Abstract
G6PD deficiency is an important cause of hemolytic anemia worldwide. Severely affected patients have chronic hemolysis with exacerbations following oxidative stress. Mutations causing severe chronic non-spherocytic hemolytic anemia commonly cluster in Exon 10, a region important for protein dimerization. An African-American male presented at age two weeks with pallor and jaundice, and was found to have hemolytic anemia with G6PD deficiency. His severe clinical course was inconsistent with the expected G6PD A- variant. DNA sequencing revealed two common mutations (A-) and a third novel Exon 10 mutation. This inherited haplotype represents a novel triple G6PD coding mutation causing chronic hemolysis.
Keywords: hemolytic anemia, G6PD deficiency
Introduction
The glucose-6-phosphate dehydrogenase (G6PD) enzyme is critically important for protecting erythrocytes from oxidative stress and intravascular hemolysis.1 G6PD deficiency is the most common erythrocyte enzymopathy, affecting over 400 million people worldwide.2 G6PD variants are divided into five classes according to severity; the degree of hemolysis is inversely related to residual enzymatic function.2,3,4
Mutations of the X-linked G6PD gene have been identified in >190 different nucleotides, resulting in hundreds of biochemical and genetic variants.2 Over 60 mutations are described as Class I variants, which comprise the most severe form of G6PD deficiency and lead to chronic non-spherocytic hemolytic anemia (CNSHA).4 CNSHA is characterized by chronic hemolytic anemia with intermittent exacerbations of acute intravascular hemolysis after exposure to oxidant stress, and typically occurs with enzyme activity <10% of normal. Rarely, a severe Class I variant will also result in a low G6PD level in circulating neutrophils.3 Due to the short lifespan of these cells and their ability to generate new enzyme, a low G6PD level in neutrophils is especially significant. Class II variants typically have <10% residual enzyme activity, but CNSHA is not present. Class III and IV variants (10–60% and 60–150% activity, respectively) result in milder phenotypes with hemolysis only after extreme oxidative stress.5,6 While mutations leading to G6PD deficiency occur throughout the entire coding region of the G6PD gene, most known mutations leading to CNSHA are clustered in Exon 10 that encodes the presumptive dimerization site, although a few such mutations have been described in Exons 7 and 8.7,8,9,10 CNSHA can occur with as much as 35% residual enzyme activity, therefore the mutation location may be the most consistent feature among Class I variants.11
Approximately 10 percent of African-American males are affected with the Class III variant G6PD A-,11 known to be a clinically mild form of G6PD deficiency that does not cause chronic hemolysis. Enzyme activity levels of approximately 10% in erythrocytes and 100% in neutrophils have been reported.3 We describe an African-American male infant who presented at two weeks of age with pallor, jaundice, and non-spherocytic hemolytic anemia and was found to have a low G6PD level. The patient had a subsequent severe clinical course with recurrent episodes of symptomatic anemia and chronic hemolysis, and a low G6PD level in both erythrocytes and granulocytes, indicating a severe phenotype inconsistent with the expected G6PD A- variant. DNA sequencing revealed an inherited novel third mutation in Exon 10, presumably explaining his severe clinical course and CNSHA. This is the first known description of a triple coding mutation in G6PD deficiency.
Case Report
A first born African-American male infant born at term to healthy parents was evaluated at age two weeks for pallor and jaundice. No splenomegaly was present on physical examination. Laboratory investigation revealed severe anemia with a hemoglobin concentration of 5.0 g/dL with 47% reticulocytes. His birth history was unremarkable. Both mother and baby were blood group and type O negative and the infant had a negative DAT. No spherocytes were identified on peripheral blood smear review. The infant received one packed red blood cell transfusion and subsequent blood counts over the next several months documented hemoglobin levels between 7 and 10 g/dL with elevated absolute reticulocyte counts (ARC) ranging from 280–645 × 109/L.
At age 7 months, the patient developed upper respiratory symptoms, pallor, and icterus and was hospitalized with severe anemia. No splenomegaly or other physical examination abnormality was identified. His hemoglobin concentration was 5.2 g/dL with serum bilirubin elevated at 5.5 mg/dL, and the DAT was negative. The G6PD enzyme level was markedly reduced at 0.5 units/gram hemoglobin (normal 4.6–13.5). He was discharged without transfusion in stable condition, with a presumed diagnosis of G6PD A- and unknown oxidative triggering event. Evaluation one month later revealed ongoing hemolytic anemia with a hemoglobin concentration of 10.7 g/dL and ARC of 282 × 109/L. During the first year of life, he had continued chronic hemolytic anemia with reticulocytosis, unlike the clinically mild course expected with G6PD A-. The child is now 3 years old with baseline hemoglobin concentration of 10–11 g/dL, ARC of 200–300 × 109/L, and total bilirubin of 1.5–2.0 mg/dL. Repeat G6PD enzyme level remains very low at ~1 unit/gram hemoglobin.
Materials/Methods
G6PD DNA sequencing
Exons 2–13 containing all coding regions of the G6PD gene were amplified. Primer sequences are included in a Supplemental Appendix. All 50uL PCR reactions were performed using a Biometra-T Gradient DNA Thermocycler with 50 ng genomic DNA, 2 µM primers, Qiagen HotStar Taq polymerase with 5x Q solution, 10x PCR buffer with MgCl2, and 2 U Taq DNA polymerase. PCR reactions were initially heated for 15 minutes at 95 C°. PCR was performed in the touch-down manner with 66 C° starting annealing temperature. Temperature was reduced by 1 C° each cycle until 57 C°. After ten cycles, the PCR profile was completed with 30 cycles of 30 seconds at 95 C°, 30 seconds at 58 C° (Exons 6–8 at 65 C°), 45 seconds at 72 C°, then 5 minutes at 72 C. Amplicons were analyzed by 2.5% agarose electrophoresis before sequencing using BigDye terminator chemistry (Applied Biosystems, Foster City CA) at the St. Jude DNA sequencing facility.
G6PD activity
Venous blood from the patient was collected in EDTA and centrifuged in lymphocyte separation media. Peripheral blood mononuclear cells were removed after separation and placed in genomic DNA lysing solution. Peripheral blood neutrophils were purified using dextran agglutination as described.12 G6PD assays were performed using quantitative ultraviolet kinetic determination with commercial reagents (Trinity Biotech, St. Louis MO).
Results and Discussion
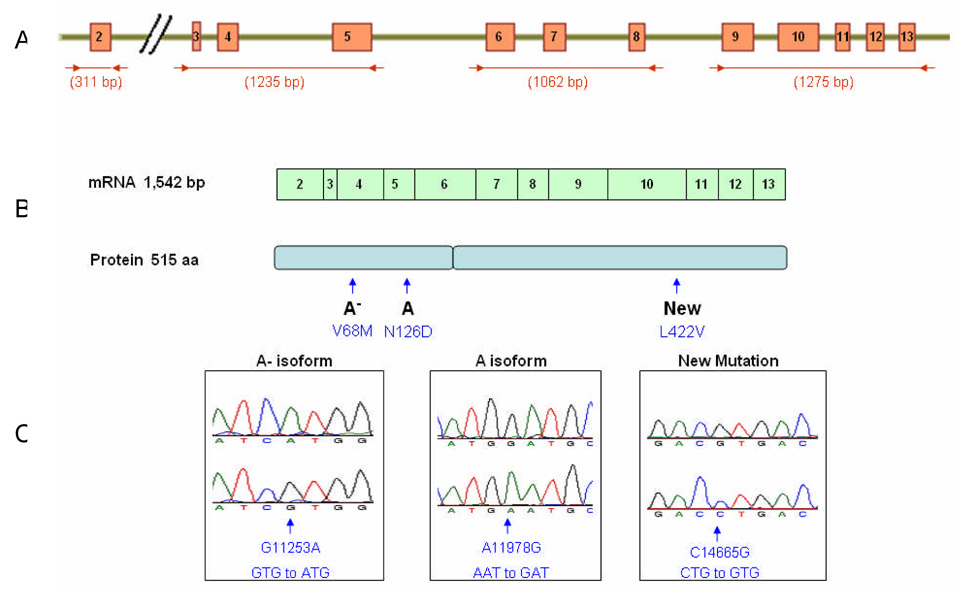
DNA analysis revealed three distinct G6PD coding mutations in this patient (Figure 2). Two previously described mutations were identified: A11978G (N126D) in Exon 5 leading to the common A isoform, and G11253A (V68M) in Exon 4, one of three known mutations leading to the A- variant.13,14,15 A novel third mutation was identified in Exon 10, C14665G, changing leucine to valine at codon 422. This new L422V mutation localizes within the domain involved in G6PD protein dimer formation5 near the NADP+ binding cleft.16 Other nearby G6PD mutations such as Japan (G410N), Atlanta (Y428Stop) and Anaheim (R393H) are also severe Class 1 variants.8,10,13,17 Current evidence suggests the severity of the Class 1 variants results from enzyme instability due to ineffective protein dimerization.4,5,18 The L422V mutation would be presumed, therefore, to have a deleterious effect on G6PD enzyme function. This novel mutation helps explain the CNSHA and reduced enzyme activity in erythrocytes and neutrophils found in our patient. The mother was heterozygous for all three coding mutations, indicating germline transmission of the abnormal haplotype.
Figure 2.
A. Four primer sets allowed sequencing of all coding exons of the G6PD gene. B. The mRNA and protein length are indicated, and the locations of the described mutations within the protein are highlighted. C. Electropherograms illustrate the 3 distinct coding mutations identified in an African-American male with congenital non-spherocytic hemolytic anemia.
There are significant implications to our findings. First, this case illustrates that G6PD screening is typically semi-quantitative and not reliable for distinguishing mild from severe variants. It is critical to follow a patient’s clinical course to determine if hemolysis is present necessitating further evaluation. Second, not all African-American males with G6PD deficiency will necessarily have the mild A- variant.19,20 Despite initial DNA sequencing indicating the presence of two common mutations that lead to the A- variant, we performed complete DNA sequencing because the infant’s clinical course suggested a severe enzyme deficiency. In areas where G6PD deficiency occurs with routine DNA-based newborn screening, 21 this case supports the need for careful clinical correlation.
Supplementary Material
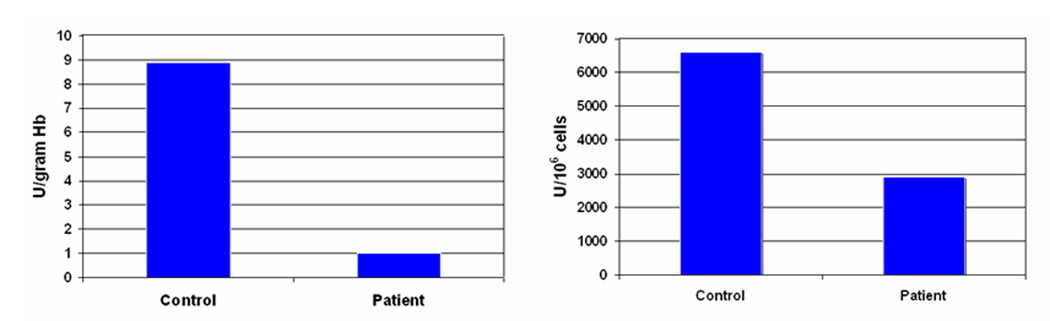
Figure 1.
G6PD level in the RBC (left) and neutrophis (right) of the patient compared to a normal adult control.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Dennis Jay for assistance with the G6PD quantitative assays, and the St. Jude Children’s Research Hospital Hartwell Center core DNA sequencing facility for performing the DNA sequencing. This publication is supported in part by NCI grant T32-CA070089 (JM) and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Fico A, Paglialunga F, Cigliano L, et al. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11:823–831. doi: 10.1038/sj.cdd.4401420. [DOI] [PubMed] [Google Scholar]
- 2.Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician. 2005;7(72):1277–1282. [PubMed] [Google Scholar]
- 3.Mason PJ, Bautista JM, Gilsanz F. G6PD deficiency: the genotype-phenotype association. Blood Reviews. 2007;21:267–283. doi: 10.1016/j.blre.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Fiorelli G, Martinez di Montemuros F, Cappellini M. Chronic non-spherocytic hemolytic disorders associated with glucose-6-phosphate dehydrogenase variants. Balliere’s Clinical Haematology. 2000;13:39–55. doi: 10.1053/beha.1999.0056. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida A, Beutler E, Motulsky AG. Human glucose-6-phosphate dehydrogenase variants. Bull WHO. 1971;45:243–253. [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood. 2008;111:16–24. doi: 10.1182/blood-2007-04-077412. [DOI] [PubMed] [Google Scholar]
- 7.Costa E, Cabeda JM, Vieira E, et al. Glucose-6 phosphate dehydrogenase Aveiro: a de novo mutation associated with chronic nonspherocytic hemolytic anemia. Blood. 2000;95:1499–1501. [PubMed] [Google Scholar]
- 8.Beutler E, Kuhl W, Gelbart T, Forman L. DNA sequence abnormalities of human glucose-6-phosphate dehydrogenase variants. J Biol Chem. 1991;7(266):4145–4150. [PubMed] [Google Scholar]
- 9.Vulliamy TJ, Kaeda JS, Ait-Chafa D, et al. Clinical and haematological consequences of recurrent G6PD mutations and a single new mutation causing chronic nonspherocytic haemolytic anaemia. Br J Haematol. 1998;101:670–675. doi: 10.1046/j.1365-2141.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 10.Vulliamy T, Beutler E, Luzzatto L. Variants of glucose-6-phosphate dehydrogenase are due to missense mutations spread throughout the coding region of the gene. Hum Mutuat. 1993;2:159–167. doi: 10.1002/humu.1380020302. [DOI] [PubMed] [Google Scholar]
- 11.Beutler E. G6PD: population genetics and clinical manifestations. Blood Rev. 1996;10:45–52. doi: 10.1016/s0268-960x(96)90019-3. [DOI] [PubMed] [Google Scholar]
- 12.Norris J, Hall S, Ware RE, et al. Glycosyl-phosphatidylinositol anchor synthesis in paroxysmal nocturnal hemoglobinuria: partial or complete defect in an early step. Blood. 1994:816–821. [PubMed] [Google Scholar]
- 13.Beutler E, Vulliamy TJ. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol. Dis. 2002;28:93–103. doi: 10.1006/bcmd.2002.0490. [DOI] [PubMed] [Google Scholar]
- 14.Town M, Bautista JM, Mason PJ, Luzzatto L. Both mutations in G6PD A-are necessary to produce the G6PD deficient phenotype. Hum Mol Genet. 1992;1:171–174. doi: 10.1093/hmg/1.3.171. [DOI] [PubMed] [Google Scholar]
- 15.Beutler E, Kuhl W, Vives-Corrons JL, Prchal JT. Molecular heterogeneity of glucose-6-phosphate dehydrogenase A. Blood. 1989;74:2250–2255. [PubMed] [Google Scholar]
- 16.Au SWN, Gover S, Lam WMS, Adams MJ. Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP+ molecule and provides insights into enzyme deficiency. Structure. 2000;8:293–303. doi: 10.1016/s0969-2126(00)00104-0. [DOI] [PubMed] [Google Scholar]
- 17.Hirono A, Miwa S, Fujii H, et al. Molecular study of eight Japanese cases of glucose-6-phosphate dehydrogenase deficiency by nonradioisotopic single-strand conformation polymorphism analysis. Blood. 1994;11(83):3363–3368. [PubMed] [Google Scholar]
- 18.Cappellini MD, Martinez di Montemuros F, DeBellis G, et al. Multiple G6PD mutations are associated with clinical and biochemical phenotype similar to that of G6PD Mediterranean. Blood. 1996;87:3953–3958. [PubMed] [Google Scholar]
- 19.Shannon K, Buchanan G. Severe hemolytic anemia in black children with glucose-6-phosphate dehydrogenase deficiency. Pediatrics. 1982;3(70):364–369. [PubMed] [Google Scholar]
- 20.Kay A, Kuhl W, Prchal J, Beutler E. The origin of glucose-6-phosphate dehydrogenase (G6PD) polymorphisms in African-Americans. Am J Hum Genet. 1992;50:394–398. [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Fontaine JM, Freer DE, Naylor EW. Alternative DNA-based newborn screening for glucose-6-phosphate dehydrogenase deficiency. Mol. Genet. Metab. 2005;86:212–219. doi: 10.1016/j.ymgme.2005.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.