine the expression of nitric oxide synthases (NOSs) and their modulation by hypoxia in human peritoneal (NF) and adhesion fibroblasts (ADF).
Design
Prospective experimental study.
Setting
University medical center.
Patient(s)
Fibroblasts from peritoneum and adhesion tissues.
Intervention(s)
Hypoxia and silencing inducible NOS (iNOS) gene expression in fibroblasts.
Main Outcome Measure(s)
We used reverse-transcriptase polymerase chain reaction to quantify messenger RNA (mRNA) levels of NOS isoforms. Griess assay was used to measure NO levels.
Result(s)
The mRNA copies/μg RNA of neuronal NOS (nNOS) and endothelial NOS (eNOS) were 6.6 × 103 in NF, 5.7 × 103 in ADF and 7.0 × 103 in NF, 6.1 × 103 in ADF, respectively. The mRNA copies/μg RNA of iNOS were 31.3 × 103 in NF and 33.0 × 103 in ADF. Hypoxia increased iNOS mRNA copies/μg RNA from 31.3 × 103 to 61.3 × 103 in NF and from 33.0 × 103 to 63.9 × 103 in ADF, whereas there were no changes in mRNA levels of nNOS and eNOS in NF and ADF. Nitric oxide levels were lower in ADF (0.94 μmol/L) than NF (1.97 μmol/L). Silencing iNOS decreased NO levels in NF (from 1.97 μmol/L to 0.41 μmol/L) and in ADF (from 0.94 μmol/L to 0.27 μmol/L).
Conclusion(s)
Nitric oxide synthases are differentially expressed in NF and ADF, with iNOS being the most expressed and the main source of NO. Hypoxia was shown to alter the expression of NOSs and NO in NF and ADF.
Keywords: Adhesions, fibroblasts, hypoxia, nitric oxide, nitric oxide synthase, peritoneum, RT-PCR, siRNA
Peritoneal adhesions are a frequent cause of abdominopelvic open pelvic and laparoscopic surgery (1–4). Adhesions result from the normal peritoneal wound healing response and develop in the first 3–5 days after injury (2, 5). We and others have found that approximately 55%–85% of women undergoing open pelvic surgery will form adhesions postoperatively (6–8). Adhesions can cause long-term morbidity, including infertility, bowel obstruction, and possibly pelvic pain (9–13). Subsequent surgeries are also likely to be prolonged and potentially incur greater risks, such as bowel injury (14). Despite their overbearing influence on surgical care, the mechanisms by which injury to the peritoneum triggers the inflammatory response leading to postoperative adhesion development remains poorly understood.
Fibroblasts underling the mesothelium play a central role in the healing of the peritoneum after surgery. Fibroblasts, which invade the wound in the first few days of healing, have multiple functions important to wound repair, such as collagen synthesis, extracellular matrix molecules reorganization, and wound contraction resulting in mature scar formation (15). Previously we have characterized differences between human fibroblasts isolated from normal peritoneum and adhesion tissues of the same patients; we have identified phenotype differences between these two cell types (16–18). Specifically, adhesion fibroblasts have significantly reduced nitric oxide (NO) levels. Although there was no difference in messenger RNA (mRNA) levels of inducible nitric oxide synthase (iNOS) between normal peritoneal and adhesion fibroblasts, there was a significant increase in the basal mRNA levels for iNOS in each response to hypoxia (19, 20). Hypoxia has a variety of effects on fibroblasts, both in vitro and in vivo (21). In previous studies we have also shown that hypoxia down-regulates iNOS catalytic activity in human normal peritoneal and adhesion fibroblasts (22).
There are three isoforms of NOS, named according to their activity or the tissue type in which they were first described: neuronal NOS (nNOS), endothelial NOS (eNOS), and iNOS. Neuronal NOS and eNOS are two constitutive isoforms, and iNOS is the induced, more persistent one. Despite the names of these enzymes, all three isoforms can be found in a variety of tissues and cell types (23, 24). The three NOSs catalyze the formation of citrulline and NO from L-arginine (L-Arg), reduced nicotinamide-adenine dinucleotide phosphate, and O2 (25). Under normal conditions, NO is typically generated in small amounts (low concentrations) and operates as a signaling molecule in many diverse physiological processes, such as blood pressure control, neurotransmission, and learning and memory; but in conditions of overproduction (at high concentrations) or deficiency, it functions as a defensive cytotoxin and may cause a series of diseases, ranging from asthma to cardiovascular diseases (23, 26–28).
To date, few studies have been performed to examine the role of NO in the development of postoperative adhesions. On the basis of our prior studies characterizing the adhesion phenotype, and in view of these observations, we raised the questions of whether the isoforms of NOSs are differentially expressed in fibroblasts isolated from normal peritoneal and adhesion tissues of the same patients, and whether hypoxia modulates the NOSs/NO expression system. The results of this study should give insight into the mechanism by which hypoxia can alter the expression of the different NOS isoforms and NO levels in the peritoneum immediately after surgery. This may lead to the design of interventional therapies targeted toward limiting or preventing the development of postoperative adhesions. In this study, we have determined for the first time the expression levels of NOSs in fibroblasts established from human normal peritoneal and adhesion tissues under normal and hypoxic conditions. In addition, we have used small interfering RNA (siRNA) technology to specifically silence iNOS expression, to determine the source of NO in these cells. We have shown that hypoxia can alter not only the basal expression level of the different NOSs but also NO levels. Hypoxia increased iNOS, whereas there was no change in nNOS and eNOS mRNA levels in normal and adhesion fibroblasts. Furthermore, hypoxia was shown to lower NO levels in normal peritoneal fibroblasts to those levels seen in adhesion fibroblasts.
MATERIALS AND METHODS
Source and Culture of Human Fibroblasts
Fibroblasts were isolated from normal peritoneum and adhesions as previously described (16). Briefly, at initiation of the surgery, after entry into the abdominal cavity, normal parietal peritoneal tissues from the anterior abdominal wall lateral to the midline incision and adherent tissues were excised from 5 different patients undergoing laparotomy for pelvic pain. Normal peritoneum was ≥3 in (7.6 cm) from any adhesions. Harvested adherent tissues come from bands of filmy and/or dense components of adhesions, which were excised with particular attention to not including peritoneum of attached organs. Patients did not have an active pelvic or abdominal infection and were not pregnant. All patients gave informed written consent to tissue collection, which was conducted under a protocol approved by the Wayne State University Institutional Review Board.
The harvested tissue samples were immediately placed in standard media (Dulbecco’s Modified Eagle Medium [DMEM] containing 10% fetal bovine serum [FBS], and 1% penicillin and streptomycin). The tissues were cut into small pieces in a sterile culture dish and transferred into another fresh T-25 flask with 3 mL of Dispase solution (2.4 U/mL; GIBCO BRL, Life Technologies, Rockville, MD). The flasks were incubated 4 hours in a 37°C incubator. The samples were then centrifuged for 5 minutes at 1400 × g, transferred into a fresh T-25 flask with prewarmed DMEM medium, and put into a 37°C incubator (95% air, 5% CO2).
The outgrowth of fibroblasts generally took 2 weeks. When confluence was reached, the cells were transferred to 100-mm tissue culture dishes and cultured in standard media with 10% FBS. Thereafter, the confluent dishes were subcultured by trypsinization (1:3 split ratio) to a maximal 12th passage. Studies were conducted using passage 3 to 5 cells to maintain comparability.
Hypoxic Culture Conditions
All hypoxic experiments were performed as described elsewhere, in an airtight modular incubator chamber (16, 29). The chamber was deoxygenated by a positive infusion of 2% O2 in a CO2-nitrogen gas mixture. Cultures were then placed in a standard humidified tissue incubator.
There were no statistically significant differences in viability by crystal violet or trypan blue exclusion (data not shown). Parallel cultures were placed in normoxic conditions for all time points. Cells were harvested at the 24-hour time point. All experiments were performed in triplicate.
Cell Culture, Transfection of siRNA for iNOS
Cells were maintained in DMEM supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin including 10% heat-inactivated FBS at 37°C in 5% CO2. For siRNA transfection, cells were grown to a confluence of 30% to 40% in 12-well plates (Becton Dickinson, Franklin Lakes, NJ) and transfected with the use of 3 μL NeoFX reagent (Ambion, Austin, TX; catalogue no. 1631), 2 μL of 20 μmol siRNA, and OptiMEM medium (Invitrogen, Carlsbad, CA; catalogue no. 31985–047) up to a final volume of 100 uL. NeoFX reagent and SiRNA were incubated at room temperature for 10 minutes and then applied onto 1.0 × 105 cells per well. Transfection mixtures were incubated with cells for 24 hours before washing cells with medium and incubating for an additional 24 hours. Ribonucleic acids were extracted from the cells, complementary DNA (cDNA) were synthesized, and quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed as described below.
siRNA Design, Synthesis, and Labeling
Small interfering RNAs were designed after determination of target sequences by aligning iNOS and myeloperoxidase sequences to an Ambion Web-based algorithm. The 21-nucleotide duplex siRNA molecules with 3-dTdT overhangs were resuspended in nuclease-free water according to the instructions of the manufacturer (Ambion). To ensure stringent controls, both a 2A-based mutated control siRNA with 2 nucleotide mismatches (siRNA-2Amut) and a scrambled control sequence (siRNA-SCR) obtained from Ambion (Silencer Negative Control No. 1 siRNA; catalogue no. 4610) were used.
Real-Time RT-PCR for nNOS, iNOS, and eNOS
Over the past several years, the development of novel chemistries and instrumentation platforms enabling detection of PCR products on a real-time basis has led to widespread adoption of real-time RT-PCR as the method of choice for quantitating changes in gene expression. The real-time RT-PCR technique was used to detect and compare mRNA levels of nNOS, iNOS, and eNOS in peritoneal and adhesion fibroblasts isolated from 5 different patients under normal and hypoxic conditions. The advantage of the real-time RT-PCR method over the conventional RT-PCR method is that it allows the determination of the absolute copy number of mRNA.
RNA isolation
Total RNA was extracted from human normal peritoneal fibroblasts and adhesions using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the protocol provided by the manufacturer.
Reverse transcription
A 20-μL cDNA reaction volume was prepared with the use of QuantiTect Reverse Transcription Kit (Qiagen), as described by the manufacturer’s protocol.
Real-time RT-PCR analysis
Optimal oligonucleotide primers for real-time RT-PCR amplification of reverse-transcribed cDNAwere selected with the aid of a commercial software program (Beacon Designer 7.0; Premier Biosoft, Palo Alto, CA). Human oligonucleotide primers, which amplify variable portions of the protein coding regions, are as follows. Beta-actin primers, which amplified a 559-base pair (bp) fragment are: sense primer AAGCAGGAGTATGACGAGTCCG, and antisense primer GCCTTCATACATCTCAAGTTGG. Endothelial NOS primers, which amplify a 160-bp fragment, are: sense primer ATTATATCCTACACAAGACTCCAG, and antisense primer TCTTCAAGTTGCCCATGTTAC. Inducible NOS primers, which amplified a 127-bp fragment, are: sense primer GTTCTCAAGGCACAGGTCTC, and anti-sense primer GCAGGTCACTTATGTCACTTATC. Neuronal NOS primers, which amplified a 165-bp fragment, are: sense primer TCTAACAGGCTGGCAATGAAG, and antisense primer TCTCTAAGGAAGTGATGGTTGAC.
Real-time RT-PCR was performed with a QuantiTect SYBR Green RT-PCR Kit (Qiagen) and a Cepheid 1.2f Detection System (Cepheid, Sunnyvale, CA). Reactions were 25-μL volumes including 12.5 μL of 2× QuantiTect SYBR Green RT-PCR Master Mix, 1 μL of cDNA template, and 0.2 μmol/L each of target-specific primers that were designed to amplify a part of each gene. To quantify each target transcript, a standard curve was constructed with a 10-fold dilution series of standard plasmid. The three-step PCR protocol applied for nNOS, iNOS, and eNOS reactions consisted of 45 cycles of 95°C for 15 seconds, 57°C (nNOS), 55°C (iNOS), 56°C (eNOS) for 30 seconds, and 72°C for 30 seconds. After PCR, a melting curve analysis was performed to demonstrate the specificity of the PCR product as a single peak. A control, which contained all the reaction components except for the template, was included in all experiments. The amount of mRNAs was then normalized to a housekeeping gene, β-actin.
Detection of NO Levels on Normal and Adhesion Fibroblasts
The nitrate/nitrite colorimetric assay (Griess assay) was used to measure the levels of stable NO byproducts in fibroblasts from normal peritoneal and adhesion tissues. Nitrate is a stable oxidation product of NO, and its measurement serves as a convenient assay for NO production. The absolute detection limit is 30–60 pmol nitrite or nitrate per 300-μL sample volume; this translates into a sensitivity of 0.1 μmol (37). Normal peritoneal and adhesion fibroblasts culture media were analyzed for NO by using a Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI) following procedures recommended by the manufacturer. Nitrite was determined at 540 nm using a microplate reader, and the concentration was calculated using nitrate standards. In other words, the normal peritoneal and adhesion fibroblasts’ iNOS gene was silenced.
Statistical Analysis
Real-time RT-PCR data were analyzed using a Kruskall-Wallis test followed by Dunn’s multiple comparison tests. Correlation analysis was performed by calculating the Pearson R2 coefficient, followed by calculation of a two-tailed P value. Statistically significant differences for comparison of means were established at P<.05. All statistical analyses were performed using commercial software (GraphPad Prism 4.0 for Windows and Mac; GraphPad Software, San Diego, CA).
RESULTS
We used real-time RT-PCR to measure mRNA levels of NOS isoforms in human normal peritoneal and adhesion fibroblasts under normoxic and hypoxic conditions. Under normoxic conditions, the constitutive NOSs (nNOS and eNOS) basal mRNA levels were 6.6 × 103/μg RNA (normal), 5.7 × 103/μg RNA (adhesion), 7.0 × 103/μg RNA (normal), and 6.1 × 103/μg RNA (adhesion), respectively (Figs. 1 and 2). The expression levels of nNOS and eNOS were lower in adhesions (nNOS 16% [P<.005], eNOS 15% [P = .001]) compared with normal fibroblasts, respectively (Figs. 1 and 2). Inducible NOS mRNA levels were 31.3 × 103/μg RNA and 33.0 × 103/μg RNA in normal peritoneal and adhesion fibroblasts, respectively (Fig. 3). Inducible NOS mRNA levels were 4.7-fold (P<.001) and 5.8-fold (P<.001) higher than those of nNOS and eNOS in normal fibroblasts and adhesions, respectively (Fig. 3). However, there was no significant difference for iNOS expression between normal peritoneal fibroblasts and adhesions (Fig. 3; P=.1367). When cells were cultured under hypoxic conditions, nNOS and eNOS mRNA levels were decreased in normal fibroblasts (nNOS 17% [P<.005], eNOS 13% [P<.005]) but not adhesions (nNOS 1% [P=0.6], eNOS 2% [P=0.23]) compared with those under normoxic conditions (Figs. 1 and 2); whereas, there were no significant changes in nNOS or eNOS expression levels in adhesions before and after hypoxia (nNOS P=0.6, eNOS P=.23) (Figs. 1 and 2). Inducible NOS expression levels were increased twofold in both normal (96%; P<.001) and adhesion fibroblasts (94%; P<.001) compared with those under normoxic conditions (Fig. 3). Similarly, under normoxic conditions, there was no significant difference in iNOS mRNA levels in normal peritoneal and adhesion fibroblasts under hypoxic conditions (61.3 × 103/μg RNA vs. 63.9 × 103/μg RNA) (Fig. 3).
FIGURE 1.
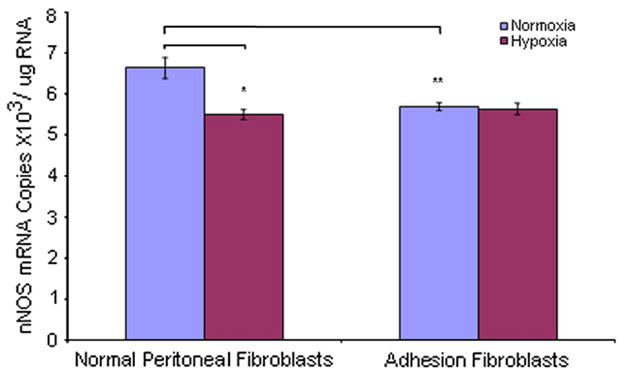
Comparison of nNOS mRNA levels in human normal peritoneal (n = 5) and adhesion fibroblasts (n = 5) before and after hypoxia (2% O2) treatment.
*P<.005, ** P<.005 vs. normal peritoneal fibroblasts cultured under normoxic conditions. Results are representative of the mean of three independent experiments.
FIGURE 2.
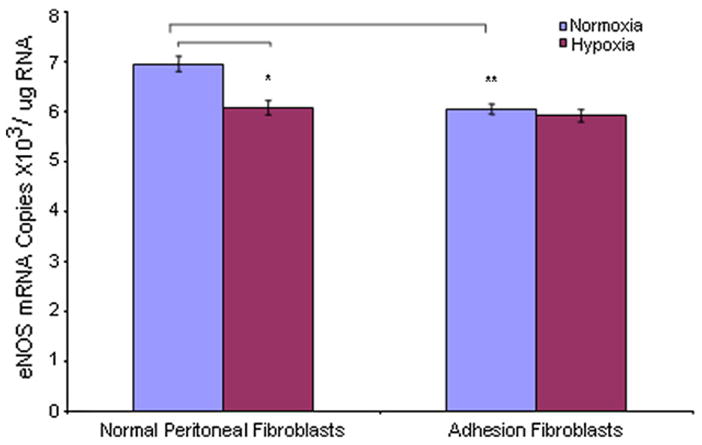
Comparison of eNOS mRNA levels in human normal peritoneal (n = 5) and adhesion fibroblasts (n = 5) before and after hypoxia (2% O2) treatment.
* P<.005, ** P=.001 vs. normal peritoneal fibroblasts cultured under normoxic conditions. Results are representative of the mean of three independent experiments.
FIGURE 3.
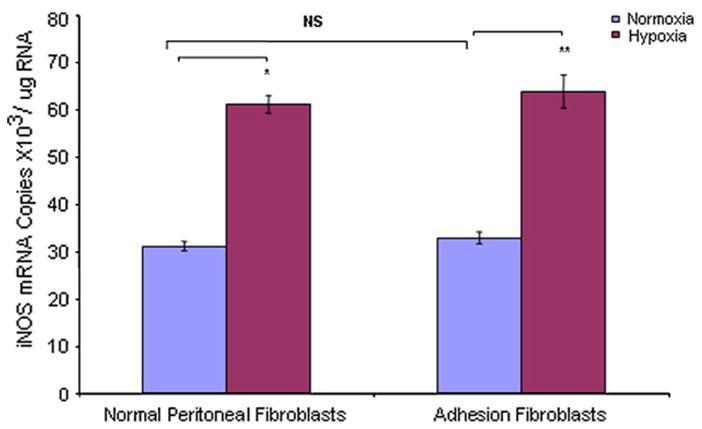
Comparison of iNOS mRNA levels in human normal peritoneal (n = 5) and adhesion fibroblasts (n = 5) before and after hypoxia (2% O2) treatment. * P<.001 vs. normal peritoneal fibroblasts cultured under normoxic conditions. ** P<.001 vs. adhesion fibroblasts cultured under normoxic conditions. Results are representative of the mean of three independent experiments.
To confirm whether NO is mainly produced by iNOS, synthesis of NO by normal peritoneal and adhesion fibroblasts before and after silencing iNOS gene expression was measured by nitrate/nitrite accumulation in the culture media, as described in Materials and Methods. Normal fibroblasts have significantly higher NO levels (1.97 μmol/L) than adhesion fibroblasts (0.94 μmol/L; P<.005) (Fig. 4). Treatment of normal fibroblasts with hypoxia decreased NO levels to those seen in adhesion fibroblasts (0.8 μmol/L; P<.005). Hypoxia had no significant effect on NO levels in adhesion fibroblasts (Fig. 4). After silencing iNOS gene expression, the NO levels decreased 79% (from 1.97 μmol/L to 0.41 μmol/L; P<.001) and 71% (from 0.94 μmol/L to 0.27 μmol/L; P<.001) in normal peritoneal fibroblasts and adhesion fibroblasts compared with the basal levels, respectively (Fig. 4).
FIGURE 4.
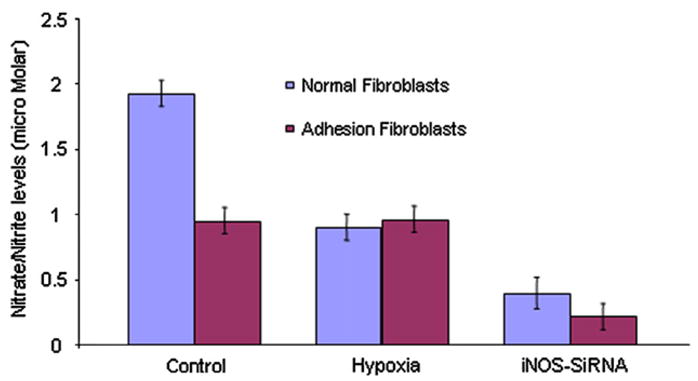
Nitric oxide levels in human normal peritoneal (n = 5) and adhesion fibroblasts (n = 5). Griess assay was performed in 24-hour culture media collected from human normal peritoneal and adhesion fibroblasts before and after iNOS gene silencing by SiRNA under normal and hypoxic conditions. Results are representative of the mean of three independent experiments.
DISCUSSION
In the present study we have shown that NOS isoforms are differentially expressed in normal peritoneal as compared with adhesion fibroblasts established from the same patient(s). Additionally we have shown that hypoxia can alter not only the basal expression level of the different NOSs but also NO levels. Hypoxia increased iNOS, whereas there was no change in nNOS and eNOS mRNA levels in normal and adhesion fibroblasts. Furthermore, hypoxia was shown to lower NO levels in normal peritoneal fibroblasts to those levels seen in adhesion fibroblasts. Determining the mechanism by which hypoxia exerts its effect on NOSs and/or NO levels should shed some light on the pathogenesis of the development of postoperative adhesions and the development of potential interventional therapies.
Basal levels of NOSs (nNOS, iNOS, and eNOS) have been observed to be expressed in various tissues that drive a critical amount of NO released under various specific circumstances. The rapid and transient production of NO by nNOS is known to function in neuronal tissues as a regulator or neurotransmitter. Similarly, the capacity of eNOS to generate low-level, intermittent release of NO in the vascular endothelium is well suited for its role in maintaining basal vascular tone (30). Thus, constitutive expression of nNOS and eNOS in various tissues is important in keeping homeostasis. Expression of iNOS has now been reported in a large number of cell types, and in most circumstances the enzyme is inducible. Inducible NOS is capable of a higher output and long-lasting release of NO, far exceeding that of nNOS and eNOS isoforms in response to various conditions, such as inflammation and hypoxia (31, 32).
In view of this importance, we measured the levels of NOS isoforms in normal peritoneal and adhesion fibroblasts to understand the roles of NOSs under physiological conditions. Our results showed that there is approximately a fivefold higher basal level of iNOS than nNOS and eNOS in both normal peritoneal and adhesion fibroblasts under normoxic conditions. This is the first report that iNOS is the predominate isoform of NOSs in normal peritoneal and adhesion fibroblasts.
In response to hypoxia, nNOS and eNOS expression levels decreased, whereas iNOS expression levels increased in normal peritoneal and adhesion fibroblasts, suggesting that hypoxia down-regulates nNOS and eNOS expression and increases iNOS expression. It is now known that the levels of gene expression of both nNOS and eNOS may also be regulated at the transcriptional, translational, and posttranslational levels under different conditions (33, 34). Increased iNOS expression levels often lead to spatial and temporal formation of high NO levels, critically involved in important functions (e.g., host defense) but nevertheless associated with the onset of deleterious effects (35). However, our results showed that hypoxia reduced NO levels in normal fibroblasts and that silencing iNOS gene expression resulted in decreased NO levels in both normal peritoneal and adhesion fibroblasts.
Griess assay results showed that NO levels were significantly decreased in adhesion fibroblasts under normoxic conditions or in both normal peritoneal and adhesion fibroblasts under hypoxic conditions. Hypoxia increases iNOS expression levels in both of the cell types but reduces NO levels in normal fibroblasts. The exact mechanism by which hypoxia lowers NO levels is not known. Factors that influence rates of NO elimination after its synthesis by NOSs may contribute to the formation of the adhesion phenotype. A possible pathway for NO scavenging in adhesion fibroblasts is the interaction of NO with superoxide, which is generated from hypoxia, yielding peroxynitrite (ONOO-), a powerful oxidant that can react with tyrosine residues to form the stable adduct nitrotyrosine. Indeed, we have recently reported that adhesion fibroblasts have increased levels of nitration compared with normal peritoneal fibroblasts (22). We therefore hypothesize that hypoxia due to trauma and injury to the peritoneal surface may be the initiation trigger for the development of postoperative adhesions through the production of high levels of superoxide.
We have reported that H4B and L-Arg levels were significantly lower in adhesion compared with normal peritoneal fibroblasts (22). Both L-Arg and H4B play a crucial role in NOS coupling, and their deficiency allows the attenuation of iNOS coupling and leads to the production of superoxide anion, in addition to NO. Consistent with these findings, administration of L-Arg intraperitoneally to rats at the end of surgery to test the effect on postoperative adhesion demonstrated that adhesion formation scores were significantly lower than in the control group after two weeks (36). These results indicate the importance of L-Arg in the development of postoperative operation adhesion.
Collectively, iNOS is the primary isoform of nitric oxide synthases in normal peritoneal and adhesion fibroblasts. The increased iNOS levels in adhesions or both fibroblasts under hypoxic conditions are associated with reduced nNOS and eNOS levels, which lead to decreased nNOS and eNOS-dependent NO release. The decrease in nNOS and eNOS-dependent NO levels and the increase in iNOS expression levels may be the trigger to the development of postoperative adhesions.
Acknowledgments
Supported in part by grant number 1RO1 GM069941-01A3 from the National Institutes of Health, Bethesda, Maryland (G.M.S.).
References
- 1.Diamond MP, Daniell JF, Feste J, Surrey MW, McLaughlin DS, Friedman S. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil Steril. 1987;47:864–6. doi: 10.1016/s0015-0282(16)59181-x. [DOI] [PubMed] [Google Scholar]
- 2.Diamond MP, Hershlag A. Adhesion formation/reformation. Prog Clin Biol Res. 1990;358:23–33. [PubMed] [Google Scholar]
- 3.Menzies D, Ellis H. Intestinal obstruction from adhesions—how big is the problem? Ann R Col Surg Engl. 1990;721:60–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Weibel MA, Majno G, Jagelman DG, Ellis H. Peritoneal adhesions and their relation to abdominal surgery: a postmortem study. Am J Surg. 1973;126:345–53. doi: 10.1016/s0002-9610(73)80123-0. [DOI] [PubMed] [Google Scholar]
- 5.diZerega GS. Biochemical events in peritoneal tissue repair. Eur J Surg Suppl. 1997;577:10–6. [PubMed] [Google Scholar]
- 6.Diamond MP, Decherney AH. Pathogenesis of adhesion formation/reformation: application to reproductive pelvic surgery. Microsurgery. 1987;8:103–7. doi: 10.1002/micr.1920080215. [DOI] [PubMed] [Google Scholar]
- 7.Saed GM, Munkarah AR, Diamond MP. Cyclooxygenase-2 is expressed in human fibroblasts isolated from intraperitoneal adhesions but not from normal peritoneal tissues. Fertil Steril. 2003;79:1404–8. doi: 10.1016/s0015-0282(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 8.Saed GM, Diamond MP. Effect of glucose on the expression of type I collagen and transforming growth factor-beta1 in cultured human peritoneal fibroblasts. Fertil Steril. 2003;79:158–63. doi: 10.1016/s0015-0282(02)04556-9. [DOI] [PubMed] [Google Scholar]
- 9.Caspi E, Halperin Y, Bukovsky I. The importance of periadnexal adhesions in tubal reconstructive surgery for infertility. Fertil Steril. 1979;31:296–300. doi: 10.1016/s0015-0282(16)43877-x. [DOI] [PubMed] [Google Scholar]
- 10.Ellis H. The clinical significance of adhesions: focus on intestinal obstruction. Eur J Surg Suppl. 1997;577:5–9. [PubMed] [Google Scholar]
- 11.Hulka JF. Adnexal adhesions: a prognostic staging and classification system based on a five-year survey of fertility surgery results at Chapel Hill, North Carolina. Am J Obstet Gynecol. 1982;144:141–8. doi: 10.1016/0002-9378(82)90615-9. [DOI] [PubMed] [Google Scholar]
- 12.Maetani S, Tobe T, Kashiwara S. The neglected role of torsion and constriction in pathogenesis of simple adhesive bowel obstruction. Br J Surg. 1984;71:127–30. doi: 10.1002/bjs.1800710217. [DOI] [PubMed] [Google Scholar]
- 13.Kresch AJ, Seifer DB, Sachs LB, Barrese I. Laparoscopy in 100 women with chronic pelvic pain. Obstet Gynecol. 1984;64:672–4. [PubMed] [Google Scholar]
- 14.Coleman MG, McLain AD, Moran BJ. Impact of previous surgery on time taken for incision and division of adhesions during laparotomy. Dis Colon Rectum. 2000;43:1297–9. doi: 10.1007/BF02237441. [DOI] [PubMed] [Google Scholar]
- 15.Williams RL, Armstrong DG. Wound healing. New modalities for a new millennium. Clin Podiatr Med Surg. 1988;15:117–8. [PubMed] [Google Scholar]
- 16.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–8. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 17.Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78:144–7. doi: 10.1016/s0015-0282(02)03146-1. [DOI] [PubMed] [Google Scholar]
- 18.Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78:137–43. doi: 10.1016/s0015-0282(02)03145-x. [DOI] [PubMed] [Google Scholar]
- 19.Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82:1198–205. doi: 10.1016/j.fertnstert.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Saed GM, Lu H, Jiang ZL, Abuolba S, Abu-Soud HM, Diamond MP. Cross-talk between inducible nitric oxide syntheses (iNOS) and myeloperoxidase (MPO) in fibroblasts isolated from normal peritoneal and adhesion tissues. Fertil Steril. 2005;84:S463–4. [Google Scholar]
- 21.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–7. [PubMed] [Google Scholar]
- 22.Saed GM, Zhao M, Diamond MP, Abu-Soud HM. Regulation of inducible nitric oxide synthase in post-operative adhesions. Hum Reprod. 2006;21:1605–11. doi: 10.1093/humrep/dei500. [DOI] [PubMed] [Google Scholar]
- 23.Colasanti M, Persichini T, Menegazzi M, Mariotto S, Giordano E, Caldarera CM, et al. Induction of nitric oxide synthase mRNA expression. Suppression by exogenous nitric oxide. J Biol Chem. 1995;270:26731–3. doi: 10.1074/jbc.270.45.26731. [DOI] [PubMed] [Google Scholar]
- 24.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–30. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 25.Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Annu Rev Pharmacol Toxicol. 1997;37:339–59. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 26.Moncada S. Nitric oxide. J Hypertens. 1994;12(10):S35–9. [PubMed] [Google Scholar]
- 27.Persson MG, Zetterstrom O, Agrenius V, Ihre E, Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–7. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 28.Darley-Usmar VM, McAndrew J, Patel R, Moellering D, Lincoln TM, Jo H, et al. Nitric oxide, free radicals and cell signaling in cardiovascular disease. Biochem Soc Trans. 1997;25:925–9. doi: 10.1042/bst0250925. [DOI] [PubMed] [Google Scholar]
- 29.Saed GM, Zhang W, Diamond MP. Effect of hypoxia on stimulatory effect of TGF-beta 1 on MMP-2 and MMP-9 activities in mouse fibroblasts. J Soc Gynecol Investig. 2000;7:348–54. [PubMed] [Google Scholar]
- 30.Ignarro LJ. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ Res. 1989;65:1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc) 1998;63:766–81. [PubMed] [Google Scholar]
- 32.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–55. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 33.Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci U S A. 1995;92:7809–13. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–90. [PubMed] [Google Scholar]
- 35.Persichini T, Cantoni O, Suzuki H, Colasanti M. Cross-talk between constitutive and inducible NO synthase: an update. Antioxid Redox Signal. 2006;8:949–54. doi: 10.1089/ars.2006.8.949. [DOI] [PubMed] [Google Scholar]
- 36.Kaleli B, Ozden A, Aybek Z, Bostanci B. The effect of L-arginine and pentoxifylline on postoperative adhesion formation. Acta Obstet Gynecol Scand. 1998;77:377–80. [PubMed] [Google Scholar]
- 37.Schulz K, Kerber S, Kelm M. Reevaluation of the Griess method for determining NO/NO2- in aqueous and protein-containing samples. Nitric Oxide. 1999;3:225–34. doi: 10.1006/niox.1999.0226. [DOI] [PubMed] [Google Scholar]


