Abstract
The purpose of the present study was to evaluate the effects of bovine serum albumin (BSA) and essentially fatty acid-free BSA (BSA-FAF) on the biliary clearance of compounds in sandwich-cultured rat hepatocytes. Unbound fraction (fu), biliary excretion index (BEI), and unbound intrinsic biliary clearance (intrinsic Cl’biliary) were determined for digoxin, pravastatin, and taurocholate in the absence or presence of BSA or BSA-FAF. BSA had little effect on the BEI or intrinsic Cl’biliary of these compounds. Surprisingly, BSA-FAF decreased both BEI and intrinsic Cl’biliary for digoxin and pravastatin, which represent low and moderately-bound compounds, respectively. The BEI and intrinsic Cl’biliary of taurocholate, a highly-bound compound, were not altered significantly by BSA-FAF. Neither BSA nor BSA-FAF had a discernable effect on the bile canalicular networks, based on carboxydichlorofluorescein (CDF) retention. The addition of physiological concentrations of calcium, or the addition of fatty acids to BSA-FAF, was unable to restore the BEI or intrinsic Cl’biliary of the model compounds to similar values in the absence or presence of BSA. Careful consideration is warranted when selecting the type of BSA for addition to in vitro systems such as sandwich-cultured rat hepatocytes.
INTRODUCTION
In vitro models are utilized widely to investigate hepatic metabolism and transport. Sandwich-cultured rat hepatocytes are one such model system. Culturing primary hepatocytes between two layers of extracellular matrix, which resembles basement membranes in vivo to which cells attach, allows the cells to maintain many in vivo structural and functional characteristics. Apical (canalicular) and basolateral (sinusoidal) membrane domains are re-established following cellular repolarization and sealing of tight junction complexes, liver specific proteins are expressed, and intact functional bile canalicular networks are formed (Hoffmaster et al., 2004; LeCluyse et al., 1994; LeCluyse et al., 2000; Liu et al., 1998; Liu et al., 1999a). This model has been established as an in vitro method to predict in vivo biliary clearance of drug candidates (Fukuda et al., 2008; Liu et al., 1999b). It is a useful in vitro model to study hepatic uptake and biliary excretion mechanisms simultaneously. Data from medium, cell, and bile compartments of sandwich-cultured hepatocytes can be used for pharmacokinetic modeling of hepatobiliary drug disposition (Turncliff et al., 2006). Sandwich-cultured rat hepatocytes also are used to predict drug and xenobiotic interactions that may occur in vivo at the level of hepatic transporters (Annaert et al., 2005; Kemp et al., 2005; McRae et al., 2006).
Albumin and α1-acid glycoprotein are the primary proteins responsible for binding low-molecular-weight compounds in plasma. As a general rule, cations bind to α1-acid glycoprotein, while anions bind primarily to albumin (Peters, 1996). Albumin is the most abundant protein present in plasma, and often is added to in vitro systems (Aungst et al., 2000; Cross et al., 2003; Krishna et al., 2001; Neuhoff et al., 2006; Yamashita et al., 2000) to prevent precipitation of water-insoluble drugs and to more closely mimic physiologic conditions (Katneni et al., 2008). Bovine serum albumin (BSA) is utilized commonly in vitro as a replacement for albumin from other species (Neuhoff et al., 2006; Saha and Kou, 2002; Taub et al., 2002).
A major determinant of drug disposition in vivo is binding to plasma proteins (Otagiri, 2005). Many drug candidates are extensively protein-bound, tend to be hydrophobic and of limited solubility, and are surface adsorptive, thus leading to experimental challenges in the drug development process (Artursson, 1990; Wilson et al., 1990). The ability to deliver a compound to, and stabilize a compound in, in vitro systems can be enhanced by the addition of protein. However, such manipulation may significantly alter pharmacokinetic/pharmacodynamic behavior (Bennhold, 1965; Fisher et al., 1999; Liu et al., 2005; Neuhoff et al., 2006; Wiseman et al., 1964). Metabolic studies utilizing human liver microsomes, fresh or cryopreserved hepatocytes, or transfected systems underpredict in vivo hepatic clearance and metabolic inhibition potential (for review, see Rowland et al., 2007). Studies investigating the effect of BSA on hepatic metabolism by UDP-glucuronosyltransferase (UGT) 2B7, UGT1A9, and cytochrome P450 2C9 in these systems suggested an increase in in vitro intrinsic clearance in the presence of BSA (for review, see Rowland et al., 2007; Rowland et al., 2008a, b). Similar results were observed for UGT2B7 despite the preparation of BSA utilized [“crude”, essentially fatty acid-free (BSA-FAF), essentially globulin-free, or essentially fatty acid-free and globulin-free; Rowland et al., 2007]. It was hypothesized that BSA, despite the preparation type, sequesters fatty acids that are inhibitory to the activity of metabolic enzymes (Rowland et al., 2007); however, not all metabolic enzymes (UGT1A1; UGT1A6) are inhibited by fatty acids (Rowland et al., 2008a).
Little work evaluating the effect of BSA on transport proteins and the hepatobiliary disposition of compounds has been published. Previous investigations using transfected cells have focused on transport proteins involved in the uptake of compounds into hepatocytes (Cui et al., 2001; Hata et al., 2003; Shi et al., 1995). The primary objective of the present study was to characterize the effect of BSA on the biliary excretion index (BEI) and biliary clearance of compounds in sandwich-cultured rat hepatocytes. Because several preparations of albumin are available commercially, we investigated the effects of BSA and BSA-FAF on these parameters. The compounds selected for investigation (digoxin, pravastatin, and taurocholate) span a range of unbound fractions: digoxin (fu~0.7; Lacy et al., 2002), pravastatin (fu~0.5; Hatanaka, 2000), and taurocholate (fu~0.15; Forker and Luxon, 1981; Hung et al., 2005). Additional studies were designed to characterize: 1) the effect of albumin preparations on bile canalicular networks, and 2) the effect of long chain fatty acids, which are present in BSA preparations, on BEI and biliary clearance.
METHODS
Materials
Collagenase (type 1, class 1) was obtained from Worthington Biochemicals (Lakewood, NJ). Dulbecco’s modified Eagle’s medium (DMEM, no phenol red), insulin, MEM non-essential amino acids solution (100x), L-glutamine, penicillin G-streptomycin solution, and 5 (and 6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDFDA) were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum, sodium taurocholate, digoxin, Triton X-100, dexamethasone, methanol, Hanks’ balanced salts solution (HBSS) modified with (H-1387) or without (H-4891) calcium chloride, bovine serum albumin (BSA), essentially fatty acid-free bovine serum albumin (BSA-FAF; A-8806), linoleic acid (18:2n-6), linolenic acid (18:3n-3), oleic acid (18:1n-9), palmitic acid (16:0), palmitoleic acid (16:1n-7), and stearic acid (18:0) were obtained from Sigma-Aldrich (St. Louis, MO). BioCoat™ collagen I plates, Matrigel™ basement membrane matrix, and ITS+™ (insulin/transferrin/selenium) culture supplement were purchased from BD Biosciences Discovery Labware (Bedford, MA). Pravastatin and d6-rosuvastatin were purchased from Toronto Research Chemicals (North York, Ontario, Canada). [3H]-taurocholate (5 Ci/mmol, >97% purity) and [3H]-digoxin (40 Ci/mmol, >97% purity) were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). Bio-Safe II™ liquid scintillation cocktail was obtained from Research Products International (Mt. Prospect, IL). Centrifree® micropartition devices were obtained from Millipore (Billerica, MA). Bicinchoninic acid (BCA) protein assay reagents and BSA for the protein assay standard were purchased from Pierce Chemical Co. (Rockford, IL). All other chemicals and reagents were of analytical grade and available from commercial sources.
Animals
Male Wistar rats (200–325 g), obtained from Charles River Laboratories, Inc. (Raleigh, NC), served as liver donors for hepatocyte isolation. Animals were maintained in a controlled environment with a 12-h light/dark cycle and had free access to water and food prior to surgery. All animal procedures were compliant with the guidelines of the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Isolation and In Vitro Culture of Primary Rat Hepatocytes
Hepatocytes were isolated from male Wistar rats using a two-step collagenase perfusion, as described previously (LeCluyse et al., 1996). Cell viability, determined by trypan blue exclusion, was >88%. Hepatocytes were seeded (~1.75 × 106 cells/well) in 6-well BioCoat™ plates in DMEM supplemented with 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium/mL, 100 μg streptomycin sulfate/mL, 1 μM dexamethasone, 5% (v/v) fetal bovine serum, and 10 μM insulin (day 0 of culture), and cultured in a humidified incubator (95% O2, 5% CO2) at 37°C. To achieve a sandwich configuration 24 h post-plating (day 1 of culture), hepatocytes were overlaid with Matrigel™ basement membrane matrix (0.25 mg/mL) in 2 mL/well cold serum-free DMEM containing 2 mM L-glutamine, 1% (v/v) MEM non-essential amino acids, 100 units penicillin G sodium/mL, 100 μg streptomycin sulfate/mL, 0.1 μM dexamethasone, and 1% (v/v) ITS+™. The culture medium was changed every 24 h until experiments were performed on day 4 of culture.
Accumulation Experiments
The method to determine substrate accumulation in sandwich-cultured rat hepatocytes has been described previously (Liu et al., 1999a). Briefly, hepatocytes were rinsed twice with 2 mL warm HBSS containing Ca2+ (standard buffer) or Ca2+-free HBSS and incubated with 2 mL of the same buffer for 10 min at 37°C (to maintain tight junction integrity and bile canalicular networks or disrupt tight junctions and open bile canalicular networks, respectively). The buffer was removed, and the cells were incubated for 10 min at 37°C with 1.5 mL of [3H]-taurocholate (1 μM), [3H]-digoxin (10 μM), or pravastatin (5 μM) in standard buffer in the presence or absence of 4% (w/v) BSA or BSA-FAF. For experiments in which long chain fatty acids were added, standard buffer with or without BSA or BSA-FAF remained at room temperature for ~1 h following the addition of long chain fatty acids (diluted in dimethyl sulfoxide) to allow binding of the fatty acids to albumin (Peters, 1996) prior to the addition of pravastatin and subsequent incubation with hepatocytes for 10 min. The fatty acids added were linoleic acid (5.48 μM), linolenic acid (0.20 μM), oleic acid (6.94 μM), palmitic acid (9.38 μM), palmitoleic acid (0.76 μM), and stearic acid (7 μM). Hepatocytes were rinsed vigorously three times with 2 mL ice-cold standard buffer following the incubation. For rinsing of [3H]-digoxin-treated hepatocytes, 10 μM unlabeled digoxin was added to the rinsing buffer to reduce nonspecific binding as described previously (Annaert et al., 2001). Additional digoxin accumulation studies were performed with or without 1 mM Ca2+ (calcium chloride) added to the digoxin-containing incubation solution. Taurocholate- and digoxin-treated hepatocytes were lysed with 1 mL 0.5% (v/v) Triton X-100 in phosphate-buffered saline by placing plates on an orbital shaker for a minimum of 20 min at room temperature. The samples were analyzed by liquid scintillation spectroscopy in a Packard Tri-Carb scintillation counter (PerkinElmer Life and Analytical Sciences). Pravastatin-treated hepatocytes were lysed with 1 mL 70% (v/v) ice-cold methanol/water, scraped off the plates, and sonicated for 20 sec with a sonic dismembrator. The samples were analyzed by liquid chromatography with detection by tandem mass spectrometry (LC/MS/MS).
LC/MS/MS Analysis
Hepatocyte lysates from pravastatin accumulation experiments were centrifuged at 12,000g for 5 min at 4°C. The resulting supernatants were diluted 1:6 with water and methanol containing the internal standard (d6-rosuvastatin). Analysis of these samples was performed with a Shimadzu solvent delivery system (Shimadzu Scientific Instruments, Columbia, MD) and a Leap HTC Pal thermostated autosampler (LEAP Technologies, Carrboro, NC) connected to an Applied Biosystems API 4000 triple quadrupole mass spectrometer with a TurboSpray ion source (Applied Biosystems, Foster City, CA). Tuning, operation, integration, and data analysis were performed in positive mode using multiple reaction monitoring (Analyst software v.1.4.1; Applied Biosystems). Ten microliters of sample were injected onto an Aquasil 5-μM C18 column (2.1 mm i.d. × 50 mm; Thermo Electron Corporation, Waltham, MA). The mobile phase consisted of 0.1% (v/v) formic acid diluted in water (Buffer A) and methanol containing 0.1% (v/v) formic acid (Buffer B). Analytes were eluted with a high-pressure linear gradient program at a flow rate of 0.75 mL/min as follows: 20% buffer B for 0.75 min, increased to 40% buffer B over 0.64 min and held for 1.91 min, rapidly increased to 90% buffer B and held for 0.7 min, and returned rapidly to 20% buffer B. The system was allowed to re-equilibrate for 1 min, with a total run time of 5 min per injection. The entire column effluent was diverted from the TurboSpray ion probe of the mass spectrometer for the first 0.75 min and last 1 min. An eight point calibration curve (2–1000 nM) was constructed for pravastatin using peak area ratios of analyte and internal standard using the following transitions: pravastatin (447.1 → 327.4) and d6-rosuvastatin (488.2 → 264.2). All points on the curve back-calculated to within ±15% of the nominal value.
Nonspecific Binding and Protein Determination
All accumulation data were corrected for nonspecific binding of the relevant substrate to Matrigel™-overlaid hepatocyte-free BioCoat™ plates. The data also were normalized to the protein concentration in each well (lysed with Triton X-100), determined in duplicate aliquots using BCA protein assay reagents as instructed by the manufacturer. BSA, as supplied by the manufacturer, was used as a standard (0.2 – 1 mg/mL). Due to the incompatibility of methanol with the BCA protein assay, the average protein concentration of samples from one liver preparation lysed with Triton X-100 was used to normalize the accumulation data of samples lysed with methanol from the same liver preparation.
Determination of Protein Binding
The unbound fraction (fu) of each compound was determined over a range of concentrations: taurocholate (0.1–100 μM), digoxin (0.1–30 μM), and pravastatin (1–10 μM). This determination was not performed to examine the binding kinetics of each compound, but rather to ensure that binding was stable and reproducible under the conditions planned for the experiment. Solutions in standard buffer were prepared in the absence or presence of 4% (w/v) BSA or BSA-FAF and incubated at 37°C for 30 min. Solutions containing long chain fatty acids were prepared in a manner similar to that described under Accumulation Experiments, prior to the addition of pravastatin and incubation for 30 min at 37°C. Media samples from sandwich-cultured rat hepatocyte experiments also were taken to determine the fu of digoxin. Samples (1 mL) were placed in Centrifree® micropartition devices and centrifuged at 1500g at 37°C for either 30 sec (non-BSA-containing samples) or 4 min (BSA-containing samples) to pass ~10% of the original volume through the filter. The absence-of-protein condition was used to assess non-specific binding. Samples were obtained from above (total concentration in the presence of protein) and below (unbound concentration) the filter. Substrate concentrations, determined by either liquid scintillation spectroscopy (taurocholate and digoxin) or LC/MS/MS (pravastatin), were used to calculate fu. The fu of digoxin in the absence or presence of BSA or BSA-FAF in the cell-free system was similar to the fu in sandwich-cultured rat hepatocyte experiments (results not shown); therefore, the fu of pravastatin and taurocholate were not determined in sandwich-cultured rat hepatocytes.
Fluorescence Microscopy
Retention of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein (CDF) in bile canalicular networks in the absence or presence of BSA or BSA-FAF was examined. Hepatocytes were rinsed twice with 2 mL standard buffer and incubated with 2 mL of the same buffer at 37°C for 10 min. The buffer was removed, and the cells were incubated for 10 min at 37°C with 1.5 mL CDFDA (2 μM) in standard buffer to preload the bile canaliculi with CDF. Subsequently, hepatocytes were incubated with standard buffer in the absence or presence of 4% (w/v) BSA or BSA-FAF for 10 min, during which time the cells and bile canaliculi were imaged with a Zeiss Axiovert 100TV inverted fluorescent microscope (Carl Zeiss Inc., Thornwood, NY).
Data Analysis
The BEI (%) and unbound intrinsic biliary clearance (intrinsic Cl’biliary, mL/min/kg) were calculated using B-CLEAR® technology (Qualyst, Inc., Raleigh, NC; Liu et al., 1999a):
| (1) |
where substrate accumulation in the cell + bile compartments was determined in hepatocytes preincubated in standard buffer; cellular accumulation of substrate was determined in hepatocytes preincubated with Ca2+-free HBSS.
| (2) |
where fu represents the unbound fraction of substrate (fu = 1 in the case of no BSA) and AUCmedia represents the area under the substrate concentration-time curve, determined by dividing the sum of the substrate concentration in the incubation medium at the beginning and end of the incubation period by 2, and multiplying by the incubation time (10 min). Intrinsic Cl’biliary values were converted to mL/min/kg based on 200 mg protein/g of liver and 40 g liver/kg of rat body weight (Seglen, 1976).
Statistical Analysis
Data are represented as mean and the associated S.E.M., S.D., or range, as appropriate. Statistical comparisons were performed with SigmaStat (SPSS Inc., Chicago, IL). The effects of BSA and BSA-FAF, at a single concentration of compound and a single concentration of BSA, on BEI and intrinsic Cl’biliary were evaluated by one-way analysis of variance (ANOVA) with Tukey’s post-hoc test in order to determine whether the presence and type of BSA affected either of these parameters. The effects on fu were evaluated by two-way ANOVA with Tukey’s post-hoc test, using log transformed data when appropriate for statistical comparisons, in order to determine whether the fu of each compound was significantly altered by the type of BSA utilized or by the concentration of compound. In all cases, p < 0.05 was considered statistically significant.
RESULTS
Effect of BSA and BSA-FAF on Digoxin Hepatobiliary Disposition
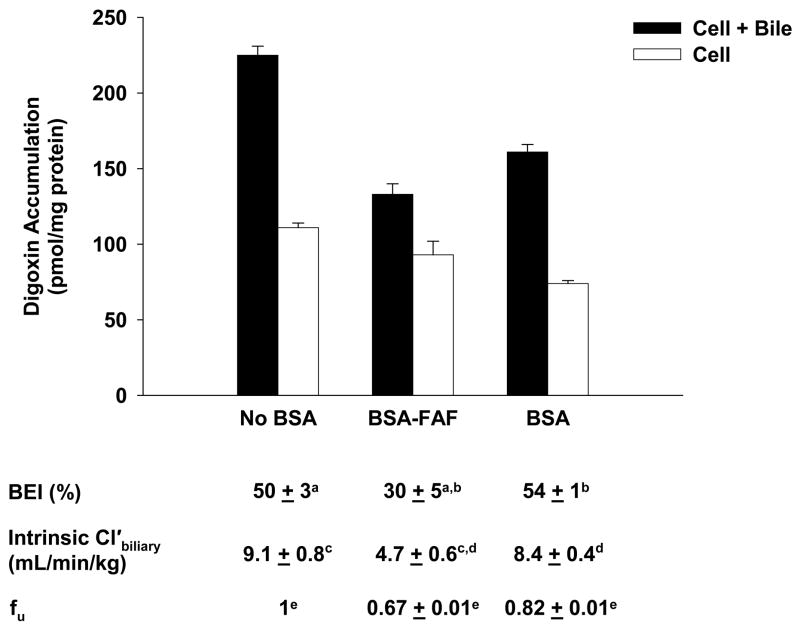
Figure 1 shows the effect of BSA and BSA-FAF on the fu, BEI and intrinsic Cl’biliary of digoxin. The fu, determined by ultrafiltration, was concentration-independent from 0.1–30 μM (results not shown), but was significantly higher in the presence of BSA compared to BSA-FAF (p < 0.05). Digoxin binding to albumin was minimal: 18% in the presence of BSA and 33% in the presence of BSA-FAF. The BEI and intrinsic Cl’biliary of digoxin were not altered by the presence or absence of BSA. However, BSA-FAF significantly decreased both BEI and intrinsic Cl’biliary compared to no BSA or BSA conditions (p < 0.05).
Figure 1. Hepatobiliary disposition of digoxin in the absence or presence of BSA or BSA-FAF.
Sandwich-cultured rat hepatocytes were incubated with [3H]-digoxin (10 μM) in the absence or presence of 4% (w/v) bovine serum albumin (BSA) or essentially fatty acid-free BSA (BSA-FAF) for 10 min following incubation in standard or Ca2+-free buffer. Filled bars represent accumulation in hepatocytes and bile canaliculi (cell + bile). Open bars represent accumulation in hepatocytes (cell). The biliary excretion index (BEI), unbound intrinsic biliary clearance (intrinsic Cl’biliary), and unbound fraction (fu) were determined as described under Methods. Data are presented as mean ± S.E.M. n = 3 livers in triplicate. Groups with the same letter indicate a statistically significant difference (p < 0.05).
Effect of BSA and BSA-FAF on Pravastatin Hepatobiliary Disposition
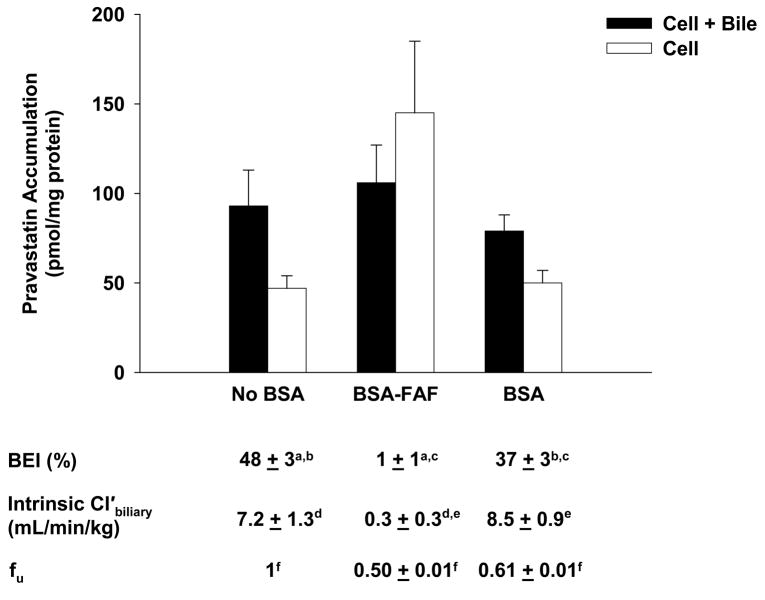
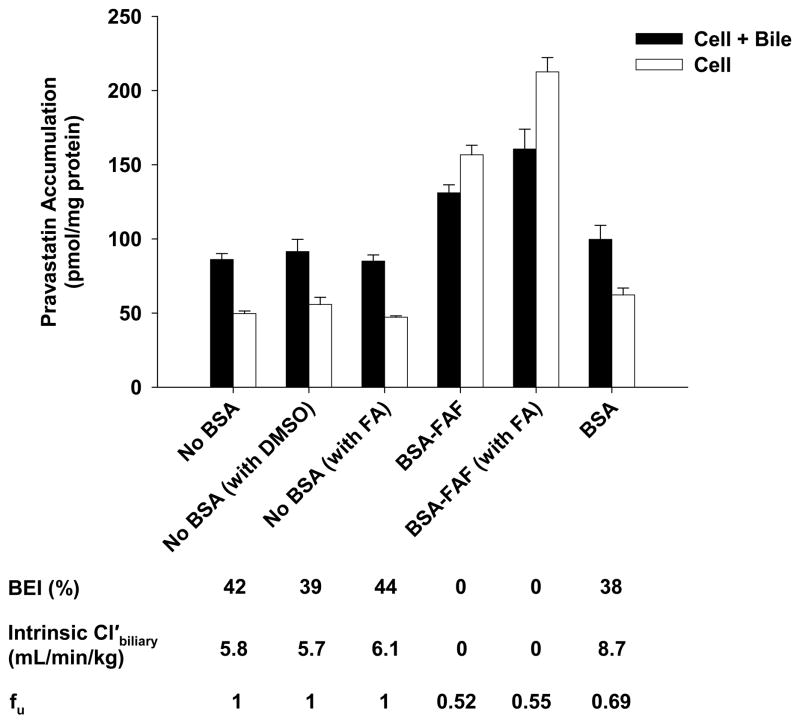
The effects of BSA and BSA-FAF on pravastatin fu, BEI, and intrinsic Cl’biliary are illustrated in Figure 2. The fu was concentration-independent from 1–10 μM (results not shown), but was significantly higher in the presence of BSA compared to BSA-FAF (p < 0.05). Pravastatin was moderately-bound to albumin: 39% in the presence of BSA and 50% in the presence of BSA-FAF. The intrinsic Cl’biliary of pravastatin was not significantly different in the presence or absence of BSA. However, BSA-FAF significantly decreased both BEI and intrinsic Cl’biliary of pravastatin compared to values in the absence or presence of BSA (p < 0.05).
Figure 2. Hepatobiliary disposition of pravastatin in the absence or presence of BSA or BSA-FAF.
Sandwich-cultured rat hepatocytes were incubated with pravastatin (5 μM) in the absence or presence of 4% (w/v) BSA or BSA-FAF for 10 min following incubation in standard or Ca2+-free buffer. Filled bars represent accumulation in hepatocytes and bile canaliculi (cell + bile). Open bars represent accumulation in hepatocytes (cell). BEI, intrinsic Cl’biliary, and fu were determined as described under Methods. Data are presented as mean ± S.E.M. n = 3 livers in triplicate. Groups with the same letter indicate a statistically significant difference (p < 0.05).
Effect of BSA and BSA-FAF on Taurocholate Hepatobiliary Disposition
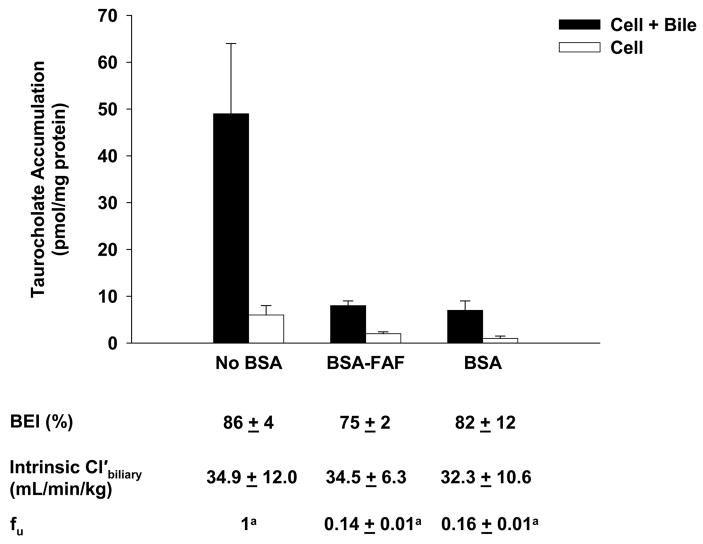
Figure 3 shows the effect of BSA and BSA-FAF on the fu, BEI, and intrinsic Cl’biliary of taurocholate. The fu was concentration-independent from 0.1–100 μM (results not shown), but was significantly higher in the presence of BSA compared to BSA-FAF (p < 0.05). Despite the fact that taurocholate was highly bound to albumin (approximately 85% in the presence of BSA or BSA-FAF), the BEI and intrinsic Cl’biliary of taurocholate were unaffected by BSA.
Figure 3. Hepatobiliary disposition of taurocholate in the absence or presence of BSA or BSA-FAF.
Sandwich-cultured rat hepatocytes were incubated with [3H]-taurocholate (1 μM) in the absence or presence of 4% (w/v) BSA or BSA-FAF for 10 min following incubation in standard or Ca2+-free buffer. Filled bars represent accumulation in hepatocytes and bile canaliculi (cell + bile). Open bars represent accumulation in hepatocytes (cell). BEI, intrinsic Cl’biliary, and fu were determined as described under Methods. Data are presented as mean ± S.E.M. n = 3 livers in triplicate. The taurocholate fu data were log-transformed for statistical comparison. Groups with the same letter indicate a statistically significant difference (p < 0.05).
Effect of BSA and BSA-FAF on CDF Retention in Bile Canaliculi
CDFDA readily diffuses into hepatocytes, where it is hydrolyzed rapidly to CDF and excreted into the bile canaliculi by multidrug resistance-associated protein (Mrp) 2. After pre-incubating hepatocytes for 10 min with CDFDA, CDF retention was evaluated over 10 min in the absence or presence of BSA or BSA-FAF to assess the maintenance of tight junction integrity (Figure 4). BSA and BSA-FAF had no discernable effects on CDF accumulation in bile canalicular networks over the 10-min period compared to the no BSA condition.
Figure 4. Retention of carboxydichlorofluorescein (CDF) in bile canaliculi in the absence or presence of BSA or BSA-FAF.
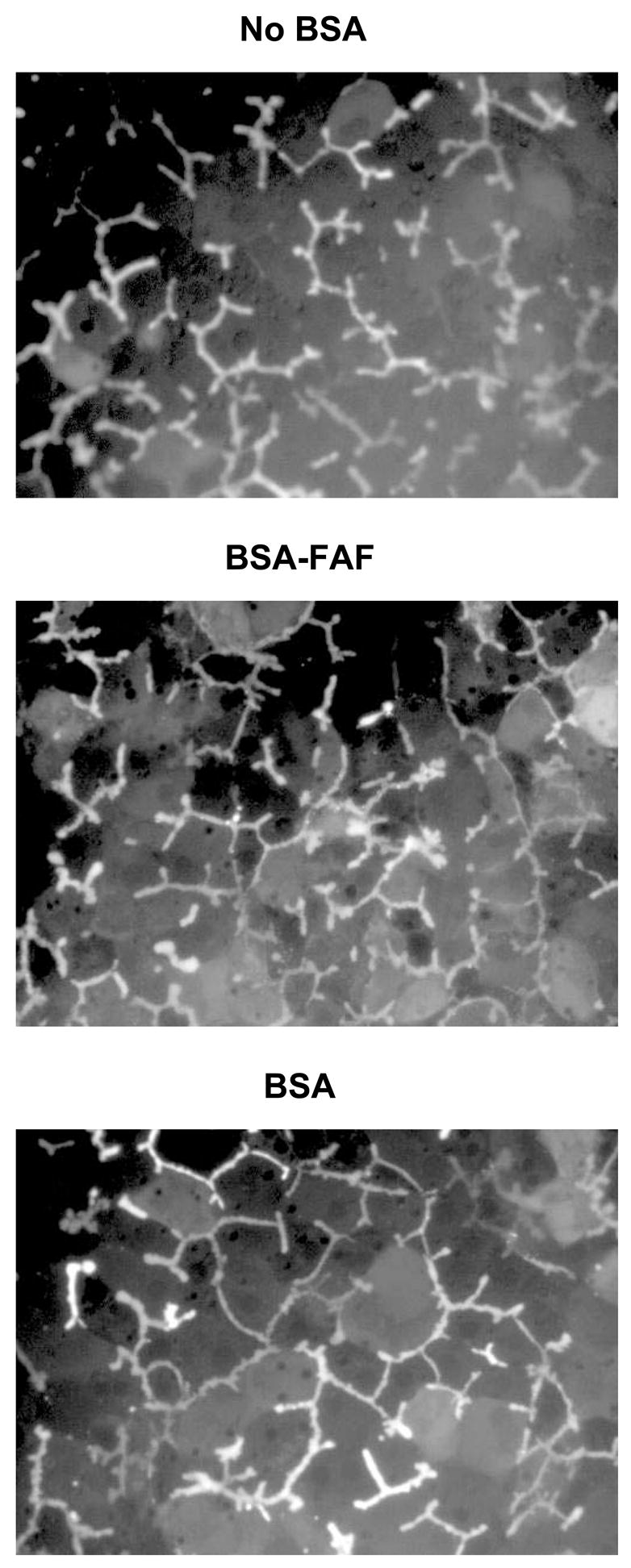
Sandwich-cultured rat hepatocytes were incubated with carboxydichlorofluorescein diacetate (CDFDA; 2 μM) in standard buffer for 10 min to preload the bile canaliculi with CDF. Subsequently, hepatocytes were imaged for 10 min in the absence or presence of BSA or BSA-FAF. Representative images of CDF fluorescence during the 10 min period are shown.
Effect of Additional Ca2+ on the Hepatobiliary Disposition of Digoxin in the Absence or Presence of BSA or BSA-FAF
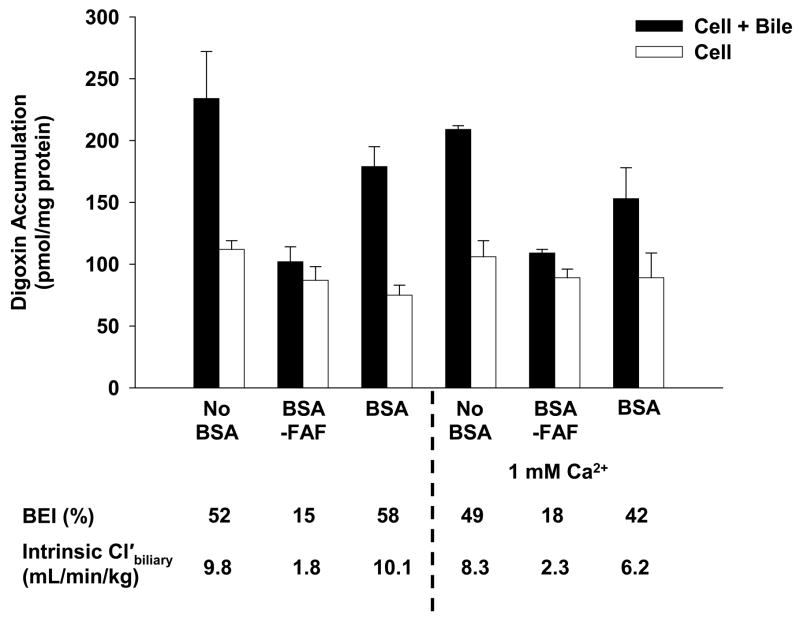
Figure 5 shows the effect of BSA and BSA-FAF on the BEI and intrinsic Cl’biliary of digoxin in the presence of additional Ca2+. Similar to the results in Figure 1, the BEI and intrinsic Cl’biliary of digoxin were not affected by BSA compared to no BSA, while BSA-FAF decreased both parameters. The addition of 1 mM Ca2+ was unable to restore digoxin BEI and intrinsic Cl’biliary values in the presence of BSA-FAF to similar values in the presence or absence of BSA. Similar findings were obtained with the addition of 2.5 mM Ca2+ (results not shown).
Figure 5. Effect of additional Ca2+ on the hepatobiliary disposition of digoxin in the absence or presence of BSA or BSA-FAF.
Sandwich-cultured rat hepatocytes were incubated with [3H]-digoxin (10 μM) in the absence or presence of 4% (w/v) BSA or BSA-FAF with or without 1 mM Ca2+ (calcium chloride) for 10 min following incubation in standard or Ca2+-free buffer. Filled bars represent accumulation in hepatocytes and bile canaliculi (cell + bile). Open bars represent accumulation in hepatocytes (cell). The BEI and intrinsic Cl’biliary were determined as described under Methods. Data are presented as mean ± range. n = 1 liver in duplicate.
Effect of the Addition of Fatty Acids to BSA or BSA-FAF on Pravastatin Hepatobiliary Disposition
Rowland et al. (2007) reported that palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1n-9), linoleic acid (18:2n-6), palmitoleic acid (16:1n-7), and linolenic acid (18:3n-3) are six of the predominant long chain fatty acids present in BSA. To determine whether addition of these fatty acids to BSA-FAF restored the BEI and intrinsic Cl’biliary of pravastatin, additional experiments were conducted (Figure 6. As described previously (Figure 2), the fu was significantly higher in the presence of BSA compared to BSA-FAF (p < 0.05). The BEI and intrinsic Cl’biliary of pravastatin in the presence of BSA were similar to values in the absence of BSA with or without the addition of fatty acids. BSA-FAF decreased the fu, BEI, and intrinsic Cl’biliary of pravastatin compared to the absence or presence of BSA, and the addition of fatty acids to BSA-FAF was unable to mitigate this effect.
Figure 6. Effect of the addition of fatty acids to BSA-FAF on pravastatin hepatobiliary disposition.
Sandwich-cultured rat hepatocytes were incubated with pravastatin (5 μM) in the absence or presence of 4% (w/v) BSA or BSA-FAF, with or without addition of long chain fatty acids or vehicle (dimethyl sulfoxide; DMSO), for 10 min following incubation in standard or Ca2+-free buffer. Long chain fatty acids [linoleic acid (5.48 μM), linolenic acid (0.20 μM), oleic acid (6.94 μM), palmitic acid (9.38 μM), palmitoleic acid (0.76 μM), and stearic acid (7 μM)] were added to standard buffer with or without BSA-FAF and preincubated at room temperature for ~1 h prior to the addition of pravastatin. Filled bars represent accumulation in hepatocytes and bile canaliculi (cell + bile). Open bars represent accumulation in hepatocytes (cell). BEI, intrinsic Cl’biliary, and fu were determined as described under Methods. Data are presented as mean ± S.E.M. n = 1 liver in triplicate.
DISCUSSION
Protein often is added to in vitro systems to facilitate delivery and improve the stability of drug candidates, to prevent precipitation of water-insoluble compounds, and to minimize adsorption to experimental apparatuses (Artursson, 1990; Katneni et al., 2008; Saha and Kou, 2002; Wilson et al., 1990). Albumin is the most abundant plasma protein, and therefore is added most frequently, typically in the form of BSA (Aungst et al., 2000; Cross et al., 2003; Krishna et al., 2001; Neuhoff et al., 2006; Yamashita et al., 2000). Different BSA preparations are commercially available and BSA-FAF is used frequently for in vitro experiments. The effects of different types of BSA on biliary clearance estimates in sandwich-cultured hepatocytes have not been reported to date. This study evaluated the effect of BSA and BSA-FAF on BEI and biliary clearance in sandwich-cultured rat hepatocytes for three compounds representing different degrees of protein binding: digoxin (low binding); pravastatin (moderate binding); and taurocholate (high binding).
The equilibrium unbound fraction of a compound is an important determinant of hepatic uptake; it is assumed that only the unbound species is available to traverse the hepatic basolateral membrane. While this is a common assumption, it might not be true in all cases. For example, if protein binding of a particular compound is labile, such that the rate constants governing binding onset and offset are rapid, uptake may appear to occur for both the bound and unbound pool if the uptake process is very efficient (i.e., if the compound could be classified as a “high extraction” substrate). The fu must be taken into account when calculating the intrinsic Cl’biliary (Equation 2) of digoxin, pravastatin, and taurocholate. As expected, the fu of each compound was significantly decreased in the presence, compared to the absence, of BSA (Figures 1–3). Our data consistently show that intrinsic Cl’biliary, when corrected for fu, is similar in the absence or presence of BSA (Figures 1–3). In addition, the BEI of digoxin and taurocholate showed comparable results in the absence and presence of BSA. Similar to the present observations, Liu et al. (2005) reported no difference in digoxin clearance between isolated rat livers perfused with Krebs-Henseleit bicarbonate (KHB) buffer alone or KHB buffer containing BSA and red blood cells, after correcting for the fu of digoxin. These results confirm the importance of accounting for the fu of a compound when calculating intrinsic Cl’biliary.
Surprisingly, the intrinsic Cl’biliary of digoxin and pravastatin in the presence of BSA-FAF was significantly decreased compared to values determined in the presence or absence of BSA when fu was taken into account (Figures 1–2); comparable differences were observed for the BEI. The fu of each compound also was significantly lower in the presence of BSA-FAF (Figures 1–2). Fatty acids may either compete for binding sites on albumin, produce electrostatic effects that influence the binding of compounds to albumin, or bind to albumin and produce conformational changes that affect the binding of other compounds (Bertucci and Domenici, 2002; Kragh-Hansen et al., 2002; Otagiri, 2005). The type of effect that occurs may be substrate-dependent. In this in vitro system, BSA-FAF decreased the fu of digoxin, pravastatin, and taurocholate (Figures 1–3). Despite the difference in fu in the presence of BSA-FAF, the increased binding of digoxin and pravastatin to BSA-FAF did not account for the differences observed in BEI and intrinsic Cl’biliary for these compounds.
Addition of BSA to incubations of HeLa cells stably transfected with rat sodium-taurocholate co-transporting polypeptide (Ntcp) or organic anion-transporting polypeptide (Oatp) 1 (Oatp1a1) decreased the uptake of [35S]-sulfobromophthalein (Hata et al., 2003; Shi et al., 1995). Similarly, Cui et al. (2001) reported that addition of human serum albumin to HEK293 cells stably expressing recombinant human OATP2 (OATP1B1) or OATP8 (OATP1B3) abolished OATP8-mediated [3H]-sulfobromophthalein uptake, but did not significantly affect OATP2-mediated [3H]-sulfobromophthalein uptake. These findings suggest that decreased substrate accumulation may depend on whether albumin inhibits the transport protein(s) involved in the uptake of a particular substrate, as well as on the relative contribution of each transport protein to uptake. The hepatic uptake of digoxin and pravastatin is Oatp-mediated (Hsiang et al., 1999; Noé et al., 1997; Sasaki et al., 2004; Shitara et al., 2002; Tokui et al., 1999). Although taurocholate is a substrate for multiple Oatps, Ntcp is the primary transport protein responsible for hepatic uptake of taurocholate in rat hepatocytes (Oude Elferink et al., 1995). In the present study, the accumulation of taurocholate was notably decreased in the presence of BSA or BSA-FAF (Figure 3). The present findings are consistent with the hypothesis that BSA and BSA-FAF inhibit Ntcp-mediated transport in sandwich-cultured rat hepatocytes. Based on these data, a decrease in the intrinsic Cl’biliary of taurocholate was expected. The observation that BSA-FAF significantly decreased BEI and intrinsic Cl’biliary values for digoxin and pravastatin, but not taurocholate, further emphasizes the complexity of the albumin effect, and suggests that the findings cannot be explained simply by direct inhibition of specific transport proteins.
One possible mechanism by which BSA-FAF might affect BEI and intrinsic Cl’biliary in sandwich-cultured rat hepatocytes involves alterations in the maintenance of tight junction integrity. To investigate this possibility, CDF retention in sandwich-cultured rat hepatocytes in the absence or presence of BSA and BSA-FAF was studied over a 10-min period (Figure 4). CDFDA is hydrolyzed to CDF within hepatocytes and is transported into the bile canalicular networks by Mrp2. CDF accumulates in these networks if the integrity of the tight junction complexes have not been compromised by the absence of Ca2+ (Liu et al., 1999a), or some other factor. No obvious differences were observed in CDF retention in bile canalicular networks in the presence of BSA or BSA-FAF compared to accumulation in networks in the absence of BSA. These results suggest that the bile canalicular networks remain sealed in the presence of BSA and BSA-FAF, and that the proteins comprising the tight junctions are not modulated.
Preparation of BSA-FAF involves the incubation of BSA with activated charcoal. In addition to removal of fatty acids, other components may be removed, including calcium and other ions. Thus, BSA-FAF may have increased potential to bind calcium. Therefore, we evaluated the addition of physiological concentrations of calcium. Additional 1 mM Ca2+ (Figure 5), or 2.5 mM Ca2+ (data not shown), did not mitigate the effect of BSA-FAF on the BEI or intrinsic Cl’biliary of digoxin, suggesting that the mechanism of the BSA-FAF effect is not through the modulation of Ca2+ levels. It is unlikely that a contaminant in the BSA-FAF preparation is chelating Ca2+ and preventing the maintenance of tight junction integrity and formation of sealed bile canalicular networks.
Fatty acids have been reported to inhibit the activity of some metabolic enzymes (Rowland et al., 2007; Rowland et al., 2008a, b), and any BSA preparation can protect against this by sequestering the inhibitory fatty acids (Rowland et al., 2007). Therefore, we evaluated whether the addition of six of the predominant long chain fatty acids present in BSA [linoleic acid, linolenic acid, oleic acid, palmitic acid, palmitoleic acid and stearic acid (Rowland et al., 2007)], would mitigate the decrease in the BEI and intrinsic Cl’biliary of pravastatin observed in the presence of BSA-FAF compared to values in the presence or absence of BSA. Addition of fatty acids did not modulate the effect of BSA-FAF on the BEI and intrinsic Cl’biliary of pravastatin (Figure 6). Further work is required to elucidate the mechanism(s) responsible for the decreased BEI and intrinsic Cl’biliary values observed in the presence of BSA-FAF.
In conclusion, the results of this study indicate that the type of BSA that is added to sandwich-cultured rat hepatocytes can affect the BEI and predicted in vivo biliary clearance values. Addition of BSA yielded results consistent with the BEI and intrinsic Cl’biliary determined in the absence of BSA. In contrast, BSA-FAF significantly decreased the BEI and intrinsic Cl’biliary of some substrates. The selection of proteins for addition to in vitro systems requires careful consideration. The present observations suggest that, when necessary, BSA rather than BSA-FAF should be added in vitro to sandwich-cultured rat hepatocytes for determination of BEI and biliary clearance values.
Acknowledgments
The authors would like to thank Yi-Wei Rong for her technical expertise in the isolation of rat hepatocytes, Dr. Arlene Bridges for LC/MS/MS analysis of pravastatin samples, and Dr. Koji Abe and Dr. Daniel Bow for insightful comments regarding this manuscript.
This work was supported by National Institutes of Health grant GM41935. KKW was supported by a postdoctoral fellowship from GlaxoSmithKline Inc. Data were presented at the American Association of Pharmaceutical Scientists Annual Meeting and Exposition in San Diego, CA, November 2007.
Footnotes
Abbreviations: BSA, bovine serum albumin; UGT, UDP-glucuronosyltransferase; BSA-FAF, essentially fatty acid-free bovine serum albumin; DMEM, Dulbecco’s modified Eagle’s medium; CDF, 5 (and 6)-carboxy-2′,7′-dichlorofluorescein; CDFDA, 5 (and 6)-carboxy-2′,7′-dichlorofluorescein diacetate; HBSS, Hanks’ balanced salts solution; BCA, bicinchoninic acid; LC/MS/MS, liquid chromatography with detection by tandem mass spectrometry; fu, unbound fraction; BEI, biliary excretion index; intrinsic Cl’biliary, unbound intrinsic biliary clearance; ANOVA, analysis of variance; Mrp, multidrug resistance-associated protein
References
- Annaert PP, Brouwer KLR. Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2005;33:388–394. doi: 10.1124/dmd.104.001669. [DOI] [PubMed] [Google Scholar]
- Annaert PP, Turncliff RZ, Booth CL, Thakker DR, Brouwer KLR. P-glycoprotein-mediated in vitro biliary excretion in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2001;29:1277–1283. [PubMed] [Google Scholar]
- Artursson P. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79:476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- Aungst BJ, Nguyen NH, Bulgarelli JP, Oates-Lenz K. The influence of donor and reservoir additives on Caco-2 permeability and secretory transport of HIV protease inhibitors and other lipophilic compounds. Pharm Res. 2000;17:1175–1180. doi: 10.1023/a:1026402410783. [DOI] [PubMed] [Google Scholar]
- Bennhold H. Transport function of the serum proteins: historical review and report on recent investigations on the transport of dyestuffs and of iron. In: Desgrez P, DeTraverse PM, editors. Transport Functions of Plasma Proteins. Elsevier Publishing Company; New York: 1965. pp. 1–12. [Google Scholar]
- Bertucci C, Domenici E. Reversible and covalent binding of drugs to human serum albumin: methodological approaches and physiological relevance. Curr Med Chem. 2002;9:1463–1481. doi: 10.2174/0929867023369673. [DOI] [PubMed] [Google Scholar]
- Cross SE, Anissimov YG, Magnusson BM, Roberts MS. Bovine-serum-albumin-containing receptor phase better predicts transdermal absorption parameters for lipophilic compounds. J Invest Dermatol. 2003;120:589–591. doi: 10.1046/j.1523-1747.2003.12083.x. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Fisher JM, Wrighton SA, Calamia JC, Shen DD, Kunze KL, Thummel KE. Midazolam metabolism by modified Caco-2 monolayers: effects of extracellular protein binding. J Pharmacol Exp Ther. 1999;289:1143–1150. [PubMed] [Google Scholar]
- Forker EL, Luxon BA. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981;67:1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Ohashi R, Tsuda-Tsukimoto M, Tamai I. Effect of plasma protein binding on in vitro-in vivo correlation of biliary excretion of drugs evaluated by sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2008;3:2008. doi: 10.1124/dmd.107.019026. Epub April. [DOI] [PubMed] [Google Scholar]
- Hata S, Wang P, Eftychiou N, Ananthanarayanan M, Batta A, Salen G, Pang KS, Wolkoff AW. Substrate specificities of rat oatp1 and ntcp: implications for hepatic organic anion uptake. Am J Physiol Gastrointest Liver Physiol. 2003;285:G829–839. doi: 10.1152/ajpgi.00352.2002. [DOI] [PubMed] [Google Scholar]
- Hatanaka T. Clinical pharmacokinetics of pravastatin – Mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397–412. doi: 10.2165/00003088-200039060-00002. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KLR. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model of opioid peptide. Pharm Res. 2004;21:1294–1302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Hung DY, Siebert GA, Chang P, Roberts MS. Hepatic pharmacokinetics of taurocholate in the normal and cholestatic rat liver. Br J Pharmacol. 2005;145:57–65. doi: 10.1038/sj.bjp.0706148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katneni K, Charman SA, Porter CJH. Use of plasma proteins as solubilizing agents in in vitro permeability experiments: Correction for unbound drug concentration using the reciprocal permeability approach. J Pharm Sci. 2008;97:209–224. doi: 10.1002/jps.20877. [DOI] [PubMed] [Google Scholar]
- Kemp DC, Zamek-Gliszczynski MJ, Brouwer KLR. Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol Sci. 2005;83:207–214. doi: 10.1093/toxsci/kfi020. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U, Chuang VTG, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002;25:695–704. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- Krishna G, Chen K, Lin C, Nomeir AA. Permeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2. Int J Pharm. 2001;222:77–89. doi: 10.1016/s0378-5173(01)00698-6. [DOI] [PubMed] [Google Scholar]
- Lacy CF, Armstrong LL, Goldman MP, Lance LL. Drug Information Handbook. Vol. 10. Lexi-Comp Inc.; Hudson: 2002. Digoxin; pp. 403–405. [Google Scholar]
- LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764–C1774. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. Pharm Biotechnol. 1996;8:121–159. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Fix JA, Audus KL, Hochman JH. Regeneration and maintenance of bile canalicular networks in collagen-sandwiched hepatocytes. Toxicol In Vitro. 2000;14:117–132. doi: 10.1016/s0887-2333(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Liu L, Mak E, Tirona RG, Tan E, Novikoff PM, Wang P, Wolkoff AW, Pang KS. Vascular binding, blood flow, transporter, and enzyme interactions on the processing of digoxin in rat liver. J Pharmacol Exp Ther. 2005;315:433–448. doi: 10.1124/jpet.105.088039. [DOI] [PubMed] [Google Scholar]
- Liu X, Brouwer KLR, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15:1533–1539. doi: 10.1023/a:1011994831139. [DOI] [PubMed] [Google Scholar]
- Liu X, Chism JP, LeCluyse EL, Brouwer KR, Brouwer KLR. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999b;27:637–644. [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LSL, Lemasters JJ, Stieger B, Meier PJ, Brouwer KLR. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999a;277:G12–G21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- McRae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KLR, Kashuba AD. Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J Pharmacol Exp Ther. 2006;318:1068–1075. doi: 10.1124/jpet.106.102657. [DOI] [PubMed] [Google Scholar]
- Neuhoff S, Artursson P, Zamora I, Ungell AL. Impact of extracellular protein binding on passive and active drug transport across Caco-2 cells. Pharm Res. 2006;23:350–359. doi: 10.1007/s11095-005-9304-3. [DOI] [PubMed] [Google Scholar]
- Noé B, Hagenbuch B, Stieger B, Meier PJ. Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci USA. 1997;94:10346–10350. doi: 10.1073/pnas.94.19.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otagiri M. A molecular functional study on the interactions of drugs with plasma proteins. Drug Metab Pharmacokinet. 2005;20:309–323. doi: 10.2133/dmpk.20.309. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RP, Meijer DK, Kuipers F, Jansen PL, Groen AK, Groothuis GM. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995;1241:215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr . All about Albumin, Biochemistry, Genetics, and Medical Applications. Vol. 1. Academic Press, Inc.; San Diego: 1996. p. 432. [Google Scholar]
- Rowland A, Elliot DJ, Knights KM, Mackenzie PI, Miners JO. The “albumin effect” and in vitro-in vivo extrapolation: sequestration of long-chain unsaturated fatty acids enhance phenytoin hydroxylation by human liver microsomal and recombinant cytochrome P450 2C9. Drug Metab Dispos. 2008b;36:870–877. doi: 10.1124/dmd.107.019885. [DOI] [PubMed] [Google Scholar]
- Rowland A, Gaganis P, Elliot DJ, Mackenzie PI, Knights KM, Miners JO. Binding of inhibitory fatty acids is responsible for the enhancement of UDP-glucuronosyltransferase 2B7 activity by albumin: implications for in vitro-in vivo extrapolation. J Pharmacol Exp Ther. 2007;321:137–147. doi: 10.1124/jpet.106.118216. [DOI] [PubMed] [Google Scholar]
- Rowland A, Knights KM, Mackenzie PI, Miners JO. The “albumin effect” and drug glucuronidation: bovine serum albumin and fatty acid-free human serum albumin enhance the glucuronidation of UDP-glucuronosyltransferase (UGT) 1A9 substrates but not UGT1A1 and UGT1A6 activities. Drug Metab Dispos. 2008a;36:1056–1062. doi: 10.1124/dmd.108.021105. [DOI] [PubMed] [Google Scholar]
- Saha P, Kou JH. Effect of bovine serum albumin on drug permeability estimation across Caco-2 monolayers. Eur J Pharm Biopharm. 2002;54:319–324. doi: 10.1016/s0939-6411(02)00089-9. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Suzuki H, Aoki J, Ito K, Meier PJ, Sugiyama Y. Prediction of in vivo biliary clearance from the in vitro transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II monolayer expressing both rat organic anion transporting polypeptide 4 and multidrug resistance associated protein 2. Mol Pharm. 2004;66:450–459. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shi X, Bai S, Ford AC, Burk RD, Jacquemin E, Hagenbuch B, Meier PJ, Wolkoff AW. Stable inducible expression of a functional rat liver organic anion transport protein in HeLa cells. J Biol Chem. 1995;270:25591–25595. doi: 10.1074/jbc.270.43.25591. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Sugiyama D, Kusuhara H, Kato Y, Abe T, Meier PJ, Itoh T, Sugiyama Y. Comparative inhibitory effects of different compounds on rat Oatp1 (Slc21a1)-and Oatp2 (Slc21a5)-mediated transport. Pharm Res. 2002;19:147–153. doi: 10.1023/a:1014264614637. [DOI] [PubMed] [Google Scholar]
- Taub ME, Kristensen L, Frokjaer S. Optimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I-IV. Eur J Pharm Sci. 2002;15:331–340. doi: 10.1016/s0928-0987(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Tokui T, Nakai D, Nakagomi R, Yawo H, Abe T, Sugiyama Y. Pravastatin, an HMG-CoA reductase inhibitor, is transported by rat organic anion transporting polypeptide, oatp2. Pharm Res. 1999;16:904–908. doi: 10.1023/a:1018838405987. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Hoffmaster KA, Kalvass JC, Pollack GM, Brouwer KLR. Hepatobiliary disposition of a drug/metabolite pair: Comprehensive pharmacokinetic modeling in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 2006;318:881–889. doi: 10.1124/jpet.106.102616. [DOI] [PubMed] [Google Scholar]
- Wilson G, Hassan IF, Dix CJ, Williamson I, Shah R, Mackay M, Artursson P. Transport and permeability properties of human Caco-2 cells: An in vitro model of the intestinal epithelial cell barrier. J Control Release. 1990;11:25–40. [Google Scholar]
- Wiseman EH, Nelson E. Correlation of in vitro metabolism rate and physical properties of sulfonamides. J Pharm Sci. 1964;53:992. doi: 10.1002/jps.2600530846. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci. 2000;10:195–204. doi: 10.1016/s0928-0987(00)00076-2. [DOI] [PubMed] [Google Scholar]