Abstract
Objective
Our objective was to investigate whether serum concentrations of a novel anti-angiogenic factor, soluble endoglin (sEng), could predict placental abruption.
Methods
In a nested case control study of nulliparous pregnancies, we examined levels of sEng in serum collected prospectively from 31 women who later developed placental abruption and from 31 normal controls. All serum specimens were collected before the onset of hypertension or abruption and before labor or delivery. Serum sEng was compared within three gestational age intervals: early- (<20 weeks), mid- (21–32 weeks), and late (≥33 weeks) pregnancy.
Results
There was no significant difference in sEng between abruption cases and controls in early pregnancy. sEng was significantly elevated among abruption cases at 21–32 weeks (10.7 versus 5.9 ng/mL, P<0.01). Subgroup analyses revealed no differences in sEng concentrations at any gestational age interval between cases with abruption without hypertension and healthy controls. Among women who developed hypertension and placental abruption, sEng was not significantly increased in early pregnancy, but was in mid-pregnancy (19.3 versus 5.5 ng/mL, P=0.002) and in late pregnancy (15.6 versus 9.5 ng/mL, P=0.04).
Conclusion
Serum levels of the anti-angiogenic factor sEng are elevated prior to the development of hypertension and placental abruption. These elevations are not apparent until the late second trimester (26 – 27 weeks, on average), but they persist from this time in gestation onward. sEng may be useful for identifying pregnant women at risk for abruption and hypertension.
Keywords: Abruptio placentae, preeclampsia, gestational hypertension, endoglin, angiogenic factors
Introduction
Abruptio placentae occurs in approximately 1% of pregnancies (Ananth et al., 1996; Ananth et al., 2005) and is a major risk factor for antepartum fetal death, preterm delivery, and fetal growth restriction, accounting for up to 25% of all perinatal deaths (Ananth et al., 1999; Ananth et al., 2001). While the precise mechanisms underlying placental abruption are unknown, it has been suggested that abruption may result from abnormalities in trophoblastic remodeling of the maternal spiral arteries. Failed transformation of the maternal uterine circulation from a high- to low-pressure system during placental development may predispose to arterial rupture, retroplacental hemorrhage, and premature separation of the placenta from the uterine wall (Dommisse & Tiltman 1992; Eskes 1997).
Angiogenesis is a critical component of normal placental development. Numerous investigators have reported that an imbalance in circulating levels of proangiogenic factors, such as placental growth factor (PlGF) and anti-angiogenic factors, such as soluble fms-like tyrosine kinase (sFlt-1), may be associated with abnormal placentation and predict the development of uteroplacental diseases such as preeclampsia and intrauterine growth restriction (IUGR) (Chaiworapongsa et al., 2005; Levine et al., 2004; Shibata et al., 2005; Taylor et al., 2003; Thadhani et al., 2004). We recently demonstrated that abnormalities in PlGF and sFlt-1 also precede the onset of hypertension and placental abruption (Signore et al., 2006).
Endoglin is a cell-surface co-receptor for transforming growth factors β1 and β3 that has anti-angiogenic effects and is highly expressed in syncytiotrophoblast (Venkatesha et al., 2006). Elevated serum levels of soluble endoglin (sEng) have been demonstrated in women prior to the clinical onset of preeclampsia (Levine et al., 2006).
We hypothesized that sEng concentrations would be elevated prior to placental abruption. We thus examined levels of sEng in serial serum samples collected prospectively from women who later developed placental abruption and from normal controls. These were the same women and specimens in whom we had previously shown that concentrations of PlGF were reduced and sFlt-1 increased before onset of hypertension and placental abruption (Signore 2006).
Materials and Methods
We conducted a nested case-control study of subjects from the Calcium for Preeclampsia Prevention (CPEP) Study. CPEP was a randomized, double-blind clinical trial performed by the National Institute of Child Health and Human Development during 1992–1996 to evaluate the effects of daily supplementation with calcium or placebo on the incidence and severity of preeclampsia. Healthy, normotensive, nulliparous women with singleton pregnancies were enrolled between 13 and 21 weeks gestation at 5 participating U.S. medical centers and followed until 24 hours postpartum, using a common protocol and identical data collection forms. Gestational age was determined by ultrasound examination. Serum specimens were requested from participants at 13–21 wks, at 26–29 wks, at 36 wks, and when hypertension or proteinuria was noted. Many women actually provided samples outside the requested gestational periods; all specimens were included.
Enrollment details for the original study are described elsewhere (Levine et al., 1996). Of 4589 CPEP participants, we excluded 253 who were lost to follow-up, 21 whose pregnancy terminated prior to 20 weeks, 13 who were missing maternal or perinatal outcome data, 4 without smoking information, 9 with hypertension not verified by team chart review, 32 with stillbirths, and one woman whose infant had a chromosomal abnormality, leaving 4257 women with adequate information and a live birth. Of these, 102 were excluded because they lacked a baseline serum specimen; another 524 were excluded because their baseline specimens had label dates more than 2 days following the date recorded by the research nurse on the laboratory specimen forms.
Of the remaining 3631 women, 2469 had remained normotensive throughout pregnancy and had delivered an appropriate- or large-for-gestational age infant. We randomly selected 120 of these women to serve as a pool from which to select normal controls. We identified all 32 women within the CPEP cohort who experienced abruptio placentae (including women with live births and stillbirths), but excluded one woman since her only serum specimen could not be located in the specimen repository. Ten of the 31 women with abruption developed hypertension during their pregnancies – seven, preeclampsia, and three, gestational hypertension. From the pool of normal controls we selected one control subject for each abruption case, matched for clinical center, current smoking status (current smoker or quit after the last menstrual period vs. never smoked or quit before the last menstrual period), and specimen collection pattern. Matching for specimen collection pattern meant that the control must have had at least as many specimens as the case in the following gestational age intervals: <20 weeks, 21–32 weeks, and ≥33 weeks. If more than one control met all matching criteria for a given case, a random selection was made. Once a control was matched to a case, it could not be used as a match for any other case. Because controls had to be matched on several factors and had to have at least as many samples as the cases, we were able to match only one control to each case. All serum specimens collected from subjects prior to the onset of hypertension or proteinuria and before labor or delivery were included in the present study.
Abruptio placentae was clinically diagnosed by physicians participating in the CPEP study, according to the study protocol and manual of operations, and was defined as the presence at delivery after 24 weeks gestation of a retroplacental blood clot and/or antecedent vaginal hemorrhage not associated with vasa previa, placenta previa, or uterine rupture. Evidence of fetal distress and/or sonographic evidence of abruption were present in some cases, but these findings were not required for diagnosis. Hypertension was defined as a diastolic blood pressure of at least 90 mm Hg on two occasions 4 to 168 hours apart in a woman with blood pressure <135/85 prior to CPEP enrollment at 13–21 weeks of gestation. Preeclampsia required, in addition, proteinuria of at least 1+ (30 mg per deciliter) on dipstick testing, each on two occasions 4 to 168 hours apart, a single dipstick indicating ≥2+ proteinuria, a protein / creatinine ratio of 0.35 or greater, or a 24-hour urine collection with >300 mg protein. Gestational hypertension was the de novo onset of hypertension after 20 weeks of gestation in the absence of proteinuria. A small for gestational age (SGA) infant was defined as an infant whose birth weight was below the 10th percentile according to U.S. tables of birth weight for gestational age that account for race, parity, and the sex of the infant (Zhang & Bowes, Jr. 1995).
Never-thawed serum specimens were randomly allocated to batches and analyzed, using an enzyme-linked immunosorbent assay (ELISA) for endoglin according to the manufacturer's instructions (R & D Systems, Minneapolis, MN). The measured interassay coefficient of variation in our laboratory was 12%. All samples were run in duplicate by technicians blinded to pregnancy outcome, and the mean values of the duplicate samples were reported.
Chi-square or t-tests were used in analyses of maternal or infant characteristics to compare categorical or continuous variables, respectively. Although arithmetic mean concentrations of sEng are reported in the text and tables, statistical tests for differences in levels of this factor were performed after logarithmic transformation. Because the number of samples in each group was relatively small, we performed both a matched and an unmatched analysis. In the matched analysis, the unit of analysis was the case-control pair; as such, in any gestational age interval in which one member of the pair had no serum specimen (for example, because she had delivered prior to that interval), no sample(s) from the other member of the pair was/were considered in the analysis. In the unmatched analysis, the unit of analysis was an individual specimen; specimens from each subject were retained in the analysis regardless of the availability of a specimen from the same gestational interval from the matched subject. Since the results of both these analytic methods were similar; the unmatched analysis is presented because the numbers were greater, giving it greater statistical power. All P values are 2-sided; a value of P<0.05 was considered significant. Analyses were conducted using SAS v 9.1 (SAS Institute, Cary, NC).
The Office of Human Subjects Research at the National Institutes of Health granted an exemption from institutional review board approval because data and specimens could not be linked to identifiable women.
Results
Characteristics of the case and control subjects are presented in Table 1. As expected, women with placental abruption were significantly older than controls. They delivered infants at a significantly earlier gestational age so that lower birth weight and preterm birth were significantly more common among infants of mothers with placental abruption. Abruption and control subjects did not differ significantly on race/ethnicity, body-mass index, or blood pressure at enrollment in the CPEP trial (mean 17.6 weeks).
Table 1.
Baseline and delivery characteristics of subjects and their infants
| Placental Abruption (n=31) | Controls (n=31) | |
|---|---|---|
| Values shown are mean ± standard deviation unless otherwise specified. P values are shown only for significant differences. Body mass index =weight in kilograms/height2in meters; LMP, last menstrual period; GH, gestational hypertension. | ||
| Maternal Characteristics | ||
| Age (years) | 22.5 ± 5.7 | 20.2 ± 2.4* |
| Height (cm) | 162.5 ± 7.5 | 165.2 ± 6.1 |
| Weight (kg) | 70.3 ± 17.2 | 70.6 ± 17.6 |
| Body-mass index (kg/m2) | 26.6 ± 6.3 | 25.8 ± 5.9 |
| Systolic blood pressure at enrollment (mm Hg) | 108.0 ± 8.6 | 106.9 ± 10.3 |
| Diastolic blood pressure at enrollment (mm Hg) | 59.4 ± 7.6 | 57.8 ± 7.7 |
| Race/Ethnicity [N (%)] | ||
| White, non-Hispanic | 12 (38.7) | 15 (48.4) |
| White Hispanic | 2 (6.5) | 3 (9.7) |
| Black | 14 (45.2) | 13 (41.9) |
| Other | 3 (9.7) | 0 (0.0) |
| Current smoker or quit after LMP [N (%)] | 12 (38.7) | 12 (38.7) |
| Alcohol use ≥ 1/week [N (%)] | 1 (3.2) | 0 (0.0) |
| Calcium treatment [N (%)] | 16 (51.6) | 17 (54.8) |
| Other pregnancy complications – no. (%) | ||
| Hypertension | 10 (32.3) | 0 (0) |
| GH | 3 (9.7) | |
| Mild preeclampsia | 3 (9.7) | |
| Severe preeclampsia | 4 (12.9) | |
| Preterm premature rupture of membranes | 7 (22.6) | 0 (0.0)* |
| Infant Characteristics | ||
| Gestational age at delivery (wks) | 35.8 ± 3.9 | 39.8 ± 1.3† |
| Birth weight (g) | 2330 ± 940 | 3409 ± 475† |
| Preterm delivery [N (%)] | 19 (61.3) | 1(3.2)† |
| Small for gestational age [N (%)] | 7 (25.9) | 0(0.0)‡ |
| Fetal death [N (%)] | 4 (12.9) | 0(0.0) |
P<0.05
P<0.001
P value not calculated because small for gestational age infants were excluded from the control group.
Maternal serum sEng levels were compared in three gestational age intervals (baseline, ≤ 20 weeks; mid-pregnancy, 21–32 weeks; and late-pregnancy, ≥ 33 weeks; Table 2). At baseline, women who developed placental abruption had somewhat higher sEng levels than normal control women. In mid-pregnancy, the difference became greater, reaching statistical significance (P<.01). Because of the high rate of preterm delivery (61 percent) among abruption cases, relatively few case subjects (N=10) contributed a specimen at ≥ 33 weeks gestation. In late pregnancy, sEng was higher among abruption cases than controls, but this difference did not reach statistical significance.
Table 2.
Soluble endoglin (sEng) levels within three gestational intervals in women who developed placental abruption and in women with normal pregnancy
| Data are presented as N or arithmetic means. ± standard error. P values refer to differences between means after log-transformation. GA, gestational age; SGA, small for gestational age. | ||
| Baseline (≤ 20 wks) | Placental Abruption | Controls |
| N | 29 | 31 |
| GA at collection (d) | 110 ± 4 | 113 ± 3 |
| sEng (ng/mL) | 9.0 ± 2.2 | 6.3 ± 0.3 |
| Mid-pregnancy (21–32 wks) | Placental Abruption | Controls |
| N | 26 | 31 |
| GA at collection (d) | 188 ± 2 | 193 ± 2 |
| sEng (ng/mL) | 10.7 ± 2.0 | 5.9* ± 0.3 |
| Late-pregnancy (≥ 33wks) | Placental Abruption | Controls |
| N | 10 | 27 |
| GA at collection (d) | 255 ± 2 | 258 ± 1 |
| sEng (ng/mL) | 15.0 ± 1.3 | 12.5 ± 1.4 |
P<0.01
Because elevated sEng concentrations have been associated with preeclampsia, we wished to determine whether women with abruption and hypertension would have greater abnormalities in concentration of this antiangiogenic factor. We therefore analyzed sEng concentrations separately for the 10 abruption cases (32 percent) who developed gestational hypertension or preeclampsia during pregnancy and for the 21 cases who remained normotensive (Table 3). sEng levels in normotensive case and control women were similar at all 3 gestational age intervals. In the group of women with hypertension and abruption, however, sEng levels were strikingly higher compared to control women. The differences reached statistical significance despite the modest numbers in mid- (P=0.002) and late (P=0.04) pregnancy.
Table 3.
Soluble endoglin (sEng) levels in women who developed placental abruption and in normal controls, stratified by hypertensive status of cases
| Data are presented as N or arithmetic means ± standard error. P values are for comparison of means after log-transformation. GA, gestational age. | ||||
| Baseline(≤ 20 wks) | Placental Abruption without Hypertension | Controls | Placental Abruption with Hypertension | Controls |
| N | 19 | 21 | 10 | 10 |
| GA at collection (d) | 110 ± 5 | 116 ± 3 | 110 ± 6 | 107 ± 5 |
| sEng (ng/mL) | 6.7 ± 0.5 | 6.5 ± 0.4 | 13.4 ± 6.2 | 5.9 ± 0.4 |
| Mid-pregnancy (21–32wks) | Placental Abruption without Hypertension | Controls | Placental Abruption with Hypertension | Controls |
| N | 18 | 21 | 8 | 10 |
| GA at collection (d) | 190 ± 3 | 193 ± 3 | 186 ± 2 | 192 ± 3 |
| sEng (ng/mL) | 6.9 ± 0.4 | 6.1 ± 0.4 | 19.3 ± 5.5 | 5.5* ± 0.3 |
| Late-pregnancy (≥ 33wks) | Placental Abruption without Hypertension | Controls | Placental Abruption with Hypertension | Controls |
| N | 8 | 18 | 2 | 9 |
| GA at collection (d) | 255 ± 2 | 257 ± 2 | 255 ± 2 | 261 ± 2 |
| sEng (ng/mL) | 14.8 ± 1.6 | 14.0 ± 2.0 | 15.6 ± 2.7 | 9.5** ± 0.9 |
P<0.01
P<0.05
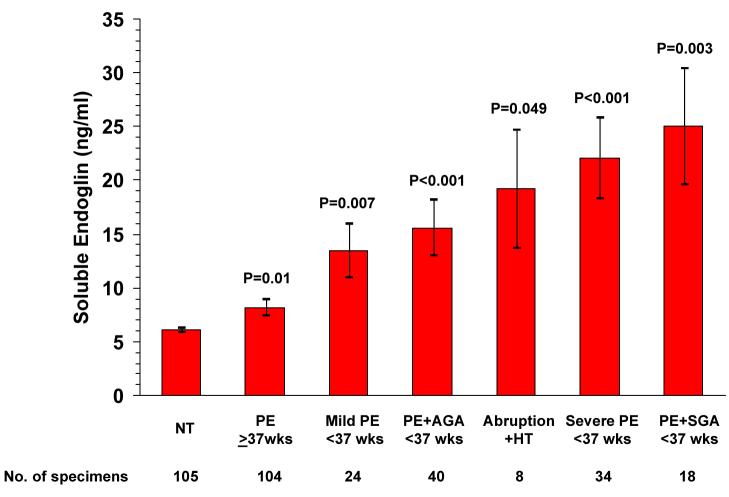
We compared concentrations of sEng at 21–32 weeks among women who later developed abruption and hypertension to sEng concentrations in other CPEP subjects who developed preeclampsia and had sEng measurements within the same gestational interval (Levine et al., 2006 and RJ Levine, personal communication). sEng levels in women with abruption and hypertension were as high as in women who subsequently developed preterm preeclampsia. They were, however, greater than levels in women who developed preeclampsia at term (19.3 vs. 8.2 ng/mL, P=0.05; Figure 1).
Figure 1. Mean Concentrations of Soluble Endoglin According to Preeclampsia Status and Severity.
The figure shows the concentrations of soluble endoglin in serum specimens obtained at 21 to 32 weeks of gestation from normotensive controls (NT) and from women who later developed term preeclampsia (PE≥37wks), mild preterm preeclampsia (Mild PE <37 wks), preterm preeclampsia with delivery of an appropriate- or large-for-gestational-age infant (PE<37 wks +AGA), placental abruption with hypertension (Abruption + HT), severe preterm preeclampsia (Severe PE <37 wks), or preterm preeclampsia with delivery of a small-for-gestational-age infant (PE<37 wks + SGA). The P values given above each bar are for the comparison with normotensive controls. The P value above the bracket is for the comparison of placental abruption with hypertension vs. term preeclampsia. No other comparisons between placental abruption and preeclampsia subgroups approached significance. I bars represent standard errors.
Precise information on the dates of abruption was not available, but in most instances abruption probably occurred very close to delivery. The mean duration from specimen collection within the 21–32 week interval to delivery was 53 days with a range of 16–83 days; and both specimens obtained after 33 weeks of gestation from women with subsequent hypertension and abruption were 14 days from delivery.
Discussion
Our data show that women who will later develop abruptio placentae have higher sEng levels in mid-pregnancy and that levels are elevated most in those who develop abruptio with preeclampsia or gestational hypertension. In this latter group, the elevation was quite striking, reaching statistical significance in mid- and late pregnancy despite the fact that many women with abruptio delivered prematurely and did not provide late pregnancy samples. These findings raise the possibility that sEng, known to be involved in the pathogenesis of preeclampsia and gestational hypertension, may also have a role in the development of abruptio placentae with hypertension in pregnancy. More work is needed to determine whether circulating angiogenic factors reflect a direct contribution of angiogenic factors to the pathogenesis of abruptio placentae or an indirect contribution through the induction of hypertension, a well-known risk factor for abruption.
Only one other study of endoglin concentrations and abruption has been reported to date (Tikkanen et al., 2007). The same commercial assay was used in that study, and the mean sEng concentration in control subjects at an average gestational age of 15.5 weeks (5.5 ng/mL) was comparable to that observed in control subjects from the present study (6.3 ng/mL) in specimens collected at ≤ 20 weeks (mean gestational age 16.1 weeks). However, in contrast to the present study, Tikkanen et al concluded that second-trimester maternal serum sEng failed to predict placental abruption. They also noted that sEng levels in hypertensive women with abruption were not different from those in normotensive women with abruption. Several factors may explain the difference in our findings. In the Tikkanen study, subjects with preeclampsia and small for gestational age infants were included in the control group. As these pregnancy complications have been associated with increased sEng (Levine et al., 2006; Romero et al., 2008), the inclusion of these subjects may have blunted the study's ability to detect a difference in sEng between abruption cases and controls. Additionally, only 4 of the 42 abruption cases in the Tikkanen study had preeclampsia or pregnancy-induced hypertension. With this small number of cases it would be difficult to detect a significant difference in sEng among hypertensive abruption cases. Importantly, Tikannen measured soluble sEng at a single point early in pregnancy (15 – 16 weeks of gestation). In our previous work, we have shown that significant differences in anti-angiogenic factors among women who develop pregnancy complications are rarely apparent prior to 17 weeks of gestation (Levine et al., 2004; Levine et al., 2006; Rana et al., 2007). In the current study, we were able to assess serum sEng at multiple time points in pregnancy, and thus demonstrated that significant elevations in sEng do occur prior to abruption, but later in the second trimester, at 26–27 weeks on average, and that these elevations persist throughout pregnancy. Furthermore, we were able to show that the interval between measurement of sEng at 21–32 weeks of gestation and delivery with abruption and hypertension averaged 7.6 weeks. Thus, abnormalities in circulating sEng occur early enough that they may be useful for the early identification of women at risk.
By comparing soluble endoglin levels reported here to those in a previous publication (Levine et al., 2006), we demonstrated that sEng concentrations at 21–32 weeks in women who later developed placental abruption and hypertension were as elevated as in women who subsequently developed preterm preeclampsia and greater than in women who developed preeclampsia at term. This provides additional evidence linking the extent of abnormalities in circulating angiogenic factors with the severity of clinical manifestations of placental disease (Signore et al., 2006).
Normal placentation requires that chorionic villous cytotrophoblasts acquire an invasive phenotype, enabling them to penetrate and remodel the maternal decidual vasculature. Endoglin appears to play an important role in these processes. Addition of a monoclonal antibody to endoglin or of antisense endoglin oligonucleotides promoted trophoblast outgrowth and migration in placental explant cultures (Caniggia et al., 1997), indicating that endoglin is an important negative regulator of trophoblast invasion and placental development. Endoglin expression is increased on the syncytiotrophoblast of preeclamptic placentae at 25 and 40 weeks compared to age-matched normotensive controls (Venkatesha et al., 2006). Soluble endoglin, a 65 kDa truncated form of endoglin which lacks the transmembrane and cytoplasmic domains, may be produced by the placenta and is known to be elevated in the sera of women with preeclampsia (Venkatesha et al., 2006). sEng inhibits the formation of capillary tubes in vitro and induces vascular permeability and hypertension with focal glomerular endotheliosis in vivo (Venkatesha et al., 2006).
Thus, endoglin and sEng are involved in multiple aspects of placental development, including trophoblast invasion and angiogenesis. sEng appears to be a promising marker for gestational diseases related to poor placentation, such as preeclampsia and placental abruption. We speculate that upregulation of membrane-bound endoglin early in pregnancy, at the time of trophoblast migration and spiral artery invasion, may impair uterine vascular remodeling, leading to persistent high-pressure uteroplacental circulation and placental hypoxia. Upregulation of membrane-bound endoglin causes increased amounts of soluble endoglin to be released into maternal blood.
Some limitations and strengths of our study should be noted. One limitation is the small number of abruption cases; however, abruptio placentae is a rare outcome. The small number of subjects available in our study group during late pregnancy is the result of the high rate of preterm delivery in abruption cases. We were able to analyze samples collected prior to the onset of symptoms in all abruption cases from a large prospective, carefully monitored cohort. Thus, we were able to minimize selection bias. Furthermore, all specimens were obtained before hypertension or placental abruption developed in any of the subjects. Because of this, we were able to demonstrate that the changes in sEng preceded, and were not the consequence of, hypertension or abruptio placentae.
In summary, we have shown that circulating levels of the anti-angiogenic factor sEng are elevated in women who will subsequently develop placental abruption and preeclampsia or gestational hypertension. Abnormal concentrations of sEng in the women who developed placental abruption and hypertension became evident at 26 – 27 weeks, on average, but they persisted from this time in gestation onward. As has been observed with preeclampsia (Levine, et al., 2006), these midgestational abnormalities in sEng concentrations occurred many weeks in advance of clinically apparent disease. Future investigations should explore the possible value of sEng as a marker for abruption risk and as a possible factor in the causal pathway of placental abruption. If our findings are confirmed by others, circulating concentrations of sEng in combination with other variables might be useful as a predictive or diagnostic test for placental disease, including hypertension complicated by placental abruption.
Acknowledgment
Supported by funds from the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. The CPEP trial was supported by contracts (N01-HD-1-3121, -3122, -3123, -3124, -3125, and -3126; N01-HD-3154; and N01-HD-5-3246) with the National Institute of Child Health and Human Development, with cofunding from the National Heart, Lung, and Blood Institute. Dr. Karumanchi is supported by R01 grant HL079594 from the National Heart, Lung, and Blood Institute.
Dr. Karumanchi reports being named coinventor on multiple provisional patents that have been filed by Beth Israel Deaconess Medical Center for the diagnosis and treatment of preeclampsia. These patents have been nonexclusively licensed to several companies. Dr. Karumanchi reports having served as a consultant to Abbott, Beckman Couleter, and Johnson & Johnson.
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282:1646–1651. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Oyelese Y, Yeo L, Pradhan A, Vintzileos AM. Placental abruption in the United States, 1979 through 2001: temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192:191–198. doi: 10.1016/j.ajog.2004.05.087. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309–318. doi: 10.1016/0029-7844(96)00088-9. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Smulian JC, Demissie K, Vintzileos AM, Knuppel RA. Placental abruption among singleton and twin births in the United States: risk factor profiles. Am J Epidemiol. 2001;153:771–778. doi: 10.1093/aje/153.8.771. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of preeclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- Eskes TK. Abruptio placentae. A “classic” dedicated to Elizabeth Ramsey. Eur J Obstet Gynecol Reprod Biol. 1997;75:63–70. doi: 10.1016/s0301-2115(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Esterlitz JR, Raymond EG, DerSimonian R, Hauth JC, Ben CL, Sibai BM, Catalano PM, Morris CD, Clemens JD, Ewell MG, Friedman SA, Goldenberg RL, Jacobson SL, Joffe GM, Klebanoff MA, Petrulis AS, Rigau-Perez JG. Trial of Calcium for Preeclampsia Prevention (CPEP): rationale, design, and methods. Control Clin Trials. 1996;17:442–469. doi: 10.1016/s0197-2456(96)00106-7. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-Like Tyrosine Kinase 1 Is Increased in Preeclampsia But Not in Normotensive Pregnancies with Small-for-Gestational-Age Neonates: Relationship to Circulating Placental Growth Factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- Signore C, Mills JL, Qian C, Yu K, Lam C, Epstein FH, Karumanchi SA, Levine RJ. Circulating angiogenic factors and placental abruption. Obstet Gynecol. 2006;108:338–344. doi: 10.1097/01.AOG.0000216014.72503.09. [DOI] [PubMed] [Google Scholar]
- Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Stenman UH, Nuutila M, Paavonen J, Hiilesmaa V, Ylikorkala O. Failure of second-trimester measurement of soluble endoglin and other angiogenic factors to predict placental abruption. Prenat Diagn. 2007;27:1143–1146. doi: 10.1002/pd.1869. [DOI] [PubMed] [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bowes WA., Jr. Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstet Gynecol. 1995;86:200–208. doi: 10.1016/0029-7844(95)00142-e. [DOI] [PubMed] [Google Scholar]