Summary of recent advances
When prostate cancers progress following androgen depletion therapy, there are currently few treatment options with only one, docetaxel, that has been shown to prolong life. Recent work has shown that castration resistant prostate cancers (CRPC) continue to depend on androgen receptor (AR) signaling which is reactivated despite low serum androgen levels. Currently available AR targeted therapy, including GnRH agonists and antiandrogens, cannot completely shut down AR signaling. Several mechanisms that enhance AR signaling in an androgen depleted environment have been elucidated. These include AR mutations that allow activation by low androgen levels or by other endogenous steroids, AR overexpression, increased local intracrine synthesis of androgens, and upregulation of tyrosine kinase pathways. This has led to the development of a number of novel agents targeting AR signaling pathway, including more effective antiandrogens, inhibitors of CYP17, an enzyme required for androgen synthesis, inhibitors of 5α-reductase, inhibitors of HSP90 which protects AR from degradation, inhibitors of histone deacetylases which is required for optimal AR mediated transcription, as well as inhibitors of tyrosine kinase inhibitors. Many of these strategies are currently being tested in clinical trials in CRPC.
Introduction
The androgen receptor (AR), located on Xq11-12, is a 110 kD nuclear receptor that, upon activation by androgens, mediates transcription of target genes that modulate growth and differentiation of prostate epithelial cells. AR signaling is crucial for the development and maintenance of male reproductive organs including the prostate gland, as genetic males harboring loss of function AR mutations and mice engineered with AR defects do not develop prostates or prostate cancer [1,2]. This dependence of prostate cells on AR signaling continues even upon neoplastic transformation, leading to the seminal discovery by Huggins and Hodges in 1941 that orchiectomy produced prostate cancer regression [3]. Androgen depletion (now using GnRH agonists) continues to be the mainstay of prostate cancer treatment. However androgen depletion is usually effective for a limited duration and prostate cancer evolves to regain the ability to grow despite low levels of circulating androgens [4]. Treatment options for castration resistant prostate cancer (CRPC) are an unmet need with docetaxel being the only agent that has been shown to prolong survival [5,6]. Interestingly, while a small minority of CRPC does bypass the requirement for AR signaling [7], the vast majority of CRPC, though frequently termed “androgen independent prostate cancer” or “hormone refractory prostate cancer,” retains its lineage dependence on AR signaling. Over the past several years, several important mechanisms of enhanced AR signaling in low serum androgen levels in CRPC have been elucidated. This has led to novel therapeutic strategies targeting AR signaling which offer promising potential in future treatment of CRPC (Figure 1).
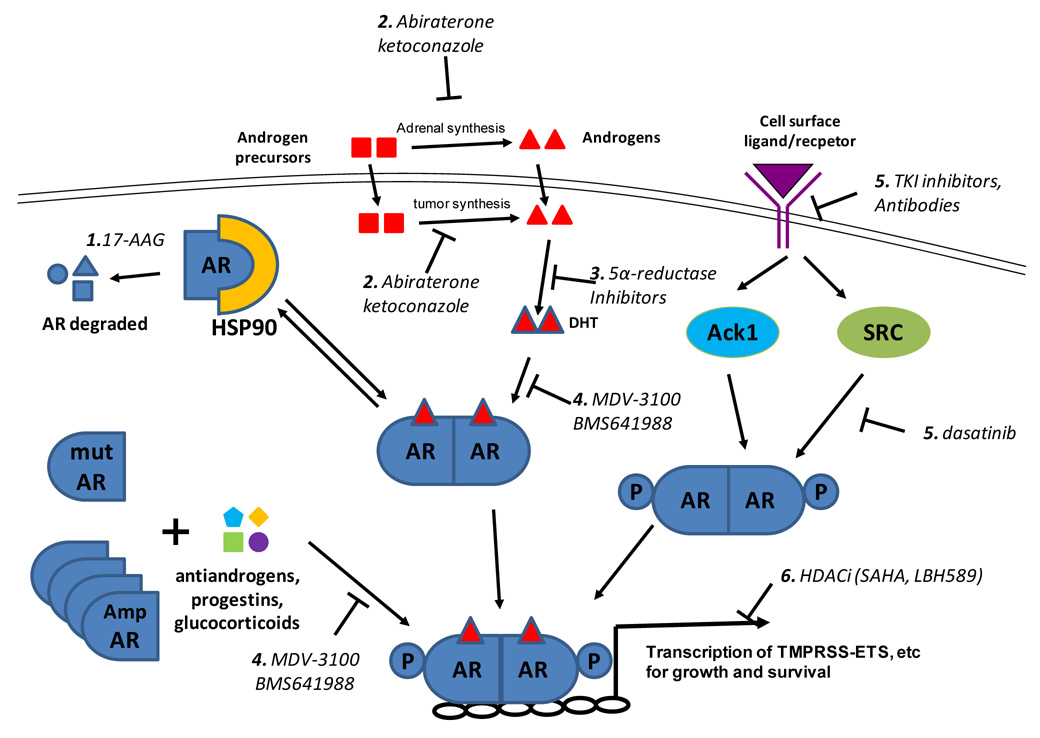
Figure 1.
Schematic of therapeutic targets of the AR pathway. 1) AR is bound to the molecular chaparonin HSP90 which prevents its degradation. HSP90 inhibitors, such as 17-AAG, cause AR degradation and decrease AR levels. 2) In men treated with GnRH agonists to shut down testicular androgen synthesis, residual serum androgens are synthesized by the adrenal glands. In additional, evidence suggests intratumoral androgen synthesis. Both can be inhibited by the non-specific p450 inhibitor ketoconazole and the 17-lyase inhibitor abiraterone. 3) Testosterone is converted to the more potent dihydrotestosterone (DHT) by 5α-reductase, which is inhibited by finasteride and dutasteride. 4) Ligands, such as DHT bind to AR and this is inhibited by antiandrogens such as bicalutamide and novel agents MDV-3100 and BMS641988. Mutation of AR as well as AR overexpression can convert endogenous steroids (e.g., progestins, estrogens, corticosteroids) and some antiandrogens into agonists. MDV-3100 was designed to suppress AR function even when AR is overexpressed. 5) Activation of receptor tyrosine kinases, in particular HER2, can lead to downstream AR activation. Two downstream kinases that directly phosphorylate AR on tyrosine are Ack1 and SRC. Other downstream pathways of receptor tyrosine kinases, including the AKT and MAP kinase pathways, are also implicated. Antibodies such as trastuzamab and pertuzumab and small molecular TKI inhibitors such as erlotinib and lapatinib target HER2. Dasatinib target SRC. 6) Proper transcription mediated by AR requires the proper chromatin state. HDAC inhibitors inhibit transcription of AR target genes by disruption of chromatin structure and inhibition of recruitment of coactivators and RNA polymerase II.
Castration resistant prostate cancer requires AR signaling
Several clinical observations have long offered clues that AR signaling is active and required in most CRPC. PSA, an exquisitely AR dependent gene, is widely used as a marker for disease activity. PSA declines after initiation of hormone depletion therapy and a subsequent rise is commonly the first sign of disease progression. This indicates that reactivation of AR signaling accompanies the development of CRPC. Both the relative and absolute level of PSA decline—markers of the degree of AR inhibition—after initial androgen depletion is predictive of outcome [8]. After development of castration resistance, further hormonal manipulations targeting AR can elicit response while treatment with exogenous androgens usually results in tumor flare. First demonstrated for flutamide, treatment with any currently available antiandrogen may result in agonist conversion, and tumor response can be observed upon andiandrogen withdrawal (AAWD) [9]. Gene expression studies of clinical prostate cancer specimens show that AR activated genes (defined as genes down-regulated after neoadjuvant androgen deprivation prior to prostatectomy) were reactivated in CRPC despite continued androgen deprivation [10]. In the laboratory, knockdown of AR results in cell death in both human and murine castration resistant prostate cancer cell lines [11–13].
Recently, it was discovered that up to 90% of all prostate cancers overexpress an ets oncogene, including ERG, ETV1, ETV5 and ETV6 via a variety of mechanisms. The most common mechanism of overexpression is fusion of the ets gene (in particular ERG) to the 5’ untranslated region of highly AR regulated TMPRSS2 gene [14,15]. Thus, in addition to the lineage dependence of prostate cells on AR signaling, prostate cancer has additional selection pressure to maintain TMPRSS2 expression and AR activity.
Mechanisms of AR activation in CRPC
Numerous mechanisms have been implicated in reactivation of AR in the castrate environment and have been extensively reviewed (Figure 1) [16–18]. Most directly, mutations of AR that allow other steroids such as corticosteroids and anti-androgens are detected in ~10% of CRPC in a CALGB clinical trial, though the actual incidence may be more frequent [19]. Other mechanisms including activation of kinase pathways that can both stabilize AR and enhance its transcriptional activity and upregulations of AR coactivators that increase AR mediated transcription. These sensitize AR to lower levels of ligand. Here, we focus on three crucial and druggable mechanisms—increased level of AR and increased level of ligand and activation of kinase pathways.
CRPC utilize several sources of ligands
Hormone ablation therapy does not completely eliminate serum androgens. Serum testosterone is reduced to a mean of 15 ng/ml from a normal range of >200 ng/ml while serum levels of adrenal androgens such as dehydroepiandrosterone (DHEA) and androsteindione are unaffected. Intraprostatic androgen concentration is reduced much less dramatically by only ~75%, and is sufficient to activate AR [20,21]. While this level of reduction is sufficient to induce response in untreated prostate cancer, cellular alterations that sensitize the AR pathway induce resistance and confer growth.
One possible source of increased intratumoral androgens are the tumor cells. Two expression profiling studies comparing metastatic CRPC with primary tumors show that enzymes involved in androgen synthesis are upregulated in CRPC. Holzbeierlein et. al. found overexpression of enzymes involved in synthesis of cholesterol, the common steroid precursor, from acetyl-CoA [10] and Stanbrough et. al. found overexpression of enzymes involved in synthesis of testosterone and the more potent androgen DHT from cholesterol [22] (Figure 2). Using mass spectrometry to directly measurement of intratumoral androgens, Montgomery and colleagues found that castration resistant metastatic tumors in men treated with GnRH have higher levels of testosterone but not dihydrotestosterone than primary tumors in untreated men. They also corroborated overexpression of enzymes in androgen synthesis measured by real-time PCR. Castration resistant xenografts similarly maintained elevated intratumoral testosterone levels in castrate mice [23]. These data suggest that intracrine androgen synthesis may allow tumors to grow despite low serum androgen levels.
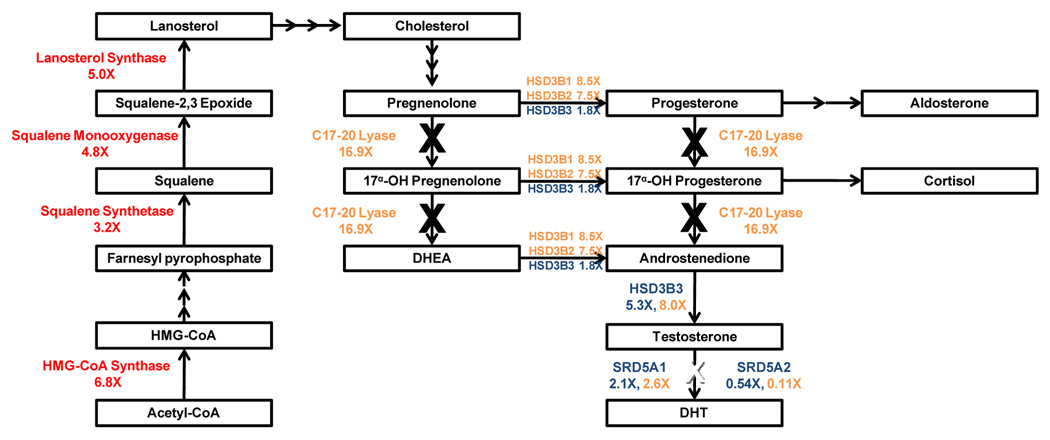
Figure 2.
Androgen synthesis pathway and therapeutic targets. Cholesterol is synthesized from Acetyl-CoA and enzymes in this pathway were found to be upregulated by Holzbeierlein et. al. (red) [10]. Subsequently, the weak androgen androstenedione, testosterone, and the potent DHT are synthesized from cholesterol and enzymes in this pathway were found to be upregulated by Stanbrough et. al. (blue) and Montgomery et. al. (orange) [22,23]. Abiraterone blocks CYP17 which contain both 17α-hydroxylase/C17,20-lyase activities (boxed orange). Dutasteride blocks both SRD5A1 and SRD5A2 (boxed green).
AR overexpression in CRPC
Overexpression of AR is common in CRPC. Compared to localized disease, CRPC has higher expression of AR based on immunochemical staining with genomic amplification seen in ~20% of cases [24,25]. Expression profiling studies consistently identify AR to be overexpressed in CRPC [10,22,26,27].
Laboratory data indicate that AR overexpression is necessary and sufficient to induce CRPC in xenograft models. To identify genes important for development of castration resistance, a panel of 7 prostate cancer xenografts was selected for castration resistance by passage in castrated mice. Comparison of the expression profiles between the 7 isogenic pairs reveal that AR is the only gene overexpressed in all resistant xenografts. Further, forced modest overexpression of AR in the parental LnCaP and LaPC4 xenografts conferred castration resistance by sensitizing cells to residual levels of androgens restoring expression of AR regulated genes. Intriguingly, overexpression of AR also converted the anti-androgen bicalutamide into a weak agonist, indicating that AR overexpression can underlie AAWD [12].
Activation of kinase pathways in CRPC
The HER2 receptor tyrosine kinase is progressively overexpressed in more advanced, castrate resistance prostate cancers, though it is seldom, if ever, amplified as seen in breast cancers [28]. In experimental systems, forced overexpression of HER2 results in increased AR activity and stability while pharmacologic inhibition or knockdown of the protein results in growth suppression [29]. One possible downstream target of HER2 activation is the Cdc42-associated tyrosine kinase Ack1. Activated Ack1 mediates AR activation through tyrosine phosphorylation of Y267 and knockdown of Ack1 or mutation of Y267 to phenylalanine abrogates HER2 mediated AR activation and growth [30].
In addition to HER2, increased signaling by a number of other growth factor receptors (e.g, EGFR, IGF-1R, IL-6R) can enhance AR signaling and confer castration resistance in preclinical models [31–33]. These receptors induce downstream activation of critical growth and survival pathways, including the AKT, MAPK, and STAT pathways. Expression of both activated AKT and BRAF, also results in castration resistance [13]. While these mechanisms of CRPC are frequently referred to as “ligand independent”, it is unknown whether AR is truly activated without ligand binding or whether AR is sensitized to lower levels of ligands since experimental systems to decrease AR ligands, such as in vitro growth in charcoal stripped serum or castration of mice in vivo leave residual ligands. This distinction between true ligand independent and hypersensitized AR is not just semantic since therapies designed to further reduce AR ligands would be active only if ligand is still required. Furthermore, AR alleles containing mutations that impair ligand binding can no longer confer resistance to castration.
Another kinase implicated in AR crosstalk is SRC. Upon ligand binding, AR binds and activates SRC and downstream events within 5 minutes in a “non-genomic” mechanism [34]. SRC can in turn tyrosine phosphorylate AR augmenting its transcriptional activity. SRC activity is substantially increased in models of CRPC [35,36]. A 10 amino acid peptide that blocks the AR-SRC interaction inhibits androgen mediated proliferation in tissue culture and xenografts [37].
Evolving Treatments for CRPC
Antiandrogens
Upon development of castration resistance, an antiandrogen, such as flutamide, bicalutamide, nilutamide, and cyproterone acetate, is typically added if it was not included in initial treatment. In some cases, this can result in prolonged disease control. However, in the majority of patients who have progressed despite androgen ablation and especially in symptomatic patients, the time to progression is usually short [38,39]. In addition, all these compounds have clinically been observed to convert to agonists [9]. A number of novel antiandrogens are currently in preclinical development.
MDV-3100, a novel antiandrogen, was rationally designed utilizing the AR crystal structure, modeling and cell based screening. Since bicalutamide is converted to an agonist in LnCaP cells that overexpress AR, candidate compounds were screened for their ability to inhibit growth and PSA secretion in these cells. Of the resulting hits, MDV-3100 has the best pharmacological properties of good oral availability and long half-life. MDV-3100 binds AR in cells with 10-fold higher affinity that bicalutamide in competition studies and similarly, it inhibits PSA secretion at 10-fold lower concentrations. Subsequent preclinical studies showed that MDV-3100 completely inhibits growth of both castration-resistant xenografts. Unlike bicalutamide, MDV-3100 impairs AR nuclear translocation and blocks DNA binding. MDV-3100 is currently undergoing Phase I/II clinical trial in CRPC. Early data is highly promising (Table 1).
Table 1.
Clinical trials AR targeting agents in CRPC
| Agent | Phase | RESIST Response | PSA Response | Reference |
|---|---|---|---|---|
| Androgen and DHT lowering compounds | ||||
| Abiraterone | II (chemo naive) | 12/21 | 18/30 (60%) | JS De Bono, abs 5005, 2008 ASCO Annual Meeting |
| II (chemo refractory) | 3/8 | 14/35 (40%) | DC Danila, abs 5019, 2008 ASCO Annual Meeting | |
| II (chemo refractory) | 4/28 | 16/31 (52%) | JS De Bono, abs 5005, 2008 ASCO Annual Meeting | |
| III (open) | ||||
| Dutasteride | II (open) | |||
| Dustasteride + ketoconazole | II (keto sensitive) | 30/57 (53%) | M Taplin, abs 5068, 2008 ASCO Annual Meeting | |
| Dustasteride + ketoconazole | II (keto refractory) | 0/10 (8/10 with PSA decline) | AO Sartor, abs 257, 2007 Prostate Cancer Symposium | |
| Antiandrogens | ||||
| MDV-3100 | I/II | 13/14 | HI Scher, abs 5006, 2008 ASCO Annual Meeting. | |
| BMS-641988 | I | |||
| EGFR and HER2 Targeting Agents | ||||
| Erlotinib | II (closed) | |||
| Erlotinib + docetaxel | II (closed) | 0/8 | 5/22 | [65] |
| Gefitinib | II (closed) | 0 | 0/23 | [66] |
| Lapatinib | II (open) | 1/21 | YE Whang, abs 16037, 2008 ASCO Annual Meeting | |
| Trastuzumab | II (closed) | [67] | ||
| Pertuzumab | II | 0/30 (5/30 SD >23 wk) | 0/41 | [68] |
| SRC Inhibitors | ||||
| Dasatinib | II | 67% disease control | 1/36 | EY Yu, abs 5156, 2008 ASCO Annual Meeting |
| HDAC inhibitors | ||||
| Depsipeptide | 1/21, (6/21 SD > 4m) | 2/31 | C Parker, abs in J Clin Oncol (2007) 25(18_suppl) 15507 | |
| LBH589 | I | 0/8 | DE Rathkopf, abs 5152, 2008 ASCO Annual Meeting | |
| SAHA+DOC | I | 2/8 | DE Rathkopf, abs 5152, 2008 ASCO Annual Meeting | |
| HSP90 Inhibitors | ||||
| 17-AAG | II | 0/15 | EI Heath, abs in J Clin Oncol (2007) 25(18_suppl) 15553 | |
BMS-641988 is a novel antiandrogen with 20-fold greater affinity for AR than bicalutamide and is effective in two xenograft models that have progressed on bicalutamide. It is currently undergoing Phase I clinical trial in CRPC.
Androgen Lowering Therapies
Since residual serum androgens as well as upregulated intracrine androgen synthesis may be sufficient to promote CRPC growth in patients on hormone ablation therapy, especially when the pathway is sensitized by AR mutation or overexpression, strategies to further lower androgen levels are under investigation. Treatment with aminoglutethimide and ketoconazole, both nonspecific p450 inhibitors that target numerous enzymes in the steroid synthesis pathway (Figure 2), results in 50% PSA reduction in 25–50% of patients with CRPC for a median duration of ~6 months [40]. Analysis of the CALGB 9583 comparing AAWD with AAWD plus ketoconazole showed that patients with higher baseline serum levels of the adrenal androgen androstenedione (Figure 2) had higher response rates and prolonged overall survival with ketoconazole treatment [40,41]. Non-selective p450 inhibitors have significant constitutional side effects, limiting quality of life and treatment duration. Additionally, they interfere with the metabolism of multiple drugs.
Abiraterone is a selective, high affinity (IC50=2nM), irreversible inhibitor of the p450 enzyme, CYP17, which contain both 17α-hydroxylase/C17,20-lyase activities, targeting both adrenal and tumor intracrine androgen synthesis (Figure 2). At tolerable doses, serum testosterone concentration fell to castrate levels in intact mice without significantly affecting serum cortisol levels, whereas ketoconazole suppressed cortisol more than testosterone [42]. While single agent abiraterone can suppress testosterone to castrate levels in the short term, there is some testosterone recovery with long term treatment due to compensatory increase in gonadotropin release. Addition of abiraterone to GnRH treatment results in substantial decrease of both testosterone and adrenal androgens (Figure 2) [43]. Preliminary data from phase I/II trials of patients with CRPC show significant activity with PSA response exceeding 60% in chemotherapy naïve and 40% in chemotherapy treated patients with median time to progression of 8 and 5.5 months respectively (JS deBono, abs 5005 and DC Danila abs 5019 presented in 2008 ASCO Annual Meeting). This has motivated an international randomized phase III randomized trial comparing abiraterone with placebo in patients CRPC who have progressed on docetaxel.
Testosterone is converted to the more potent DHT by two isoforms of 5α-reductase, SRD5A1 and SRD5A2, in peripheral androgen dependent tissues (Figure 2). SRD5A2 is the predominant isoform in the benign prostate and finasteride, a specific inhibitor of SRD5A2 reduces PSA and prostate weight and is approved for use in benign prostatic hypertrophy. However, progressive prostate cancer is characterized by increased SRD5A1 and decreased SRD5A2 [22,44]. Dutasteride, a potent inhibitor of both SRD5A1 and SRD5A2, inhibits cancer growth in the R-3327H rat prostate cancer model and the probasin large-T antigen (TRAMP) mouse prostate tumor model [45,46]. Because dutasteride causes feedback increase in testosterone levels, simultaneous castration may increase its efficacy. In the LnCaP xenograft model, dutasteride but not finasteride inhibits tumor growth more than castration alone [46]. Thus, dutasteride is in phase II clinical trials of various stages of prostate cancer, including CRPC (Table 1).
HSP90 Inhibitors
HSP90, a molecular chaparonin, is required for the refolding of denatured proteins. In addition, it is required to maintain the proper baseline folding of several important proteins in oncogenesis, including AR, Her2, AKT, and mutagenic BRAF. Geldanamycin, a natural compound produced by Streptomyces hygroscopicus, binds the ATP-binding pocket of HSP90 and causes degradation of client proteins [47]. Tanespimycin (17-AAG,), a more stable geldanamycin derivative, inhibits AR positive prostate cancer xenografts without significant toxicity to the murine hosts. Treatment resulted in 80% loss of AR expression and 97% loss of HER2 expression [48]. In an independent unbiased validation that HSP90 inhibition targets AR activity, Hieronymus el. al. screened a library of chemical compounds that decreased expression in AR regulated genes. Two compounds that scored in this screen, celastrol and gedunin, were subsequently discovered to be HSP90 inhibitors and cause the same gene expression perturbations as 17-AAG [49].
In a recent phase I trial, 17-AAG plus trastuzumab (Herceptin) showed significant clinical activity in patients with HER2 positive metastatic breast cancer that have progressed on trastuzumab based therapy, offering a proof of principle that clinically achievable levels HSP90 inhibitors can be active [50]. However, 17-AAG did not show significant clinical activity in CRPC (EI Heath, abstract in J Clin Oncol 2007, 18_suppl:15553). One limitation with 17-AAG is its insolubility in water and thus difficulty obtaining high serum concentrations. More water-soluble geldanamycin analogs, such as 17-DMAG (alvespimycin) as well as combination strategies are currently under clinical investigation.
HDAC Inhibitors
Gene transcription depends on a complex interplay between transcription factors, such as AR and a chromatin state that support active gene transcription [51]. The fundamental unit of chromatin, the nucleosome, consists of 146 base pairs of DNA wrapped around an octamer of highly conserved core histone proteins (two each of H2A, H2B, H3, and H4). All four core histones have a number of positively charged lysine residues in their amino-terminal tail that form tight interactions with negatively charged DNA. The majority of these lysines can undergo acetylation. Acetylation of lysine residues is regulated by the balance of histone aceyltransferases (HAT) and histone deacetylases (HDAC), akin to the regulation of phosphorylation by kinases and phosphatases. More recently, numerous other proteins have been found to be acetylated, including p53, HSP90 and AR.
Naturally occurring antibiotics including trichostatin A, depsipeptide and the synthetic polar compound suberoylanilide hydroxamic acid (SAHA) were found to induce differentiation and to be selective more toxic in tumor cells. These agents were found to inhibit HDAC, and as expected, induce hyperacetylation of histones and transcriptional changes. HDAC inhibitors have demonstrated encouraging anti-tumor activity and SAHA has been approved in the treatment of cutaneous T-cell lymphomas [52].
HDAC inhibitors have been noted to have greater anti-proliferative effects on AR-positive prostate cancer cells than their AR-negative counterparts, and inhibit xenograft growth in both castration sensitive and resistant models [53,54]. One proposed mechanism is that HDAC inhibitors target HDAC6 which deacetylates HSP90 and decreases AR stability [55]. Furthermore, HDAC inhibitors directly suppress AR transcription [54,56].
While these mechanisms are important, we have further observed that HDAC inhibitors directly inhibit the transcription of approximately half of AR target genes, even in CRPC models where AR is overexpressed and present at high levels that cannot be decreased by HSP90 or AR transcriptional inhibition. These genes include the TMPRSS-ERG fusion implicated in prostate oncogenesis as well as well characterized AR response genes such as PSA and KLK2 (DS Welsbie et. al., unpublished).
Several HDAC inhibitors, including depsipeptide, SAHA, and LBH589 are in phase I/II clinical trials in CRPC. So far, the observed activity is modest at best (Table I). The low activity may be due to suboptimal dosing as low concentration of HDAC inhibitor may paradoxically activate AR in tissue culture models [57]. This has motivated trials designed to increase HDAC inhibitor concentration with shorter exposure as well as combination trials with chemotherapy.
Kinase Inhibitors
Agents targeting EGFR or HER2, including small molecule kinase inhibitors (gefitinib, erlotinib, lapatinib) and monoclonal antibodies (trastuzumab, pertuzamab) have been studied clinically in CRPC but showed disappointing results (Table 1). Since these inhibitors are most active in tumors where the target is mutated or amplified (e.g., EGFR mutation in lung cancer, HER2 amplification in breast cancer), one explanation is that EGFR/HER2 is not a relevant target in CRPC despite showing activity in preclinical models. Alternatively, there is the mounting evidence that loss of PTEN mediates resistance to EGFR and HER2 targeted therapies in both breast cancer and glioblastoma [58–61]. PTEN loss is common in high grade and metastatic CRPC and mediates early development of castration-resistance in mouse models [62,63]. Therefore, combination treatment with novel PI3K inhibitors with ERFR/HER2 inhibitors may be warranted.
Dasatinib, currently approved as an ABL kinase inhibitor for treatment of chronic myelogenous leukemia [64], is also a namomolar SRC inhibitor. Phase II clinical trials of dasatinib in CRPC are ongoing.
Conclusion
With increasing appreciation that AR signaling remain crucial in CRPC, a number of new therapeutic strategies have evolved. Phase I–II clinical data show that some have very promising activity while others have been disappointing. As expected from the fact that multiple mechanisms can underlie AR activation, no single therapeutic agent is active in all patients. The ability to dissect AR pathway aberrations in patients would allow individualized therapy targeting the particular aberration.
Acknowledgements
HIS would like to thank the MSKCC Prostate Cancer SPORE grant, the Prostate Cancer Foundation. CLS is a Doris Duke Distinguished Clinical Scientist and an Investigator of the Howard Hughes Medical Institute and would like to thank grants from the US National Cancer Institute and the UCLA Prostate SPORE seed grant. YC would like to thank the Ruth L. Kirschstein National Research Service Awards (5T32CA009207), the AACR BMS Oncology Fellowship, and the ASCO YIA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Annotations
- 1.McPhaul MJ. Androgen receptor mutations and androgen insensitivity. Mol Cell Endocrinol. 2002;198:61–67. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 2.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huggins C, Hodges CV. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 4.Smaletz O, Scher HI. Outcome predictions for patients with metastatic prostate cancer. Semin Urol Oncol. 2002;20:155–163. doi: 10.1053/suro.2002.32938. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Slovin SF. Neuroendocrine differentiation in prostate cancer: a sheep in wolf's clothing? Nat Clin Pract Urol. 2006;3:138–144. doi: 10.1038/ncpuro0435. [DOI] [PubMed] [Google Scholar]
- 8.Fleming MT, Morris MJ, Heller G, Scher HI. Post-therapy changes in PSA as an outcome measure in prostate cancer clinical trials. Nat Clin Pract Oncol. 2006;3:658–667. doi: 10.1038/ncponc0664. [DOI] [PubMed] [Google Scholar]
- 9.Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607–609. doi: 10.1016/s0022-5347(17)36163-3. [DOI] [PubMed] [Google Scholar]
- ••10.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4.Authors performed genome wide expression analysis on clinical prostate cancer specimens and found that many AR regulated genes that were suppressed when patients initially started hormone ablation therapy are re-expressed upon progression to CRPC. They also found AR and enzymes in androgen synthesis to be frequently overexpressed.
- 11.Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, Bubley GJ, Lu ML, Balk SP. Androgen receptor remains critical for cell-cycle progression in androgenindependent CWR22 prostate cancer cells. Am J Pathol. 2006;169:682–696. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••12.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972.Authors selected seven prostate cancer xenografts in castrate mice and found that in all the castrate resistant subclones, AR was overexpressed. Further, forced over-expression was able to confer castrate resistant growth and converted bicalutamide from an antagonist to an agsonist.
- 13.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–14482. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••14.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448:595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- ••15.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679.Using bioinformatics tool called "outlier analysis", Tomlins and colleagues found that members of the ets transcription factors, including Erg, ETV-1, ETV-5, and ETV-6 were highly overexpressed in a subset of prostate cancers. There are a number of mechanisms of overexpression, including most commonly fusion with the AR regulated TMPRSS2 gene.
- 16.Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004;11:459–476. doi: 10.1677/erc.1.00525. [DOI] [PubMed] [Google Scholar]
- 17.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 18.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 19.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, et al. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21:2673–2678. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 20.Mizokami A, Koh E, Fujita H, Maeda Y, Egawa M, Koshida K, Honma S, Keller ET, Namiki M. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004;64:765–771. doi: 10.1158/0008-5472.can-03-0130. [DOI] [PubMed] [Google Scholar]
- 21.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- •22.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000.Authors compared expression profile of laser dissected primary prostate cancer with that of castrate resistant bone marrow metastatic disease. They found upregulation of AR and multiple genes involved in androgen synthesis.
- ••23.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249.Using mass spectrometry to measure tissue androgen levels, the group found that CRPC tumors in men treated with androgen depletion therapy had higher testosterone levels than primary tumors in untreated men, despite the former group having lower circulation testosterone levels. Using quantitative rtPCR, they found overexpression of a number of key enzymes in androgen synthesis.
- 24.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, Willi N, Mihatsch MJ, Sauter G, Kallioniemi OP. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–806. [PubMed] [Google Scholar]
- 25.Edwards J, Krishna NS, Grigor KM, Bartlett JM. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 27.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- 29.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, Earp HS, Whang YE. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 32.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 33.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 34.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 35.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–11054. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 37.Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26:6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- 38.Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, Kolvenbag G, Shapiro L, Schwartz M. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol. 1997;15:2928–2938. doi: 10.1200/JCO.1997.15.8.2928. [DOI] [PubMed] [Google Scholar]
- 39.Fossa SD, Slee PH, Brausi M, Horenblas S, Hall RR, Hetherington JW, Aaronson N, de Prijck L, Collette L. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol. 2001;19:62–71. doi: 10.1200/JCO.2001.19.1.62. [DOI] [PubMed] [Google Scholar]
- 40.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22:1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Ryan CJ, Halabi S, Ou SS, Vogelzang NJ, Kantoff P, Small EJ. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 42.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–273. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, Harland S, Robbins A, Halbert G, Nutley B, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi: 10.1038/sj.bjc.6601879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas LN, Douglas RC, Lazier CB, Too CK, Rittmaster RS, Tindall DJ. Type 1 and type 2 5-alpha-reductase expression in the development and progression of prostate cancer. Eur Urol. 2008;53:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 45.Shao TC, Li H, Ittmann M, Cunningham GR. Effects of dutasteride on prostate growth in the large probasin-large T antigen mouse model of prostate cancer. J Urol. 2007;178:1521–1527. doi: 10.1016/j.juro.2007.05.118. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12:4072–4079. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 47.Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Curr Top Med Chem. 2006;6:1205–1214. doi: 10.2174/156802606777812068. [DOI] [PubMed] [Google Scholar]
- 48.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, Heller G, Tong W, Cordon-Cardo C, Agus DB, et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–993. [PubMed] [Google Scholar]
- 49.Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, Nieto M, Du J, Stegmaier K, Raj SM, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 51.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 52.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 53.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 54.Rokhlin OW, Glover RB, Guseva NV, Taghiyev AF, Kohlgraf KG, Cohen MB. Mechanisms of cell death induced by histone deacetylase inhibitors in androgen receptor-positive prostate cancer cells. Mol Cancer Res. 2006;4:113–123. doi: 10.1158/1541-7786.MCR-05-0085. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Meng S, Wang H, Bali P, Bai W, Li B, Atadja P, Bhalla KN, Wu J. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol Cancer Ther. 2005;4:1311–1319. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- 56.Marrocco DL, Tilley WD, Bianco-Miotto T, Evdokiou A, Scher HI, Rifkind RA, Marks PA, Richon VM, Butler LM. Suberoylanilide hydroxamic acid (vorinostat) represses androgen receptor expression and acts synergistically with an androgen receptor antagonist to inhibit prostate cancer cell proliferation. Mol Cancer Ther. 2007;6:51–60. doi: 10.1158/1535-7163.MCT-06-0144. [DOI] [PubMed] [Google Scholar]
- 57.Korkmaz CG, Fronsdal K, Zhang Y, Lorenzo PI, Saatcioglu F. Potentiation of androgen receptor transcriptional activity by inhibition of histone deacetylation--rescue of transcriptionally compromised mutants. J Endocrinol. 2004;182:377–389. doi: 10.1677/joe.0.1820377. [DOI] [PubMed] [Google Scholar]
- 58.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 59.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 60.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Sawyers CL. In: Molecular Biology of Prostate Cancer. edn 8. Devita VT, Hellman S, Rosenberg S, editors. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 63.Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C. Emergence of androgen independence at early stages of prostate cancer progression in nkx3.1; pten mice. Cancer Res. 2006;66:7929–7933. doi: 10.1158/0008-5472.CAN-06-1637. [DOI] [PubMed] [Google Scholar]
- 64.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O'Brien S, Nicaise C, Bleickardt E, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 65.Gross M, Higano C, Pantuck A, Castellanos O, Green E, Nguyen K, Agus DB. A phase II trial of docetaxel and erlotinib as first-line therapy for elderly patients with androgen-independent prostate cancer. BMC Cancer. 2007;7:142. doi: 10.1186/1471-2407-7-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curigliano G, De Braud F, Teresa Sandri M, Renne G, Zorzino L, Scardino E, Rocco B, Spitaleri G, De Pas T, Noberasco C, et al. Gefitinib combined with endocrine manipulation in patients with hormone-refractory prostate cancer: quality of life and surrogate markers of activity. Anticancer Drugs. 2007;18:949–954. doi: 10.1097/CAD.0b013e328136c162. [DOI] [PubMed] [Google Scholar]
- 67.Ziada A, Barqawi A, Glode LM, Varella-Garcia M, Crighton F, Majeski S, Rosenblum M, Kane M, Chen L, Crawford ED. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate. 2004;60:332–337. doi: 10.1002/pros.20065. [DOI] [PubMed] [Google Scholar]
- 68.Agus DB, Sweeney CJ, Morris MJ, Mendelson DS, McNeel DG, Ahmann FR, Wang J, Derynck MK, Ng K, Lyons B, et al. Efficacy and safety of single-agent pertuzumab (rhuMAb 2C4), a human epidermal growth factor receptor dimerization inhibitor, in castration-resistant prostate cancer after progression from taxane-based therapy. J Clin Oncol. 2007;25:675–681. doi: 10.1200/JCO.2006.07.0649. [DOI] [PubMed] [Google Scholar]