Summary
We showed recently that contact of human vaginal epithelial cells (VECs) by Trichomonas vaginalis and incubation with trichomonad proteins in conditioned medium induced expression of VEC genes. We performed 2-D SDS-PAGE followed by MALDI-TOF to identify the major secreted proteins. Based on protein abundance and separation of spots in 2-D gels, 32 major secreted proteins were examined, which gave 19 proteins with accession numbers. These proteins included known secreted cysteine proteinases. In addition, other secreted proteins were enzymes of carbohydrate metabolism, adhesin protein AP65, heat shock proteins, thioredoxin reductase and coronins. We confirmed that the secreted trichomonad proteins induced expression of VEC genes, including interleukin 8 (IL-8), COX-2 and fibronectin. Purified AP65 added to VECs had a pronounced effect only on IL-8 gene expression, which was inhibited in the presence of 12G4 monoclonal antibody to AP65. Moreover, AP65 expressed episomally within epithelial cells was found to enhance the expression of IL-8 and COX-2. This may be the first report of analysis of the secreted proteins of T. vaginalis and of the host epithelial cell response to these proteins and to the prominent adhesin AP65.
Introduction
Trichomonas vaginalis causes trichomonosis, the number one, non-viral sexually transmitted infection (STI) worldwide. This STI is considered a health disparities disease (Sorvillo et al., 1998) that remains poorly studied. Infection by T. vaginalis is associated with serious adverse health consequences to women that include infertility (El-Shazly et al., 2001), atypical pelvic inflammatory disease (Moodley et al., 2002), preterm birth and low birth weight infants (Cotch et al., 1997), and predisposition to cervical neoplasia (Viikki et al., 2000). Trichomonosis among men can cause non-chlamydial, non-gonococcal urethritis (Bennett et al., 1989; Bakare et al., 1999) and, more recently, serum antibody in men to T. vaginalis was found to be related with prostate cancer (Sutcliffe et al., 2006). For both men and women, trichomonosis increases predisposition to HIV seroconversion (Guenthner et al., 2005; Mason et al., 2005; Rughooputh and Greenwell, 2005). These sequelae are especially significant given the astounding high rates and long-term duration of this STI in both women and men (Van Der Pol et al., 2005).
The complex profile of both the parasite and host responses following cytoadherence and during infection remains poorly characterized. We showed that brief contact of trichomonads with vaginal epithelial cells (VECs), but not HeLa cells, produced dramatic changes in parasite morphology, as evident from the parasite transformation from an ellipsoid to amoeboid form (Arroyo et al., 1993), suggesting host-specific signalling of parasites by VECs. Five different parasite surface proteins (AP120, AP65, AP51, AP33 and AP23) mediate adherence (Arroyo et al., 1992; Moreno-Brito et al., 2005), and genes encoding adhesins are upregulated in expression during attachment to VECs (Garcia et al., 2003; Kucknoor et al., 2005a). In a separate study using different forms of parasites grown in culture flasks, α-actinin was shown to be overexpressed in amoeboid parasites compared with batch-cultured ellipsoid trichomonads (Addis et al., 1998).
While overall immune responses during trichomonosis are largely unknown, high levels of interleukin-8 (IL-8) and leukotreine B4 (LTB4) have been found in the vaginal secretions from symptomatic patients with trichomonosis (Shaio et al., 1994; 1995; Shaio and Lin, 1995). There are also reports of IL-8 produced in response to T. vaginalis stimulation by human neutrophils (Ryu et al., 2004) and human monocytes (Shaio et al., 1995). Further, in vitro studies have revealed that IL-8 production is regulated through NF-κB and MAP kinase signalling pathways. Nonetheless, little is known about the host responses resulting immediately from infection by T. vaginalis.
Justifiably, studies have focused on examining the secreted proteins of microbial pathogens (Bumann et al., 2002; Knudsen et al., 2005; Medina et al., 2005; Trost et al., 2005). The secreted proteins may mediate important pathogen-host interactions, including eliciting symptoms, immune evasion and pathogenesis. Much remains to be learned about the trichomonad virulence factors involved in symptomatology and pathogenesis among some women and men. In a recent study, we showed that T. vaginalis adherence to VECs upregulated expression of numerous genes that may play a role in pathogenesis (Kucknoor et al., 2005b). Moreover, trichomonad proteins present in conditioned medium also induced expression of VEC genes. Therefore, because secreted proteins may promote survival of T. vaginalis in the vagina and to better understand the secreted proteins of T. vaginalis that interact directly with the vaginal epithelium during infection, we wanted to characterize these proteins. By two-dimensional (2-D) electrophoresis and MALDI-TOF mass spectroscopy we identified secreted proteins to be proteases, the prominent adhesin AP65, heat shock proteins (HSPs), enzymes of carbohydrate metabolism, thioredoxin reductase and coronins. We further show that secreted proteins induce expression of IL-8, COX-2 and FN-1 in VECs. Purified extracellular AP65 induced expression of only IL-8, and, for the first time, we demonstrate the episomal expression in HeLa cells of AP65 and increased synthesis by host cells of IL-8 and COX-2, suggesting a major role of this adhesin in host responses.
Results
Upregulation of host genes induced by secreted medium
We wanted to confirm that secreted T. vaginalis proteins induaced expression of VEC genes, as before (Kucknoor et al., 2005b). We therefore selected the genes encoding the cytokine IL-8, cyclooxygenase-2 (COX-2), fibronectin (FN1) and monocyte chemoattractant protein (MCP-1). The levels of specific mRNAs of known genes were analysed by semiquantitative reverse transcription polymerase chain reaction (RT-PCR). IL-8, COX-2 and FN-1 are known to be elevated by conditioned growth medium (Kucknoor et al., 2005b). We normalized expression of each gene to VEC glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and compared expression of immortalized MS-74 VECs incubated with the secreted proteins to control VECs handled identically except without added trichomonal extract or with control medium. RT-PCR products after agarose gel electrophoresis and staining are shown in Fig. 1A. Except for the MCP-1 gene, the genes for IL-8, COX-2 and FN1 had two- to threefold increased amounts of RT-PCR product compared with controls. As before, the MCP-1 gene was not induced by the secreted proteins, and this suggests that only parasite contact with VECs (Kucknoor et al., 2005b) elicits a complete host response. As a control, medium alone gave a basal level expression of the four genes. These data suggest that secreted proteins and/or contact by T. vaginalis (Kucknoor et al., 2005b) induce host cell responses. Figure 1B presents the levels of increased expression relative to GAPDH as quantified by the Scion image beta program, further affirming the net increased expression compared with the controls. These data show that trichomonad proteins from cell-free supernatants signal for elevated expression of VEC genes.
Fig. 1.
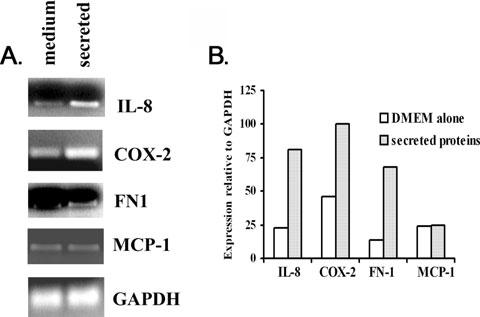
Representative results from a semiquantitative RT-PCR experiment showing induction of host gene expression patterns stimulated by T. vaginalis secreted proteins. Total RNA from MS-74 VECs incubated with growth medium alone or with secreted proteins was isolated (Experimental procedures). RNA was reverse-transcribed using oligo(dT) primer and PCR performed using gene-specific primers.
A. Showing the RT-PCR products separated on ethidium bromide (EtBr)-stained gels after electrophoresis in 2% agarose.
B. Illustrating the extent of gene expression pattern relative to the housekeeping GAPDH gene used as a control. The values were obtained by scanning the intensity of bands from pictures of the gels in A using the Scion image beta program. The expression for each gene was relative to baseline density for GAPDH plotted on the graph. This experiment was repeated on four separate occasions with similar results.
Visualization and evaluation of secreted proteins
To visualize and evaluate the quality of the secreted proteins, we then electrophoresed the proteins by SDS-PAGE. Figure 2A shows protein profiles after electrophoresis of total trichomonal lysate (T, lane 1), and of TCA-precipitated secreted proteins (S), and of TCA-precipitated proteins secreted by parasites adherent to VECs (S1). Not unexpectedly because of the known upregulated expression of trichomonad proteins by contact with VECs (Kucknoor et al., 2005a), the intensity of some secreted proteins was increased after contact compared with the secreted proteins of parasites alone. Under these conditions, control supernatant from VECs alone had few detectable proteins (data not shown). As a control to monitor contamination of secreted proteins resulting from trichomonal lysis, duplicate gels were blotted onto nitrocellulose (Fig. 2B) and probed with mAbs specific to α-tubulin and L-64 to a 30 kDa cytoplasmic protein (Alderete et al., 1987). Proteins corresponding to α-tubulin and to the 30 kDa cytoplasmic protein were detected only in the trichomonal lysate (lanes 1 and 3) but not in the secreted protein preparation (lanes 2 and 4), indicating maintenance of parasite integrity during growth.
Fig. 2.
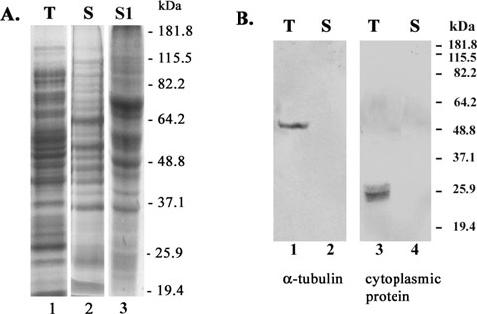
The secreted proteins of T. vaginalis. Preparations of total protein from whole cell lysates (T), secreted proteins (S) and secreted proteins by parasites adherent to VECs (S1) were separated by SDS-PAGE using 10% acrylamide gels.
A. Presenting the complex patterns of proteins after Coomassie brilliant blue staining of gels.
B. Showing the immunoblots probed with mAbs to α-tubulin and L64 to a 30 kDa cytoplasmic protein. Numbers on the right side refer to electrophoretic mobilities of protein standards in kDa.
Two-dimensional SDS-PAGE of secreted proteins and protein identification
Figure 3 presents a representative 2-D profile of total secreted proteins. This pattern after 2-D electrophoresis was considerably less complex than that seen in total protein preparations (Alderete and Garza, 1984; Alderete et al., 1986; Khoshnan et al., 1994). Protein spots arbitrarily chosen based on the protein abundance and clear spot separation for MALDI-TOF mass spectroscopy analysis are indicated by the numbered arrows. Table 1 lists the proteins, and as can be seen, a total of 32 spots analysed yielded the identity of 19 proteins. We had difficulty identifying proteins < 30 kDa because of the presence of multiple species within the digested peptides used for Mascot analysis. Among the major secreted proteins were several enzymes of carbohydrate metabolism, two cysteine proteinases, the adhesin AP65, an endoplasmic reticulum HSP, cytoplasmic HSP, an enzyme involved in arginine metabolism, thioredoxin reductase and cytoskeleton-related proteins actin and coronins. In the spot 4 region of the 2-D gel, there are other AP65 proteins that have been recently characterized on 2-D gels that react with the 12G4 mAb (Garcia et al., 2003). These data are consistent with our earlier report on the complex profile of secreted or released proteins in cell-free culture supernatants under conditions where no lysis is detected (Alderete and Garza, 1984).
Fig. 3.
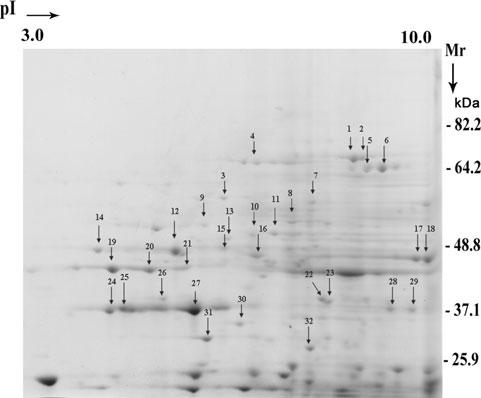
The secreted proteins ofT. vaginalis analysed by 2-D SDS-PAGE. The proteins were precipitated by TCA (Experimental procedures) for electrophoresis, and visualization of spots was by Coomassie brilliant blue staining of gels. Thirty-two proteins in the range of pH 3-10 were randomly chosen for characterization and identification by MALDI-TOF mass spectroscopy and are indicated on the gel by numbered arrows.
Table 1.
List of proteins corresponding to numbered spots in two-dimensional SDS-PAGE analysis of secreted proteins.a
| Spota | Protein IDb | MW | pI | Accession number | Peptidesc |
|---|---|---|---|---|---|
| 1, 2, 3d | Endoplasmic reticulum HSP 70 | 68253 | 5.1 | 3510734 | 24, 17, 26 |
| 4 | Adhesion protein AP65-1 | 63269 | 7.2 | 687630 | 14 |
| 5, 6 | Cytoplasmic HSP 70 | 70259 | 5.5 | 3510736 | 21, 23 |
| 7 | Unknown | 54346 | 5.5 | 851069 | 12 |
| 8 | Enolase | 51283 | 5.8 | 58429954 | 11 |
| 9, 10, 11 | Coronin | 37427 | 5.4 | 7160326 | 12, 21, 8 |
| 12 | Coronin | 48174 | 5.5 | 7160324 | 11 |
| 13 | Chaperonin subunit CCT zeta | 57974 | 6.0 | 10567604 | 13 |
| 14 | Malic enzyme | 42501 | 5.9 | 33243008 | 11 |
| 15 | Glyceraldehyde-3-phosphate dehydrogenase | 39085 | 6.8 | 539406 | 15 |
| 16 | Elongation factor 2 | 21 | |||
| 17, 18 | Actin | 40680 | 5.2 | 1480822 | 19, 11 |
| 19-21 | Alcohol dehydrogenase 1 | 34171 | 6.3 | 21217455 | 17, 12, 10 |
| 22, 23 | Cysteine protease | 34641 | 6.4 | 452294 | 21, 16 |
| 24-27 | Fructose-1-6-biphosphate aldolase | 36239 | 5.8 | 14719270 | 33, 21, 4 |
| 28 | Cysteine protease | 31164 | 6.6 | 452296 | 11 |
| 29 | Unknown | 38437 | 5.5 | 7677551 | 8 |
| 30 | Carbamate kinase | 33906 | 5.6 | 4105707 | 17 |
| 31, 32 | Thioredoxin reductase | 32348 | 6.0 | 23095907 | 17 |
The numbered protein designations are those presented in Fig. 2.
Protein ID refers to the known protein identity determined by Mascot search using Swiss-Prot database.
Peptides refers to number of peptide digests used in the analyses and multiple numbers indicates the peptides used for identifying the proteins that gave multiple spots in the two-dimensional analysis.
Multiple numbers indicate spots where the MALDI-TOF analysis gave amino acid sequences to identical proteins with the same accession numbers.
ID, identity of protein; pI, isoelectric point; HSP, heat shock protein.
Upregulation of host genes by purified AP65
As reported by others (Addis et al., 1997), AP65 was detected in the secreted protein preparation (Table 1). Because adherence is mediated by the prominent adhesin AP65 (Garcia et al., 2003; Mundodi et al., 2004), and parasite contact induces signalling of VEC genes (Kucknoor et al., 2005b), we hypothesized that purified native AP65 signalled VECs for induced expression of genes. Figure 4A shows the immunoblots probed with 12G4 mAb to AP65 purified from a trichomonad lysate (lane 1) versus the secreted proteins (lane 2). VECs were incubated with purified AP65 for 2 h, and RNA from the VECs was isolated. Figure 4B presents agarose gels and Fig. 4C shows the Scion image quantification of semiquantitative RT-PCR products after electrophoresis. Five-fold higher amounts of IL-8 transcript were detected in VECs incubated with AP65 compared with control. Incubation of VECs with purified AP65 in the presence of 12G4 mAb resulted in control amounts of IL-8 transcript (Fig. 4B, lane 3), suggesting IL-8 induction by AP65 signalling. Interestingly, there was only a slight increase in band intensities after agarose electrophoresis (Fig. 4B) and Scion image quantification (Fig. 4C) for genes encoding for COX-2 or FN1, which were not reduced in the presence of 12G4 mAb (not shown), perhaps suggesting non-specific effects. The GAPDH gene control confirms that equal amounts of RNA were added to the samples for RT-PCR (Fig. 4B). We then confirmed that elevated levels of IL-8 mRNA correlated with amounts of IL-8 (Fig. 4D). We quantified IL-8 by enzyme-linked immunosorbant assay (ELISA) (Experimental procedures) and found 176 pg ml-1 after 60 min incubation with AP65, which was reduced to control amounts (94 pg ml-1) in the presence of 12G4 mAb. A similar trend was seen for the 30 min time point. These data suggest strongly that AP65 signals VECs for increased IL-8 gene expression.
Fig. 4.
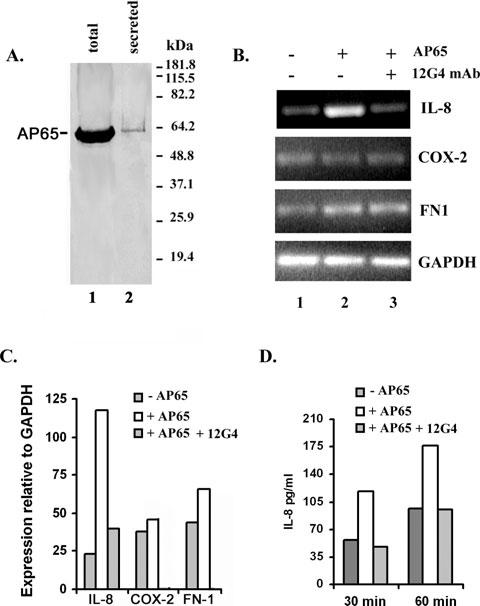
Induction of host gene expression by addition of purified AP65 to VECs.
A. Showing the detection of AP65 in total protein lysates of T. vaginalis (lane 1) versus the secreted proteins (lane 2). Lysates were prepared from whole cells and secreted supernatant as described in Experimental procedures prior to SDS-PAGE and blotting onto nitrocellulose membranes. The blot was probed with the 12G4 mAb to AP65 (Garcia et al., 2003). The numbers indicate the molecular weight standards in kDa.
B. Showing representative results from one of four experiments. EtBr-stained bands after agarose electrophoresis of RT-PCR products for the four genes show the increased expression of IL-8 transcript (lane 2). GAPDH is included as the internal control unaffected by AP65 and to show that identical amounts of RNA were added to each reaction.
C. Illustrating quantitatively the gene expression pattern relative to the GAPDH gene as analysed by the Scion image beta program.
D. Showing the effect of AP65 on IL-8 production. Samples included confluent monolayers of control VECs or VECs stimulated with purified AP65. Additionally, VECs were treated with AP65 and 12G4 mAb. At selected times after incubation, supernatants were collected and assayed for IL-8. Four independent experiments were carried out and shown here is a representative experiment with the mean of quadruplicate samples. Samples did not vary more than 5% of the mean.
Transient transfection of T. vaginalis AP65 in HeLa cells
We performed indirect immunofluorescence using 12G4 mAb to determine if AP65 is internalized after incubation with purified AP65 or adherence by T. vaginalis. Figure 5A shows that AP65 is in fact detected in permeabilized VECs in both conditions (panels a1 and a2). Similar fluorescence is neither seen with non-permeabilized VECs after removal of adherent organisms nor with VECs alone (panel a3). Alexa Fluor 568-conjugated goat anti-mouse IgG secondary antibody with or without treatment with 12G4 mAb had no fluorescence in controls (panel a3). Based on this result, we hypothesized that transfection with AP65 would provide additional evidence and an alternative way to assess its role in host cell signalling for increased expression of genes without interference by other factors, as has been done in another microbial model (Kim et al., 2005). Therefore, we transfected HeLa cells and confirmed episomal synthesis of AP65 by immunoblotting and probing with 12G4 mAb. AP65 is detected on nitrocellulose blots of total proteins of transfected HeLa cells after SDS-PAGE (Fig. 5B, lane 2) compared with no bands in control cells or cells with plasmid without insert (lane 1). The multiple bands were the result of partial degradation of AP65 by HeLa cells. Figure 5C illustrates the intensity of RT-PCR products after agarose electrophoresis and staining to detect the transcript levels of the three select genes, as above (Fig. 4). Greater than twofold expression was apparent for both IL-8 and COX-2, but not FN-1, as evident by both intensity of bands and Scion image scan comparisons (Fig. 5C and D). These data suggest different signalling pathways depend on the presentation of parasite proteins.
Fig. 5.
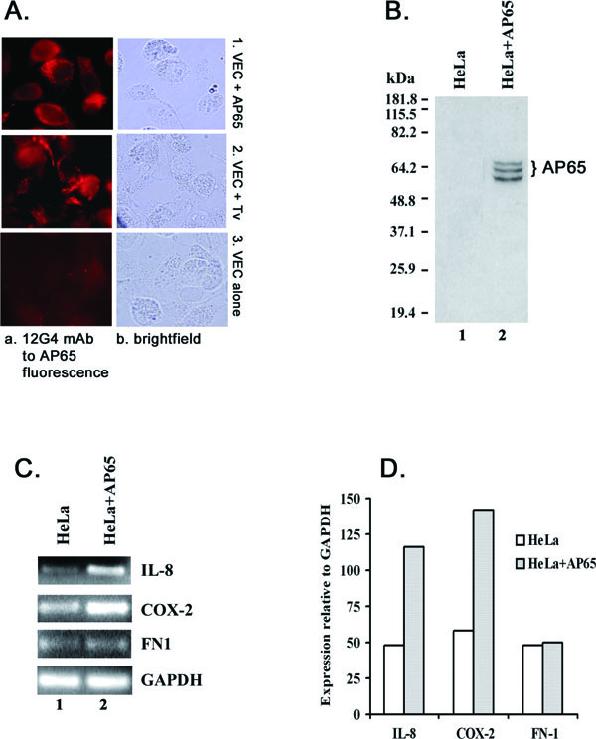
Detection of AP65 in VECs incubated with purified AP65 and live trichomonads (A and B) and induction of host gene expression in HeLa cells expressing AP65 (C and D).
A. Shows the photomicrographs of VECs from immunofluorescence using 12G4 mAb. VECs were grown on chambered culture slides and incubated with purified AP65 (panel a1) or with live T. vaginalis organisms (panel a2) for 40 min and washed well (Experimental procedures). VECs were fixed and permeabilized before incubation with mAb. The immunostained cells were observed by microscopy in oil immersion with a final magnification of 1000×.
B. Showing the nitrocellulose blot after SDS-PAGE of total proteins from control (lane 1) and transfected HeLa cells (lane 2) probed with 12G4 mAb. HeLa cells were transiently transfected with the gene encoding ap65-1. The multiple bands are the result of degradation of AP65 in the cells. No immunocrossreactive proteins were ever detected in immunoblots of control HeLa cell total proteins or control cells and cells transfected with plasmid without insert.
C. Illustrating the PCR products after agarose gel electrophoresis and EtBr staining of thetranscripts of the four genes of HeLa cells episomally expressing AP65.
Part D presents quantitative Scion image scans relative to GAPDH.
As we detected higher transcript levels of IL-8 and COX-2 mRNAs in AP65-transfected HeLa cells, it was important to confirm elevated amounts of protein. We performed immunofluorescence to detect AP65 and COX-2 in the transfected HeLa cells. As seen in Fig. 6A, the synthesis and cytoplasmic localization of AP65 within transfected cells was evident (panel 1b), and COX-2 was readily visualized in the perinuclear region in transfected HeLa cells (panel 2b). Neither control HeLa cells labelled individually with mAbs to AP65 or COX-2 nor transfected HeLa cells without primary antibody handled identically had detectable fluorescence. Likewise, cells transfected with plasmid without insert were non-reactive by fluorescence. Finally, we then showed a threefold increase in the amount of IL-8 synthesized in transfected cells compared with the control (Fig. 6B). These data demonstrate that upregulation of IL-8 and COX-2 gene expression is related to synthesis of AP65 in the transfected cell.
Fig. 6.
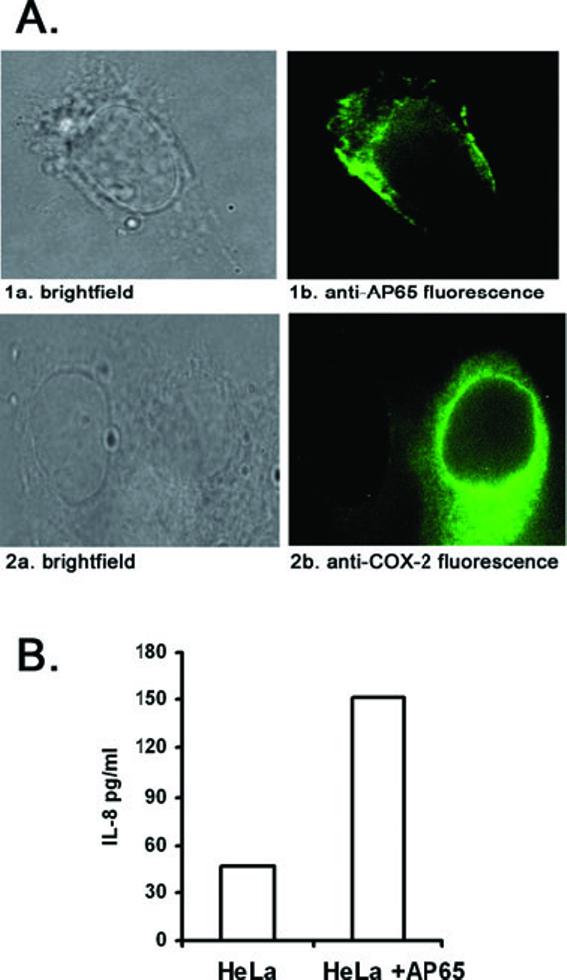
Detection of AP65, COX-2 and IL-8 protein expression in AP65 transiently transfected HeLa cells.
A. Shown is of HeLa cells transfected with the plasmid, and after 24 h, transfected cells were washed well and incubated with either 12G4 mAb to AP65 (panel 1b) or COX-2 mAb (panel 2b) followed by FITC-conjugated anti-mouse secondary IgG antibody. The immunostained cells were observed by oil immersion microscopy at 1000 × final magnification. Panels 1a and 2a are brightfield microscopy of the identical cells presented in the fluorescence panels. No fluorescence was detected in the presence of either mAb or with secondary antibody in control HeLa cells.
B. Showing the quantification of IL-8 by ELISA (Experimental procedures) in culture supernatants of transiently transfected HeLa cells. Four independent experiments were carried out and shown here is a representative experiment with the mean of quadruplicate samples. Samples did not vary more than 5% of the mean.
Discussion
The non-self-limiting nature of trichomonosis occurs in the exceedingly complex and constantly changing urogenital tract of women. The menstrual cycle with the fluctuations in pH, iron and other nutrients and the desquamation of the VECs may represent external cues by which the infecting T. vaginalis organisms respond. Trichomonads must penetrate the mucus layer (Lehker and Sweeney, 1999) before contact with VECs (Arroyo et al., 1992) and the possible penetration into the basement membrane for binding to extracellular matrix proteins (Alderete et al., 2002). Trichomonal cytotoxicity of vaginal and cervical epithelial cells and the vaginal discharge following infection may be critical obstacles for successful host colonization by the parasite. It is known that secretions of patients contain numerous trichomonad proteins, including high molecular weight immunogenic proteins (Alderete et al., 1991) and cysteine proteinases (Alderete and Provenzano, 1997). Further, that numerous proteins are readily released and/or secreted during growth and multiplication without lysis of organisms has been established (Alderete and Garza, 1984). Recently, we showed that parasite contact with VECs induced expression of numerous VEC genes (Kucknoor et al., 2005b). Further, we found that spent supernatant from overnight-grown batch culture induced expression of genes after incubation with immortalized VECs as did live trichomonads. Therefore, it seems reasonable to hypothesize that secreted proteins are important mediators of host and parasite responses to infection that may promote either or both parasitism and pathogenesis.
Given the complex total protein composition of T. vaginalis by 2-D analysis (Alderete et al., 1986; Provenzano and Alderete, 1995) and the characterization of proteins released by trichomonads into cell-free culture supernatants (Alderete and Garza, 1984), we predicted that the profile of secreted proteins would be complex. From the complex 2-D patterns (Fig. 3), 32 major spots were characterized, which represented 19 unique proteins with corresponding accession numbers (Table 1). Most of these spots represent proteins with molecular weights to known sequences, indicating that, despite the known presence of cysteine proteinases, there was diminished or no degradation of the secreted proteins. Importantly, the proteins were not released due to damaged or lysed organisms, as before (Alderete and Garza, 1984), indicating that the 2-D patterns are an accurate portrait of the overall secreted proteins.
It is intriguing that six secreted proteins among those characterized are metabolic enzymes, and this includes AP65 with identity to the decarboxylating malic enzyme (Engbring et al., 1996). This finding is in agreement with the work of others on the release of AP65 in culture supernatants (Addis et al., 1997). Indeed, that this adhesin-enzyme, and not other proteins of the hydrogenosome organelle, is a member of the secreted protein family supports the fact that lysis is not responsible for proteins in growth medium. This confirms that AP65 is not solely a protein of the hydrogenosome organelle, and in fact it has been established that in fresh clinical isolates, iron upregulates expression of AP65 (Lehker et al., 1991) and other adhesins, and also modulates compartmentalization of these proteins outside the hydrogenosome organelle (Garcia et al., 2003).
More recently, we showed the trichomonad enzymes enolase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), among others, to be upregulated in expression following cytoadherence (Kucknoor et al., 2005a). Interestingly, GAPDH, fructose bis-phosphate aldolase (FBA), enolase and malate dehydrogenase have also been identified in the secreted proteomes of Plasmodium falciparum (Vincensini et al., 2005) and Schistosoma mansoni (Knudsen et al., 2005). Moreover, FBA, and GAPDH are highly immunogenic proteins effective in eliciting protection against Streptococcus pneumoniae (Ling et al., 2004), S. mansoni (Argiro et al., 2000) and Onchocerca volvulus (McCarthy et al., 2002). The literature is now replete with reports of microbial pathogens with anchorless, surface-associated metabolic enzymes (SAEs) (Alderete et al., 2001), and SAEs have functional diversity.
Microbial pathogens secrete proteins involved in the modification of disulphide bonds. Helicobacter pylori, for example, appears to modulate the disulphide bonds in its surroundings (Bumann et al., 2002), and, as suggested, the secretion of thioredoxin may play a role through changing the viscosity of mucus (McGee et al., 2006). We now show that thioredoxin reductase that may likewise be involved in the modification of protein disulphide bonds is secreted. Protein disulphide isomerase (PDI) was also found to be upregulated in trichomonads after contact with VECs (Kucknoor et al., 2005a). Thus, altering disulphide bonds within the vaginal microenvironment during infection may promote long-term colonization in numerous ways. In addition, coronins constitute an evolutionary family of WD-repeat containing actin-binding proteins, which are cytoskeletal and membrane trafficking multifunctional regulators (Rybakin and Clemen, 2005). The amoeboid transformation of trichomonads upon cytoadherence requires cytoskeleton rearrangements, and thus the combined role for actin, coronins and HSPs as chaperones seems logical. Interestingly, actins and HSP70 are also secreted and delivered to the erythrocyte surface in P. falciparum (Vincensini et al., 2005) and to the parasite tegument in S. mansoni (Knudsen et al., 2005). Lastly, carbamate kinase, an enzyme involved in arginine metabolism is also secreted. Interestingly, this pathway is largely restricted to prokaryotes and has been found only in the two primitive eukaryotes Giardia lamblia (Edwards et al., 1992) and T. vaginalis (Linstead and Cranshaw, 1983). The carbamate kinase gene represents a complex genetic history that spans the evolutionary time period from the archaea to the primitive eukaryotes Giardia and Trichomonas (Minotto et al., 2000). Importantly, the absence of any human orthologues to this gene and its importance as a secreted metabolic enzyme in T. vaginalis makes carbamate kinase a primary target for novel drug design and/or a vaccine candidate with any limited side-effects in host.
We searched for known eukaryotic signal sequences among the secreted proteins using the SPdb, a signal peptide database (Choo et al., 2005). Given the high occurrence of lateral gene transfer in T. vaginalis (de Koning et al., 2000), we felt it appropriate to also search for a bacterial LPXTG motif or choline-binding repeats and/or secretory pathways associated with these secreted proteins. No signal peptide sequences were found. It is therefore conceivable that these metabolic enzymes are secreted from the parasites and are re-associated on the surface of the pathogen, thereby functioning as virulence factors by binding to host proteins (Pancholi and Chhatwal, 2003) and/or modifying host proteins to activate the signalling cascade to enable the progression of pathogenesis. Finally, it will be of interest whether the secreted metabolic enzymes in the trichomonad secreted protein family have alternative functions yet to be determined.
We now show that purified AP65 incubated with VECs increased expression of only IL-8 and not COX-2 and FN1, two genes upregulated by parasite contact with VECs (Kucknoor et al., 2005b). Moreover, the dramatic increased IL-8 synthesis confirms the earlier report by us (Kucknoor et al., 2005b) as well as work by others that patients have elevated levels of IL-8 in secretions (Shaio et al., 1994). It is possible that the extent of IL-8 found in secretions of patients (Shaio et al., 1994) is a function of the presentation to host cells of AP65, and this would have a pronounced effect on the extent of lymphocytic infiltration in vaginal secretions. A recent report showed episomal expression of the H. pylori CagA in gastric epithelial cells gave signalling for IL-8 induction (Kim et al., 2005). We therefore hypothesized that episomal AP65 within HeLa cells, that also accommodate T. vaginalis adherence, would likewise induce IL-8 expression and give results without interference from unknown factors thereby be a more direct means of establishing a role for AP65 in host cell signalling. Importantly, during the course of this study, we verified the synthesis and cytoplasmic localization of AP65 within the transfected HeLa cells. Indeed, increased expression of both IL-8 and COX-2 was observed in these transfected cells (Fig. 5). These data provide evidence that different signalling pathways for expression of host genes result from presenting parasite proteins externally or within the host cells. Although requiring further experimental verification, that host cells during infection may sequester intracellularly parasite proteins is not inconceivable.
In our earlier report (Kucknoor et al., 2005b), we concluded that T. vaginalis organisms possessed some soluble factor to induce COX-2 gene expression in VECs. These data now indicate that compared with intracellular AP65 (Fig. 5), extracellular AP65 requires additional factors for signalling for optimal COX-2 gene expression (Fig. 1). We confirmed the presence of COX-2, which was readily visualized in the perinuclear region in ap65-transfected HeLa cells (Fig. 6). That AP65 affects the expression of cytokines or other growth factors that in turn induce COX-2 cannot be discounted. Further, COX-2 and DAD1, a member of Bcl2 gene family (Cory et al., 2003), which is also upregulated by trichomonads (Kucknoor et al., 2005b), are antiapoptotic. As such, these observations may be relevant in light of the non-self-limiting nature of trichomonosis, the significance of which may be related to cervical cancer (Yap et al., 1995; Zhang et al., 1995; Sayed el-Ahl et al., 2002) and prostate cancer (Sutcliffe et al., 2006). Therefore, these observations make evident the need to continue to characterize this family of secreted proteins of T. vaginalis.
Because parasite contact with VECs also upregulates expression of numerous trichomonad genes, including the prominent adhesin AP65 (Mundodi et al., 2004) and metabolic enzymes (Kucknoor et al., 2005a), it seemed reasonable to expect that the preparation of secreted proteins would be complex in total number of proteins. Given the fact that a functional trichomonad protein, like the AP65 adhesin (Arroyo et al., 1993; Garcia et al., 2003), induces VEC gene expression (Figs 4-6), it is also conceivable that other secreted proteins play important roles in the biology of this host-parasite interaction. This is the first attempt to characterize the secreted proteins of T. vaginalis. Much remains to be learned about the parasite virulence factors involved in symptomatology and pathogenesis among some women and men. Characterization of the secreted proteins may lead to an understanding of soluble factors in secretions of patients that play important roles in modulating the overall host response to trichomonosis, as evidenced by the role of AP65 in IL-8 and COX-2 upregulation of gene expression.
Experimental procedures
Parasites and host cells
Trichomonas vaginalis isolate T016 was grown in trypticase-yeast extract-maltose (TYM) medium supplemented with 10% heat-inactivated horse serum at 37°C (Diamond, 1957). Mid-to late-logarithmic phase trichomonads were used for all experiments. Immortalized MS-74 human VECs and HeLa epithelial cells in confluent monolayer cultures were prepared for adherence experiments, as before (Garcia et al., 2003). These epithelial cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) medium supplemented with 10% fetal bovine serum, at 37°C in presence of 5% CO2.
Precipitation of extracellular proteins
Trophozoites (107 ml-1) at ∼18 h of growth were resuspended in RPMI medium (Invitrogen) and incubated for an additional 1 h at 37°C. Parasites were monitored throughout the growth and incubation periods to assure absence of cell lysis and viability using trypan blue exclusion assay and microscopic observations. Further, the protocol used here was from our earlier work, which carefully demonstrated that the soluble trichomonad proteins in cell-free culture supernatants was not due to lysis of parasites by various criteria (Alderete and Garza, 1984). Briefly, supernatant was clarified by gentle centrifugation at 500 g at 4°C, as before (Alderete and Garza, 1984). The resulting supernatant was filtered through a 0.22-μm-pore-size filter to remove insoluble debris. Filtered supernatant was immediately precipitated using 10% TCA (w/v) and incubated overnight at 4°C. The precipitate was centrifuged for 10 min at 10 000 g. The pellet was washed twice in 10 ml of acetone and air dried.
Two-dimensional sodium dodecylsulphate-polyacrylamide gel electrophoresis (2-D SDS-PAGE)
Isoelectric focusing gels and total protein samples were prepared using standard protocols (Hochstrasser et al., 1988). Protein samples were solubilized for 30 min at room temperature in 125 μl of rehydration buffer (9.8 M urea-100 mM DTT-4% CHAPS, and 0.2% Bio-lytes pI 3-10 in 0.001% bromophenol blue). For the resolution of protein samples, a Protean IEF Cell System (Bio-Rad) was used. One hundred μg of protein was separated on 11-cm immobilized pH gradient (IPG) strips (Bio-Rad) with a 3 to 10 linear pH gradient. IEF was conducted at 20°C for 12 h at a maximum voltage of 8000 V and maximum current of 50 μ amp/gel. The IPG strips were equilibrated twice with SDS equilibration buffer (3M urea, 2% SDS, 1% DTT and 10% glycerol in 125 mM Tris-HCl, pH 8.8). The strips were then transferred to precast 10% Tris-Glycine SDS-PAGE gels (Bio-Rad). Gels were always in triplicate, and all gels used samples from three independent batches of cell culture supernatants.
MALDI-TOF/MS analysis
Identification of protein spots after 2-D SDS-PAGE was accomplished by mass spectrometry. Protein spots were visualized by staining of gels with Bio-Safe Coomassie (Bio-Rad) and imaged with the GS-800 densitometer (Bio-Rad). Thirty-two protein spots were selected for analysis based on protein abundance and clear separation of individual spots. Selected spots were excised with a biopsy needle in situ digestion with trypsin, according to standard protocols (Shevchenko et al., 1996). The resulting digests were analysed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS) using an Applied Biosystems Voyager-DE STR (Framingham, MA) operated in reflector mode using delayed extraction. The peptide mass maps produced by MALDI-TOF/MS were searched against published databases using Mascot (Matrix Science) to provide information about the identity of the protein(s) in each spot. Sequence information and characterization of selected digests were accomplished with capillary-HPLC-electrospray tandem mass spectrometry (HPLC-ESI-MS/MS) on a Thermo Finnigan LCQ ion trap mass spectrometer coupled to a Michrom BioResources Paradigm MS4 micro HPLC by means of a home-built microspray interface. A data-dependent acquisition protocol was employed consisting of one survey scan followed by 4 collision-induced dissociation spectra. The CID spectra were searched primarily against the SWISSPROT followed by the TIGR data-base, which consisted of partially annotated T. vaginalis genome sequences (http://tigrblast.tigr.org/er-blast/index.cgi/project=tvg) using Mascot (Matrix Science; in-house license). A 95% confidence level threshold was used for Mascot protein scores (MALDI-TOF/MS) or peptide scores (ESI-MS/MS).
Isolation of total RNA
The MS-74 VECs were used for interaction experiments with trichomonads, as before (Garcia et al., 2003). Briefly, 1 × 105 VECs were seeded onto 6-well tissue culture plates and allowed to form a confluent monolayer. VECs were then washed with a medium mixture of DMEM: TYM (2:1, v/v) without serum. Secreted proteins from supernatant of trichomonal medium after growth was added to the MS-74 monolayer at different concentrations and incubated at 37°C for 2 h. In some experiments, purified AP65 protein obtained by affinity chromatography with monoclonal antibody (12G4 mAb) to AP65 was added to the monolayer at a concentration of 10 μg/well. Total RNA from MS-74 cells was isolated using Trizol reagent (Invitrogen).
Semiquantitative RT-PCR analysis of selected VEC genes
Total RNA (1 μg) was reverse transcribed with oligo (dT) primer using Superscript II reverse transcriptase (Invitrogen), according to the manufacturer’s protocol. PCR amplification of cDNA was carried out using gene-specific primers (Kucknoor et al., 2005a). The gene encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. PCR products were separated on 2% agarose gels with ethidium bromide. The band intensity was quantified using the Scion image beta program (http://www.scioncorp.com/pages/scion_image_windows.htm). PCR reactions were carried out at four different times to confirm reproducibility.
SDS-PAGE and immunoblotting
Equal amounts of TCA-precipitated secreted proteins and total T. vaginalis lysates were separated by SDS-PAGE on 10% acrylamide gels, and the proteins transferred onto nitrocellulose membranes (Bio-Rad). Nitrocellulose blots were blocked in 0.1% Tween 20 containing 5% BSA and probed with the 12G4 mAb to AP65 (Garcia et al., 2003), B512 mAb to α-tubulin (Sigma), and L64 mAb to an ∼30 kDa cytoplasmic protein (Alderete et al., 1987). These antibodies served as controls for the various experiments. For example, mAb B512 to α-tubulin was to show membrane integrity. The 30 kDa cytoplasmic protein detected by mAb L64 reacts only with the cytosol fraction by immunoblotting and with the cytoplasm by fluorescence using permeabilized trichomonads. Thus, both these mAbs confirmed absence of lysis. AP65 in the preparation of secreted proteins was detected by 12G4 mAb. The blots were further incubated with secondary anti-mouse IgG conjugated with horseradish peroxidase (Bio-Rad). The blots were washed well and incubated in horseradish peroxidase substrate (Bio-Rad) to visualize proteins.
Episomal expression of AP65 in HeLa cells and visualization by microscopy
In order to express the T. vaginalis ap65-1 gene in HeLa cells, the ap65-1 open reading frame was amplified by PCR and cloned into the pC1Neo vector (Promega). The resulting plasmid termed pC1Neo-AP65 was purified using Qiagen Maxi kit. For RNA and protein analysis, HeLa cells were transfected in 6-well tissue culture plates. Transient transfection was carried out with 1 μg of purified plasmid using TransIT-HeLaMONSTER transfection Kit (Mirus). For microscopic observation, HeLa cells were seeded on BD Falcon culture slides (Becton Dickinson) and grown to ∼70% confluent monolayers for transfection, as above. After 24 h, the monolayer was washed and incubated in blocking buffer (PBS-5% BSA) followed by the addition of 1:1000 dilution of COX-2 mAb (Cayman Chemicals) and 12G4 mAb for 1 h. Fluoresceine isothiocyanate-conjugated anti-mouse IgG antibody was then added for 30 min at 4°C.
For detection of AP65 in VECs, MS74 cells were grown on BD Falcon culture slides. Purified AP65 or trichomonads was added to the monolayer (10:1 ratio) and incubated for 40 min. The wells on the slides were washed 5 times with PBS to remove the unbound protein as well as the bound trichomonads. The VECs were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. The cells were incubated with 12G4 mAb (1:1000) for one h followed by Alexa Fluor 568-conjugated goat anti-mouse IgG secondary antibody (Invitrogen) for 30 min. The monolayer was washed with PBS prior to removing the chambers on the slides. Slides were processed for observation using an epifluorescence Olympus BX41 microscope.
Enzyme linked immunosorbant assay for the detection of IL-8
IL-8 from cell culture supernatant was analysed by ELISA using BD OptEIA Human IL-8 Kit II (BD Pharmingen), according to the instructions supplied by the manufacturer. Assay standardization and standard curves for IL-8 were carried out according to the kit instructions.
Acknowledgements
We thank Christopher Carroll, Institutional Mass Spectrometry Laboratory, UTHSCSA for excellent assistance with the MALDI-TOF analyses. Members of the laboratory are acknowledged for their suggestions and discussion of our work. This work was supported by Public Health Service Grants AI43940 and AI45429 from the National Institutes of Health.
References
- Addis MF, Rappelli P, Cappuccinelli P, Fiori PL. Extracellular release by Trichomonas vaginalis of a NADP+ dependent malic enzyme involved in pathogenicity. Microb Pathog. 1997;23:55–61. doi: 10.1006/mpat.1996.0128. [DOI] [PubMed] [Google Scholar]
- Addis MF, Rappelli P, Delogu G, Carta F, Cappuccinelli P, Fiori PL. Cloning and molecular characterization of a cDNA clone coding for Trichomonas vaginalis alpha-actinin and intracellular localization of the protein. Infect Immun. 1998;66:4924–4931. doi: 10.1128/iai.66.10.4924-4931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Garza GE. Soluble Trichomonas vaginalis antigens in cell-free culture supernatants. Mol Biochem Parasitol. 1984;13:147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Provenzano D. The vagina has reducing environment sufficient for activation of Trichomonas vaginalis cysteine proteinases. Genitourin Med. 1997;73:291–296. doi: 10.1136/sti.73.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Garza G, Smith J, Spence M. Trichomonas vaginalis: electrophoretic analysis and heterogeneity among isolates due to high-molecular-weight trichomonad proteins. Exp Parasitol. 1986;61:244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Demes P, Gombosova A, Valent M, Yanoska A, Fabusova H, et al. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987;55:1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Newton E, Dennis C, Engbring J, Neale KA. Vaginal antibody of patients with trichomoniasis is to a prominent surface immunogen of Trichomonas vaginalis. Genitourin Med. 1991;67:220–225. doi: 10.1136/sti.67.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 2001;3:359–370. doi: 10.1046/j.1462-5822.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Benchimol M, Lehker MW, Crouch ML. The complex fibronectin - Trichomonas vaginalis interactions and Trichomonosis. Parasitol Int. 2002;51:285–292. doi: 10.1016/s1383-5769(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Argiro LL, Kohlstadt SS, Henri SS, Dessein HH, Matabiau VV, Paris PP, et al. Identification of a candidate vaccine peptide on the 37 kDa Schistosoma mansoni GAPDH. Vaccine. 2000;18:2039–2048. doi: 10.1016/s0264-410x(99)00521-6. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Bakare RA, Ashiru JO, Adeyemi-Doro FA, Ekweozor CC, Oni AA, Okesola AO, Adebayo JA. Non-gonococcal urethritis (NGU) due to Trichomonas vaginalis in Ibadan. West Afr J Med. 1999;18:64–68. [PubMed] [Google Scholar]
- Bennett JR, Barnes WG, Coffman S. The emergency department diagnosis of Trichomonas vaginitis. Ann Emerg Med. 1989;18:564–566. doi: 10.1016/s0196-0644(89)80845-5. [DOI] [PubMed] [Google Scholar]
- Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, Sabarth N, et al. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002;70:3396–3403. doi: 10.1128/IAI.70.7.3396-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo KH, Tan TW, Ranganathan S. SPdb - a signal peptide database. BMC Bioinformatics. 2005;6:249. doi: 10.1186/1471-2105-6-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. [see comment] Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Edwards MR, Schofield PJ, O’Sullivan WJ, Costello M. Arginine metabolism during culture of Giardia intestinalis. Mol Biochem Parasitol. 1992;53:97–103. doi: 10.1016/0166-6851(92)90011-8. [DOI] [PubMed] [Google Scholar]
- El-Shazly AM, El-Naggar HM, Soliman M, El-Negeri M, El-Nemr HE, Handousa AE, Morsy TA. A study on Trichomonas vaginalis and female infertility. J Egypt Soc Parasitol. 2001;31:545–553. [PubMed] [Google Scholar]
- Engbring JA, O’Brien JL, Alderete JF. Trichomonas vaginalis adhesin proteins display molecular mimicry to metabolic enzymes. Adv Exp Med Biol. 1996;408:207–223. doi: 10.1007/978-1-4613-0415-9_25. [DOI] [PubMed] [Google Scholar]
- Garcia AF, Chang TH, Benchimol M, Klumpp DJ, Lehker MW, Alderete JF. Iron and contact with host cells induce expression of adhesins on surface of Trichomonas vaginalis. Mol Microbiol. 2003;47:1207–1224. doi: 10.1046/j.1365-2958.2003.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner PC, Secor WE, Dezzutti CS. Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for the sexual transmission of HIV-1. Infect Immun. 2005;73:4155–4160. doi: 10.1128/IAI.73.7.4155-4160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser D, Augsburger V, Pun T, Weber D, Pellegrini C, Muller AF. ‘High-resolution’ mini-two-dimensional gel electrophoresis automatically run and stained in less than 6 h with small, ready-to-use slab gels. Clin Chem. 1988;34:166–170. [PubMed] [Google Scholar]
- Khoshnan A, Provenzano D, Alderete JF. Unique double-stranded RNAs associated with the Trichomonas vaginalis virus are synthesized by viral RNA-dependent RNA polymerase. J Virol. 1994;68:7108–7114. doi: 10.1128/jvi.68.11.7108-7114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Lee Y-C, Kim HK, Blaser MJ. Helicobacter pylori CagA transfection of gastric epithelial cells induces interleukin-8. Cell Microbiol. 2005;8:97–106. doi: 10.1111/j.1462-5822.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Knudsen GM, Medzihradszky KF, Lim KC, Hansell E, McKerrow JH. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics. 2005;4:1862–1875. doi: 10.1074/mcp.M500097-MCP200. [DOI] [PubMed] [Google Scholar]
- de Koning AP, Brinkman FS, Jones SJ, Keeling PJ. Lateral gene transfer and metabolic adaptation in the human parasite Trichomonas vaginalis. Mol Biol Evol. 2000;17:1769–1773. doi: 10.1093/oxfordjournals.molbev.a026275. [DOI] [PubMed] [Google Scholar]
- Kucknoor AS, Mundodi V, Alderete JF. Adherence to human vaginal epithelial cells signals for increased expression of Trichomonas vaginalis genes. Infect Immun. 2005a;73:6472–6478. doi: 10.1128/IAI.73.10.6472-6478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucknoor A, Mundodi V, Alderete JF. Trichomonas vaginalis adherence mediates differential gene expression in human vaginal epithelial cells. Cell Microbiol. 2005b;7:887–897. doi: 10.1111/j.1462-5822.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75:231–238. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker MW, Arroyo R, Alderete JF. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med. 1991;174:311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling E, Feldman G, Portnoi M, Dagan R, Overweg K, Mulholland F, et al. Glycolytic enzymes associated with the cell surface of Streptococcus pneumoniae are antigenic in humans and elicit protective immune responses in the mouse. Clin Exp Immunol. 2004;138:290–298. doi: 10.1111/j.1365-2249.2004.02628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstead D, Cranshaw MA. The pathway of arginine catabolism in the parasitic flagellate Trichomonas vaginalis. Mol Biochem Parasitol. 1983;8:241–252. doi: 10.1016/0166-6851(83)90046-4. [DOI] [PubMed] [Google Scholar]
- McCarthy JS, Wieseman M, Tropea J, Kaslow D, Abraham D, Lustigman S, et al. Onchocerca volvulus glycolytic enzyme fructose-1,6-bisphosphate aldolase as a target for a protective immune response in humans. Infect Immun. 2002;70:851–858. doi: 10.1128/IAI.70.2.851-858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee DJ, Kumar S, Viator RJ, Bolland JR, Ruiz J, Spadafora D, et al. Helicobacter pylori thioredoxin is an arginase chaperone and guardian against oxidative and nitrosative stresses. J Biol Chem. 2006;281:3290–3296. doi: 10.1074/jbc.M506139200. [DOI] [PubMed] [Google Scholar]
- Mason PR, Fiori PL, Cappuccinelli P, Rappelli P, Gregson S. Seroepidemiology of Trichomonas vaginalis in rural women in Zimbabwe and patterns of association with HIV infection. Epidemiol Infect. 2005;133:315–323. doi: 10.1017/s0950268804003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina ML, Haynes PA, Breci L, Francisco WA. Analysis of secreted proteins from Aspergillus flavus. Proteomics. 2005;5:3153–3161. doi: 10.1002/pmic.200401136. [DOI] [PubMed] [Google Scholar]
- Minotto L, Edwards MR, Bagnara AS. Trichomonas vaginalis: characterization, expression, and phylogenetic analysis of a carbamate kinase gene sequence. Exp Parasitol. 2000;95:54–62. doi: 10.1006/expr.2000.4507. [DOI] [PubMed] [Google Scholar]
- Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34:519–522. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- Moreno-Brito V, Yanez-Gomez C, Meza-Cervantez P, Avila-Gonzalez L, Rodriguez MA, Ortega-Lopez J, et al. A Trichomonas vaginalis 120 kDa protein with identity to hydrogenosome pyruvate: ferredoxin oxidoreductase is a surface adhesin induced by iron. Cell Microbiol. 2005;7:245–258. doi: 10.1111/j.1462-5822.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- Mundodi V, Kucknoor AS, Alderete JF. Silencing the ap65 gene reduces adherence to vaginal epithelial cells by Trichomonas vaginalis. Mol Microbiol. 2004;53:1099–1108. doi: 10.1111/j.1365-2958.2004.04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V, Chhatwal GS. Housekeeping enzymes as virulence factors for pathogens. Int J Med Microbiol. 2003;293:391–401. doi: 10.1078/1438-4221-00283. [DOI] [PubMed] [Google Scholar]
- Provenzano D, Alderete JF. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect Immun. 1995;63:3388–3395. doi: 10.1128/iai.63.9.3388-3395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rughooputh S, Greenwell P. Trichomonas vaginalis: paradigm of a successful sexually transmitted organism. Br J Biomed Sci. 2005;62:193–200. doi: 10.1080/09674845.2005.11732710. [DOI] [PubMed] [Google Scholar]
- Rybakin V, Clemen CS. Coronin proteins as multifunctional regulators of the cytoskeleton and membrane trafficking. BioEssays. 2005;27:625–632. doi: 10.1002/bies.20235. [DOI] [PubMed] [Google Scholar]
- Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min DY. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–1332. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed el-Ahl SA, el-Wakil HS, Kamel NM, Mahmoud MS. A preliminary study on the relationship between Trichomonas vaginalis and cervical cancer in Egyptian women. J Egypt Soc Parasitol. 2002;32:167–178. [PubMed] [Google Scholar]
- Shaio MF, Lin PR. Leucotriene B4 levels in the vaginal discharges from cases of trichomoniasis. Ann Trop Med Parasitol. 1995;89:85–88. doi: 10.1080/00034983.1995.11812934. [DOI] [PubMed] [Google Scholar]
- Shaio MF, Lin PR, Liu JY, Tang KD. Monocyte-derived interleukin-8 involved in the recruitment of neutrophils induced by Trichomonas vaginalis infection. J Infect Dis. 1994;170:1638–1640. doi: 10.1093/infdis/170.6.1638. [DOI] [PubMed] [Google Scholar]
- Shaio MF, Lin PR, Liu JY, Yang KD. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect Immun. 1995;63:3864–3870. doi: 10.1128/iai.63.10.3864-3870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Kovacs A, Kerndt P, Stek A, Muderspach L, Sanchez-Keeland L. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495–500. doi: 10.4269/ajtmh.1998.58.495. [DOI] [PubMed] [Google Scholar]
- Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, Zenilman JM, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Can Epidemiol Biomarkers Prev. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- Trost M, Wehmhoner D, Karst U, Dieterich G, Wehland J, Jansch L. Comparative proteome analysis of secretory proteins from pathogenic and nonpathogenic Listeria species. Proteomics. 2005;5:1544–1557. doi: 10.1002/pmic.200401024. [DOI] [PubMed] [Google Scholar]
- Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. [See comment] J Infect Dis. 2005;192:2039–2044. doi: 10.1086/498217. [DOI] [PubMed] [Google Scholar]
- Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncologica. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- Vincensini L, Richert S, Blisnick T, Van Dorsselaer A, Leize-Wagner E, Rabilloud T, Braun Breton C. Proteomic analysis identifies novel proteins of the Maurer’s clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol Cell Proteomics. 2005;4:582–593. doi: 10.1074/mcp.M400176-MCP200. [DOI] [PubMed] [Google Scholar]
- Yap EH, Ho TH, Chan YC, Thong TW, Ng GC, Ho LC, Singh M. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin Med. 1995;71:402–404. doi: 10.1136/sti.71.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZF, Graham S, Yu SZ, Marshall J, Zielezny M, Chen YX, et al. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann Epidemiol. 1995;5:325–332. doi: 10.1016/1047-2797(94)00101-x. [DOI] [PubMed] [Google Scholar]


