Abstract
Studies examining psychophysiologic markers of infant emotional development abound. However, few studies have used skin conductance (SC), though it measures sympathetic activity, and none have measured SC on infants sitting up and actively engaged with another person, a significant challenge given the measures sensitivity to movement artifact. In this pilot/feasibility study, we present a procedure for measuring infant SC during active engagement with another person who executes a series of startling claps to elicit an SC response. We measured SC on the plantar surface of the foot of 17 five-month old infants. We found unconditioned SC responses that were related to the intensity of physical startle reactions for each clap trial. We also found anticipatory, conditioned SC responses that occurred within 5s before each clap that occurred when the researcher raised his clasped hands. These conditioned SC responses grew linearly in intensity over trials. We conclude that SC may be a useful addition to the infant researcher’s armamentarium and may indeed be used to measure physiologic reactivity in infants even when actively engaged with another person. Addition of SC measurement to research on infant emotion and emotional communication is likely to advance our understanding of the psychophysiologic foundations of infant emotional development.
Keywords: Infants, Psychophysiology, Skin Conductance, Startle
Introduction
The measurement of skin conductance (SC), also known as electrodermal activity (EDA), began over 100 years ago (Dawson, Schell, & Filion, 2000) and continues to be used in psychophysiologic research. SC measures the electrical conductivity of the skin, which changes in relation to eccrine sweat gland activity. These sweat glands are innervated by the sympathetic nervous system. Thus, SC provides a measure of sympathetic arousal and response (Dawson et al, 2000). It is also possible to monitor direct sympathetic nervous system activity by using Laser Doppler monitoring of changes in microcirculation instead of electrical conductivity (Wallin, 1990). However, microcirculation is very influenced from environmental temperature and is therefore not suitable to use in clinical practice. SC activity can signify a wide variety of neurophysiologic processes (e.g., thermoregulation, deep breathing, general affective processes, orienting, attention, and increased muscle tone). Of particular significance for psychophysiologic studies, SC is related to emotional arousal and anxiety (Dawson et al., 2000). Measurement of SC could complement other widely-used physiologic indices, such as salivary cortisol and respiratory sinus arrhythmia. Salivary cortisol is an estimate of HPA-axis function, which is a central component of the neuroendocrine system that regulates the body’s stress response (Stansbury & Gunnar, 1994). Respiratory sinus arrhythmia (RSA) is an aspect of heart rate variability that provides an estimate of parasympathetic activity and tone, another important component of the body’s stress response system (Porges, Doussard-Roosevelt, & Maiti, 1994). Adding SC to these measures of reactivity would enrich our understanding of the physiologic underpinnings of emotion regulation by adding a measure of sympathetic activity. Such an addition would be especially important because different components of the psychophysiologic system are not tightly coupled to each other or to other systems (Bauer, Quas, & Boyce, 2002) and their relations and their development are not well understood, which effectively constrains our understanding of arousal and emotion as they unfold over time in different systems (Dawson et al., 2000; Stern, Ray & Quigley, 2001).
There have been few studies using SC in psychophysiologic research on infant emotion (also noted by Fowles, Kochanska, & Murray 2000). Moreover the reasons for its lack of use are not fully understood but there is a general sentiment in the field that SC measurement is too difficult with infants (personal communication, Rachel Keen and Stephen Porges, 2006). Gladman and Chiswick (1990) studied mean SC levels in newborns and found higher SC levels when infants were awake versus asleep and after heel pricks. Storm and her colleagues (Hellerud & Storm, 2002; Storm 2000, 2001) reported that preterm and term infants produced significant SC responses to heel pricks that correlated with their basic behavioral states (e.g., sleep, awake and calm, awake and very active, or crying) and that even non-painful tactile stimulation from routine nursery handling (e.g., lifting, changing diapers or washing with a cloth) produced SC responses equal to or higher than those in response to painful stimuli. In addition they found that in full-term, healthy infants SC responses to loud noises continue to mature during the first 10 weeks of life, at which time the SC response profile becomes similar to adults (Hernes, Morkrid, Fremming, Odegarden, Martinsen, & Storm, 2002). A limitation of all these studies is that they did not measure SC in more ecologically valid situations relevant to the study of infant emotions, particularly in the context of human interaction. For example, much of infant emotion research involves the infant sitting upright, facing an engaged adult, and sharing, communicating and conjointly regulating affective states (Tronick, 1989).
Thus, this paper presents data to support that SC can be reliably and validly measured in 5-month old infants sitting up and actively engaged with another person. SC responses were measured in response to a series of startling claps executed by a researcher sitting in front of the infant. We also wanted to see if we could overcome the methodological problem of movement artifact, which is a common concern in physiologic studies, but is further exacerbated because infants are very active and cannot be instructed to stay still. Thus, this feasibility study represents an important initial step in developing a way to introduce the measurement of SC into the field of infant emotion research.
Methods
Participants
A total of 30 5-month old infants were recruited. The first 11 infants were used to solve technical problems regarding the best type of electrodes and their placement to minimize movement artifact and maximize infant tolerance for them. Data for two other infants were lost to technical problems with data collection. Therefore, a total of 17 infants (11 males) provided usable data for the clap startle paradigm.
All infants were full-term, products of uneventful pregnancies, and clinically normal at birth. Mean age for mothers was 33 years (SD = 5). Maternal ethnicity was diverse (6 American Caucasians, 4 Western Europeans, 3 African Americans, 2 Asians, 2 Caribbeans, 1 African, and 1 Puerto Rican).
Physiological Measure
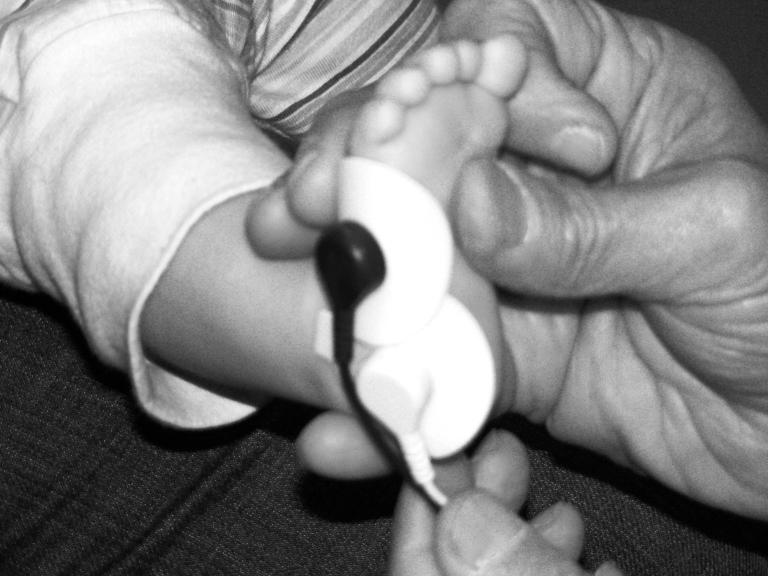
SC data was collected and analyzed using PowerLab 8SP and Chart 4.2 (ADInstruments, Sydney, Australia) running on a PC-based laptop computer. The PowerLab uses dry metal, finger electrodes to measure skin conductance in adults. However, these electrodes do not fit infant fingers or any other part of the infant’s body and are very sensitive to movement artifact. Therefore, AdInstruments created new leads which were fitted with disposable, self-adhesive, pre-gelled, AgAg-Cl snap electrodes with circular contact areas 1 cm in diameter (Biopac Systems, Inc., product no. EL507). We experimented with different electrode placements (e.g., palmer surface [too much artifact], forehead [too weak a signal], thighs [too much artifact and a weak signal]) and chose the plantar surface of the infant foot (heel and outer edge; see figure 1). To increase the stability of electrode contact, we also secured the electrodes with an Ace® brand self-adhering 2-inch bandage. The leads emerged from the wrap at the heel of the infant and ran under the seat of the chair so they were out of sight. The wrapping also did not draw the attention of the infants.
Figure 1.
Placement of SC electrodes on infant foot.
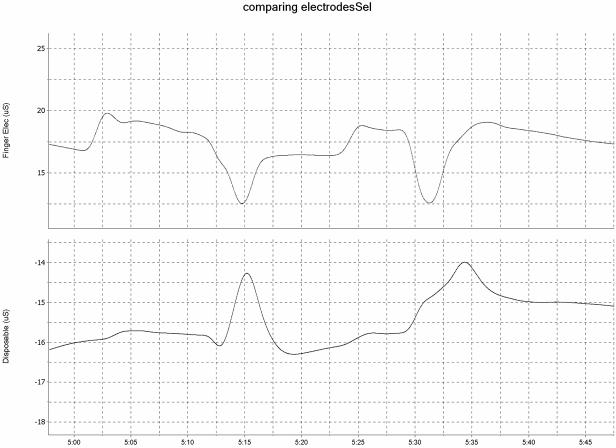
We compared the disposable, adhesive electrodes with the original metal electrodes by attaching each to a separate infant foot and recording simultaneously from each lead. Measurement was collected using one recording instrument with two channels so as to avoid equipment interference. In figure 2, the top image provides an example of movement artifact created by the original electrodes, which is represented by a sudden drop in SC level, and the bottom image shows the recording from the disposable, adhesive electrode made at the same time with the same infant.
Figure 2.
Simultaneous Recording using Standard Metal Electrodes (Top) and Disposable, Wet Electrodes (Bottom)
Procedures
Infants were recruited through a database of families at the Child Development Unit of Children’s Hospital Boston. Families were called during or immediately before the infant’s fifth month of age. Upon arrival to the lab, families were given the opportunity to settle into the waiting room and insure that the infant was comfortable and alert. During this time, study procedures were described to mothers and informed consent was obtained. Mothers were told that there was no risk to her or her infant, that her infant may cry or fuss during the protocol, and that she could stop the study at any time if she or her infant were too uncomfortable to continue. All mothers signed informed written consent, and the Institutional Review Board of Children’s Hospital Boston approved this study.
Next, mother and infant were escorted into the observation room. The infants were then strapped into a car seat secured to a table. SC electrodes were placed on the infant’s foot and secured, while mothers distracted the infant. Placement of the SC electrodes and wrapping took about 30 seconds and none of the infants were distressed by the procedure. Video cameras and physiologic software were then started and synchronized.
Clap Startle Paradigm
Once set-up was complete and the infant was in an alert state, one of the authors sat in front of the infant and made one sudden hand clap for up to five trials. For each clap trial, the researcher raised his hands in a clasped position in the infant’s line of vision, slowly spread them apart and clapped. Each trial lasted about five seconds. The next trial was begun after the infant calmed and/or his or her attention was captured again.
Infant behavioral and emotional responses were coded from the videotapes immediately after and within 5s of each clap trial. Responses were coded in two domains: Physical Startle, which rated the intensity of the infant’s observable startle reflex into three categories: (0) No Reaction, (1) Mild/Moderate Reaction (one or both arms may move or the head may move back, but the whole body does not), and (2) High Reaction (the whole body jerks along with the arms)); and (b) Distress, which rated the infant’s response to being startled into three categories: (0) no visible distress, (1) fussy or visibly distressed but still attentive, or (2 highly distressed and averting gaze). Two observers coded the reactions separately and then jointly reviewed and resolved disagreements.
SC responses were measured 5s before and 5s after each clap stimulus. SC responses were measured if they began within the 5s window. The amplitude of the SC response was measured as the difference between the peak of the SC response and the start of the initial rise of the response.
Statistics
Using the Mixed Models module of SPSS 12.0, random regression analysis was used to assess linear trends across trials because individual observations were clustered within infants. Random regression can also accommodate missing observations in trials. Nine of the 85 observations (11%) were missing. Three separate regression models were run using time of each trial as the independent variable and Conditioned Skin Conductance Response, Physical Startle, and Emotional Distress as the dependent variables. In this model, a statistically significant coefficient for trial time indicates a linear trend across trials. Four regression models were also run using Physical Startle and Distress as independent variables and Conditioned SC Response and Unconditioned SC Response as the outcome variables. We used a first-order autoregressive covariance structure for error terms in all regression models.
Results
Physical Startle Reactions and Emotional Distress to the Clap Stimuli
Table 1 shows the mean intensity ratings for physical startle reactions and emotional distress after each clap trial. It shows that clap stimuli produced physical startle reactions in infants that were larger for the first clap and diminished over trials. However, this trend was not statistically significant using mixed model regression analysis.
Table 1.
Mean (SD) values for all the dependent measures. Behavioral variables (Physical Startle and Emotional Distress) are mean ratings of the intensity of the startle reaction and emotional distress coded by observers. Skin Conductance (SC) variables represent mean response amplitudes in microsiemens (μs). F-statistic represents the significance of the linear pattern of the variable across clap trials using mixed model regressions with time of clap as the independent variable
| Clap 1 | Clap 2 | Clap 3 | Clap 4 | Clap 5 | F | |
|---|---|---|---|---|---|---|
| N | 18 | 18 | 17 | 17 | 11 | |
| Behavioral Variables | ||||||
| Physical Startle | 0.50 (0.79) | 0.28 (0.58) | 0.35 (0.70) | 0.24 (0.56) | 0.18 (0.41) | F(1,39.53)=0.00 |
| Emotional Distress | 0.11 (0.32) | 0.22 (0.55) | 0.24 (0.44) | 0.41 (0.62) | 0.27 (0.47) | F(1,28.25)=5.06* |
| Skin Conductance | ||||||
| Unconditioned SC Response (μs) | 0.25 (0.46) | 0.10 (0.18) | 0.25 (0.39) | 0.16 (.040) | 0.27 (0.50) | F(1,56.02)=0.11 |
| Conditioned SC Response (μs) | -- | 0.12 (0.24) | 0.22 (0.48) | 0.22 (0.50) | 0.39 (0.69) | F(1,28.43)=6.65* |
p<.05
For the ratings of emotional distress, most infants were not very distressed by the clap protocol. Nine of the 17 infants showed no visible distress or just one rating of it. Only two ratings of ‘highly distressed and gaze averting’ were made for all clap trials. ‘Fussy or visibly distressed but attentive’ was coded for 20% of the trials (16 of 81), and ‘no visible distress’ was rated for 78% of clap trials (63 of 81 total claps). Infant emotional distress after each clap trial progressively increased and the linear trend was significant in mixed model regression with time of clap as the independent variable (F(1,28.25)=5.06, p < .05).
Skin Conductance Responses to the Clap Stimuli
Before analyzing SC data, all SC graphs were visually scanned for any major signs of movement artifact, defined as sudden large drops in SC amplitude. There were none. Table 1 shows the mean amplitude of both unconditioned and conditioned SC responses for each clap trial. As expected, we found unconditioned SC responses across all clap stimuli, ranging in amplitude from 0 to 1.74 μs (mean (SD) = 0.20 (0.38) μs). There was no obvious linear pattern in unconditioned SC responses across clap stimuli. We also found conditioned SC responses that occurred when the researcher raised his clasped hands. These responses ranged in amplitude from 0 to 2.12 μs (mean (SD) = 0.17 (0.43) μs). These responses did appear to have a linear pattern of increasing amplitude across claps in mixed model regression with time of clap as the independent variable (F(1,28.43)=6.65, p < .05).
Relations between Behaviors and SC Responses
The intensity of physical startle reactions after each clap trial was positively related to the amplitude of unconditioned SC response (F(1,55.89) = 6.67, p < .05). Distress after each clap was not related to either conditioned or unconditioned SC responses.
Discussion
In this initial pilot study, we demonstrate that skin conductance can indeed be reliably and validly measured in infants who are sitting up and actively engaged with another person. By placing electrodes on the plantar surface of the infant’s foot and wrapping them with Ace® Wrap bandages, we were able to minimize movement artifact, a significant deterrent which had undermined earlier work using SC. We found that five-month old infants produced unconditioned SC responses to startling claps made by a researcher sitting in front of them. We also found that infants developed conditioned SC responses that anticipated the claps and were paired to when the researcher raised his clasped hands in preparation to clap. The amplitude of the unconditioned and conditioned SC responses were generally comparable to the SC responses to loud auditory stimuli found by Hernes et al. (2002), who reported mean (SD) amplitudes of 2.62 (20) μs for six-month-old infants. With regards to the connection between behavioral/physical reaction and SC reaction, we found that the intensity of the infant’s physical startle reaction was positively related to the amplitude of the unconditioned SC response. However, infant emotional distress was not related to either unconditioned or conditioned SC responses.
There are limitations to this pilot study. The sample size is small. The clap is not a standardized stimulus. Nevertheless, for our purposes this pilot/feasibility study suggests that infant SC can be reliably and validly measured even with sitting infants actively engaged with another person and that issues such as movement artifact can be overcome. Furthermore, this study has demonstrated for us that measuring infant SC is feasible enough to add to other studies of infant emotional development and communication. We acknowledge that there are also other measures of the sympathetic nervous system, such as the pre-ejection period of cardiac activity (Alkon, Goldstein, Smider, Essex, Kupfer, Boyce, & MacArthur Assessment Battery Working Group, 2003; Quigley & Stifter, 2006), laser doppler flowmetry of cutaneous blood flow (Galland, Taylor, Bolton, & Sayers, 2000; Inwald, Hathorn, & Costeloe, 1996), and salivary alpha-amylase (Granger, Kivlighan, Blair, El-Sheikh, Mize, Lisonbee, 2006). To the best of our knowledge, these measures have not all been validated with infants or have not been used with active infants in ecologically valid situations interacting with others. Thus, their utility in infant emotional research remains an empirical question. Given the fact that different individuals respond to stimuli differently and with different physiologic profiles (individual response stereotypy; Dawson et al., 2000; Salomon, Matthews, & Allen, 2000), it will be important to utilize many physiologic indices to capture the complex orchestration of physiologic responses that reflect the autonomic foundation of emotional expression and functioning, as suggested by Kutas and Federmeier (1998). We hope this study serves to invite others in the field of infant emotion to consider the use of SC to help advance our understanding of the psychophysiologic foundations of infant emotional development.
Acknowledgements
We thank Scott Orr for his guidance on collecting and measuring SC, Marina Fuertes for her help with developing and scoring the behavioral codes, and Ramin Mojtabai for his help with the statistical analysis. The study was undertaken as part of a post-doctoral research fellowship sponsored by the Clinical Research Training Program of Judge Baker Children’s Center, Boston, and was supported by an Institutional NRSA T32 MH 16259-26 (PI: Ed Tronick) from NIMH, the Sackler Foundation Scholarship for Research in Psychobiology from Harvard University (PI: Jacob Ham), and the Livingston Award from Harvard Medical School (PI: Jacob Ham).
References
- Alkon A, Goldstein LH, Smider N, Essex MJ, Kupfer DJ, Boyce WT, MacArthur Assessment Battery Working Group Developmental and contextual influences on autonomic reactivity in young children. 2003;42(1):64–78. doi: 10.1002/dev.10082. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multi-system approach. Journal of Developmental and Behavioral Pediatrics. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, editors. Handbook of Psychophysiology. 2nd Edition Cambridge University Press; Cambridge, U.K: 2000. pp. 200–223. [Google Scholar]
- Fowles DC, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37:777–787. [PubMed] [Google Scholar]
- Galland BC, Taylor BJ, Bolton DPG, Sayers RM. Vasoconstriction following spontaneous sighs and head-up tilts in infants sleeping prone and supine. Early Human Development. 2000;58(2):119–132. doi: 10.1016/s0378-3782(00)00070-0. [DOI] [PubMed] [Google Scholar]
- Gladman G, Chiswick ML. Skin conductance and arousal in the newborn. Archives of Disease in Childhood. 1990;65:1063–1066. doi: 10.1136/adc.65.10_spec_no.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Stroud LR, Schwartz EB, Handwerger K. Integrating the measurement of salivary a-amylase into studies of child health, development, and social relationships. Journal of Personal and Social Relationships, Special Issue: Physiology and Human Relationships. 2006;23:267–290. [Google Scholar]
- Hellerud BC, Storm H. Skin conductance and behaviour during sensory stimulation of preterm and term infants. Early Human Development. 2002;70:35–46. doi: 10.1016/s0378-3782(02)00070-1. [DOI] [PubMed] [Google Scholar]
- Hernes KG, Morkrid L, Fremming A, Odegarden S, Martinsen OG, Storm H. Skin conductance changes during the first year of life in full-term infants. Pediatric Research. 2002;52(6):837–843. doi: 10.1203/00006450-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Inwald D, Hathorn MKS, Costeloe K. The deep breath vasoconstriction reflex - a new tool for autonomic assessment in infancy? Early Human Development. 1996;45:55–61. doi: 10.1016/0378-3782(95)01713-5. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Minding the body. Psychophysiology. 1998;35:13–150. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2-3):167–186. [PubMed] [Google Scholar]
- Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43:357–365. doi: 10.1111/j.1469-8986.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- Salomon K, Matthews KA, Allen MT. Patterns of sympathetic and parasympathetic reactivity in a sample of children and adolescents. Psychophysiology. 2000;37(6):842–849. [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:108–134. [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. 2nd Edition Oxford University Press, Inc.; New York, NY: 2001. [Google Scholar]
- Storm H. Skin conductance and the stress response from heel stick in preterm infants. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2000;83:143–147. doi: 10.1136/fn.83.2.F143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm H. Development of emotional sweating in preterms measured by skin conductance changes. Early Human Development. 2001;62:149–158. doi: 10.1016/s0378-3782(01)00129-3. [DOI] [PubMed] [Google Scholar]
- Tronick EZ. Emotions and emotional communication in infants. American Psychologist. 1989;44(2):112–119. doi: 10.1037//0003-066x.44.2.112. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Neural control of human skin blood flow. Journal of the Autonomic Nervous System. 1990;30(Supplement):185–190. doi: 10.1016/0165-1838(90)90128-6. [DOI] [PubMed] [Google Scholar]