Abstract
We have characterized FIAT, a 66 kDa leucine zipper (LZ) protein that dimerizes with activating transcription factor 4 (ATF4) to form inactive dimers that cannot bind DNA. Computer analysis identifies three putative LZ motifs within the FIAT amino acid sequence. We have used deletion- and/or site-specific mutagenesis to individually inactivate these motifs in order to identify the functional LZ that mediates the FIAT-ATF4 interaction. Amino acids 194-222 that encode the FIAT LZ2 were deleted (mutant FIAT ZIP2 DEL). We inactivated each zipper individually by replacing two or three leucine residues within each zipper by alanine residues. The engineered mutations were L142A/L149A (mutant M1, first zipper), L208A/L215A/L222A (mutant M2, second zipper), and L441A/L448A (mutant M3, third zipper). MC3T3-E1 osteoblastic cells with an integrated 1.3 kb mouse osteocalcin gene promoter fragment driving expression of luciferase were transfected with expression vectors for ATF4 and the various FIAT deletion- or site-specific mutants. Inhibition of ATF4-mediated transcription was compared between wild-type (WT) and LZ FIAT mutants. The deletion mutant FIAT ZIP2 DEL and the sequence-specific M2 mutant did not interact with ATF4 and were unable to inhibit ATF4-mediated transcription. The M1 or M3 mutations did not affect the ability of FIAT to contact ATF4 or to inhibit its transcriptional activity. Stable expression of WT FIAT in osteoblastic cells inhibited mineralization, but not expression of the FIAT ZIP2 DEL and M2 mutants. This structure-function analysis reveals that FIAT interacts with ATF4 and modulates its activity through its second leucine zipper motif.
Keywords: FIAT, ATF4, OSTEOCALCIN GENE TRANSCRIPTION, OSTEOBLAST, BZIP TRANSCRIPTION FACTORS
Activating transcription factor 4 (ATF4) was recently characterized as a crucial player in bone development [Yang et al., 2004]. It is an osteoblast-enriched [Yang and Karsenty, 2004] basic domain-leucine zipper (bZIP) transcription factor that belongs to the ATF/CREB (cAMP response element binding protein) family. The structure of the protein allows it to dimerize through its leucine zipper domain to form a large variety of homodimers and/or heterodimers and bind to DNA via its basic region. It has been shown to form homodimers in vitro and to heterodimerize with Fos/Jun family members extensively [Hai and Curran, 1991; Hai and Hartman, 2001]. In addition, HTLV-1, Tax, GPE1-binding protein, IGEBP1, nuclear factor-interleukin 6 (NF-IL-6), NF-E2-related factor (NRF) 2, and c-maf have been shown to heterodimerize with ATF4 [Masuoka and Townes, 2002]. The dimerization partner of ATF4 confers the complex its distinguishable DNA binding specificity and therefore allows ATF4 to coordinate a variety of different intracellular signals [Hai and Curran, 1991]. It is known that ATF4 is an integral component in a number of pathways responsible for amino acid metabolism, transport, and redox chemistry induced by oxidative stress [Fawcett et al., 1999; Chen et al., 2004], and this central mediation in amino acid transport also confers ATF4 an essential role in bone formation by regulating the synthesis of type I collagen, the most abundant matrix protein in mineralized bone [Yang et al., 2004].
The importance of ATF4 in osteoblast development was first revealed when it was identified as the osteoblast-specific transcription factor 1 (OSF1) that binds to the osteoblast-specific element 1 (OSE1) site in the proximal osteocalcin gene promoter to activate osteocalcin gene transcription [Ducy and Karsenty, 1995; Schinke and Karsenty, 1999]. This potent activation of the osteocalcin gene is crucial for osteoblast differentiation and proper bone matrix mineralization, and it requires cooperative interaction of ATF4 with RUNX2 and SATB2 at the osteocalcin promoter [Xiao et al., 2005; Dobreva et al., 2006]. The importance of ATF4 in bone formation was further confirmed by its ability to induce osteoblast-specific gene expression in non-osteoblastic cells [Yang and Karsenty, 2004] and by the severely osteoporotic phenotype of ATF4-deficient mice due to decreased osteoblast-specific gene expression, delayed osteoblast differentiation, and impaired type I collagen synthesis [Yang et al., 2004]. These functions of ATF4 require phosphorylation by the ribosomal S6 kinase-2 (RSK2) at serine 251, and compound heterozygous Rsk2+/-; Atf4+/- mice have a low bone mass skeletal dysplasia similar to Rsk2+/- mice, demonstrating that the two molecules reside within a common genetic pathway [Yang et al., 2004].
Not only does ATF4 regulate osteoblast development by activating genes essential for osteoblast differentiation, such as osteocalcin and bone sialoprotein, but upon phosphorylation of residue serine 254 by protein kinase A (PKA) [Elefteriou et al., 2005], ATF4 also induces the expression of receptor activator of nuclear factor κB ligand (RANKL) on the surface of osteoblasts, which serves as a potent stimulator for osteoclast differentiation [Elefteriou et al., 2005]. Thus, ATF4 controls bone formation through regulating both osteoblast function and osteoclast differentiation, with a dominating role in promoting osteoblast activity since ATF4-null mice have an osteoporotic phenotype.
Factor Inhibiting ATF4-mediated Transcription (FIAT) is a novel leucine zipper factor identified in a differential screen for proteins expressed in osteoblasts, whose name was coined for its interaction with ATF4 and subsequent blockage of ATF4-directed osteocalcin gene transcription [Yu et al., 2005]. Fiat encodes for a 1.8 kb coding sequence which translates into a 66 kDa polypeptide that mainly localizes in the nucleus of osteoblasts. It is predicted to form a long coiled-coil at its COOH terminus, a structure that favors protein-protein interactions. Another group has characterized an identical protein named γ-taxilin on the basis of its extended COOH-terminal coiled-coil and showed that it interacts with syntaxin family members and αNAC [Nogami et al., 2004; Yoshida et al., 2005]. However, to alleviate the text, the acronym FIAT, which defines the function of the protein studied here, will be used throughout.
Computer modeling predicts that FIAT contains three putative leucine zipper domains but no identifiable basic DNA recognition sequences (Fig. 1). It can heterodimerize with ATF4 through one of these zippers and thereby prohibits ATF4 from binding to its cognate DNA sequences [Yu et al., 2005]. Transgenic mice overexpressing FIAT under the control of the osteoblast-specific α1(I) collagen promoter are osteopenic [St-Arnaud and Yu, 2005; Yu et al., 2005, 2006], characterized by reduced bone mineral density, lowered trabecular volume, and decreased bone rigidity, which was shown to be caused by impaired osteoblastic activity without changes in osteoblast proliferation and apoptosis. The phenotype of the osteoblast-specific FIAT transgenic mice mimics several aspects of the ATF4-deficient mice. Altogether, these observations suggest that FIAT regulates osteoblast functions through modulating ATF4-dependent activities.
Fig. 1.

Leucine zipper motifs in the FIAT protein sequence. The 524-amino acids FIAT protein contains three identifiable leucine zipper domains (boxed in black) with the leucines in each zipper highlighted. Amino acids 194-222 were deleted to create the FIAT ZIP2 DEL mutant. The leucine-to-alanine substitutions engineered to generate mutants M1 (L142A/L149A), M2 (L208A/L215A/L222A), and M3 (L441A/L448A) are indicated above the boxed leucine zipper domains.
In this study, we sought to identify the functional domain of FIAT responsible for interacting with ATF4. Deletion mutation of the second leucine zipper of FIAT rendered FIAT unable to repress ATF4-induced osteocalcin transcription, while mutation of the first and third leucine zipper of FIAT had no effect on its function. Moreover, point-specific mutations that changed three leucines to alanines within the second leucine zipper were sufficient to disrupt FIAT-ATF4 complex formation, resulting in elevated ATF4-induced osteocalcin gene transcription, and increased matrix mineralization. These results confirm that the second leucine zipper is necessary for FIAT to interact with and inhibit the functions of ATF4.
MATERIALS AND METHODS
DNA CLONING AND SEQUENCE MUTAGENESIS
Site-specific and deletion mutations of FIAT were generated by a PCR-based strategy [Elion, 1993]. The following mutations were engineered: deletion of amino acids 194-222 that encode the FIAT leucine zipper (LZ) 2 (mutant FIAT ZIP2 DEL); L142A/L149A (mutant M1, LZ1); L208A/L215A/L222A (mutant M2, LZ2); and L441A/L448A (mutant M3, LZ3). For site-specific mutagenesis, primers that contained specific leucine to alanine mutations in the leucine zippers of FIAT were generated, and copies of mutated sequences were amplified using those specific primers, and subsequently ligated in frame with the V5-His epitope tag within the pcDNA3.1/V5-His TOPO vector (Stratagene, La Jolla, CA). For deletion mutation, two FIAT sequences flanking LZ2 were amplified by PCR, phosphorylated by calf intestinal alkaline phosphatase, and ligated together to create a FIAT LZ2 deletion mutation. This mutated DNA sequence was also cloned in the pcDNA3.1/V5-His TOPO vector in frame with the epitope tag. All mutations were confirmed by complete sequencing of all inserts. Information on the primer sequences used for mutagenesis is available upon request. As for the mutant sequences, ATF4 (gift from G. Karsenty, Columbia University, New York, NY) and wild-type (WT) FIAT sequences were cloned in the pcDNA3.1/V5-His TOPO vector for mammalian expression.
TRANSIENT AND STABLE TRANSFECTION EXPERIMENTS
An MC3T3-E1 osteoblastic cell line with an integrated osteocalcin promoter-luciferase reporter allele [Xiao et al., 1997] was used for transient transfections. In transient transfection, cells were cultured in αMEM containing 10% fetal bovine serum and were plated at 1.0 × 105 cells/well in a 6-well plate. Cells were transfected with a total of 2 μg of vectors expressing ATF4, FIAT, FIAT M1, FIAT M2, FIAT M3, FIAT ZIP2 DEL or empty vector using the GenePORTER Transfection Reagent (Genlantis, San Diego, CA), following the instructions provided by the manufacturer. At 24 h post-transfection, cells were lysed and 20 μl of cell lysate were used to measure luciferase activity. Data represent means + SEM of four independent transfection experiments performed in duplicate. For stable transfection, MC3T3-E1 cells were cultured in αMEM containing 10% fetal bovine serum, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate and were plated at 1.0 × 105 cells/well of a 6-well plate. Cells were transfected with 2 μg of the empty pcDNA3.1/V5-His TOPO vector or expression vectors transcribing WT FIAT, FIAT M1, FIAT M2, FIAT M3, and FIAT ZIP2 DEL using the GenePORTER Transfection Reagent (Genlantis). At 48 h post-transfection, cells were trypsinized and re-plated at a 1:40 ratio for selection with 400 μg/ml G418. Cell populations were established as pools of stably transfected clones and thus represent the most frequent integration event.
CO-IMMUNOPRECIPITATION AND WESTERN BLOTTING
For co-immunoprecipitation, MC3T3-E1 cells transfected with FIAT, FIAT M1, FIAT M2, and FIAT M3 were pre-treated for 5 h with 1 μg/ml of Proteasome Inhibitor III (Calbiochem, Gibbstown, NJ) prior to harvesting in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1.0% Nonidet NP-40, 0.5% sodium deoxycholate, 0.1% SDS with inhibitors of proteases: 5 μg/ml leupeptin, aprotinin, pepstatin A, and 1 mM PMSF). Total cell extract proteins (1.5 mg) were pre-cleared with 25 μl of protein A-sepharose slurry (Amersham Biosciences, Baie d’Urfe, Quebec) for 1 h at 4°C with gentle rocking. Following centrifugation, the cleared extract was incubated over-night at 4°C with gentle rocking, with or without 2 μg of anti-ATF4 (Santa Cruz Biotechnology, Santa Cruz, CA) or unrelated antibody and 25 μl of protein A-agarose slurry (Santa Cruz Biotechnology). The extracts were centrifuged at 10,000g for 1 min, and the precipitates were washed twice with RIPA buffer, then twice with PBS, prior to re-suspension in 25 μl of 2× Laemmli SDS-PAGE sample buffer. Following boiling for 3 min and burst centrifugation, the precipitates were separated on 7.5% SDS-PAGE, transferred to nitrocellulose membranes, and the blots were probed with 1:300 dilution of anti-V5 antibody (Invitrogen Life Technologies, Burlington, Ontario), then with anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences). Proteins were detected by chemifluorescence with Immobilon Western ECL detection reagents (Millipore Corporation, Billerica, MA).
MINERALIZATION ASSAYS
Vector-transfected MC3T3-E1 cells and pools of MC3T3-E1 cells stably expressing FIAT, FIAT M1, FIAT M2, FIAT M3, and FIAT ZIP2 DEL were plated at 1.0 × 105 cells/well of a 6-well plate and cultured in αMEM containing 10% fetal bovine serum, 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate. At 14 days post-confluence, cells were fixed with 4% PFA for 20 min and stained with 5% silver nitrate in dH2O for von Kossa staining [Dickson, 1984] until coloring appeared. Mineralized nodules were photographed with bright-field microscopy.
RESULTS
Three putative leucine zipper domains within the 524-amino acids (a.a.) FIAT protein sequence were identified by computer modeling, which were designated LZ1 (a.a. 135-156), LZ2 (a.a. 194-223), and LZ3 (a.a. 434-455), respectively (Fig. 1). We first engineered a full deletion of LZ2 (mutant ZIP2 DEL) and tested the repressive effect of this mutation on ATF4-induced osteocalcin transcription in osteoblasts. Osteoblastic MC3T3-E1 cells with an integrated osteocalcin promoter-luciferase reporter allele [Xiao et al., 1997] were transfected with expression vectors for either ATF4 alone, ATF4 and WT FIAT, or ATF4 and FIAT ZIP2 DEL (Fig. 2A). Expression of the transfected recombinant molecules was monitored by Western blotting (lower panel). As expected, ATF4 up-regulated transcription from the osteocalcin promoter (by approximately 10-fold), and WT FIAT significantly inhibited ATF4-mediated osteocalcin transcription (Fig. 2A). ATF4-induced transcription from the osteocalcin promoter in cells co-expressing FIAT ZIP2 DEL remained unchanged (Fig. 2A), suggesting that the LZ2 of FIAT is responsible for suppressing the activity of ATF4 and that deletion of this domain renders FIAT non-functional.
Fig. 2.
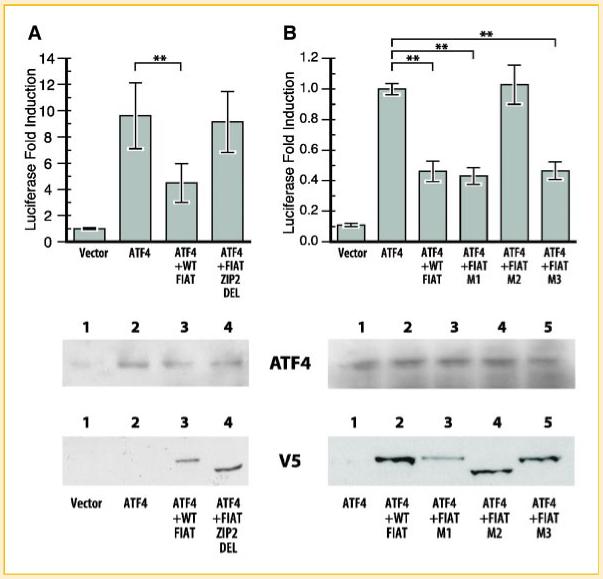
The second leucine zipper within the FIAT sequence is responsible for repressing ATF4-induced transcriptional activity. Transient transfection assays in MC3T3-E1 osteoblastic cells with expression vectors for ATF4 and wild-type or mutant FIAT. A: Transfection with wild-type (WT) or ZIP2 DEL FIAT. B: Transfection with WT or the site-specific M1, M2, or M3 mutants. The expression of the recombinant proteins was monitored by Western blotting at the bottom of each panel. The M2 mutant reproducibly exhibited faster migration than WT FIAT or the M1 and M3 mutants in SDS-PAGE. Mutation within the second leucine zipper of FIAT rendered FIAT unable to inhibit ATF4-mediated gene transcription. **P<0.01. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To further confirm that the impairment of FIAT transcriptional repression of ATF4 by FIAT ZIP2 DEL is due to the mutation of LZ2 and not to the disruption of FIAT tertiary protein structure caused by the deletion, point-specific mutation of each zipper were generated by changing internal leucine residues to alanine (Fig. 1). This strategy has been successfully used to inactivate LZ motifs in structure-function analysis of bZIP proteins [Kouzarides and Ziff, 1988; Schuermann et al., 1989]. As shown in Figure 2B, FIAT M1, bearing the L142A/L149A mutations in LZ1, and FIAT M3, containing the L441A/L448A mutations in LZ3, were able to inhibit ATF4-induced gene transcription as effectively as WT FIAT. However, FIAT M2 (L208A/L215A/L222A) did not inhibit ATF4-induced osteocalcin promoter activity, further demonstrating that LZ2 mediates FIAT interaction with ATF4. Expression levels of all proteins were similar as monitored by immunoblotting. It should be noted that the M2 mutant reproducibly exhibited faster migration than WT FIAT or the M1 and M3 mutants in SDS-PAGE (Fig. 2B, lower panel).
The leucine zipper domain is a structure that allows protein dimerization, based on the alignment of 4-5 leucine heptads along the same face of a coiled-coil structure which optimizes for interaction with another leucine zipper [Vinson et al., 2002]. We have previously shown that FIAT heterodimerizes with ATF4 in the yeast two-hybrid system, and interaction of the two proteins was further demonstrated in osteoblasts by co-immunoprecipitation [Yu et al., 2005, 2006]. We tested the protein-protein interaction between ATF4 and FIAT mutants by co-immunoprecipitation in MC3T3-E1 cells co-transfected with expression vectors for ATF4 and the various FIAT proteins. Cell extracts of transfected cells were immunoprecipitated by either sepharose beads alone, a non-specific antibody, or anti-ATF4 antibody. Precipitated proteins were transferred to an immunoblot and probed with an anti-V5 antibody to reveal the presence of the different epitope-tagged recombinant FIATs. Sepharose beads and non-specific antibody were unable to precipitate any V5-tagged FIAT (data not shown). There was no background associated with anti-ATF4 immunoprecipitation in untransfected cells (Fig. 3A, lane 1). Recombinant WT FIAT, FIAT M1, and FIAT M3 were co-precipitated by the anti-ATF4 antibody (Fig. 3A). The FIAT M2 and ZIP2 DEL mutants did not co-immunoprecipitate with ATF4 (Fig. 3A). Comparable expression levels of each recombinant FIAT molecule was ascertained by Western blotting (Fig. 3B). These results prove that a functional LZ2 is necessary for FIAT-ATF4 complex formation.
Fig. 3.
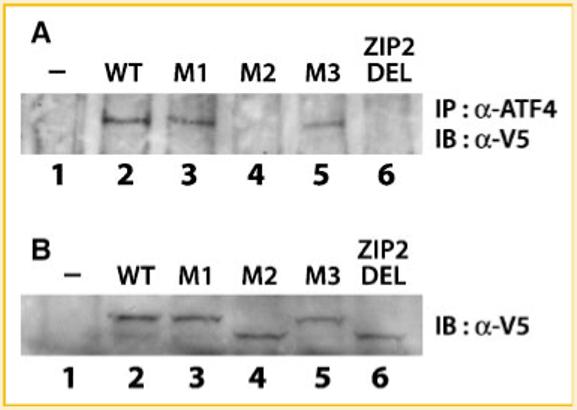
Mutation in the second leucine zipper of FIAT abrogates the FIAT-ATF4 interaction. MC3T3-E1 osteoblastic cells were transiently transfected with expression vectors for ATF4 or wild-type (WT) or mutant FIAT. A: Total cell extracts were co-immunoprecipitated with anti-ATF4 antibodies and probed with the anti-V5 antibody to detect the epitope-tagged FIAT molecules. B: Expression of all recombinant FIAT proteins was confirmed by Western blotting with the anti-V5 antibody. The M2 mutant reproducibly exhibited faster migration than WT FIAT or the M1 and M3 mutants in SDS-PAGE. IP, immunoprecipitation; IB, immunoblotting; α-, anti-. Mutation within the second leucine zipper of FIAT (ZIP2 DEL or M2) abrogated interaction with ATF4.
We further investigated the biological consequence of perturbing the FIAT-ATF4 interaction on osteoblast function, by assessing mineralization in MC3T3-E1 cells stably expressing either WT FIAT, FIAT ZIP2 DEL, FIAT M1, FIAT M2, or FIAT M3. The transfected cells were cultured in the presence of 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate to allow matrix mineralization. The cultures were stained by the von Kossa method [Dickson, 1984] to detect mineralized matrix at day 14 post-confluence. Figure 4A shows that WT FIAT completely suppressed matrix mineralization, while the empty vector or FIAT ZIP2 DEL had no effect. Similarly, no mineralization was observed in cultures of osteoblasts stably expressing FIAT M1 (Fig. 4B). As predicted based on the transcription and interaction data, mineralization was not inhibited in cells expressing FIAT M2. Surprisingly, mineralization was not inhibited in FIAT M3-expressing cells (Fig. 4B). Immunoblotting confirmed comparable expression of the transfected FIAT molecules (Fig. 4). The expression of the WT or mutant FIAT proteins did not significantly alter the expression of ATF4 (Fig. 4A,B) or of other transcriptional regulators of osteocalcin transcription such as Runx2 or Satb2 (Fig. 4B). These results show that an intact LZ2 domain is necessary for FIAT to modulate osteoblast function and inhibit mineralization. The data also reveal that the LZ3 domain may participate in the biological role of FIAT to inhibit matrix mineralization.
Fig. 4.
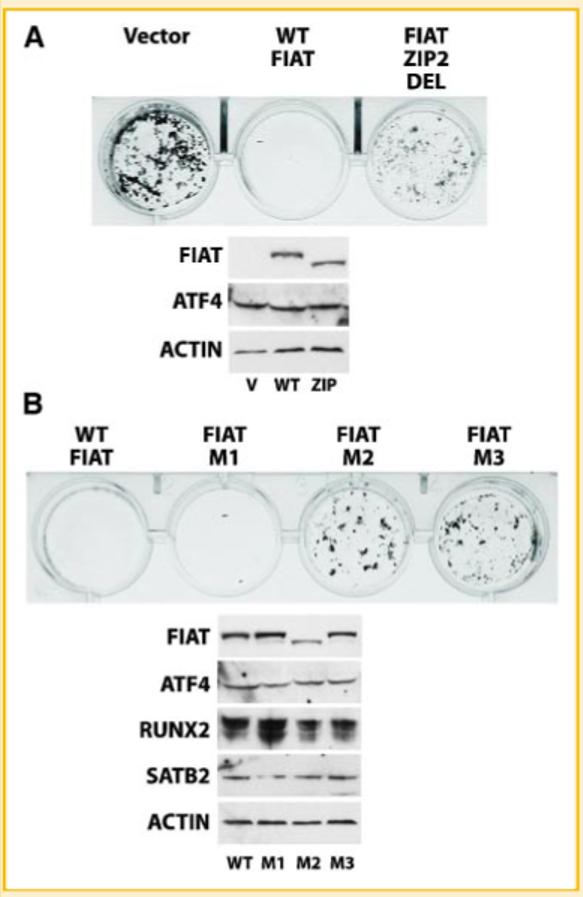
FIAT suppression of mineralization requires the second or third leucine zippers. MC3T3-E1 osteoblastic cells were stably transfected with the empty vector or with expression vectors for WT FIAT (A,B), the ZIP2 DEL mutant (A), or the site-specific M1, M2, or M3 mutants (B). The cells were cultured for 14 days in the presence of ascorbic acid and β-glycerophosphate to allow matrix mineralization and the cultures were stained by the von Kossa method. Immunoblots of the transfected FIAT molecules, ATF4, Runx2, Satb2, or actin are shown below the von Kossa-stained cultures in each panel. Transfection of WT FIAT or the M1 mutant inhibited mineralization, while the ZIP2 DEL, M2, and M3 mutants were compromised in their ability to block mineralization. V, vector; WT, wild-type; zip, FIAT ZIP2 DEL mutant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
We have previously shown that FIAT suppresses bone formation through ATF4-dependent pathways by characterizing the phenotype of osteoblast-specific FIAT transgenic mice [Yu et al., 2005]. Here, we investigated the mechanism by which FIAT represses ATF4 activity in osteoblasts. We showed that FIAT barricades the functions of ATF4 by interacting with ATF4 through the second leucine zipper, which leads to attenuated osteocalcin gene transcription, and that this interaction eventually impacts matrix mineralization.
ATF4 is an essential bZIP transcription factor necessary for bone formation. It regulates osteoblast differentiation by inducing osteoblast-specific genes transcription, and controls the synthesis of type I collagen, the most abundant protein that contributes to bone matrix mineralization [Yang and Karsenty, 2004; Yang et al., 2004]. It also indirectly regulates osteoclast differentiation by inducing RANKL expression [Elefteriou et al., 2005, 2006]. FIAT is a ZIP domain-containing transcriptional regulator that reduces bone mass accrual [Yu et al., 2005]. Based on several observations, we propose that one of the mechanisms by which FIAT regulates bone formation is by interfering with ATF4-dependent pathways. First, the FIAT-ATF4 interaction was confirmed by yeast two-hybrid assays and co-immunoprecipitation experiments in primary osteoblasts [Yu et al., 2005]. Second, FIAT represses ATF4-induced osteocalcin gene transcription in osteoblasts [Yu et al., 2005]. Third, stable inhibition of FIAT expression in osteoblasts by RNA interference influenced ATF4-dependent activities, leading to enhanced binding of ATF4 to the osteocalcin promoter, increased osteocalcin gene transcription, augmented type I collagen production, and exuberant mineralization (Yu et al., unpublished observations). Fourth, transgenic mice overexpressing FIAT under the control of the osteoblast-specific α1(I) collagen promoter are osteopenic with decreased transcription of the osteocalcin gene, lowered trabecular volume, and reduced bone mineral density [Yu et al., 2005], phenotypic traits reminiscent of those observed in ATF4-deficient mice. Both deletion- and site-specific mutagenesis have now determined that the FIAT-ATF4 interaction involves FIAT’s second leucine zipper motif. Since FIAT does not contain a DNA-binding basic domain flanking this zipper motif, its interaction with ATF4 leads to the formation of an inactive dimer that cannot bind the ATF4 response element [Yu et al., 2005].
Interestingly, the ATF4 primary sequence also contains two leucine zipper domains: one located in the amino-terminal domain and one forming the carboxy-terminal bZIP motif [Liang and Hai, 1997]. The putative function of the amino-terminal zipper motif of ATF4 remains unclear as site-specific inactivation of this motif by replacing leucine residues by glycine residues (mutation L104G/L111G) did not affect interaction with WT FIAT (Gauthier and St-Arnaud, unpublished observations).
One surprising finding of our study was the biological impact of the M3 mutation. The FIAT M3 mutant behaved as the WT protein in the transcription assay and in the co-immunoprecipitation assay, but it was compromised in its ability to inhibit mineralization (Fig. 4). One possible interpretation of these results is that the third leucine zipper of FIAT acts as a subordinate interaction domain that strengthens the FIAT-ATF4 complex. This is unlikely since single inactivation of LZ2 appears to completely abolish the FIAT-ATF4 interaction (Fig. 3). Alternatively, it remains a formal possibility that FIAT interacts with additional bZIP proteins that control osteoblast activity. Attractive candidates include the fos family members Fra-1 and ΔFosB that have been shown to regulate osteoblast function and bone mass accrual [Jochum et al., 2000; Sabatakos et al., 2000]. These putative additional FIAT partners may interact with FIAT through LZ3. Characterization of all the mechanisms through which FIAT regulates osteoblast activity and bone formation will require further experimentation and should reveal why a modest perturbation in the level of FIAT expression translates into a significant bone phenotype [St-Arnaud and Yu, 2005; Yu et al., 2005, 2006]. As such, FIAT represents an interesting drug development target since even a partial modulation of its activity would presumably significantly impact on bone mass.
ACKNOWLEDGMENTS
We thank Dr. Renny T. Franceschi (University of Michigan) for his generous gift of the MC3T3-E1 subclone stably transfected with the osteocalcin-luciferase reporter. Mark Lepik prepared the figures. V. W.C. Yu was a Canada Bone Scholar supported by the CIHR Skeletal Health Training Grant. This study was supported by NIH (grant no. 1R01AR053287-01A1 to R. St-A).
Grant sponsor: NIH; Grant number: 1R01AR053287-01A1.
REFERENCES
- Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem. 2004;279:50829–50839. doi: 10.1074/jbc.M409173200. [DOI] [PubMed] [Google Scholar]
- Dickson GR. Methods of calcified tissue preparation. Elsevier; New York, NY: 1984. [Google Scholar]
- Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, Karsenty G, Grosschedl R. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125:971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LF, Karsenty G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4:441–451. doi: 10.1016/j.cmet.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion EA. Constructing recombinant DNA molecules by the polymerase chain reaction. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. Wiley and Sons; NY: 1993. pp. 3.17.1–3.17.10. [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: Activating transcription factor proteins and homeostasis. Gene. 2001;273:1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochum W, David JP, Elliott C, Wutz A, Plenk H, Jr., Matsuo K, Wagner EF. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med. 2000;6:980–984. doi: 10.1038/79676. [DOI] [PubMed] [Google Scholar]
- Kouzarides T, Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988;336:646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Liang G, Hai T. Characterization of human activating transcription factor 4, a transcriptional activator that interacts with multiple domains of cAMP-responsive element-binding protein (CREB)-binding protein. J Biol Chem. 1997;272:24088–24095. doi: 10.1074/jbc.272.38.24088. [DOI] [PubMed] [Google Scholar]
- Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–745. doi: 10.1182/blood.v99.3.736. [DOI] [PubMed] [Google Scholar]
- Nogami S, Satoh S, Tanaka-Nakadate S, Yoshida K, Nakano M, Terano A, Shirataki H. Identification and characterization of taxilin isoforms. Biochem Biophys Res Commun. 2004;319:936–943. doi: 10.1016/j.bbrc.2004.05.073. [DOI] [PubMed] [Google Scholar]
- Sabatakos G, Sims NA, Chen J, Aoki K, Kelz MB, Amling M, Bouali Y, Mukhopadhyay K, Ford K, Nestler EJ, Baron R. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- Schinke T, Karsenty G. Characterization of Osf1, an osteoblast-specific transcription factor binding to a critical cis-acting element in the mouse Osteocalcin promoters. J Biol Chem. 1999;274:30182–30189. doi: 10.1074/jbc.274.42.30182. [DOI] [PubMed] [Google Scholar]
- Schuermann M, Neuberg M, Hunter JB, Jenuwein T, Ryseck RP, Bravo R, Muller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989;56:507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R, Yu VW. Inhibition of bone mass accrual by the FIAT transcriptional repressor. Med Sci (Paris) 2005;21:1020–1021. doi: 10.1051/medsci/200521121020. [DOI] [PubMed] [Google Scholar]
- Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M. Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol. 2002;22:6321–6335. doi: 10.1128/MCB.22.18.6321-6335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: Requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Ge C, Zhao Z, Lai Y, Boules H, Phimphilai M, Yang X, Karsenty G, Franceschi RT. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J Biol Chem. 2005;280:30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- Yang X, Karsenty G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J Biol Chem. 2004;279:47109–47114. doi: 10.1074/jbc.M410010200. [DOI] [PubMed] [Google Scholar]
- Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nogami S, Satoh S, Tanaka-Nakadate S, Hiraishi H, Terano A, Shirataki H. Interaction of the taxilin family with the nascent polypeptide-associated complex that is involved in the transcriptional and translational processes. Genes Cells. 2005;10:465–476. doi: 10.1111/j.1365-2443.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- Yu VW, Ambartsoumian G, Verlinden L, Moir JM, Prud’homme J, Gauthier C, Roughley PJ, St-Arnaud R. FIAT represses ATF4-mediated transcription to regulate bone mass in transgenic mice. J Cell Biol. 2005;169:591–601. doi: 10.1083/jcb.200412139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VWC, Gauthier C, St-Arnaud R. Inhibition of ATF4 transcriptional activity by FIAT/γ-taxilin modulates bone mass accrual. Ann NY Acad Sci. 2006;1068:131–142. doi: 10.1196/annals.1346.027. [DOI] [PubMed] [Google Scholar]


