Abstract
Objective
Following unblinding of the Diabetes Prevention Program (DPP) results, a 16-session lifestyle intervention program was offered to all study participants, including those who had initially been randomized to lifestyle treatment. This study compares the effects of the lifestyle program between participants who had previous exposure and those who had not.
Design
A 16-session behavioral intervention was conducted in groups at each of the 27 DPP sites during a transitional (bridge) period from the DPP trial to the DPP Outcomes Study (DPPOS). Session participation for this 6-month behavioral weight loss program was confirmed by originally randomized treatment groups.
Subjects and measurements
Independently assessed weight measurements were available within a 7-month period before and after the program for 2808 ethnically diverse participants.
Results
Participants from the lifestyle group in the DPP were the least likely to attend a repeat offering of a 16-session behavioral weight loss program conducted in groups. Weight loss during the transitional lifestyle program was strongly related to the duration of attendance in the three groups that were participating in the program for the first time (metformin, placebo and troglitazone), but not related to amount of earlier weight loss.
Conclusion
Individuals who were naive to the behavioral program lost a greater amount of weight and this was strongly related to their degree of participation. A second exposure to a behavioral weight loss program resulted in unsatisfactory low attendance rates and weight loss.
Keywords: behavior modification, metformin, weight loss, maintenance
Introduction
Over the past 40 years, behavioral weight loss treatments, using a combination of low-fat, low-calorie diet, physical activity and lifestyle modification strategies, have produced significant weight loss on first use. These interventions have resulted in initial weight losses of up to 10% and dropout rates of less than 20%.1,2 Initial weight loss achieved with behavioral treatment tends to be somewhat less in population-based, multi-center clinical trials, with 5–7% reductions during the first 6 months of intervention and good adherence rates for up to 3 years, on average.3,4
A key area in behavioral weight management involves identification of effective long-term weight maintenance approaches.5–7 However, reviews of many well-controlled studies suggest that although longer duration of treatment appears to be associated with better weight loss maintenance, or at least delay of weight regain, participants may eventually tire of coming to scheduled treatment visits.1,8 Moreover, data on rates of joining, level of participation and weight loss outcomes for new rounds of structured treatment are limited. Beyond 2–4 years of initial intervention, optimal behavioral adherence and weight loss maintenance strategies are simply not known.
Most approaches to long-term weight loss management offer some form of continued contact, such as periodic ‘boosters’ or ‘restarts’. It is important to characterize the degree to which participants engage in such offerings and, if so, to what degree weight loss can be achieved or maintained. It is also critical to gain better understanding of the demographic data, previous weight loss outcomes and other individual variables that may influence subsequent attendance and adherence in the ongoing weight loss programs and long-term clinical trials.
Participant response to the Diabetes Prevention Program (DPP) lifestyle intervention has been described previously.9 After the DPP results were announced and treatments were unmasked, a 16-session, 6-month group program called Healthy Lifestyle Program (HELP) was offered to all study participants as part of a bridge period protocol because it was deemed ethically appropriate and desirable. The bridge period represents the transition from the DPP trial to the DPP Outcomes Study (DPPOS). As such, this phase of DPP offers a unique opportunity to study the degree to which participants will engage in repeat treatment offerings and their weight loss response.
This paper examined the effects of offering the original 16-session individual weight loss program in a group format to all study participants after the DPP trial results were announced in August 2001. Specifically, we looked at rates of participation and weight loss response to a new round of behavioral weight loss treatment in this large, diverse, multiethnic sample of DPP participants by original treatment group assignment and by weight change before program participation.
Methods
Design
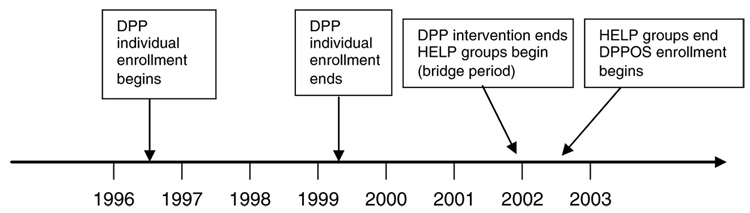
Figure 1 presents a timeline that defines the intervention periods from randomization through the end of DPP, the transitional bridge period and the start of DPPOS.
Figure 1.
Timeline of intervention periods. Treatment period timeline and DPP main trial enrollment to DPPOS. All DPP participants (n=3819) were randomized by June, 1999, resulting in an average follow-up period of 3.2 years. Bridge period HELP program was administered during a fixed 6-month period and offered to all remaining participants (n=3727).
The Diabetes Prevention Program examined the effects of four treatment arms to prevent or delay type 2 diabetes in high-risk adults. Overweight or obese individuals with elevated fasting glucose and impaired glucose tolerance were randomly assigned to an individually administered intensive lifestyle weight loss program (ILS), metformin (MET), troglitazone (TROG) or placebo (PLAC) arm. Descriptions of the treatments and major outcomes have been previously reported.9
The first phase of DPP was terminated early after 3.2 years of average follow-up when ILS efficacy was clearly demonstrated. Briefly, 95% of all participants, randomized to the lifestyle arm of the DPP, completed the individually administered 16-session core curriculum with a median time to program completion of 24 weeks. The mean weight loss for this treatment period was 6.5±4.7 kg. After 3.2 years, ILS reduced the incidence of diabetes by 55%, compared with PLAC; and MET reduced incidence of diabetes by 31%, compared with PLAC. Treatment with TROG was stopped after an average of only 0.9 years of follow-up (due to toxicity) and after drug treatment was withdrawn, this group was offered lifestyle sessions and handouts with no additional behavioral coaching on self-management strategies. Subsequent analyses of the DPP data have shown weight loss to be the dominant factor in the efficacy of ILS.10
DPP intervention protocols
All individually administered DPP interventions are summarized briefly as participants from these original treatment arms form the basis of comparison for the effects of HELP group treatment during the transitional bridge period.
Intensive lifestyle group
The goal of ILS treatment was to achieve ≥7% of body weight loss and ≥150 min per week of physical activity (mainly brisk walking) during the initial 6 months of weekly treatment and then endeavor to maintain this weight loss and level of physical activity through the remaining treatment period. A low-fat, low-calorie dietary approach was used. The DPP intervention consisted of a 16-week individually administered state-of-the-art behavioral weight management core curriculum, followed by at least monthly visits with a lifestyle coach for the purpose of self-management, problem solving and one-on-one support. Periodic group classes and motivational campaigns were used to reduce weight regain and maintain activity levels.
Metformin and placebo group
Metformin was initiated at a dose of 850mg or matching placebo taken orally once per day. By 1 month, the goal was to increase the dose to twice daily unless gastrointestinal symptoms warranted a longer titration period. Participants were maintained on this regimen and medication adherence support was provided quarterly by a medication case manager. More than 80% of the participants were compliant with the metformin and placebo medications. Standard lifestyle recommendations and some written information on healthy eating, healthy weight and physical activity were provided annually to all participants.
Troglitazone group
Treatment was initiated at 400mg daily, but stopped early (0.9 years follow up on average) due to severe adverse side effects. After TROG participants were informed and the drug was withdrawn, they were offered quarterly group lifestyle sessions to learn the educational content of the ILS core curriculum but received no additional behavioral coaching or support. TROG also proceeded with the regular schedule of assessment visits.
Healthy lifestyle program group treatment during the bridge transition period
After the DPP was terminated in July 2001, active efforts were made to maintain all participants in their treatment arms through the unmasking and debriefing phase. Debriefing consisted of meetings with all remaining participants (that is, those who had not died, or withdrawn from the study) to provide them with their individual outcome data and review the basis for the decision to end the trial. A 16-session HELP group program was offered at two to three different times per week over a period of 6 months from January through July 2002. Groups consisted of 10–20 participants (maximum) and the group leaders were primarily the same lifestyle coaches who implemented the DPP lifestyle treatment, in addition to some program coordinators, at each clinic site.
The 16 HELP sessions were nearly identical in content, including all participant materials and supplemental handouts, to the original DPP 16-session core curriculum that has been described in detail elsewhere.11 However, as HELP was conducted in a group setting, individualized problem solving and support were limited and no additional incentives were offered (for example, providing a low-fat cookbook or a discount coupon for sneakers contingent on a participant making progress toward their DPP goals). A final difference was treatment duration with the original DPP lifestyle participants being coached in the core curriculum lessons for up to 1 year compared with 6 months in the HELP program. All participants including those who had not received any behavioral treatment (PLAC & MET) as well as those previously exposed (ILS & TROG) were encouraged to attend HELP (a second round of core treatment sessions) for weight loss, weight maintenance or restart following weight regain regardless of their earlier treatment responses.
Participants
A total of 3819 participants from 27 clinics in the United States were enrolled in the DPP between 1996 and 1999. To be eligible participants were 25 years of age or older, had a minimum body mass index of 24 kg/m2 (22 kg/m2 in Asian Americans), elevated fasting glucose and impaired glucose tolerance during an oral glucose tolerance test based on American Diabetes Association or World Health Organization criteria. A plasma glucose concentration of 95 to 125mg per 100 ml (5.3–6.9mmoll−1) in the fasting state (≤125mg per 100 ml in the American Indian clinics) and 140 to 199mg per 100 ml (7.8–11.0mmoll−1) 2 hours after a 75 g oral glucose load defined impaired glucose tolerance. These concentrations are elevated but are not diagnostic of diabetes according to the 1997 criteria of the American Diabetes Association. Before June 1997, the criterion for plasma glucose in the fasting state was 100–139mg per 100 ml (5.6–7.7mmoll−1), or ≤139mg per 100 ml in the American Indian clinics. Participants were excluded if they had an earlier diagnosis of diabetes or had any other conditions or medications that would impair their ability to participate or affect weight loss outcomes. All participants gave written informed consent after approval by local Institutional Review Boards. At the termination of DPP there was 2.5% attrition in the randomized cohort, due to death or withdrawal from the study, with 3727 participants remaining. Participants were recruited for the HELP program through home mailings and in person during regular clinic visits as the consent process for the transitional bridge period was being conducted. All participants who provided informed consent were strongly encouraged to attend one of the HELP groups.
Bridge period cohort and outcome measures
Definition of the bridge cohort
Only those individuals, who had valid major clinic visits and had weight data measured within 7 months of the first and last HELP sessions, were defined as members of the bridge cohort (75% or 2808 out of 3727 DPP participants met this definition). Table 1 shows the demographic characteristics of the bridge cohort (n=2808). The remaining DPP participants are characterized as ‘not bridge’ (n=1011).
Table 1.
Baseline characteristics of bridgea (n=2808) versus not bridge (n=1011) cohorts, HELP attendees (n=1428) versus HELP non-attendees (n=1380)
| Clinical Characteristics | Bridgea (n=2808) | Not bridge (n=1011) | HELP attendeesb 1–16 sessions (n=1428) | HELP non-attendees 0 session (n=1380) |
|---|---|---|---|---|
| Age at randomization | 51.4±10.2c | 48.6±11.1 | 51.8±9.9 | 51.0±10.6 |
| Mean weight at randomization (kg) | 93.2±19.5c | 96.6 (21.1) | 93.1 (19.5) | 93.3 (19.4) |
| Mean weight | ||||
| Pre-Bridge (kg) | 91.7 (20.7) | 91.8 (20.7) | ||
| Gender | ||||
| Male | 915 (32.6%) | 329 (32.5%) | 395 (27.7%) | 520 (37.7%) |
| Female | 1893 (67.4%) | 682 (67.5%) | 1033 (72.3%) | 860 (62.3%) |
| Race/ethnicity | ||||
| White | 1568 (55.8%) | 550 (54.4%) | 811 (56.8%) | 757 (54.9%) |
| African-American | 531 (18.9%) | 220 (21.8%) | 259 (18.1%) | 272 (19.7 %) |
| Hispanic | 445 (15.8%) | 164 (16.2%) | 230 (16.1%) | 215 (15.6%) |
| American Indian | 139 (5.0%) | 42 (4.2%) | 67 (4.7%) | 72 (5.2%) |
| Asian | 125 (4.5%) | 35 (3.5%) | 61 (4.3%) | 64 (4.6%) |
| Diabetes status | ||||
| Pre-Bridge | 367(25.7%) | 358 (25.9%) |
Abbreviations: DPP, Diabetes Prevention Program; HELP, Healthy Lifestyle Program.
Bridge cohort is defined as those DPP participants having valid weight data proximal to the HELP sessions. The remainder is characterized as not Bridge.
Tests comparing HELP attendees vs HELP non-attendees are all non-significant (P>0.05).
Tests comparing Bridge and not Bridge cohorts are significant for baseline age and weight only (Bridge cohort was older and weighed less at randomization; P<0.0001).
Rates of joining
An individual was defined as joining the HELP program if he or she attended at least one session. This was examined as an indicator of interest in the program being offered.
Rates of participation
To further reflect the continuum of a participant’s engagement in the HELP program the number of HELP sessions attended was divided into four groups as follows: 0, 1–5, 6–11 and 12–16.
Change in weight from baseline to initiation of the HELP sessions
To examine the influence of previous weight loss and weight gain history during the DPP, we calculated the pre-HELP weight change as the clinic assessment weight just before the initiation of HELP in January of 2002 minus baseline DPP weight. Clinic assessment weights were measured every 6 months. As DPP participants were initially randomized over a period of 3 years (1996–1999), the duration of the pre-HELP intervention period is anywhere from 2.3 to 5.2 years (mean=3.7 years).
Weight change during the HELP program: clinic assessments
Weight change during transition from DPP to DPPOS was calculated as the first available clinic assessment weight following the end of the HELP sessions in July 2002 minus the last available clinic assessment weight before beginning the HELP sessions. Only weights measured within 7 months of the first and last HELP sessions were used to calculate this weight change, as outside of this window other well-defined treatment periods (first DPP, then DPPOS) were in effect. In summary, a bridge cohort of 2808 participants was constructed, regardless of whether or not these participants attended any HELP sessions.
Statistical methods
For baseline characteristics, means and standard deviations were reported for continuous variables whereas numbers and percentages were reported for the categorical variables. In comparing the rates of joining (number of sessions attended=0, 1–16) and the rates of participation (number of sessions attended=0, 1–5, 6–11 or 12–16) in HELP, among the four treatment groups, Pearson’s χ2 test was used. Because of non-normal distribution of HELP sessions, the Kruskal–Wallis test12 was used to test if there is any association between pre-HELP weight change and rates of participation in HELP. A linear trend in post-treatment weight change by categories of HELP session participation was tested. The trend test was constructed by multiplying the mean weight change in each of the four ordered categories of HELP participation (low to high) with −3, −1, 1 and 3, respectively, and testing whether the sum is equal to zero. The SAS system version 8.2 was used for all analyses (SAS Institute, Inc. Cary, NC, USA).
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the use of human volunteers were followed during the Bridge transition period from DPP to DPPOS.
Results
In Table 1 the first two data columns indicate that among the DPP baseline demographic characteristics for the bridge period cohort studied (n=2808) participants differed only in mean age and weight at randomization compared with the remaining non-examined DPP participants. Tests comparing the bridge and not-bridge cohorts indicate that the bridge group (those with proximal weight data available) was a few years older and weighed significantly less at randomization (P<0.0001).
In addition, Table 1 shows the demographic characteristics and diabetes status of HELP program participants (n=1428) attending anywhere from 1 to 16 sessions versus HELP program non-participants (n=1380) who attended 0 sessions. Results indicate that there were no significant DPP baseline differences in the clinical characteristics between these two groups. Finally, Table 1 also shows that neither the weight assessed at the most recent clinic visit just before HELP, nor the participant’s diabetes status at that time, differed between those who chose to attend versus those who did not.
The rates of joining (coming to at least one session) were significantly lower among the previously treated lifestyle group and highest among the naive groups: metformin and placebo (ILS=40%; MET=58%; TROG=48%; PLAC=57%; P<0.0001). Table 2 further examines the total amount of participation in HELP sessions by the four treatment groups. There are significantly fewer lifestyle participants in the 6–11 and 12–16 session attendance categories compared with the other three treatment groups (P<0.0001).
Table 2.
Rates of joining and participation in HELP, by treatment groups
| Treatment group | 0 sessions | 1–5 sessions | 6–11 sessions | 12–16 sessions |
|---|---|---|---|---|
| Lifestyle | 497 (60%)a | 142 (17%) | 102 (12%)b | 87 (11%)b |
| Metformin | 324 (42%) | 119 (15%) | 134 (17%) | 199 (26%) |
| Troglitazone | 223 (52%) | 51 (12%) | 54 (13%) | 97 (23%) |
| Placebo | 336 (43%) | 108 (14%) | 128 (16%) | 207 (27%) |
Abbreviations: HELP, Healthy Lifestyle Program; ILS, lifestyle weight loss program; MET, metformin treatment arm; PLAC, placebo treatment arm; TROG, troglitazone treatment arm.
ILS compared with MET, TROG, PLAC (grey shaded area) has significantly lower rate of joining 1–16 sessions (P<0.0001).
ILS has significantly lower participation in the 6–11 and 12–16 session attendance categories compared with the other three treatment arms. (P<0.0001).
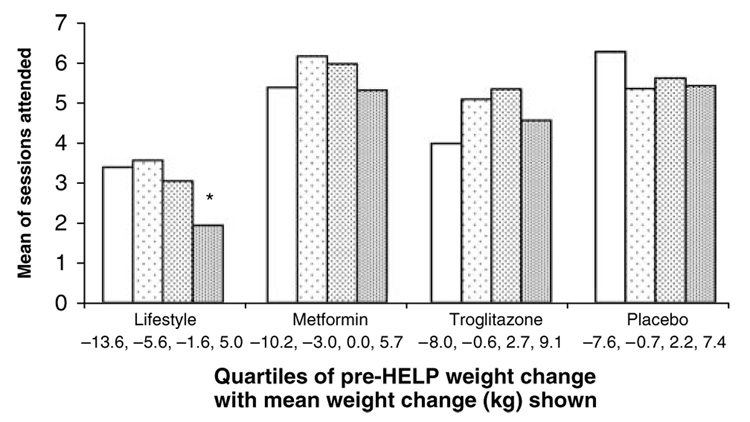
Figure 2 shows the average number of HELP sessions attended within the quartiles of pre-HELP weight change for each of the four originally randomized treatment groups. The data indicate that the amount of previous weight change during DPP, before the initiation of HELP sessions, did not predict session attendance in the program for the metformin, placebo or troglitazone groups (P>0.05). However, in the lifestyle group, weight change before beginning HELP sessions did have a significant association with a lower rate of sessions attended (P<0.05). Lifestyle participants who had gained the most weight since their DPP baseline (an average of 5.0 kg) had the lowest rate of participation.
Figure 2.
HELP session participation (out of 16) by pre-HELP weight change. From left to right, the four bars in each treatment group represent participant quartiles of pre-HELP weight change (kg), from greatest weight loss to greatest weight gain, by the mean of sessions attended out of 16. The average of pre-HELP weight change in each quartile is shown at the bottom. Weight change before beginning HELP was not associated with joining for MET, PLAC, TROG (NS; P>0.05). *Lifestyle participants who had gained the most weight since baseline (average=5.0 kg) had a significantly lower rate of joining HELP (P<0.05) when compared with the participants in the first and second quartiles (average weight loss at 13.6 and 5.6 kg).
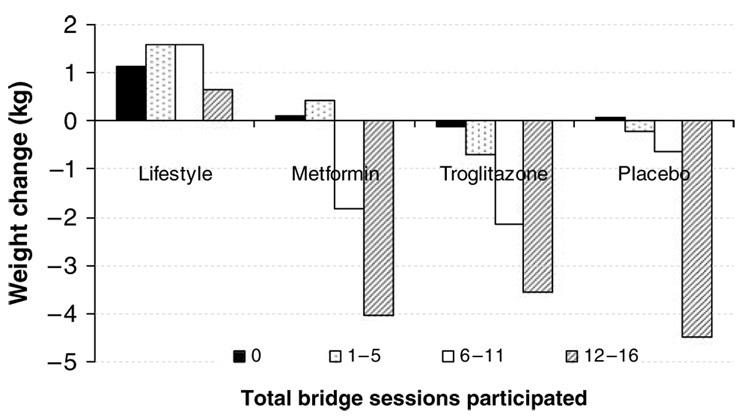
Figure 3 presents quartiles of session attendance (0, 1–5, 6–11 or 12–16) in relation to weight loss during the HELP sessions for the four original treatment groups. It shows a significant increase in weight loss for each of the other three lifestyle-naive groups (MET, TROG and PLAC, P-value for linear trend <0.0001) that paralleled their increased level of participation. Attendance was not significantly related to weight loss outcome in the ILS group.
Figure 3.
Bridge participation and weight change. From left to the right, the four bars in each treatment group represent rate of HELP session participation (either 0, 1–5, 6–11, or 12–16) by weight change in kg.
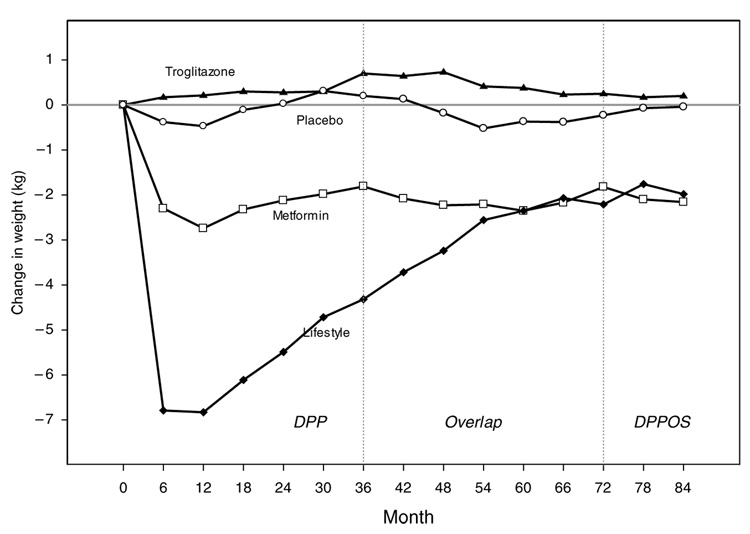
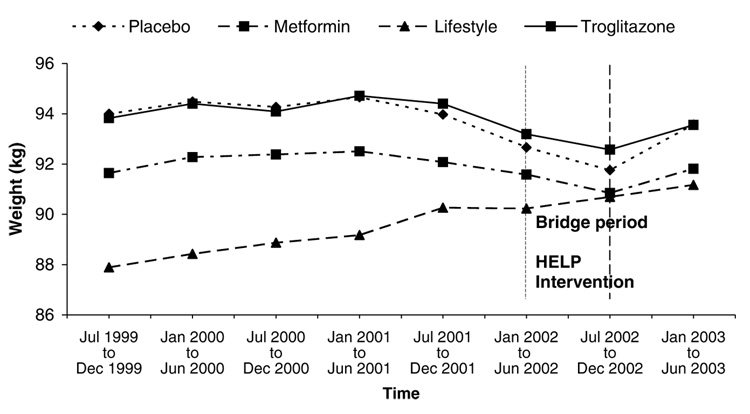
Figure 4 shows the longitudinal weight loss results from DPP baseline through the overlapping bridge period to the beginning of DPPOS by treatment group (ILS, MET, TROG and PLAC). Given that individual participant recruitment for DPP occurred over a 3-year time period (1996–1999) and does not reflect calendar time it is difficult to see the effect of the HELP program, which was implemented in a fixed 6-month period from January through July 2002. Figure 5, therefore, was constructed based on calendar time and examines the weight loss results from the last 6 months of 1999 (when all DPP participants were randomized) to the first 6 months of 2003 (when the majority of participants had moved through the transitional bridge period and had again signed new consents for DPPOS). This graph demonstrates a clear effect of the HELP group program in all groups except ILS, for whom it was a repeat exposure. Nonetheless, it should be noted that the average long-term weight loss for the DPP ILS group at the end of the HELP program (more than 5 years of follow-up on average), is still greater than any weight loss maintenance results previously reported in the literature. Average weight loss in the ILS group at this time was 2.69 kg with 26.1% of participants maintaining a 7% weight loss or better.
Figure 4.
Long-term weight change in DPP and beginning of DPPOS. Weight change per 6-month visit interval by the four DPP treatment groups are represented as follows: triangle—troglitazone; open circle—placebo; open square—metformin; diamond—lifestyle.
Figure 5.
Weight change by treatment group based on calendar time. Calendar time weight change (from the end of randomization) by the four DPP treatment groups are represented as: diamond with dotted line—placebo; square with dashed line—metformin; triangle with dashed line— lifestyle; square with solid line—troglitazone.
Discussion
This paper has described the adherence and weight loss outcomes when all participants in the DPP were offered a group lifestyle program during transition between the original intervention period and the ongoing DPPOS. It highlights the importance of session attendance and the differential effects of having previously been exposed to a similar weight loss program. Since the introduction nearly 40 years ago of behavioral modification for the treatment of obesity,13 lifestyle programs have undergone a number of changes in an effort to improve longer term weight loss outcomes. Core learning programs have been lengthened from 12 to as much as 24 weeks and have been extended further by periods of maintenance intervention.1 The DPP Healthy Lifestyle Program (HELP) described in this paper was patterned on the 16 week, individually administered intervention delivered to the intensive lifestyle group before the DPP trial was terminated and after treatment results were unmasked. Subsequently, all trial participants were offered the program in a group format, including the lifestyle group who had previously received it, because it was considered ethically appropriate to do so. The program was not intended to be a long-term maintenance intervention per se, nonetheless HELP represented a second bout of treatment for the ILS group, that could be compared with an initial bout of treatment for three of the DPP groups (MET, TROG, PLAC).
As previous studies have shown,1 being adherent to treatment sessions over time improves weight loss outcome. Not surprisingly we confirm this by showing that those participants who were naive to the lifestyle intervention and stayed in the HELP program for more sessions, lost more weight, although not as much as the individually treated lifestyle group did during DPP. Among those attending 12–16 sessions, weight loss was 3.5–4.5 kg compared with 0.5–2.2 kg in those attending 6–11 sessions and 0.2–1.8 kg for those with only 1–5 weeks of attendance.
There is limited data on whether overweight individuals will volunteer to participate in known treatment programs a second time around, and, if so, how they will fare in such programs. Does the old adage, ‘If at first you don’t succeed, try, try again’ apply to individuals struggling with weight management? It would appear so, given the perennial search for new ways to lose weight, after periods of regain, which Polivy and Herman14 have described as the ‘false hope syndrome’. However, in this study, it is clear those who had already experienced the lifestyle program differed from those who had not in their efforts at joining, engagement and weight loss success.
First, those who had used the program previously had a much lower rate of joining again. From 48 to 58% of the naive DPP participant groups signed up for the HELP program, compared with 40% of those from the ILS group and they were significantly less likely to participate in the full number of sessions available. Furthermore, in the ILS group it was those participants who had gained the most weight since the DPP started who were the least likely to try again. The naive participants lost weight whereas those from the original lifestyle group gained weight, on average, regardless of how many sessions they attended. Only one other report that we know of, using very low calorie diets,14 indicated that less than 50% of a treatment cohort is likely to volunteer for a second round of treatment.
It should be noted that the average weight loss achieved by lifestyle participants who completed 16-sessions the first time around, was considerably more than that was lost by any of the other groups during the HELP program.9 Individualized scheduling in the DPP trial gave participants in the lifestyle arm a wider range of time in which to complete the 16 sessions of the program and in fact the original treatment period was about 24 weeks in duration. Additionally, a limitation of this study is that the weight outcome data for the HELP program is limited by the fact that the weight measures used were taken as much as 7 months before and after the delivery of the 16 session program and may not represent the acute effects of the intervention.
In summary, for the ILS group, it appears that a repeat bout of the core behavioral sessions, and in the company of participants experiencing the process for the first time, is neither inviting nor effective. Participants who regained the most weight since their initial intervention began are the least likely to return, once again highlighting a basic conundrum of care management that those who need the help most are often the least likely to pursue it. Long-term models for weight management must find novel methods to engage participants over time beyond simply repeating the core behavioral treatment program. Participants may perceive that they have already learned the basic training material, they may be discouraged by the experience of weight regain, or they may be content with their current weight status. In any case they may see little value in continuing. Previously adherent participants who are experiencing regain have commented, ‘I know exactly what I need to be doing to lose weight and stay active, I just need to be doing it’.
Finally, participants who have started out with a one-on-one lifestyle treatment relationship and a regimen of individually scheduled follow-up visits may be reluctant to switch completely to group-based format. Future investigations should examine flexible, tailored regimens that allow for continued contact and support (for example, individual and/or group visits, phone, mail, web-based contacts), gain a better understanding of the process of treatment fatigue and experiment with methods for weight loss reinvigoration.
Acknowledgments
Supported by NIH U01DK.
References
- 1.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12 suppl:51S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR, Phelan S. Long term weight loss maintenance. Am J Clin Nutr. 2005;82 suppl:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 5.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psych. 2001;69:722–726. [PubMed] [Google Scholar]
- 6.Milsom VA, Perri MG, Rejeski WJ. Guided group support and the long term management of obesity. In: Latner JD, Wilson GT, editors. Self-help Approaches for Obesity and Eating. New York: Disorders: Research and Practice Guilford Press; 2007. pp. 205–222. [Google Scholar]
- 7.Wing RR, Tate DF, Gorin AA. A self-regulation program for maintenance of weight. N Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 8.Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–359. doi: 10.2337/diacare.21.3.350. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Prevention Program Research Group. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann EL. Nonparametric Statistical Methods Based on Ranks. New York: McGraw-Hill; 1975. [Google Scholar]
- 13.Stuart RB. A three-dimensional program for the treatment of obesity. Behav Res Ther. 1971;9:177–186. doi: 10.1016/0005-7967(71)90003-9. [DOI] [PubMed] [Google Scholar]
- 14.Polivy J, Herman CP. If at first you don’t succeed. False hopes of self-change. Am Psychol. 2002;57:677–689. [PubMed] [Google Scholar]