Abstract
It has been shown that mammalian cytochrome P450scc can metabolize vitamin D3 to 20-hydroxyvitamin D3 (20(OH)D3) and 20,22(OH)2D3. To define the biological significance of this pathway, we tested the effects of 20(OH)D3 on the differentiation program of keratinocytes and on the expression of enzymes engaged in vitamin D3 metabolism. Immortalized HaCaT and adult human epidermal keratinocytes were used as a model and the effects of 20(OH)D3 were compared with those of 25(OH)D3 and 1,25(OH)2D3. 20(OH)D3 inhibited proliferation and caused G2/M arrest. 20(OH)D3 stimulated involucrin and inhibited cytokeratin 14 expression. The potency of 20(OH)D3 was comparable to that of 1,25(OH)2D3. 20(OH)D3 decreased the expression of cytochrome P450 enzyme (CYP)27A1 and CYP27B1, however, having only slight effect on CYP24. The effect of 20(OH)D3 was dependent on the vitamin D receptor (VDR). As shown by electrophoretic mobility shift assay, 20(OH)D3 stimulated the binding of nuclear proteins to the VDRE. Transfection of cells with VDR-specific siRNA decreased 20(OH)D3-stimulated transcriptional activity of the VDRE promoter and the expression of involucrin and CYP24 mRNA. Therefore, the above studies identify 20(OH)D3 as a biologically active secosteroid that induces keratinocyte differentiation. These data imply that the previously unreported pathway of vitamin D3 metabolism by P450scc may have wider biological implications depending, for example, on the extent of adrenal gland or cutaneous metabolism.
INTRODUCTION
Recent studies have revealed that mammalian cytochrome P450scc (cytochrome P450 enzyme (CYP)11A1), in addition to its role in the conversion of cholesterol to pregnenolone for steroid synthesis, can also metabolize vitamins D2 and D3, as well as their provitamin precursors ergosterol and 7-dehydrocholesterol (Guryev et al., 2003; Slominski et al., 2004, 2005a, b, 2006). P450scc converts vitamin D3 to 20-hydroxyvitamin D3 (20(OH)D3) and di- and tri-hydroxyvitamin D3 in a sequential and stereospecific manner, with the initial formation of 20(OH)D3 (Slominski et al., 2005b). 20(OH)D3 is the major product of the reaction, indicating that it can be released from the active site of the enzyme, with only a minor portion remaining (or rebinding) for further hydroxylation. It is also the only product of vitamin D3 hydroxylation detected in incubations of isolated adrenal mitochondria. Thus, in organs expressing high levels of P450scc, such as adrenal cortex, corpus luteum, follicles, and placenta, production of 20(OH)D3 could possibly have systemic effects, whereas in organs expressing low levels of P450scc, such as skin (Slominski et al., 2004), it could serve local para-, auto-, or intracrine roles.
In humans, vitamin D3 is derived predominantly from its precursor 7-dehydrocholesterol, which is localized to the plasma membrane of basal epidermal keratinocytes (Holick, 2003b). The UVB component (290–320 nm) of solar radiation induces 7-dehydrocholesterol photolysis to previtamin D3, which then undergoes internal rearrangement to form vitamin D3 at normal skin temperature (Holick et al., 1980; Holick, 2003b). After entering the circulation, vitamin D3 can be hydroxylated in the liver to 25-hydroxyvitamin D3 (25(OH)D3) by mitochondrial CYP27A1 (Holick, 2003b; Feldman et al., 2005). Several microsomal cytochromes P450 (CYP2R1, CYPC11, CYP3A4, CYP2D25, and CYP2J3) also exhibit 25-hydroxylase activity on vitamin D3 (Ohyama and Yamasaki, 2004; Prosser and Jones, 2004). 25(OH)D3 is then hydroxylated in the kidney to the active form, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), by mitochondrial CYP27B1 (Feldman et al., 2005). At the cellular level, 1,25(OH)2D3 binds to specific vitamin D receptors (VDRs) that heterodimerize with the retinoid X receptors. Receptor–vitamin complexes affect expression of genes that have vitamin D response elements (VDREs) in their promoter (Christakos et al., 2003). 1,25(OH)2D3 is also synthesized locally by epidermal keratinocytes, which contain both CYP27A1 and CYP27B1 (Bikle et al., 1986; Lehmann, 2005; Bikle, 2006; Holick, 2006). The 1α-hydroxylase activity required to convert 25(OH)D3 to 1,25(OH)2D3 has also been detected in many other peripheral tissues (Bikle et al., 1986; Lehmann, 2005). CYP24 hydroxylates 1,25(OH)2D3 as well as 25(OH)D3 to yield metabolically inactive products in the kidney or in a plethora of peripheral tissues (Ohyama and Yamasaki, 2004; Ebert et al., 2006). 1,25(OH)2D3 stimulates CYP24 gene expression and inhibits the expression of both CYP27B1 and CYP27A1 genes (reviewed by Feldman et al., 2005; Bikle, 2006; Ebert et al., 2006; Holick, 2006).
Although the biological role of 20(OH)D3 is unknown, it is well documented that 1,25(OH)2D3 and its derivatives have immune and neuroendocrine activities, and tumorostatic and anticarcinogenic properties, affecting proliferation, differentiation, and apoptosis in cells of different lineages, and protecting DNA against oxidative damage (Wiseman, 1993; Holick, 2003a, b; Bikle, 2004). 1,25(OH)2D3 and its derivatives also have significant local actions on the formation and functional differentiation of adnexal structures and the epidermis, modulation of skin immune system, and protection against UVB-induced DNA damage (Holick, 2000, 2003a, b; Bikle et al., 2001; Bikle, 2004). For example, 1,25(OH)2D3 is recognized as an inhibitor of keratinocyte proliferation and as a stimulator of keratinocyte differentiation (Bikle, 2004; Bikle et al., 2004). It is known to induce both G1/0 and G2/M arrest in keratinocytes (Kobayashi et al., 1994; Dai et al., 2004). Keratinocyte differentiation is characterized by step-by-step morphological changes, which are accompanied by gradual changes in the expression of genes coding for constituents of the keratinocyte cytoskeleton and accessory envelope cross-linkage-related proteins (Bikle, 2004; Eckert et al., 2004). Early keratinocyte differentiation involves a decrease in the expression of cytokeratins 5 and 14, and increased expression of cytokeratins 1 and 10, and involucrin.
Thus, the information listed above has mandated testing for the phenotypic activity of 20(OH)D3 in comparison to the well-defined biological effects of 1,25(OH)2D3. As a model, we used immortalized human HaCaT keratinocytes and primary cell lines of adult human epidermal keratinocytes. Effects of 1,25(OH)2D3 on both the expression of genes involved in its metabolism and, as mentioned above, in the differentiation program, have previously been studied in these cells (Bikle, 2004; Bar et al., 2007). We also compared the effects of 20(OH)D3 with those of 25(OH)D3 to establish the role of the hydroxy group at C20, as opposed to C25, on biological activity.
RESULTS AND DISCUSSION
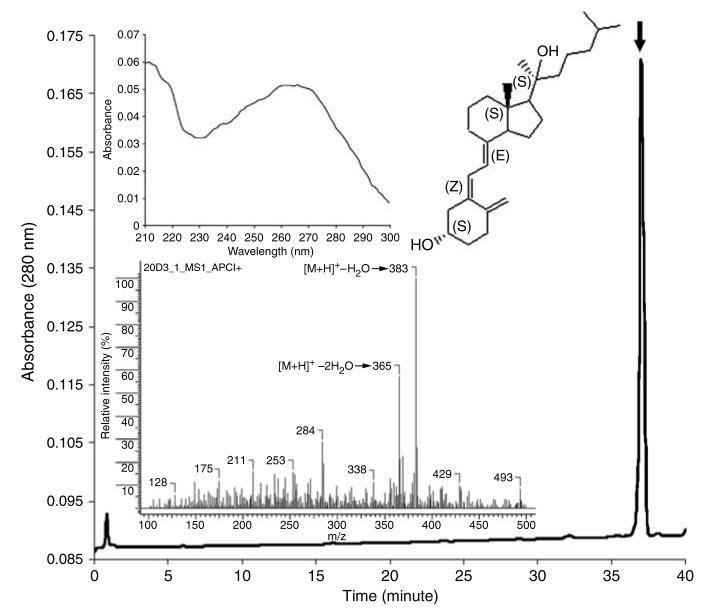
20(OH)D3 was obtained by enzymatic hydroxylation of vitamin D3 by bovine cytochrome P450scc, as described previously (Slominski et al., 2005b). The product identity and purity was confirmed by reversed-high-performance liquid chromatography (HPLC), UV spectrometry, and mass spectroscopic analyses (Figure 1). Specifically, 20(OH)D3 was eluted as a single peak with retention time of 37 minutes and UV spectrum with maximum at the 260–270 nm region characteristic for vitamin D3 derivatives. The predicted molecular mass of 20(OH)D3 (400 Da) corresponded to detected ions at m/z 383 ([M+H]+−H2O) and m/z 365 ([M+1]+−2H2O).
Figure 1. Identity and purity of 20(OH)D3 used for biological testing.
The final reversed-phase-HPLC product with a retention time of ∼37 minutes (thick arrow) had UV (top inset) and mass spectra (lower inset) characteristic of 20(OH)D3. The structure of this compound was previously defined by nuclear magnetic resonance analysis as 20(OH)D3 (5Z, 7E-9,10-seco-5,7,10(19)-cholestatriene-3,20S-diol) (see inset) (Slominski et al., 2005b).
20(OH)D3 inhibits proliferation of epidermal keratinocytes
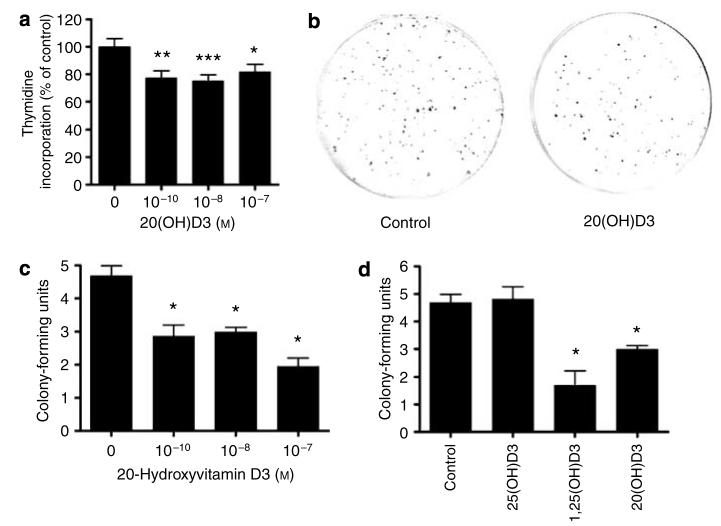
HaCaT keratinocytes were incubated for 48 hours in DMEM medium. DNA synthesis was then measured with a [3H]thymidine assay. As shown in Figure 2a, 20(OH)D3 inhibited DNA synthesis at the concentrations of 10−8 and 10−7 m. To further define the antiproliferative effect of the ligands, HaCaT keratinocytes were incubated for 10 days in DMEM in the presence or absence of vitamin D3 hydroxy derivatives and colony-forming potential was measured. As shown in Figure 2b and c, 20(OH)D3 inhibited colony formation by HaCaT cells. 20(OH)D3 at 10−8 m inhibited colony formation by 36% and at 10−7 m by 58%. 1,25(OH)2D3 at 10−8 m inhibited colony formation by 64%, whereas 25(OH)D3 had no significant effect (Figure 2d). Thus, 20(OH)D3 shows antiproliferative potency comparable to but lower than 1,25(OH)2D3.
Figure 2. 20(OH)D3 inhibits keratinocyte proliferation.
HaCaT keratinocytes were treated for 48 hours in DMEM containing 5% charcoal-treated FBS. [3H]Thymidine was added for last 12 hours of incubation and then DNA synthesis was assessed (a). HaCaT keratinocytes were cultured for 10 days in DMEM containing 5% charcoal-treated FBS. Then colonies were fixed, stained with crystal violet, and counted. Representative pictures of HaCaT treated with 20(OH)D3 at 10−8 m or controls are shown (b). Graphs showing the dose-dependent effect (c) and comparison with the effects of other secosteroids at 10−8 m (d) are presented. Data are presented as mean±SEM ([3H]thymidine assay, n=36; colony-forming assay, n=4); *P<0.05, **P<0.005, and ***P<0.0005.
Next, we tested the effect of 20(OH)D3 on normal epidermal keratinocytes using the technique of flow cytometry. The cells were seeded into Petri dishes and, after 48 hours of treatment with 20(OH)D3 or vehicle, were collected, fixed, stained with propidium iodide, and submitted for flow-cytometric analysis. Control cells were distributed as follows: 37±10% in G1/0, 38±14% in S, and 25±6% in the G2/M phase of the cell cycle (n=3). Treatment of cells for 24 hours with 10 nm 1,25(OH)2D3 resulted in significant G1/0 (52±2%, P<0.05) and G2/M (35±5%, P<0.05) arrests (S phase: 13±7%, P<0.05). Similarly, treatment of cells for 48 hours with 10 nm 20(OH)D3 resulted in G1/0 (52±5%) and G2/M (35±8%, P<0.05) arrests (S phase: 13±12%, P<0.05).
20(OH)D3 affects the expression of genes involved in keratinocyte differentiation
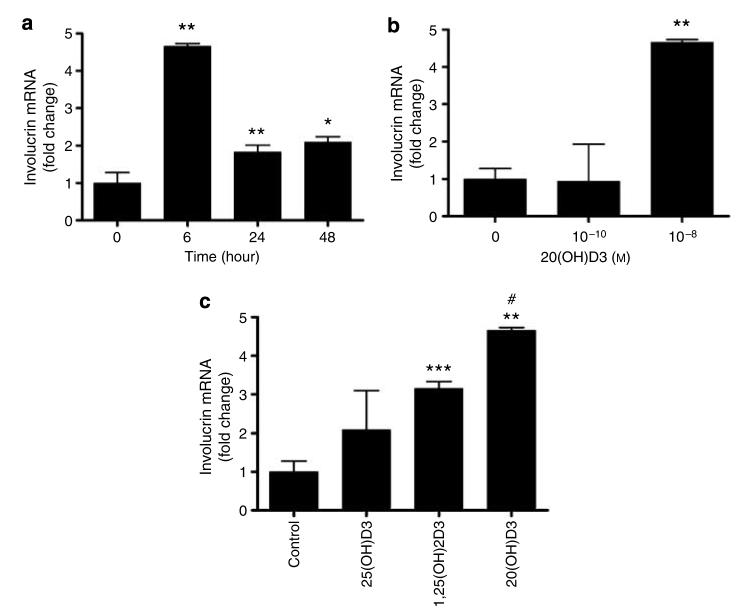
Since 1,25(OH)2D3 is a recognized stimulant of keratinocyte differentiation and its effects on the expression of proteins engaged in the formation of mature keratinocyte phenotype have been studied widely (Bikle, 2004), we compared the action of 20(OH)D3 with that of 1,25(OH)2D3 on the expression of involucrin and cytokeratin 14 genes in normal epidermal keratinocytes. 20(OH)D3 inhibited the expression of cytokeratin 14 and stimulated the expression of involucrin in a dose- and time-dependent manner. 20(OH)D3 at 10−10 m inhibited the expression of cytokeratin 14 mRNA. The effect was maximal 1 hour after treatment (for example, decreased to 45% of the control value) and started to fade at 6 hours, reaching 62% of the control. The inhibitory effect was significant at both 10−10 and 10−8 m concentrations. Of note, 20(OH)D3 showed significantly higher inhibitory effect on cytokeratin 14 mRNA expression than the 1,25(OH)2D3. As shown in Figure 3, 20(OH)D3 (at 10−8 m but not at 10−10 m) stimulated the expression of involucrin mRNA. The effect was maximal at 6 hours when 4.7-fold stimulation was observed, and started to fade by 24 hours when stimulation was only 1.8-fold (Figure 3a). Again, 20(OH)D3 has higher potency in inhibiting involucrin mRNA expression in comparison with 1,25(OH)2D3 (∼4.7- versus ∼3.1-fold stimulation, P<0.05) (Figure 3c). 25(OH)D3 increased the expression of involucrin ∼2.1-fold; however, the effect was statistically insignificant (Figure 3c).
Figure 3. 20(OH)D3 stimulates the expression of involucrin mRNA in normal human epidermal keratinocytes.
Keratinocytes were incubated in EpiLife medium containing EpiLife defined growth supplement with either 20(OH)D3, 25(OH)D3, or 1,25(OH)2D3, then lysed, and total RNA was extracted and reverse transcribed. Involucrin mRNA levels were measured with reagent Hs00846307_s1 according to the manufacturer's protocol (Applied Biosystems) and normalized to 18SrRNA content. Data are presented as mean±SEM (n=3). *P<0.05 versus control; **P<0.005 versus control; ***P<0.0005 versus control; and #P<0.05 versus 1,25(OH)2D3. (a) Time response (at 10−8 m). (b) Dose response (at 6 hours). (c) Comparison of vitamin D3 hydroxy derivatives (at 10−8 m and 6 hours).
20(OH)D3 stimulates involucrin production and increases keratinocyte size and granularity
Having established that 20(OH)D3 acts at the transcriptional level, we examined whether the changes in the gene expression were reflected in the keratinocyte differentiated phenotype. We thus measured the expression of involucrin using both flow cytometry and fluorescence microscopy. As shown in Figure 4, treatment of HaCaT keratinocytes with 20(OH)D3 at 10−8 m for 24 hours resulted in an ∼3.7-fold increase in the expression of involucrin (control differential mean fluorescence intensity 19±9; treatment differential mean fluorescence intensity 71±8, n=4, P<0.05). Interestingly, the effects of 1,25(OH)2D3 and 25(OH)D3 were negligible in this experiment. Moreover, we measured the effects of 20(OH)D3 on forward and side scatter of cells, parameters that reflect cell size and granularity, respectively. Both parameters are known to increase during keratinocyte differentiation. Mean signal intensity (MSI) of forward scatter in control cells was 235±7 and of side scatter was 175±7 (n=3). 20(OH)D3 at 0.1 nm significantly increased both forward (MSI: 251±2, P<0.05) and side scatter of HaCaT keratinocytes (MSI: 210±6, P<0.05). 1,25(OH)2D3 acted similarly but only the effect on side scatter was statistically significant (MSI: 219±4, P<0.05). 25(OH)D3 also increased both parameters, although only the effect on forward scatter was statistically significant (MSI: 254±0.06, P<0.05).
Figure 4. 20(OH)D3 stimulates the expression of involucrin in keratinocytes.
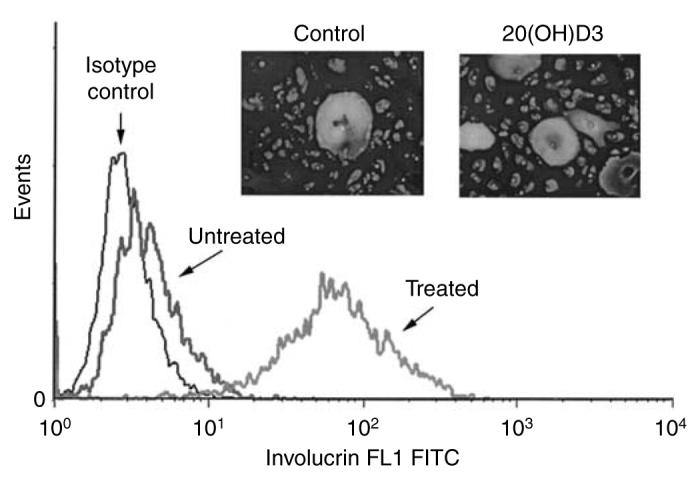
HaCaT keratinocytes were incubated for 48 hours in DMEM medium containing 5% FBS with 20(OH)D3 or vehicle and then fixed and stained with involucrin antibody followed by secondary antibody linked to FITC. Cells were then read with a flow cytometer as described previously. Data are presented as mean±SEM (n=4). Inset: Normal epidermal keratinocytes were treated with 20(OH)D3 or vehicle and then stained with anti-involucrin antibody followed by secondary antibody linked to FITC. Cells were photographed as described under Materials and Methods. Original magnification × 20.
Most of the actions of 1,25(OH)2D3 on keratinocyte differentiation we report here have been documented previously (Bikle, 2004). As far as the actions of 25(OH)D3 are concerned, we show that this compound generally acts similarly to 1,25(OH)2D3, albeit at lower potency. This agrees with previous studies, which show that 25(OH)D3 inhibits keratinocyte proliferation and stimulates keratinocyte differentiation (Itin et al., 1994; Pillai et al., 1996). This study shows for the first time that 20(OH)D3 has similar effects on programmed keratinocyte differentiation to 1,25(OH)2D3 and also has comparable potency.
20(OH)D3 inhibits the expression of CYP27B1 and CYP27A1 genes
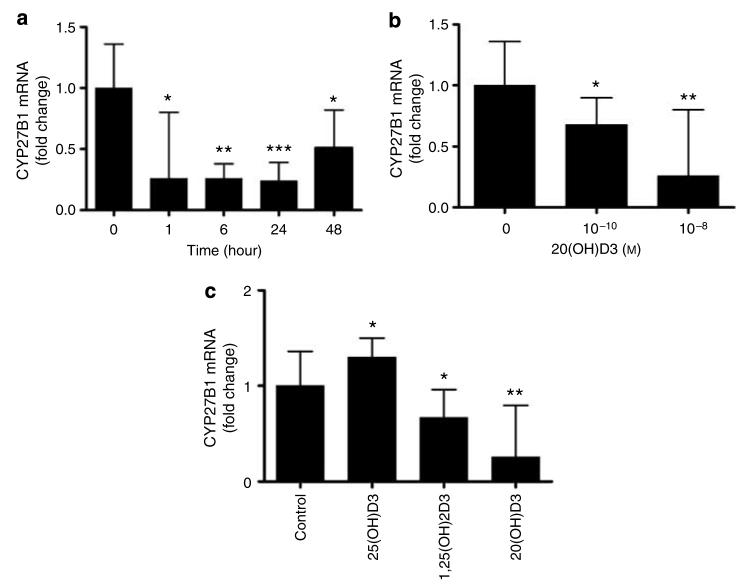
Since expression of CYP27B1 and CYP27A1 genes is inhibited by 1,25(OH)2D3 in the kidney and liver, respectively (Feldman et al., 2005; Holick, 2006), we compared the action of 20(OH)D3 with that of 1,25(OH)2D3 on the expression of these genes in normal epidermal keratinocytes. 20(OH)D3 inhibited the expression of CYP27B1 and CYP27A1 in a dose- and time-dependent manner (Figure 5). As shown in Figure 5, 20(OH)D3 at 10−8 m inhibited the expression of CYP27B1 ∼4-fold. The effect was detected at 1 hour after treatment, maintained thereafter, and faded at 48 hours when inhibition decreased to ∼2-fold (Figure 5a). Interestingly, 20(OH)D3 showed slightly higher potency than 1,25(OH)2D3 on CYP27B1 expression (for example, 1,25(OH)2D3 at 10−8 m inhibited gene expression by ∼1.5-fold) (Figure 5c). In contrast, 25(OH)D3 increased the expression of CYP27B1 by ∼1.3-fold.
Figure 5. 20(OH)D3 inhibits the expression of CYP27B1 mRNA.
Normal epidermal keratinocytes were incubated in EpiLife medium containing EpiLife defined growth supplement with either 20(OH)D3, 25(OH)D3, or 1,25(OH)2D3, then lysed, and total RNA was extracted and reverse transcribed. CYP27B1 mRNA levels were measured with reagent Hs00168017_m1 according to manufacturer's protocol (Applied Biosystems) and normalized to 18SrRNA content as described. Data are presented as mean±SEM (n=3). *P<0.05 versus control; **P<0.005 versus control; and ***P<0.0005 versus control. (a) Time response (at 10−8 m). (b) Dose response (at 1 hour). (c) Comparison of relative potencies (at 10−8 m and 1 hour).
20(OH)D3 at 10−8 m inhibited the expression of CYP27A1 gradually. The effect was observable at 1 hour after treatment, with maximum inhibition of ∼3.5-fold occurring at 24 hours. The effect started to fade at 48 hours when inhibition decreased to ∼2.6-fold. Similar to the effect on CYP27B1, the effect of 20(OH)D3 on CYP27A1 expression was also higher than that of 1,25(OH)2D3 (∼2.9-fold at 24 hours with 10−8 m 1,25(OH)2D3). 25(OH)D3 also decreased the expression of CYP27A1 by ∼1.9-fold.
Our results confirm the general actions reported for 1,25(OH)2D3 and 25(OH)D3 on CYP27B1 and CYP27A1 (Feldman et al., 2005; Bikle, 2006; Holick, 2006) using adult human epidermal keratinocytes. However, other researchers have reported that 1,25(OH)2D3 does not affect the expression of CYP27B1 and CYP27A1 in keratinocytes (Bar et al., 2007). This discrepancy might be explained by the fact that we studied adult keratinocytes as opposed to the neonatal keratinocytes used by Bar et al. Adult keratinocytes may have a fully developed apparatus for 1,25(OH)2D3 synthesis, metabolism, and regulation, and thus provide a better model for these studies than neonatal keratinocytes. Nevertheless, the major result of our studies is the demonstration for the first time that 20(OH)D3 can act as a potent inhibitor of both genes involved in 1,25(OH)2D3 synthesis.
20(OH)D3 has significantly lower potency on CYP24 transcription than 25(OH)D3 or 1,25(OH)2D3
Since CYP24 is an important physiological target of 1,25(OH)2D3 in the kidney and peripheral tissues, including skin (Holick, 2003b, 2006; Feldman et al., 2005; Lehmann, 2005; Bikle, 2006), we compared the action of 20(OH)D3 with those of 1,25(OH)2D3 and 25(OH)D3 on the transcriptional activity of the CYP24 promoter in normal epidermal keratinocytes. Normal epidermal keratinocytes were transfected with either a luciferase reporter construct driven by CYP24 promoter or a promoterless luciferase construct. Transcriptional activity of the CYP24 promoter was stimulated ∼21-fold, ∼12-fold, and only ∼2.5-fold by 1,25(OH)2D3, 25(OH)D3, and 20(OH)D3, respectively. None of these substrates affected the activity of the promoterless (pLuc) construct. Moreover, we tested the effect of 20(OH)D3 on the expression of CYP24 mRNA. As shown in Figure 6, 10 nm 20(OH)D3 at 24 hours increased CYP24 mRNA levels only 1.3-fold but at 10−6 m, ∼247-fold stimulation was observed. The effect on CYP24 was thus significantly weaker, since it required a much higher concentration of 20(OH)D3. The active form of vitamin D3 (1,25(OH)2D3) stimulates the expression of CYP24 in the kidney (Feldman et al., 2005), in cultured human neonatal keratinocytes (Bar et al., 2007), and in other skin and non-skin cells (Holick, 2003a; Lehmann, 2005; Bikle, 2006; Ebert et al., 2006). Our study confirms these findings and shows for the first time that 20(OH)D3 affects CYP24 transcriptional activity to a significantly lower degree in adult epidermal keratinocytes as compared with the potency of 1,25(OH)2D3 and 25(OH)D3. In regards to the effect at 10 nm, a slight discrepancy between the results obtained with the reporter assay and real-time PCR can be explained by a higher sensitivity of the promoter-driven construct in our system. In separate experiments using HaCaT keratinocytes, 20(OH)D3 failed to stimulate the CYP24 promoter activity, which indicates that the stimulatory effect depends on the type of cells used for the experiment. The above finding suggests that this previously unidentified compound (20(OH)D3) may play a minor role in regulating the inactivation of the active forms of vitamin D3, which is in opposition to its inhibitory actions on expression of CYP27A1 and CYP27B1 genes (see above). The discrepancy between the typical set of responses to 1,25(OH)2D3 and to 20(OH)D3 can be explained by different conformations of ligand–receptor complex eliciting different cellular responses. This biological mechanism has been documented in case of the peroxisome proliferatoractivated receptor-γ and its different ligands (Guo et al., 2006). Furthermore, 20(OH)D3 can be metabolized to different compounds, including di- and tri-hydroxyvitamin D3 that potentially may interact with the receptor (Slominski et al., 2005b).
Figure 6. 20(OH)D3 stimulates the expression of CYP24 mRNA in keratinocytes.
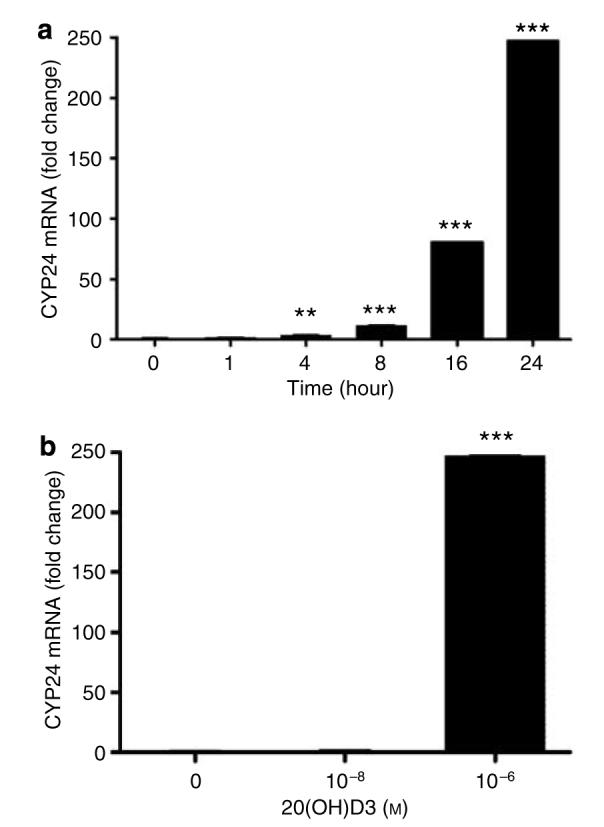
Normal epidermal keratinocytes were incubated in EpiLife medium containing EpiLife defined growth supplement with 20(OH)D3, then lysed, and total was RNA extracted and reverse transcribed. CYP24 mRNA levels were measured as described under Materials and Methods. Data are presented as mean±SEM (n=3). **P<0.005 versus control; and ***P<0.0005 versus control. (a) Time response (at 10−6 m). (b) Dose response (at 24 hours).
20(OH)D3 stimulates the VDRE and involucrin and CYP24 mRNAs through the VDR in HaCaT keratinocytes
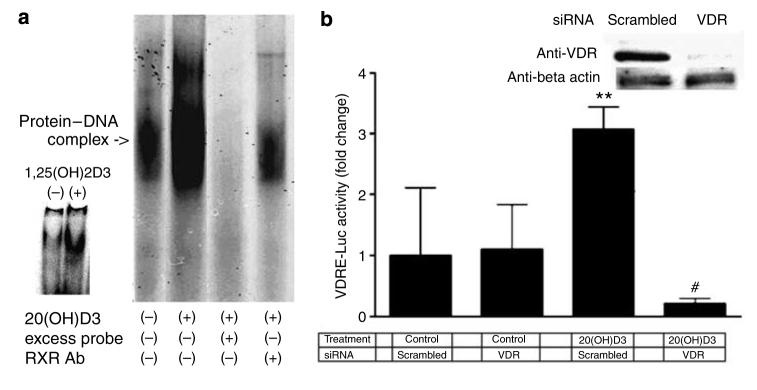
HaCaT keratinocytes were stimulated for 24 hours with 10 nm 20(OH)D3, and then nuclear extracts were prepared and incubated with labeled VDRE probe. As shown in Figure 7a, 20(OH)D3 stimulated the binding activity of protein complexes to the VDRE probe. The binding was specific since excess unlabeled VDRE caused complete disappearance of the signal. Moreover, addition of retinoid X receptor antibody resulted in a significant decrease in the signal, which provides evidence that the retinoid X receptor protein is part of a 20(OH)D3-stimulated protein complex that binds to the VDRE. As shown in the inset in Figure 7a, the positive control, 1,25(OH)2D3, stimulated binding of protein complexes to the VDRE.
Figure 7. 20(OH)D3 stimulates VDRE through the VDR in HaCaT keratinocytes.
(a) Cells were stimulated for 24 hours with 10 nm 20(OH)D3, and then nuclear extracts prepared and incubated with labeled VDRE. Arrows indicate the protein–DNA complex that contains the retinoid X receptor. The result is representative of three experiments. Inset: Control cells and cells stimulated with 1,25(OH)2D3. (b) Cells were transfected with VDRE-Luc and with scrambled or VDR siRNA, and then incubated for 24 hours with 10 nm 20(OH)D3 or with the vehicle (control). Data are presented as mean±SEM (n=4). **P<0.005, versus untreated control; #P<0.00005, versus scrambled siRNA and treatment with 20(OH)D3. Inset: Cells were transfected with scrambled or VDR siRNA and after 24 hours whole-cell lysates were prepared and expression of VDR and β-actin was assessed by western blot using the same amount of proteins.
Cells were transfected with VDRE-Luc and with scrambled or VDR small interfering RNA (siRNA). As shown in the inset of Figure 7b, VDR siRNA caused almost complete disappearance of VDR expression at the protein level 24 hours after transfection. Keratinocytes were then incubated with 10 nm 20(OH)D3 or the vehicle control for 24 hours. As shown in Figure 7b, transfection of HaCaT keratinocytes with VDR siRNA had no effect on basal VDRE-driven transcriptional activity (cells transfected with scrambled siRNA). Treatment of cells transfected with scrambled siRNA with 10 nm 20(OH)D3 resulted in ∼3-fold increase in VDRE-driven luciferase activity (P<0.005, versus control). On the other hand, transfection of keratinocytes with VDR siRNA decreased the 20(OH)D3-stimulated VDRE activity (P<0.00005). Of note, there was no statistical significance in transcriptional activity between cells transfected with scrambled siRNA and treated with the vehicle and cells transfected with VDR siRNA and treated with 20(OH)D3. Furthermore, we have transfected HaCaT keratinocytes with scrambled or VDR siRNA and then directly measured the mRNA levels of involucrin and CYP 24. Control experiments confirmed that VDR gene silencing attenuates 1,25(OH)2D3-mediated stimulation of involucrin and CYP24 gene expression (not shown), which is in agreement with the literature (Bikle et al., 2002; Bikle, 2006). As shown in Figure 8, transfection of HaCaT keratinocytes with VDR siRNA had no effect on basal mRNA levels. Treatment of cells transfected with scrambled siRNA with 10 nm 20(OH)D3 for 24 hours resulted in ∼3.5-fold increase in involucrin mRNA (P<0.0005, versus control). Treatment of cells transfected with scrambled siRNA with 1 μm 20(OH)D3 for 24 hours resulted in ∼14-fold increase in CYP24 mRNA (P<0.0005, versus control). Transfection of keratinocytes with VDR siRNA decreased 20(OH)D3-stimulated mRNA levels (P<0.0005) as compared with cells transfected with scrambled siRNA.
Figure 8. 20(OH)D3 stimulates involucrin and CYP24 expression through the VDR in HaCaT keratinocytes.
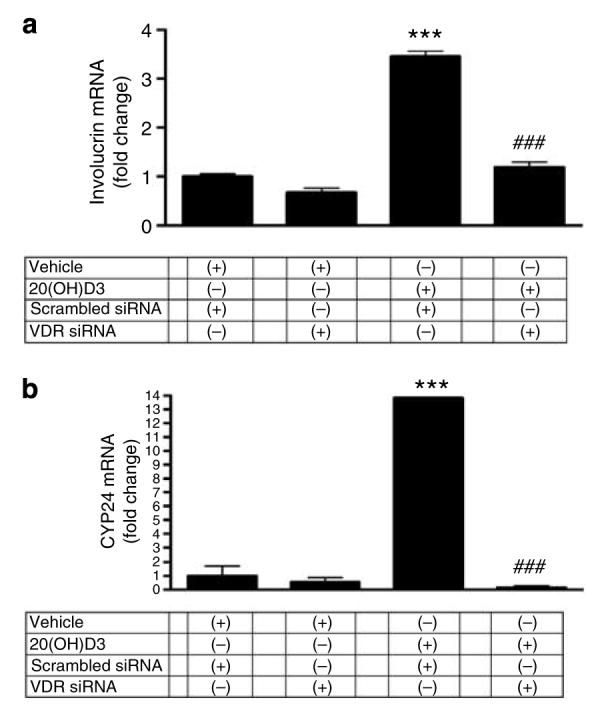
Cells were transfected with scrambled or VDR siRNA and then incubated with 10 nm (a) or 1 μm (b) of 20(OH)D3, or with the vehicle (control). mRNA levels were measured after 24 hours. Data are presented as mean±SEM (n=3); ***P<0.0005, versus untreated control; ###P<0.0005, versus scrambled siRNA and treatment with 20(OH)D3.
Thus, these experiments clearly demonstrate the involvement of VDR and VDRE in the phenotypic effects mediated by 20(OH)D3. However, we cannot entirely exclude a possibility that additional receptors, such as membrane-associated receptors, with respective downstream signaling pathways may also contribute, as suggested by others (Johansen et al., 2003; Khanal and Nemere, 2007).
CONCLUSIONS
We have previously reported that hydroxy derivatives of plant-derived ergosterol and ergocalciferol (vitamin D2), produced by the action of P450scc (CYP11A1), have biological actions on skin cells cultured in vitro (Slominski et al., 2005a). In this paper, we report for the first time that the major product of vitamin D3 metabolism catalyzed by P450scc, 20(OH)D3 (Guryev et al., 2003; Slominski et al., 2005b), acts as an inhibitor of cell proliferation and a stimulator of keratinocyte differentiation, with a potency comparable to that of 1,25(OH)2D3. Furthermore, its action on the expression of CYP27B1, CYP27A1, and CYP24 genes suggests a potential role in the regulation of 1,25(OH)2D3 production, which may depend on the cell type used. 20(OH)D3 acts through the VDR pathway, although activation of other pathways may also be involved. To conclude, our report identifies for the first time 20(OH)D3 as a biologically active secosteroid that is a potent stimulator of epidermal keratinocyte differentiation. Thus, this secosteroid produced by P450scc, in an alternate pathway of vitamin D3 metabolism (Slominski et al., 2004, 2005b), may play an important role in cutaneous biology.
MATERIALS AND METHODS
The medical ethical committee of University of Tennessee Health Science Center approved all described studies.
Synthesis and reversed-phase-HPLC purification of 20(OH)D3
Cytochrome P450scc purified from bovine adrenal glands was used to hydroxylate vitamin D3 as described previously (Slominski et al., 2005b) The main product, 20(OH)D3, was purified by preparative thin-layer chromatography. Prior to use, the remaining impurities were removed using an RF-HPLC system (Waters, Milford, MA) equipped with C18 column (10 cm × 5 mm, particle size 4 μm; Waters). An isocratic elution was performed with 82% methanol and 18% water at a flow rate of 1.5 ml min−1 at 40 °C. UV spectra were collected at 280 nm using 2487 Waters Dual λ Absorbance Detector. Fractions with vitamin D3 purity of at least at least 99% were combined, dried under nitrogen, and stored at −70 °C. In order to confirm the purity of the 20(OH)D3, the sample was dissolved in absolute ethanol (15 μl), reapplied to the HPLC column and eluted with a 50–100% of methanol gradient in water, at flow rate of 1.5 ml min−1 at 40 °C. UV spectra were collected from 210 to 300 nm using Waters 991 PDA Detector. Mass spectra were collected using a Bruker Esquire 3000 Plus Ion trap Mass Spectrometer MSD-Trap-XCT system (Agilent Technologies, Palo Alto, CA). The system was operated with the atmospheric pressure chemical ionization source in the positive ion mode. The scans (average of five spectra) were collected between 100 and 500 m/z.
Proliferation and clonogenicity assays
HaCaT and human adult epidermal keratinocytes were cultured and DNA synthesis experiment were performed as described previously (Slominski et al., 2003). Cells were plated in six-well plates at a density of 20 cells cm−2 in DMEM (Cellgro, Herndon, VA) containing 5% charcoal-treated fetal bovine serum (FBS) (Hyclone, Logan, UT), 1% antibiotic solution (PSA; Sigma, St Louis, MI), and vehicle or secosteroids. Cells were incubated at 37 °C for 10 days, with media being changed every 3 days. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline overnight, stained with 0.5% crystal violet in phosphate-buffered saline for 15 minutes, rinsed, and air-dried. The number and size of the colonies were measured using an ARTEK counter 880 (Dynex Technologies Inc., Chantilly, VA). Colony-forming units were calculated by dividing the number of colonies (size>0.5 mm) by the number of cells plated and then multiplying by 100.
DNA content and involucrin expression
Flow-cytometric DNA content and flow-cytometric and microscopic analyses of involucrin expression were performed as described previously (Slominski et al., 2003; Zbytek and Slominski, 2005).
Real-time reverse transcriptase–PCR for cytokeratin 14, involucrin, CYP27A1, and CYP27B1
RNA was extracted using an Absolutely RNA RT–PCR Miniprep kit (Stratagene, La Jolla, CA). Real-time PCR and reverse transcription products were purchased from Applied Biosystems (Foster City, CA). Reverse transcription was performed using Taqman reverse transcription reagents. The following PCR products were used: cytokeratin 14, Hs00265033_m1; involucrin, Hs00846307_s1; CYP27A1, Hs00168003_m1; CYP27B1, Hs00168017_m1; and 18SrRNA, Hs99999901_s1. The reaction was performed with Taqman Universal PCR Master Mix; data were collected with an ABI Prism 7700 and analyzed with Sequence Detector 1.9.1. Specific gene amounts were related to 18SrRNA by comparative Ct method.
Real-time reverse transcriptase–PCR for CYP24
RNA was extracted as above. Reverse transcription was performed with the Transcriptor first-strand cDNA synthesis kit (Roche, Nutley, NJ). The primers (right: 5′-GCA GCT CGA CTG GAG TGA C-3′; left: 5′-CAT CAT GGC CAT CAA AAC AAT-3′) and probe (cat. no. 04689135001) were designed with the Universal Probe Library (Roche). Real-time PCR was performed using TaqMan PCR Master Mix at 50 °C for 2 minutes, 95 °C for 10 minutes, and then performing 50 cycles (95 °C for 15 seconds, 60 °C for 1 minute). The data were collected with a Roche Light Cycler 480. The amounts of CYP24 were normalized by comparative Ct method, using cyclophilin D as a housekeeping gene.
CYP24-Luc transfection
The CYP24-Luc construct was a generous gift from Dr Tai Cheng (Boston University Medical Campus, Core Lab Director). It was originally developed by Vaisanen et al. (2005). The details of the pLuc construct and transfection have been described previously (Pisarchik and Slominski, 2004; Zbytek et al., 2006).
VDRE electrophoretic mobility shift assay
HaCaT keratinocytes were treated for 24 hours. Nuclear extracts were prepared as described previously (Zbytek et al., 2006). The synthetic IRDye-labeled oligonucleotide (LI-COR Inc., Lincoln, NE) used for the DNA mobility shift assay contained the wild-type VDRE sequence, the sp1 promoter, and part of the involucrin sequence (5′-GCGGGAGGCAGATCTGGCAGATACTGA-3′), as described (Bikle et al., 2002). The DNA-binding reaction was set up using 2.5 μg of the nuclear extract, oligonucleotide, and buffer consisting of 2.5 mmol l−1 dithiothreitol, 0.25% Tween-20, and 0.25 mg ml−1 poly(dI):poly(dC). Samples were incubated at room temperature in the dark for 30 minutes and then run on 5% Tris/borate/EDTA gel at 80 V for 1 hour. The gel was scanned using Odyssey Infrared Imaging System (LI-COR).
VDRE-Luc and siRNA transfection
HaCaT keratinocytes were transfected with VDRE-Luc (gift from Dr Thai Chen; Vaisanen et al., 2005) and/or with scrambled or VDR siRNA (Dharmacon Inc., Lafayette, MO), on-Target plus smart pool human VDR L-003448-00, on-Target plus siControl non-targeting pool D-001810-10-05), using Lipofectamine Plus (Invitrogen, Carlsbad, CA) in DMEM medium. PhRL-TK (expressing Renilla luciferase) served as a normalization control (Promega, Madison, WI). After transfection, cells were incubated for 24 hours in DMEM with 5% FBS. Cells were then transferred to fresh media containing the compounds to be tested or vehicle (ethanol) and incubated for 24 hours. Levels of VDR and β-actin 24 hours after transfection were assessed by western blot (VDR(D-6) antibody, sc-13133, 1:400; Santa Cruz Inc., Santa Cruz, CA) performed as described previously (Hanissian et al., 2005).
Statistical analysis
Data are presented as mean±SEM and have been analyzed with Student's t-test (for two groups) or one-way analysis of variance, and appropriate post hoc test (for more than two groups), using Excel (Microsoft) and Prism 4.00 (GraphPad Software, San Diego, CA). Statistically significant differences are denoted by asterisks as follows: *P<0.05, **P<0.005, and ***P<0.0005.
ACKNOWLEDGMENTS
This work was supported by NIH Grant R01AR052190 to AS.
Abbreviations
- 20(OH)D3
20-hydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- CYP
cytochrome P450 enzyme
- HPLC
high-performance liquid chromatography
- MSI
mean signal intensity
- siRNA
small interfering RNA
- VDR
vitamin D receptor
- VDRE
vitamin D response element
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Bar M, Domaschke D, Meye A, Lehmann B, Meurer M. Wavelength-dependent induction of CYP24A1-mRNA after UVB-triggered calcitriol synthesis in cultured human keratinocytes. J Invest Dermatol. 2007;127:206–13. doi: 10.1038/sj.jid.5700493. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D: production, metabolism and mechanism of action. In: Arnold A, editor. Diseases of Bone and Calcium Metabolism. South Dartmouth, MA: 2006. www.endotext.com. [Google Scholar]
- Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–44. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–66. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Oda Y, Hanley K, Feingold K, Xie Z. The vitamin D response element of the involucrin gene mediates its regulation by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2002;119:1109–13. doi: 10.1046/j.1523-1747.2002.19508.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–71. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol. 2004;89–90:355–60. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- Dai X, Yamasaki K, Yang L, Sayama K, Shirakata Y, Tokumara S, et al. Keratinocyte G2/M growth arrest by 1,25-dihydroxyvitamin D3 is caused by Cdc2 phosphorylation through Wee1 and Myt1 regulation. J Invest Dermatol. 2004;122:1356–64. doi: 10.1111/j.0022-202X.2004.22522.x. [DOI] [PubMed] [Google Scholar]
- Ebert R, Schutze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–59. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, et al. Regulation of involucrin gene expression. J Invest Dermatol. 2004;123:13–22. doi: 10.1111/j.0022-202X.2004.22723.x. [DOI] [PubMed] [Google Scholar]
- Feldman D, Pike JW, Glorieux FH. Vitamin D. 2nd ed. Elsevier Academic Press; Oxford, UK: 2005. 1952. [Google Scholar]
- Guo L, Zhang L, Sun Y, Muskhelishvili L, Blann E, Dial S, et al. Differences in hepatotoxicity and gene expression profiles by antidiabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol Divers. 2006;10:349–60. doi: 10.1007/s11030-006-9038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–9. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanissian SH, Teng B, Akbar U, Janjetovic Z, Zhou Q, Duntsch C, et al. Regulation of myeloid leukemia factor-1 interacting protein (MLF1IP) expression in glioblastoma. Brain Res. 2005;1047:56–64. doi: 10.1016/j.brainres.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Holick MF. Calcium and vitamin D. Diagnostics and therapeutics. Clin Lab Med. 2000;20:569–90. [PubMed] [Google Scholar]
- Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003a;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003b;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Jr, Anderson RR, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- Itin PH, Pittelkow MR, Kumar R. Effects of vitamin D metabolites on proliferation and differentiation of cultured human epidermal keratinocytes grown in serum-free or defined culture medium. Endocrinology. 1994;135:1793–8. doi: 10.1210/endo.135.5.7956903. [DOI] [PubMed] [Google Scholar]
- Johansen C, Kragballe K, Henningsen J, Westergaard M, Kristiansen K, Iversen L. 1Alpha,25-dihydroxyvitamin D3 stimulates activator protein 1 DNA-binding activity by a phosphatidylinositol 3-kinase/Ras/ MEK/extracellular signal regulated kinase 1/2 and c-Jun N-terminal kinase 1-dependent increase in c-Fos, Fra1, and c-Jun expression in human keratinocytes. J Invest Dermatol. 2003;120:561–70. doi: 10.1046/j.1523-1747.2003.12095.x. [DOI] [PubMed] [Google Scholar]
- Khanal R, Nemere I. Membrane receptors for vitamin D metabolites. Crit Rev Eukaryot Gene Expr. 2007;17:31–47. doi: 10.1615/critreveukargeneexpr.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Hashimoto K, Yoshikawa K. Dephosphorylation of the retinoblastoma gene product induced by differentiation and its relevancy to growth inhibition in normal human keratinocytes. J Dermatol Sci. 1994;8:171–7. doi: 10.1016/0923-1811(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Lehmann B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem Photobiol. 2005;81:1246–51. doi: 10.1562/2005-02-02-IR-430. [DOI] [PubMed] [Google Scholar]
- Ohyama Y, Yamasaki T. Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci. 2004;9:3007–18. doi: 10.2741/1455. [DOI] [PubMed] [Google Scholar]
- Pillai S, Cho S, Mahajan M, Frew L, Rawlings AV. Synergy between vitamin D precursor 25-hydroxyvitamin D and short chain ceramides on keratinocyte proliferation and differentiation. J Investig Dermatol Symp Proc. 1996;1:39–43. [PubMed] [Google Scholar]
- Pisarchik A, Slominski A. Molecular and functional characterization of novel CRFR1 isoforms from the skin. Eur J Biochem. 2004;271:2821–30. doi: 10.1111/j.1432-1033.2004.04216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–73. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196:144–53. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, et al. Enzymatic metabolism of ergosterol by cytochrome p450scc to biologically active 17alpha,24-dihydroxyergosterol. Chem Biol. 2005a;12:931–9. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005b;272:4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–88. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatiotemporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-dihydroxyvitamin D3. J Mol Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–8. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- Zbytek B, Wortsman J, Slominski A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone–proopiomelanocortin system in human melanocytes. Mol Endocrinol. 2006;20:2539–47. doi: 10.1210/me.2006-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]