Abstract
Aims
Rotigaptide is proposed to exert its anti-arrhythmic effects by improving myocardial gap-junction communication. To directly investigate the mechanisms of rotigaptide action, we treated cultured neonatal murine ventricular cardiomyocytes with clinical pharmacological doses of rotigaptide and directly determined its effects on gap-junctional currents.
Methods and results
Neonatal murine ventricular cardiomyocytes were enzymatically isolated and cultured for 1–4 days. Primary culture cell pairs were subjected to dual whole cell patch-clamp procedures to directly measure gap-junctional currents (Ij) and voltage (Vj). Rotigaptide (0–350 nM) was applied overnight or acutely perfused into 35 mm culture dishes. Rotigaptide (35–100 nM) acutely and chronically increased the resting gap-junction conductance (gj), and normalized steady-state minimum gj (Gmin) by 5–20%. Higher concentrations produced a diminishing response, which mimics the observed therapeutic efficacy of the drug. The inactivation kinetics was similarly slowed in a therapeutic concentration-dependent manner without affecting the Vj dependence of inactivation or recovery. The effects of 0–100 nM rotigaptide on ventricular gj during cardiac action potential propagation were accurately modelled by computer simulations which demonstrate that clinically effective concentrations of rotigaptide can partially reverse conduction slowing due to decreases in gj and inactivation.
Conclusion
These results demonstrate that therapeutic concentrations of rotigaptide increase the resting gap-junction conductance and reduce the magnitude and kinetics of steady-state inactivation in a concentration-dependent manner. Rotigaptide may be effective in treating re-entrant forms of cardiac arrhythmias by improving conduction and preventing the formation of re-entrant circuits in partially uncoupled myocardium.
KEYWORDS: Antiarrhythmic agents, Cell communication, Connexins, Gap junctions, Rotigaptide
1. Introduction
Uniform coupling of myocardial cells by gap junctions is essential to the rapid, synchronous electrical activation and initiation of contraction vital to cardiac function. The heterogeneous loss of ventricular Cx43 expression not only compromises the electrophysiological activation and refractory properties of the myocardium to produce a highly arrhythmogenic substrate, but also the contractile function of the heart.1–3 Rotigaptide is a novel antiarrhythmic peptide that exerts its effects on re-entrant forms of ventricular tachycardias by improving electrical cell–cell coupling.4–6 Rotigaptide, a D-isomer analogue of the antiarrhythmic hexapeptide AAP10, increases Cx43-mediated gap-junction communication, reduces ischaemia-induced infarct size and conduction slowing, and prevents spontaneous ventricular arrhythmias in various cell and animal models.7–10 Rotigaptide has been advanced into clinical development and the safety evaluation from phase I studies in healthy volunteers looks very promising.11
To further elucidate the possible mechanisms of action of rotigaptide on cardiac gap junctions, we investigated the effects of rotigaptide treatment on the gating of ventricular gap junctions using the dual whole cell action potential voltage clamp method.12 Previously, the kinetic modelling of ventricular gap-junction gating over the duration of the ventricular action potential at various frequencies revealed that significant inactivation can develop during the first 25 ms of an action potential that can contribute to further conduction slowing and eventual conduction block.13 It was further demonstrated that ventricular gap junctions recovered from the inactivation induced by the action potential-derived transjunctional voltage (Vj) gradients beginning with phase 3 repolarization. Gap-junction conductance (Gj) actually increases above initial peak values during the final recovery phase at a time when the ventricular myocardium is most susceptible to re-entrant or triggered activity that gives rise to spontaneous ectopic activity.14,15 In the present study, we demonstrate that rotigaptide has a concentration-dependent effect on the magnitude and kinetics of ventricular gap-junction conductance and inactivation. These effects were observed at clinically relevant concentrations of rotigaptide and declined with increasing doses above 100 nM. Finally, we developed model parameters for the action of rotigaptide on the inactivation of ventricular gap junctions. Computer simulations of action potential propagation demonstrate that, by increasing gap-junction conductance and slowing inactivation, rotigaptide can counteract conduction slowing in partially uncoupled myocardium during discontinuous propagation.
2. Methods
All experiments were performed on enzymatically dissociated neonatal C57Bl/6 murine ventricular myocytes cultured for 48–72 h according to published procedures.12 The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). A 100 µM rotigaptide stock solution was prepared fresh weekly and stored at 4°C. This stock solution was added to the M199/10% foetal bovine serum myocyte culture media and the cells incubated overnight at 37°C in a humidified 5% CO2 atmosphere after >24 h in culture. Rotigaptide was also present during all patch-clamp experiments. Dual whole cell patch-clamp experiments were performed at room temperature (20–22°C) using a static or perfused (1 mL/min) bath volume of 3 mL HEPES-buffered, balanced salt saline (pH 7.4) according to established procedures.16
A series of three distinct voltage clamp protocols were performed on rotigaptide-treated ventricular myocytes. The 1/s ventricular action potential waveform and 200 ms/mV transjunctional voltage (Vj) ramps were performed on ventricular myocytes treated with 0, 20, 35, 50, 100, 200, or 350 nM rotigaptide.12 A minimum of six different cell pair gap-junction current (Ij) recordings were obtained for each test concentration and the results were averaged for curve fitting analysis of the action potential kinetic and steady-state Vj-dependent gating parameters. Each experimental Ij result represented the ensemble average of 100 action potential waveforms or five complete ± Vj ramp protocols. The actual applied Vj was determined for these ensemble averaged Ij traces using Eq. (1):
| 1 |
The junctional conductance (gj) was calculated as gj = −ΔI2/Vj.16 These procedures account for the series resistance voltage drop (Vseries = Icell·Rel) across the whole cell patch electrode resistance (Rel) as a function of the whole cell current (I1 or 2) for each cell relative to the command potential (V1 or 2). Pooled data for each experimental group and Vj protocol were normalized either to the junctional conductance (Gj) value at the peak of action potential or the linear slope conductance of the Ij–Vj relationship at low Vj values.12
Analysis of the first-order inactivation kinetics required ensemble averaged signals from 5 to 10 square Vj pulses ranging from +70 to +140 mV. The ensemble averaged Ij traces were fit with biexponential decaying functions to obtain the decay time constants (τdecay) from which the fast and slow closing (on)-rates for the Vj-dependent inactivation processes were calculated. The inactivation on-rates (kon) were calculated using the expression [Eq. (2)]:
| 2 |
The open probability, Po, was calculated as the remaining fraction of Ij at the end of the Vj pulse (=steady-state Ij/peak Ij). The calculated fast and slow on-rates were plotted as a function of Vj and fit with a single exponential function of the form [Eq. (3)]:
| 3 |
where A is the rate amplitude, Vk,on the predicted voltage constant for the inactivation rate, and C the minimum rate amplitude. This inactivation kinetic analysis was performed for only control and low or optimal rotigaptide concentrations. Examples of the voltage clamp protocols used in the kinetic and steady-state gj analyses are provided in the Supplementary material, online.
3. Results
3.1. Voltage-dependent gating of ventricular gap junctions
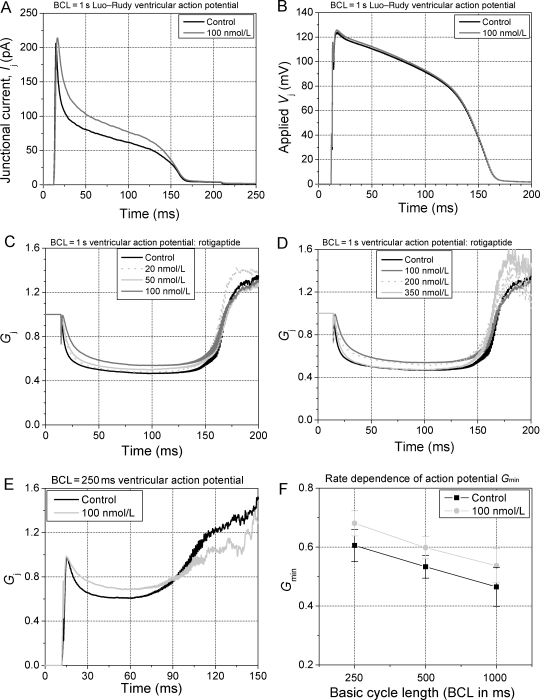
Continuous exposure to a constant concentration of rotigaptide produced dose-dependent changes in the gating of ventricular gap junctions during pacing with the 1 s basic cycle length (BCL) Luo–Rudy model ventricular action potential waveform.17 The ensemble-averaged Ij responses to the Vj gradient resulting from a train of 100 action potentials applied to cell 1 with cell 2 clamped to the resting potential (−89.8 mV) are shown for control and 100 nM rotigaptide conditions (Figure 1A and B). The action potential derived Vj gradients were essentially identical for both experiments and the peak gj was also similar between these two representative experiments. The time course of the inactivation is different between the control and the rotigaptide treatment groups, indicative of a pharmacological effect on the gating of cardiac gj. After normalization to the peak gj from each experiment (Gmax = 1), the averaged Gj (=normalized gj) data for each test concentration of rotigaptide are displayed in Figure 1C and D. The mean gj values (± SEM) were 2.86 ± 0.72 nS, 3.37 ± 1.04 nS, 2.84 ± 0.85 nS, 2.64 ± 0.39 nS, 3.44 ± 0.81 nS, 1.56 ± 0.46 nS, and 1.56 ± 0.59 nS for the 0, 20, 35, 50, 100, 200, and 350 nM rotigaptide experiments. The slowing of inactivation and the reduction in plateau inactivation during the action potential increased with increasing [rotigaptide] between 20 and 100 nM and declined nearly back to control values as [rotigaptide] was increased further to 350 nM. This biphasic dose–response curve indicates an optimal effective range of [rotigaptide] on the inhibition of gj inactivation of ≤100 nM under standard cell culture conditions. This observation is in agreement with previous findings of a bell-shaped dose–response curve for the effect of rotigaptide on conduction velocity in atrial strips experiencing metabolic stress.8
Figure 1.
Gating of ventricular gap junctions during an action potential. (A) Ensemble averaged Ij traces from two separate experiments of similar gj, one untreated (black) and one treated ventricular myocte cell pair with 100 nM rotigaptide (grey). Ij was increased in the rotigaptide experiment relative to the control recording. (B) The applied Vj gradients were virtually identical for both experiments. (C) Continuous rotigaptide treatment consistently reduced the rate and magnitude of inactivation in a concentration-dependent manner from 0 to 100 nM. (D) Above 100 nM, the rotigaptide effect was diminished, returning almost to control values at 350 nM. N = 6 experiments/concentration. (E) Reduction of Gj inactivation by 100 nM rotigaptide during rapid pacing using the 250 mS basic cycle length (BCL) ventricular action potential. (F) 100 nM rotigaptide decreased the magnitude of Gj inactivation at all stimulation frequencies >1 Hz.
In a separate set of experiments, the rate dependence of 100 nM rotigaptide was examined using the 250 and 500 ms BCL action potentials (Figure 1E and F). Again, there were no significant differences in the mean gj values between control and 100 nM rotigaptide groups. The average gj was 2.83 ± 1.00 (n = 6) and 3.88 ± 1.54 nS (n = 6) for the control and 3.44 ± 1.21 (n = 6) and 3.49 ± 1.46 nS (n = 7) for the 100 nM rotigaptide experiments at BCL = 250 and 500 ms, respectively. These data demonstrate that rotigaptide had a similar effect on the 2 or 4 Hz action potentials as on the standard 1 Hz ventricular action potential waveform. Slowing of inactivation and an increase in Gmin is evident at all stimulation frequencies. Slower frequencies were not examined since no further increase in inactivation was previously observed with longer action potential durations (APDs) and BCLs.12 Constant pacing at BCL = 1000 ms with the short, triangular neonatal murine action potential waveform also demonstrated an enhancement of Ij with 100 nM rotigaptide treatment (see Supplementary material online, Figure S4).18
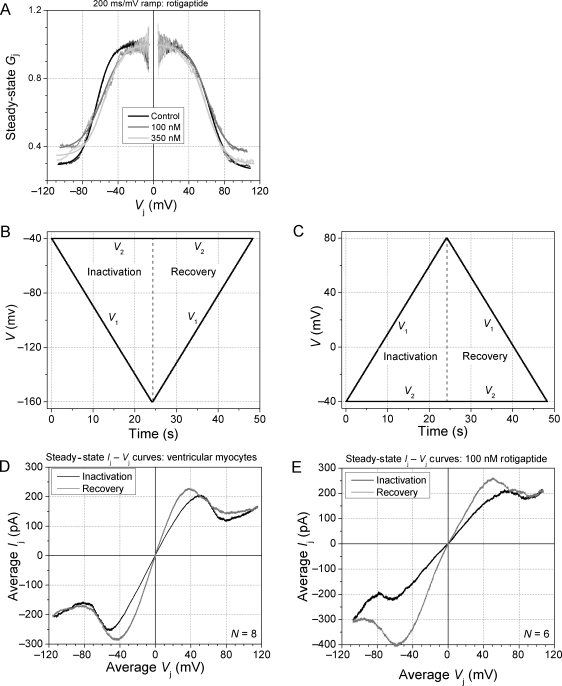
The effect of rotigaptide on the steady-state Gj–Vj relationship was also examined using a 200 ms/mV continuous Vj ramp of ± 120 mV. This provides a measure of the magnitude of the time-independent Gj inactivation. A Boltzmann equation fit of the experimental curves provides a measure of the half-inactivation voltage (V1/2), the Vj-sensitivity (valence, z), and the Vj-insensitive portion of the total Gj (Gmin, Figure 2A; see also Supplementary material, online). Gmax was taken as the linear slope conductance during the rising phase of Vj for each polarity. Reversing the direction of the Vj ramp from its maximum value provides a similar description of the steady-state Gj recovery and, most notably, the facilitation of Gj originally observed in cardiomyocyte gap junctions composed predominantly of Cx43 (Figure 2B–E).12 The Ij–Vj curves displayed in Figure 2D and E again demonstrate a reduction in the magnitude of ventricular gap-junction inactivation by rotigaptide with no apparent effect on the increased slope Gj during the return phase (i.e. facilitation = Gmax, rec) of the slow Vj ramp.
Figure 2.
Effects of rotigaptide on steady-state ventricular Gj inactivation and recovery. (A) 100 nM rotigaptide produced the maximal effect of increasing the Gmin of the steady-state Gj–Vj curves whereas this effect was almost completely reversed by a 350 nM dose. (B and C) Command voltage protocols for the negative (B) and positive (C) Vj inactivation and recovery ramps. (D) The normalized Ij (=Gj·Vj)–Vj relationship for the rising phase (black) and the falling phase (grey) of the ± 120 mV Vj ramp reveals the increased slope conductance, called facilitation, during the recovery from inactivation in ventricular myocytes. (E) The same Vj protocol applied to 100 nM rotigaptide-treated ventricular myocytes demonstrates the reduction of inactivation during the rising phase of the ramp, but a negligible effect on the return (recovery) phase of the Vj ramp.
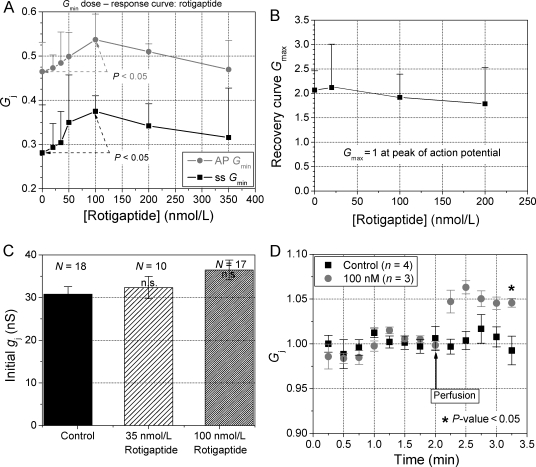
The concentration-dependent effects of rotigaptide on the Vj-insensitive Gmin of the inactivation and the Gmax, rec of the steady-state Gj–Vj curves are summarized in Figure 3. The 10% increase in Gmin was statistically different from control values for the maximal effective dose of 100 nM rotigaptide (P < 0.05, student’s t-test) and was similar for the action potential and Vj ramp voltage clamp protocols (Figure 3A). Since all gj values were normalized to the initial slope gj for each experiment (i.e. Gmax = 1), the value of Gmax, rec for the steady-state Gj–Vj curve provides the most reliable quantitative measure of the amount of facilitation present in each ventricular cell pair recording. Averaging the Gmax, rec values obtained with both Vj polarities, the mean (± SEM) Gmax, rec ranged from 1.79 ± 0.33 to 2.12 ± 0.40 for both respective control and rotigaptide treatment groups, thus indicating no statistical difference in facilitation (P > 0.20, n ≥ 5, Figure 3B). In agreement with previous reports,19 a trend towards increasing initial gj values measured immediately after achieving the dual whole cell configuration, was also observed (Figure 3C). A 5 or 18.5% increase in initial gj, up from 30.8 ± 1.7 nS, was observed with 35 or 100 nM rotigaptide treatments. These initial gj measurements were limited by the whole cell electrode series resistances and, thus, underestimate the actual gj. To further test for increases in resting gj, 100 nM rotigaptide was perfused onto ventricular cell pairs that exhibited a stable gj after the rundown period (Figure 3D). Ventricular gj, measured during +20 mV Vj pulses, was unchanged during control bath saline perfusion compared with acute rotigaptide treatments, which significantly increased gj, by +4.6 ± 0.1% (P-value < 0.05). The average ventricular gj was 14.7 ± 4.2 nS (n = 4) and 19.5 ± 5.2 nS (n = 3) for the control and 100 nM rotigaptide experiments, respectively. No changes in single channel conductances (γj) were observed (data not shown).
Figure 3.
Rotigaptide increases the Gmin of ventricular gap junctions. (A) The Vj-insensitive fraction of the total ventricular normalized junctional conductance (Gmin) was plotted relative to the rotigaptide concentration for each set of experiments using either the action potential or steady-state Vj ramp voltage clamp protocols. Similar results for both protocols demonstrate that rotigaptide effectively increases the ventricular Gmin between concentrations of 20–100 nM levels. Data points are mean ± SD. The maximal 10% increase in Gmin was statistically significant from control values (P < 0.05, n = 7). (B) Average Gmax of the steady-state Gj–Vj recovery curves (=Gmax, rec), plotted relative to rotigaptide concentration, illustrates the lack of dose-dependent effects on the magnitude of facilitation. (C) Increase in initial gj observed with overnight treatment with the indicated concentrations of rotigaptide. Measured immediately upon establishment of the dual whole cell patch configuration, initial gj was elevated by an average of 5% at 35 and nearly 20% at 100 nM rotigaptide, although none of these increases were statistically significant (one-way ANOVA, P > 0.05). (D) Acute exposure to 100 nM rotigaptide by rapid bath superfusion increased gj by 4.6% compared with control saline.
3.2. Kinetic analysis of the effects of rotigaptide
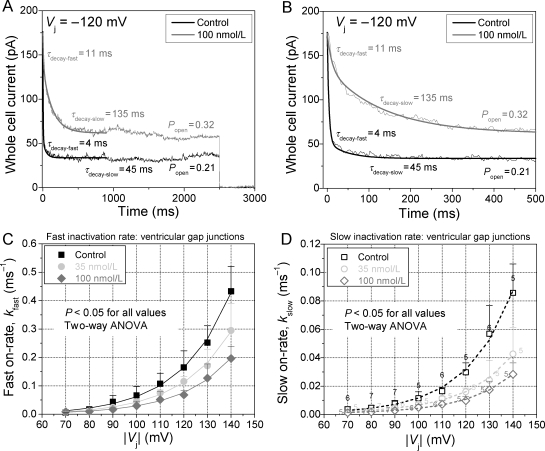
In addition to the rotigaptide-induced increase in Gmin, the first order inactivation kinetics of ventricular gap junctions was fully examined. The ensemble averaged Ij from 5 to 10 square Vj steps of fixed magnitude and duration were fit with a biexponential decaying function to determine the fast and slow decay time constants (τfast and τslow) for each Vj. The closing on-rates for the fast and slow inactivation gating mechanisms were then calculated from the respective τdecay and Po values (see Methods). The ventricular gap junction fast and slow inactivation kinetics is summarized for control, 35, or 100 nM rotigaptide treatment groups in Figure 4. The fast and slow inactivation components were similarly affected by rotigaptide treatment and were well described by an exponentially increasing function with similarly constant Vj-dependencies of 21.0 ± 0.2 and 19.7 ± 0.3 mV, respectivly (see Supplementary material online, Table S2). The average Vj constant for both inactivation components was 20.3 ± 0.7 mV, identical to the previously reported value obtained from mono-exponential fits of Ij τdecay.12 These data are the first description of the Vj-dependence of the macroscopic fast and slow inactivation gating mechanisms for ventricular gap junctions, which are in agreement with our previous report of the Vj-sensitivity of the slow inactivation rates.12 The Vj-dependence is similar for the fast and slow inactivation components and is not altered by rotigaptide treatment. Two-way ANOVA analysis of the fast and slow on-rates for each Vj examined indicated a statistically significant (P < 0.05) slowing of both inactivation rates for Vj > 70 mV when comparing the control, 35 nM, and 100 nM rotigaptide treatment groups (P < 0.05). The Vj-dependent changes in both inactivation rates were statistically significant when Vj > 100 mV. The concentration-dependent effects of rotigaptide on the fast and slow inactivation rates were well described by the monoexponential functions (ms−1):
Figure 4.
Effects of rotigaptide on fast and slow ventricular Gj inactivation kinetics. (A) Biexponential fit of the decay in whole cell 2 current to obtain the fast and slow decay time constants in response to a −120 Vj step. The fast and slow inactivation on-rates were calculated under control and 100 nM rotigaptide treatments for these ventricular myocyte cell pairs of similar gj. (B) The same current traces in (A) plotted on an expanded time scale to better illustrate the exponential fit of the fast inactivation component. (C) The Vj-dependent fast inactivation rates of ventricular Gj inactivation was progressively slowed by increasing doses of rotigaptide. (D) The slow inactivation component was similarly affected by rotigaptide treatment. The number above each symbol indicates the number of experiments for the data in (C) and (D). The parameters for the fitted curves are provided in Supplementary material online, Table S2.
| 4 |
and
| 5 |
between the concentrations of 0–100 nM.
3.3. Modelling the actions of rotigaptide on ventricular gap junctions
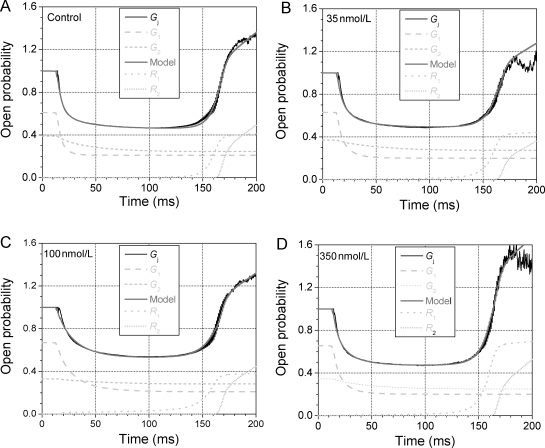
In order to develop a realistic mathematical model for the action of rotigaptide on the gating kinetics of ventricular gap junctions, the Gj- time curves for the BCL = 1000 ms action potential were fit with the equations for the previously published dynamic ventricular gap-junction model.12 In Figure 5, the average time-dependent Gj curves for 0, 35, 100, and 350 nM rotigaptide illustrated in Figure 1 were fit with a series of expressions developed to accurately describe the two observed inactivation and recovery components of ventricular Gj (see Supplementary material, online). Figure 5 results demonstrate that accurate time- and Vj-dependent descriptions of the 1/s action potential Gj curve can be achieved using this model based primarily on the inactivation kinetics described in Eqs. (4) and (5). The rotigaptide concentration-dependent values of A1 and A2 were determined from the above-given expressions for Afast and Aslow with the exceptions of the 200 and 350 nM values which were determined by fitting the Gj–time curves by eye (see Supplementary material, online). These data demonstrate that the effects of rotigaptide on the gating of cardiac gap junctions can be adequately modelled by the concentration-dependent changes in the inactivation kinetics plus a slight concentration-dependent shift in the early recovery phase of ventricular Gj towards higher declining Vj values. All other model-dependent parameters remained essentially constant and independent of [rotigaptide].
Figure 5.
Dynamic ventricular gap-junction model description of rotigaptide action. The output of the current dynamic ventricular gap-junction model (grey line) accurately fitted to the control Gj (A), the 35 nM rotigaptide Gj (B), 100 nM rotigaptide Gj (C), and the 350 nM rotigaptide Gj (D) curves. The model fits were obtained by changing the amplitude of the fast and slow inactivation kinetics (G1 and G2, light grey dashed and short dashed lines) with only minor adjustments to the threshold voltage for the initiation of the early recovery process (R1, light grey dotted line). The experimental Gj value was set equal to 1 when Vj = 0 and the summed output of the model was defined to be equal to this peak Gj during the diastolic period. See Supplementary material, online, for additional details.
3.4. Modelling ventricular action potential propagation
A major premise of this study was to determine the mechanisms by which rotigaptide preserves conduction velocity (θ) during episodes of metabolic inhibition.8 One hypothesis is that during slow, discontinuous propagation at low levels of coupling (low gj), Vj-dependent inactivation produces further reductions in θ than a static decrease in gj alone. To model this behaviour, the dynamic ventricular gap-junction equations for fast and slow inactivation were programmed into a linear cable model of a cardiac strand (see Supplementary material, online).12 Each 11 µm diameter, 100 µm long model segment (cell) was programmed with the updated Luo–Rudy II ventricular action potential and all 100 segments were uniformly coupled by a resting (initial) gj value.20 The resting gj value was either kept constant (static), or allowed to inactivate during action potential propagation according to the fast and slow inactivation kinetics described in the original dynamic ventricular gap-junction model (2005 model) or in the control experiments defined herein in the absence of rotigaptide (dynamic) (see Supplementary material, online). To simulate the effects of rotigaptide, the 0–100 nM concentration-dependent reductions in the fast and slow inactivation rates were calculated according to Eqs. (4) and (5).
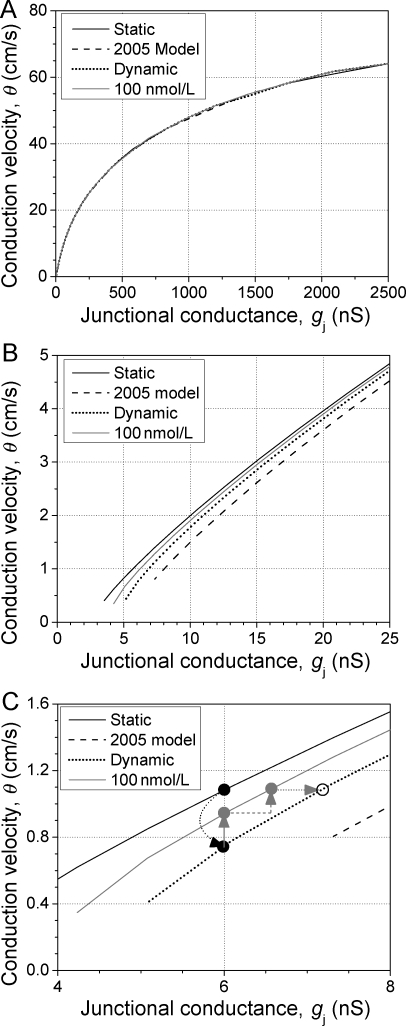
The calculated θ for the stable propagating action potential agrees closely with previous simulations using a constant reduction in gj to model the effects on conduction.21 The basis for the 20% higher maximum θ of 64 cm/s is that the newest version of the Luo–Rudy II20 model has a higher maximum upstroke velocity (Vmax) than the original version.16 At first glance, it appears that the relationship between θ and gj is not affected by the introduction of Vj-dependent gating (Figure 6A). However, it was postulated that inactivation would only affect gj below 10 cm/s since this is the value of θ required to impose the entire action potential upstroke across a single cardiac gap junction.12 At these lower gj values, disparities are observed in θ between the various models (Figure 6B). The basis for the higher θ value in the control-gating model is that the fast inactivation rates were experimentally determined in the present study whereas they were extrapolated from the slow inactivation rate constants in the previous dynamic gap-junction model. Compared with the static gj model, inactivation reduces θ by nearly a third at 6 nS of gj (Figure 6C). The optimal therapeutic dose of 100 nM rotigaptide would require an increase in gj of 10% from the resting level, in addition to the slowing of inactivation, to completely reverse the effects of inactivation on θ. A 20% increase in resting gj, as observed experimentally, would increase θ by 14% according to this most recent dynamic gj gating model and by 23% in the case of a static gj. Thus, the combination of a reduction in the rate of inactivation and an increase in resting gj of 10–20% is sufficient to reverse the effects of dynamic changes in gj and reduced levels of electrical coupling on myocardial θ.
Figure 6.
Effect of changing gj on action potential conduction velocity (θ). (A) Ventricular θ slows from a maximum of 64 to <1 cm/s as gj is reduced from 2500 to <5 nS. At high gj values, no variations in θ are observed as a result of Vj-dependent gap-junction inactivation. (B) Discrepancies in θ are apparent at low resting gj values, depending on the gap-junction inactivation rates that are utilized, and block develops at higher values than if gj is kept constant during action potential propagation. (C) The optimal dose of 100 nM rotigaptide can completely prevent the conduction slowing produced by Vj-dependent gj gating (black dotted arrow) via a reduction in the inactivation kinetics (solid grey arrow) coupled with a 10% increase in resting gj (dashed grey arrow). A 20% increase in resting gj is also sufficient to reverse the conduction slowing without alteration of the gj inactivation kinetics (dotted grey arrow).
4. Discussion
Myocardial gap junctions formed by Cx43 and Cx40 play an important role in the establishment and modulation of arrhythmias, and their functional expression and distribution are altered by infarction, heart failure, and chronic arrythmias.14,15,22,23 During ischaemia, intracellular resistance can triple in value and longitudinal conduction velocity can slow by 2.5-fold within 20 min.24,25 These effects are thought to occur as a result of myocardial uncoupling secondary to intracellular Na+ and Ca2+ accumulation, and may affect transverse θ more than longitudinal θ due to the relative paucity of gap junctions in the transverse direction.26–30 In a recent study, 50 nM rotigaptide increased myocardial θ by 20% in rapidly paced guinea pig ventricles and pretreatment totally prevented the ischaemia-induced 20% increase in θ and suppressed the development of arrhythmogenic discordant T-wave alternans.31 Yet there is almost no pharmacological therapy for these alterations in the treatment of cardiac arrhythmias. Rotigaptide and a newly synthesized Cx43 CT domain RXP-E binding peptide are the only known compounds thought to directly act on cardiac gap junctions to preserve their function.6,32,33 Rotigaptide reportedly inhibits the rundown of myocardial conduction velocity and the occurrence of spontaneous re-entrant tachycardias during acute episodes of metabolic stress including ischaemia/reperfusion by improving cardiac gj.4,8,34 Rotigaptide exposure also reduces chronic infarct size and the vulnerability to acute atrial fibrillation (AF).9,10,34 However, chronic dilated or heart failure models of AF and ventricular tachycardias of focal origin were not affected by rotigaptide administration.34–37 In the present study, we assessed the effect of therapeutic doses of rotigaptide on the conductance and Vj-dependent kinetics of cardiac gap junctions.
Increasing concentrations of rotigaptide produced a dose-dependent increase in Ij during the 4/s, 2/s, and 1/s ventricular action potential for concentrations ≤100 nM (Figure 1). These same rotigaptide concentrations increased the non-inactivating Gmin portion of the steady-state ventricular Gj–Vj relationships, but had no effect on the increased Gj observed during the recovery phase of the steady-state Gj–Vj curves (Figures 2 and 3). A trend towards higher resting gj values was also observed in ventricular cell pairs treated overnight with 35 or 100 nM rotigaptide compared with untreated controls, although the results did not achieve statistical significance (Figure 3C). The rotigaptide-induced increase in ventricular gj was confirmed by acute exposure to 100 nM concentrations by bath superfusion (Figure 3D). The relative increase in Ij during the action potential could be explained by the concentration-dependent effects of rotigaptide on the fast and slow inactivation rates of ventricular gj (Figure 4). Further kinetic analysis revealed that both inactivation processes possess nearly identical Vj-dependencies that were unaltered by rotigaptide (see Supplementary material, online). We had previously characterized the Vj-dependence of only the slow inactivation rate of ventricular gap junctions, which increased e-fold for every 20.3 mV increase in Vj.12 The values obtained in this study in the presence or absence of rotigaptide were essentially the same, changing e-fold for every 21.0 ± 0.2 mV or 19.7 ± 0.3 mV, respectively, for the fast or slow inactivation rates. This Vj-dependence results in significant increases in both gap-junction inactivation rates when Vj > 100 mV, consistent with our previous findings, and were significantly altered by 35 or 100 nM rotigaptide.12,38 Despite the shorter APDs of higher frequencies of stimulation, ischaemia, or intrinsic ionic currents, reductions in gj can occur as a result of inactivation and, therefore, be antagonized by rotigaptide treatment (Figure 1E and F and Supplementary material online, Figure S4).18
These results were sufficient to provide a mathematical basis for calculating the effects of rotigaptide on ventricular Gj inactivation during the normal action potential. From a functional perspective, the inactivation process corresponds to the on-rates for the fast and slow inactivation gates that close the ventricular gap-junction channels in response to large Vj gradients. The recovery phase of Gj corresponds to the reopening of these channels from inactivation, which apparently occurs by a different protein conformational shift since hysteresis was evident between the inactivation and recovery Gj–Vj curves (Figure 2).12 The original dynamic ventricular gap-junction model,12 consisting of two inactivation and recovery components for Gj, was utilized to fit the Gj-time plots for the various test concentrations of rotigaptide. It was determined that the concentration-dependent effects of rotigaptide on the gating of ventricular gap junctions could be accurately described by alterations in the fast and slow inactivation kinetics and an increase in the Vj-sensitivity of the early (R1) recovery process. It should be noted that these predictions are based on the inactivation kinetics at 20°C, not normal body temperature. We expect the effects of gj inactivation at 37°C to be greater than those observed in these experiments and simulations since protein conformational processes typically exhibit a Q10 of >2 as opposed to a diffusional process, which typically has a Q10 of <1.5. The temperature-dependence of cardiac gap-junction inactivation kinetics are currently being examined.
Rotigaptide, like AAP10, is thought to increase cardiac gj by PKC activation.39 The present results indicate that another mechanism by which rotigaptide can preserve myocardial gj is by altering the gap-junction inactivation kinetics. There was no obvious effect of 0–100 nM rotigaptide treatments on ventricular gap-junction formation in cardiomyocyte cultures (see Supplementary material, online). Whether or not PKC stimulation and inhibition can explain the action of rotigaptide on the gating kinetics of cardiac gap junctions is currently being investigated. An additional explanation is that alternative phosphorylation sites also participate in ischaemia-induced uncoupling and that rotigaptide delays the dephosphorylation of the PKC-dependent S368 and other previously unidentified sites on Cx43.40
One prediction of the dynamic ventricular gap-junction model is that the gating induced by finite intercellular conduction delays will produce further slowing of myocardial conduction velocity, thus promoting slow, discontinuous propagation at higher resting gj values than that previously modelled using static gj values (Figure 6).21 The slow θ that can be achieved only by reductions in gj can promote the formation of unidirectional conduction block and re-entrant arrhythmias within the inhomogeneous myocardium.14,41,42 Our present results using the updated dynamic ventricular gap-junction model, and a previous computer simulation study of a dynamic junctional resistance, both indicate that Vj-dependent inactivation will enhance these low gj conduction delays and produce conduction block at higher initial gj values than would occur without gating.12,43 The major question being asked by this study is whether the observed changes in myocardial gj are sufficient to account for the preservation of θ observed in ischaemic or diseased cardiac tissues or whole heart preparations by rotigaptide administration.8,33,35,36 Our computer simulations, using the gap-junction inactivation kinetics described in this study, reveal that Vj-dependent gating of ventricular gap junctions will gradually slow action potential propagation at velocities below 10 cm/s. More importantly, the effect of therapeutic doses of rotigaptide on the inactivation kinetics is sufficient to prevent 60% of this conduction slowing (Figure 6C). Combined with a slight increase in gj of 10%, rotigaptide can effectively abolish the conduction slowing observed under partially uncoupled conditions. Our computer simulations also indicate that the same improvement in myocardial θ can be achieved by a 20% increase in resting gj, consistent with an experimentally observed trend that did not quite achieve statistical significance (P = 0.07 at 100 nM rotigaptide, N = 17). This preservation of myocardial gj by rotigaptide may be sufficient, under certain circumstances, to prevent the formation of unidirectional block or lengthen the required re-entrant pathway beyond a sustainable limit needed for the formation of re-entrant tachycardias. This latter hypothesis will require further investigation. In conclusion, experimental evidence and computer simulations demonstrating the dynamic alterations of the gating of ventricular gap junctions provides further insight into the mechanisms by which rotigaptide can preserve myocardial conduction during acute ischaemic episodes. Additional experiments are required to determine the molecular bases for the kinetic modulation of cardiac gap junctions by rotigaptide and the mechanisms responsible for myocardial uncoupling and conduction slowing during episodes of metabolic stress.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
National Institutes of Health, National Heart, Lung and Blood Institute grant HL-042220 (R.D.V.) and Wyeth Research, Cardiovascular and Metabolic Diseases Division, Collegeville, PA, USA (R.D.V.).
Supplementary Material
Acknowledgements
We would like to acknowledge the contribution of the 1 Hz neonatal murine ventricular action potential waveform by Ms Linda J. Wang, Harvard Medical School, Boston, MA, USA and Dr Eric A. Sobie, Mount Sinai School of Medicine, New York, NY, USA.
Conflict of interest: this work was performed with rotigaptide provided by permission from Wyeth Research and licensed for use in US by Wyeth from Zealand Pharma A/S, Glostrup, Denmark. R.D.V. is a paid consultant of Wyeth Research. J.K.H. is an employee of Wyeth Research. J.S.P. is the Chief Scientific Officer and Vice President of Zealand Pharma A/S.
References
- 1.Poelzing S, Akar FG, Baron E, Rosenbaum DS. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol. 2004;286:H2001–H2009. doi: 10.1152/ajpheart.00987.2003. [DOI] [PubMed] [Google Scholar]
- 2.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, et al. Heterologous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 3.Gutstein DE, Danik SB, Lewitton S, France D, Liu F, Chen FL, et al. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol. 2005;289:H1091–H1098. doi: 10.1152/ajpheart.00095.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing D, Kjølbye AL, Nielsen MS, Petersen JS, Harlow KW, Holstein-Rathlou NH, et al. ZP123 increases gap junctional conductance and prevents reentrant ventricular tachycardia during myocardial ischemia in open chest dogs. J Cardiovasc Electrophysiol. 2003;14:510–520. doi: 10.1046/j.1540-8167.2003.02329.x. [DOI] [PubMed] [Google Scholar]
- 5.Herve JC, Dhein S. Pharmacology of cardiovascular gap junctions. Adv Cardiol. 2006;42:107–131. doi: 10.1159/000092565. [DOI] [PubMed] [Google Scholar]
- 6.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Disc. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 7.Clark TC, Thomas D, Petersen JS, Evans WH, Martin PEM. The antiarrhythmic pewptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin43. Brit J Pharmacol. 2006;147:486–495. doi: 10.1038/sj.bjp.0706631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haugan K, Olsen KB, Hartvig L, Petersen JS, Holstein-Rathlou NH, Hennan JK, et al. The antiarrhythmic peptide analog ZP123 prevents atrial conduction slowing during metabolic stress. J Cardiovasc Electrophysiol. 2005;16:537–545. doi: 10.1111/j.1540-8167.2005.40687.x. [DOI] [PubMed] [Google Scholar]
- 9.Haugan K, Marcussen N, Kjølbye AL, Nielsen MS, Hennan JK, Petersen JS. Treatment with the gap junction modifier rotigaptide (ZP123) reduces infarct size in rats with chronic myocardial infarction. J Cardiovasc Pharmacol. 2006;47:236–242. doi: 10.1097/01.fjc.0000200990.31611.6e. [DOI] [PubMed] [Google Scholar]
- 10.Hennan JK, Swillo RE, Morgan GA, Keith JC, Jr, Schaub RG, Smith RP, et al. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias reduces infarct size during myocardial ischemia/reperfusion injury in open-chest dogs. J Pharmacol Exp Ther. 2006;317:236–243. doi: 10.1124/jpet.105.096933. [DOI] [PubMed] [Google Scholar]
- 11.Kjølbye AL, Haugan L, Hennan JK, Petersen JS. Pharmacological modulation of gap junction function with the novel compound Rotigaptide: a promising new principle for prevention of arrhythmias. Basic Clin Pharmacol Toxicol. 2007;101:215–230. doi: 10.1111/j.1742-7843.2007.00123.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Gemel J, Beyer EC, Veenstra RD. Dynamic model for ventricular junctional conductance during the cardiac action potential. Am J Physiol Heart Circ Physiol. 2005;288:H1113–H1123. doi: 10.1152/ajpheart.00882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weingart R, Maurer P. Action potential transfer in cell pairs isolate from adult rat and guinea pig ventricles. Circ Res. 1988;63:72–80. doi: 10.1161/01.res.63.1.72. [DOI] [PubMed] [Google Scholar]
- 14.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 15.Nattel S, Maguy A, Le Bouter S, Yeh T-S. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 16.Veenstra RD. Voltage clamp limitations of dual whole-cell gap junction current and voltage recordings. I. Conductance measurements. Biophys J. 2001;80:2231–2247. doi: 10.1016/S0006-3495(01)76196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo C-H, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74:1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- 18.Wang LJ, Sobie EA. Mathematical model of the neonatal mouse action potential; Am J Physiol Heart Circ Physiol; 2008; Epub ahead of print 11 April 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhein S. Pharmacology of gap junctions in the cardiovascular system. Cardiovasc Res. 2004;62:287–298. doi: 10.1016/j.cardiores.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Faber GM, Rudy Y. Action potential and contractility changes in. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J. 2000;78:2392–2404. doi: 10.1016/S0006-3495(00)76783-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–741. doi: 10.1161/01.res.81.5.727. [DOI] [PubMed] [Google Scholar]
- 22.Luke RA, Saffitz JE. Remodeling of ventricular conduction pathways in healed canine infarct border zones. J Clin Invest. 1991;87:11594–11602. doi: 10.1172/JCI115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabo C, Yao J, Boyden PA, Chen S, Hussain W, Duffy HS, et al. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res. 2006;72:241–249. doi: 10.1016/j.cardiores.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Kléber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987;61:271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- 25.Cascio WE, Yang H, Johnson TA, Muller-Borer BJ, Lemasters JJ. Electrical properties and conduction in reperfused papillary muscle. Circ Res. 2001;89:807–814. doi: 10.1161/hh2101.098612. [DOI] [PubMed] [Google Scholar]
- 26.De Mello WC. Effect of intracellular injection of calcium and strontium on cell communication in the heart. J Physiol. 1975;250:231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Mello WC. Uncoupling of heart cells produced by intracellular sodium injection. Experentia. 1975;31:460–461. doi: 10.1007/BF02026379. [DOI] [PubMed] [Google Scholar]
- 28.Weingart R. The actions of ouabain on intercellular coupling and conduction velocity in mammalian ventricular muscle. J Physiol. 1977;264:341–365. doi: 10.1113/jphysiol.1977.sp011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spach MS, Kootsey JM, Sloan JD. Active modulation of electrical coupling between cardiac cells of the dog. A mechanism for transient and steady state variations in conduction velocity. Circ Res. 1982;51:347–362. doi: 10.1161/01.res.51.3.347. [DOI] [PubMed] [Google Scholar]
- 30.Kléber AG. Consequences of acute ischemia for the electrical and mecjhanical function of the ventricular myocardium. A brief review. Experentia. 1990;46:1162–1167. doi: 10.1007/BF01936928. [DOI] [PubMed] [Google Scholar]
- 31.Kjølbye AL, Dikshteyn M, Eloff BC, Deschênes I, Rosenbaum DS. Mantenance of intercellular couopling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol. 2008;294:H41–H49. doi: 10.1152/ajpheart.01089.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shibayama J, Lewandowski R, Kieken F, Coombs W, Shah S, Sorgen PL, et al. Identification of a novel peptide that interferes with the chemical regulation of connexin43. Circ Res. 2006;98:1365–1372. doi: 10.1161/01.RES.0000225911.24228.9c. [DOI] [PubMed] [Google Scholar]
- 33.Eloff BC, Gilat E, Wan X, Rosenbaum DS. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- 34.Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhytmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 35.Guerra JM, Everett TH, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 36.Haugen K, Miyamoto T, Takeishi Y, Kubota I, Nakayama J, Shimojo H, et al. Rotigaptide (ZP123) improves atrial conduction slowing in chronic volume overload-induced dilated atria. Basic Clin Pharmacol Toxicol. 2006;99:71–79. doi: 10.1111/j.1742-7843.2006.pto_432.x. [DOI] [PubMed] [Google Scholar]
- 37.Xing D, Kjølbye AL, Petersen JS, Martins JB. Pharmacological stimulation of cardiac gap junction coupling does not affect ischemia-induced focal ventricular tachycardia or triggered activity in dogs. Am J Physiol Heart Circ Physiol. 2005;288:H511–H516. doi: 10.1152/ajpheart.00720.2004. [DOI] [PubMed] [Google Scholar]
- 38.Lin X, Crye M, Veenstra RD. Regulation of connexin43 gap junctional conductance by ventricular action potentials. Circ Res. 2003;93:e63–e73. doi: 10.1161/01.RES.0000093379.61888.35. [DOI] [PubMed] [Google Scholar]
- 39.Weng S, Lauven M, Schaefer T, Polontchouk L, Grover R, Dhein S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J. 2002;16:1114–1116. doi: 10.1096/fj.01-0918fje. [DOI] [PubMed] [Google Scholar]
- 40.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou N-H, et al. Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Molec Cell Cardiol. 2006;40:790–798. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Quan W, Rudy Y. Unidirectional block and reentry of cardiac excitation: a model study. Circ Res. 1990;66:367–382. doi: 10.1161/01.res.66.2.367. [DOI] [PubMed] [Google Scholar]
- 42.Joyner RW, Sugiura H, Tan RC. Unidirectional block between isolated rabbit ventricular cells coupled by a variable resistor. Biophys J. 1991;60:1038–1045. doi: 10.1016/S0006-3495(91)82141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriquez AP, Vogel R, Muller-Borer BJ, Henriquez CS, Weingart R, Cascio WE. Influence of dynamic gap junction resistance on impulse propagation in ventricular myocardium: a computer simulation study. Biophys J. 2001;81:2112–2121. doi: 10.1016/S0006-3495(01)75859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.