Abstract
The pregnane X receptor (PXR, NR1I2) is a member of the nuclear receptor superfamily that is activated by a myriad of clinically used compounds and natural products. Activation of PXR in liver regulates the expression genes encoding proteins that are intimately involved in the hepatic uptake, metabolism, and elimination of toxic compounds from our bodies. PXR-mediated herb-drug interactions can have undesirable effects in patients on combination therapy. This can be especially important in cancer patients that self-administer over-the-counter herbal remedies together with conventional anti-cancer chemotherapeutics. Tian xian is a traditional Chinese herbal anti-cancer remedy that activates human PXR in cell-based reporter gene assays. Moreover, tian xian alters the strength of interaction between the human PXR protein and transcriptional cofactor proteins. A novel line of humanized PXR mice are described and used here to show that tian xian increases expression of Cyp3a11 in primary cultures of rodent hepatocytes. Tian xian also induces expression of CYP3A4 in primary cultures of human hepatocytes. Taken together, these data indicate that co-administration of tian xian is likely contraindicated in patients undergoing anti-cancer therapy with conventional chemotherapeutic agents. These data are of particular importance due to the fact that this herbal remedy is currently marketed as an adjunct therapy that reduces the side-effects of conventional chemotherapy and is available without a prescription. Future studies should be conducted to determine the extent to which co-administration of this Chinese herbal remedy alters the pharmacokinetic and pharmocodynamic properties of conventional anti-cancer therapy.
Introduction
Nuclear receptors comprise a large superfamily of transcription factors that are characterized by a conserved N-terminal zinc-finger type DNA-binding domain and a carboxy-terminal ligand-binding domain. They are involved in a variety of physiological, developmental, and toxicological processes (Mangelsdorf et al., 1995). Pregnane X receptor (PXR, NR1I2) was first cloned in 1998 by a research group at GlaxoWellcome as a part of an effort to identify new members of the nuclear receptor superfamily based upon homology and the mouse genome sequencing project (Kliewer et al., 1998).
Since then, PXR has been identified in various species, including human, monkey, cow, pig, rabbit, rat, mouse, chicken, fish, and worms (Blumberg et al., 1998; Lehmann et al., 1998; Moore et al., 2002). In mammals, PXR is highly expressed in the major organs that are important in xenobiotic-biotransformation including the liver and intestine (Kliewer et al., 1998). Numerous studies show that activation of PXR in the liver and intestine produces increased expression of a group of genes that encode proteins involved in the uptake, metabolism, and elimination of potentially toxic compounds (Staudinger et al., 2001a; Staudinger et al., 2001b; Maglich et al., 2002; Rosenfeld et al., 2003; Staudinger et al., 2003).
It is well-established that PXR is a key regulator of xenobiotic-inducible CYP3A gene expression (Goodwin et al., 2002; Kliewer et al., 2002). In addition, PXR regulates the inducible expression of other genes involved in the metabolism of xenobiotic compounds such as CYP2B, CYP2C, CYP24, glutathione S-transferases, sulfotransferases, and glucuronosyltransferases (Maglich et al., 2002; Sonoda et al., 2002; Wei et al., 2002; Chen et al., 2003; Pascussi et al., 2005). In rodents, PXR also regulates the expression of genes encoding the drug transporter genes organic anion transporting polypeptide 1A4, P-glycoprotein/Mdr1, multi-drug resistance-associated protein 2, and multi-drug resistance-associated protein 3 (Geick et al., 2001; Kast et al., 2002; Staudinger et al., 2003). Therefore, PXR activation has a complex nature. While it protects cells from toxic insults, it also represents the molecular basis for an important class of drug-drug interactions.
For example, if one drug activates PXR, it can be predicted that administration of this drug will promote the elimination of other co-administrated drugs that are also metabolized and eliminated by PXR-target gene products, thereby reducing the efficacy of many drug therapies in patients on combination therapy. Additionally, if one drug is a administered as a pro-drug, as is the case with certain anti-cancer therapeutic agents, and a PXR agonist is then co-administered, the resulting increased biotransformation of the pro-drug would likely produce profound and unwanted toxic side effects. This phenomenon is also observed with numerous herbal remedies including St. John's Wort, coleus forskohli, guggulsterone and many others that contain constituents that activate PXR (Staudinger et al., 2006).
Tian xian (also known as Tien Hsein and pronounced “Dianne Sean”) products are herbal dietary supplements manufactured in China by the China-Japan Feida Union Co., Ltd. (www.cjfu.com/en/Main.php). Tian xian products are distributed world-wide and are aggressively marketed as anti-cancer herbal therapy through several websites including www.tianxian.co.uk, www.cancer-tian-xian.com, www.original-tianxian.com, and www.tianxian.com. These products are also marketed as herbal therapies that alleviate the unpleasant side effects associated with western-style anti-cancer treatments (www.tianxian.com/products/products.asp#3). The main supportive information regarding their therapeutic efficacy as anti-cancer agents comes in the form of online testimonials, many of which can be found as web links to the online distributors of these products (for an example see: www.cancer-central.com/).
Currently there are no published studies or clinical trials in the scientific literature establishing the efficacy of these herbal remedies as treatments for cancer, or for their effectiveness as agents that can reduce the side effects of conventional chemotherapy in patients. However, there are three published studies from one laboratory that were performed at the School of Dentistry in the College of Medicine at National Taiwan University in Taipei, Taiwan on the biological effects of tian xian liquid (Sun et al., 2004; Sun et al., 2005a; Sun et al., 2005b). The authors conclude that a liquid formulation of tian xian modulates antigen-stimulated cytokine production by T-cells isolated from patients with recurrent aphthous ulcerations, inhibits cell growth, and induces apoptosis in a wide variety of human cancer cells in cell-based assays.
Multiple tian xian product lines exist on the market including several powder formulations contained in gelatin capsules, a liquid extract, plaster, suppositories, and an ointment (www.cjfu.com/en/2_Products/). A careful examination of the information available on the websites reveals that the main product marketed as a treatment for cancer patients is derived from the ‘original’ formulation of Tien-Hsien Capsule No.1. The dosing regimen for this powdered capsule product is three to six capsules three times daily with warm water after meals. The herbal ingredients for tian xian capsule #1, their proportion, and the purported therapeutic effect can be found at the manufacturers' website (http://www.cjfu.com/en/2_Products/). The herbs and their proportions are listed here: Radix Trichosanthis (10%), Radix Clematidis (10%), Radix Ginseng (15%), Radix Astragali seu Hedysari (10%), Ji Xing Zi (10%), Venenum Bufonis (3%), Radix Gentianae (7%), Caculus Bovis (5%), Polyporus Umbellatus (10%), and Radix Pulsatillae (20%). The high potential for herb-drug interactions in patients undergoing conventional chemotherapy is of great concern. We therefore sought to determine the extent to which these agents could potentially alter the pharmacokinetic and pharmacodynamic properties of co-administered CYP substrates.
Here, we use cell-based reporter gene assays and primary cultures of rodent and human hepatocytes to determine the extent to which an extract of tian xian produces alterations in the expression of CYP3A, a clinically important anti-cancer drug metabolizing enzyme in liver. We also describe the creation, validation, and use of a novel line of genetically engineered transgenic mice that express a FLAG-tagged human PXR protein selectively in the liver of mice lacking the murine Pxr gene.
Materials and Methods
Animal Care
All rodents were maintained on standard laboratory chow and allowed food and water ad libitum. The studies reported here have been carried out in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Compounds and Plasmids
Unless otherwise stated, all chemical compounds were purchased from Sigma (St. Louis, MO). The pSG5-hPXR and the pSG5-mPXR was previously described (Ding and Staudinger, 2005a). The GAL4-SRC1, GAL4-PBP, and GAL4-NCoR1 expression vectors were previously described (Ding and Staudinger, 2005a). The full-length human PXR was fused to the VP16 transcriptional activation domain as described (Ding and Staudinger, 2005a). The pFR-LUC reporter gene which is responsive to GAL4-fusion proteins is commercially available (BD Biosciences, Palo Alto, CA). The pFLAG-hPXR vector was constructed by excising the human PXR cDNA from pSG5-hPXR using EcoRI and SalI sites and inserting it into pCMV-TAG2B vector (Stratagene, La Jolla CA).
Extract Preparation
An extract of tian xian Capsule No.1 (Green and Gold International, Manilla, Phillipines) was prepared using one capsule (250 mgs powder) and 1 ml of absolute ethanol. The mixture was placed in a 1.5 ml centrifuge tube and extracted overnight at 4°C on a rotating shaker. The mixture was centrifuged at 16,000 X g for 5 min. The ethyl alcohol supernatant was decanted and kept at -20°C until use.
Cell Culture and Transient Transfection Analysis
The XREM-LUC reporter gene assays were performed as described (Brobst et al., 2004). The mammalian two-hybrid system analysis was performed as previously described (Ding and Staudinger, 2005a).
Generation of a TTR-FLAG-Tagged hPXR Mini-gene
The plasmid containing the TTR mini-gene shown in figure 1A was digested with Stu I and subsequently treated with calf intestinal alkaline phosphatase. The FLAG-tagged human PXR cDNA was excised from pFLAG-hPXR using NotI and XhoI, treated with Klenow DNA polymerase and dNTPs, and was ligated together with the StuI digested pTTR mini-gene. A graphical representation of the TTR-FLAG-tagged human PXR transgene is shown in figure 1B.
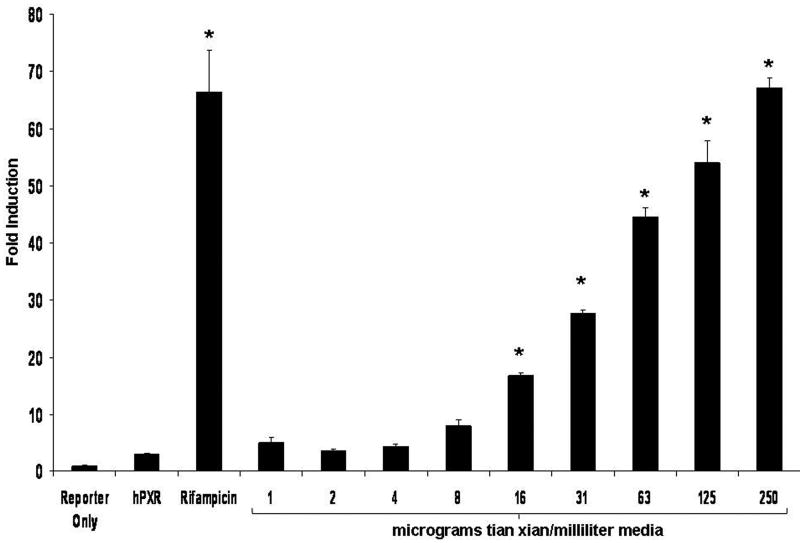
Figure 1. Tian Xian Induces PXR Activity in XREM-Luc Reporter Gene Assays.
(A) CV-1 cells were transfected with the expression vector for human PXR and the CYP3A4-derived XREM-Luc reporter gene. Cells were treated with vehicle (Veh, 0.1% Ethanol) or 10 microM rifampicin. Two-fold serial dilutions of a stock extract of tian xian (250 mg/ml) were used to perform the concentration-response analysis. All compounds were added as 1000X stock to each individual well. All cells were treated for 24 h. The data represent the mean of replicates ± SD (n=8) and are normalized against β-galactosidase activity, and are expressed as fold induction over vehicle control. * = Statistically different from vehicle control (p<0.05).
Transgenic Mouse Production and Genotyping
The TTR-FLAG-tagged hPXR transgene was excised with HindIII. The resulting 6 kb fragment was gel-purified using the QIAEX II (Qiagen, Valencia, CA) DNA purification kit. The transgene was then injected into single-cell B6C3f1 mouse zygotes. Transgene positive mice were screened using polymerase chain reaction. Briefly, a forward primer derived from the TTR promoter (5′ cctggtgcacagcagtgcatc 3′) and a reverse primer derived from human PXR (5′ cctccgacttcctcatctgcg 3′) were used to amplify a 424 bp sequence that would not be present in wild-type mice. Cycling conditions used for the genotyping reactions were as follows: 95°C for 15 seconds, 65°C for 15 seconds, and 68°C for 15 seconds for 35 cycles.
Detection of hPXR Protein and Expression-profiling of the hPXR Transgene
Approximately 250 milligrams of liver tissue was homogenized using a dounce Teflon homogenizer in 3 ml of lysis buffer containing 50 mM Tris-HCL pH 7.4, 150 mM NaCl, 1 mM EDTA, and 1% Triton X-100 and protease inhibitors. The homogenate was placed in a centrifuge at 2500 × g for 10 min. The supernatant was pre-cleared using 20 μl protein-A agarose. The resulting supernatant was immunoprecipitated using agarose linked to the M2 monoclonal antibody that recognizes the FLAG epitope. Following SDS-PAGE, the proteins were transferred to nitrocellulose membrane that was probed with our anti-hPXR antibody.
To determine expression profile of the FLAG-tagged human PXR transgene, RNA was isolated from the heart, lung, and liver using wild type and transgenic mice as described (Staudinger et al., 2001a). Following DNase I treatment, 1μg of RNA was reverse transcribed and real-time quantitative polymerase chain reaction was performed to detect the human PXR transgene (left primer- 5′ caggaggaaattgatgcagtttt 3′; right primer- 5′ gtcaagatactccatctgtagcacagt; fluorogenic probe- 5′ cccaataaggcaccacccacctatga 3′). All values were normalized to signal from 18S (left primer- 5′ ccagtaagtgcgggtcataa 3′; right primer 5′ ggttcacctacggaaacctt 3′; fluorogenic probe- 5′ cgattggatggtttagtgaggccc 3′ as described (Staudinger et al., 2003).
‘Humanized’ PXR mouse production
PXR-knockout (PXR-KO) mice were generated as described previously (Staudinger et al., 2001b). The transgenic mice harboring the FLAG-tagged hPXR mini-gene were crossed with the PXR-KO mice to obtain a mouse line expressing human PXR in a PXR-KO mouse background (TTR-hPXR). Following successful generation of ‘humanized’ mice lacking the mouse Pxr gene, the transgenic mice were backcrossed to C57Bl6 mice and then intercrossed to generate a homozygous and congenic line of mice that express the FLAG-tagged hPXR gene selectively in liver.
Statistical Analyisis
Differences between reporter gene and messenger RNA levels were determined using a one-way ANOVA followed by the Duncan's multiple range post-hoc test.
Results
Activation of PXR by Tian Xian in Cell-based Assays
To determine the extent to which the extract of tian xian activated human PXR we used a previously described cell-based reporter gene assay (Brobst et al., 2004). In CV-1 cells, 10 microM rifampicin (RIF) activated the XREM-LUC reporter gene in the presence of transfected human PXR (Fig. 1). A stock extract of tian xian (250 mg/ml) was used to treat transfected CV-1 cells. A clear concentration-response was also observed with increasing amounts of tian xian extract (Fig. 1). Thus, in CV-1 cells an extract of tian xian produced efficacious activation of human PXR. The activation of PXR by tian xian at the highest concentrations examined was comparable to that of 10 μιχρoM rifampicin, a well known PXR ligand.
Modulation of PXR-cofactor Interactions in the Mammalian Two-hybrid System
The interaction between accessory protein cofactors and PXR is modulated by the presence of activating ligands in cells. Specifically, in the absence of activating ligands PXR exhibits a strong association with the nuclear receptor corepressor protein - NCoR (Ding and Staudinger, 2005c). Conversely, in the presence of activating ligands PXR strongly associates with members of the steroid receptor coactivator family including SRC1 and SRC2 (Ding and Staudinger, 2005b; Ding et al., 2006). CV-1 cells were transfected with expression vectors encoding the GAL4 DNA-binding domain fused to the respective nuclear receptor-interacting domains in the coactivator proteins SRC1 and SRC2 together with an expression vector encoding VP16-tagged full-length human PXR. The GAL4-responsive luciferase reporter gene, pFR-LUC, was used to determine the extent to which tian xian extract modulated interaction between human PXR and protein cofactors in cell-based assays. Similar to rifampicin, treatment with increasing concentrations of tian xian extract (4, 31, and 250 μg/ml) recruited VP16-tagged human PXR to GAL4-SRC1 and GAL4-SRC2 (Fig 2A), but displaced VP16-tagged human PXR from GAL4-NCoR (Fig. 2B). These data strongly suggest that the extract of tian xian contains biologically active molecules that modulate PXR-cofactor interactions in cell-based assays. While this is useful information, activation of human PXR in a reporter gene assay does not always correlate with the ability to activate PXR in the context of hepatocytes.
Figure 2. Differential Modulation of PXR-SRC-1/2 and PXR-NCoR Interactions by Tian Xian.
Receptor-interaction domains fused to the GAL4-DNA-binding domain were used to determine whether tian xian altered PXR association with (A) SRC1/2 and (B) NCoR in the mammalian two-hybrid system. Transient transfection of CV-1 cells was performed as described in materials and methods. Twenty-four hr post-transfection, CV-1 cells were treated with vehicle (Veh, 0.1% Ethanol) or 10 microM rifampicin. A stock extract (250 mg/ml) was used to treat cells with three different dilutions of tian xian (1:64,000; 1:8,000, and 1:1,000). All compounds were delivered as 1000X (1 μl/ml) and all wells were treated for 24 hr. The data represent the mean of replicates ± SD (n=8) and are normalized against β-galactosidase activity, and are expressed as percent full reporter gene activity. In (A) * = Statistically different from GAL4 fusion alone control (p<0.05). In (B) * = Statistically different from GAL4-NCoR + VP16-hPXR control (p<0.05).
Construction and In Vivo Hepatic Expression of a FLAG-tagged Human PXR Mini-gene in PXR Knockout Mice
The best characterized PXR-target gene in mouse liver encodes the Cyp3a11 enzyme, the heme-containing steroid mono-oxygenase and functional orthologue of human CYP3A4. We have produced a novel line of transgenic mice in which expression of the FLAG-tagged human PXR cDNA is under the control of an enhancer region isolated from the transthyretin promoter. As shown in figure 3A, this transgene drives expression of the FLAG-tagged human PXR transgene in a liver-selective manner. This is consistent with the previous use of the same transthyretin enhancer region in other lines of transgenic mice (Ye et al., 1999). We subsequently crossed this line of mice with the previously described PXR knockout mice (Staudinger et al., 2001b), and then back-crossed these mice into the C57BL/6 line of mice to create a novel strain of mice that are homozygous for the transgene and lack the murine pxr gene. Using this strategy we have created a novel line of mice that express the FLAG-tagged human PXR protein in a liver-specific manner in the absence of the murine Pxr gene (Fig. 3B and inset).
Figure 3. Humanized PXR Transgenic Mouse Production and Expression Profiling.
(A). A 3,000 bp upstream fragment of the transthyretin promoter was used to drive expression of the FLAG-tagged human PXR cDNA. The location of the PCR primers used for genotyping is shown with the forward primer (fp) located in the transthyretin promoter region. The reverse primer (rp) is derived from the human PXR cDNA sequence. The sequence of each primer is listed in Materials and Methods. The resulting transgenic mouse line was crossed to the PXR knockout mice and then bred to homozygosity for both the Pxr knockout allele as well as the transgenic allele. Nine successive backcrosses were performed into the C57Bl6 strain of mice to obtain a congenic line of mice that are homozygous for the transgene and nullizygous for the wild type Pxr allele. (Inset). The anti-FLAG M2 monoclonal antibody was used to precipitate immuno-reactive proteins from wild type and humanized PXR transgenic livers. Proteins were resolved using SDS-PAGE on identical gels. One was stained with coomassie blue (left) and the other was transferred to PVDF membrane. The membrane was subsequently probed using anti-human PXR antibodies (right). (B). Total RNA was isolated from liver, heart, and lung tissue. The RNA was DNase-treated and reverse transcribed as described in Materials and Methods. Real-time quantitative PCR analysis was used to detect expression levels of the human PXR transgene. Data are expressed as relative transgene expression over wild type liver control and are normalized to 18S values obtained as described in Materials and Methods.
The ‘Humanized’ PXR Transgenic Mice Exhibit Species-specific Responses to Known Species-specific PXR Activators
It is well known that PXR exhibits a species-specific response to certain CYP3A inducers (Lehmann et al., 1998). Indeed, several humanized PXR transgenic mouse models have already been developed that are currently being used to assess the potential for drug-drug interactions commercially and in academic laboratory settings (Xie et al., 2000; Gonzalez, 2007). The hallmark experiment that determines the utility of these mouse models is the administration of rifampicin, a selective human PXR activator, and pregnenolone 16α carbonitrile (PCN), a selective mouse PXR activator to distinguish the functional difference between wild type and humanized PXR mice.
We therefore administered 10 microM concentrations of these two compounds for 48 hr to primary cultures of hepatocytes isolated from wild type, PXR-KO, and humanized PXR mice (Fig 4A). As expected, PCN induced the expression of Cyp3a11 in wild type hepatocytes, while rifampicin had only a minimal effect. Also as expected, neither rifampicin nor PCN had any effect on the expression of Cyp3a11 in hepatocytes isolated from PXR-KO mice. In contrast, treatment of primary cultures of hepatocytes isolated from humanized PXR mice with rifampicin produced marked induction of Cyp3a11 gene expression, while treatment with PCN produced only minimal increased expression of this known PXR-target gene.
Figure 4. The Expression of Cyp3a11 is Induced by Tian Xian in a PXR-dependent Manner and in Humanized PXR Mouse Hepatocytes.
(A). Primary cultures of hepatocytes were isolated from transgenic humanized PXR, PXR knockout, and wild type mice. Cultures were treated with vehicle (Veh, 0.1% DMSO) or 10 microM of Rifampicin or PCN. All cells were treated for 48 h before RNA isolation. Total RNA was isolated and used in real time quantitative PCR analysis. The data are normalized to 18S levels and are expressed as average values (n=3) ± SD. * = Statistically different from vehicle control group (p<0.05). (B). Primary cultures of hepatocytes were isolated from wild type and PXR-KO mice. Cultures were treated with vehicle (Veh, 0.1% DMSO), 10 microM PCN, and increasing concentrations of tian xian extract. All cells were treated for 48 h before RNA isolation. Total RNA was isolated and used in real time quantitative PCR analysis. The data are normalized to 18S levels and are expressed as average values (n=3) ± SD. * = Statistically different from vehicle control group. (C). Primary cultures of hepatocytes were isolated from transgenic humanized PXR mice. Cultures were treated with vehicle (Veh, 0.1% Ethanol), 10 μιχρoM of Rifampicin, or with three different dilutions of tian xian (1:64,000; 1:8,000, and 1:1,000). All compounds were delivered as 1000X (1 μl/ml) and all wells were treated for 24 hr. All cells were treated for 24 h before RNA isolation. Total RNA was isolated and used in real time quantitative PCR analysis. The data are normalized to 18S levels and are expressed as average values (n=3) ± the SD. * = Statistically different from vehicle control group.
To determine the extent to which PXR activity is required for induction of Cyp3a11 gene expression we treated primary cultures of hepatocytes isolated from wild type and PXR-KO mice with 10 microM PCN and increasing concentrations of tian xian (4, 31, and 250 μg/ml). Treatment with PCN produced robust and PXR-dependent induction of Cyp3a11 gene expression, while treatment with tian xian also increased Cyp3a11 gene expression in a concentration- and PXR-dependent manner (Fig. 4B). Primary cultures of hepatocytes isolated from humanized PXR mice were treated with 10 microM rifampicin and increasing concentrations of tian xian (4, 31, and 250 μg/ml). Tian xian treatment produced increased levels of Cyp3a11 gene expression in a concentration-dependent manner, similar to that obtained with rifampicin, the prototypical human PXR activator (Fig 4C). These data indicate that compounds contained within the tian xian extract activate both mouse and human PXR and produce increased expression of a known PXR-target gene, Cyp3a11, in the context of cultured hepatocytes.
Tian Xian Induces the Expression of CYP3A4 in Primary Cultures of Human Hepatocytes
The relative expense and low availability of primary cultures of human hepatocytes has recently led to a large effort to find suitable alternatives to test the potential for drug-drug and herb-drug interactions. One of the more positive aspects of the PXR reporter gene assay and the use of humanized mouse models includes the genetic uniformity and technical convenience of both cell-based systems and engineered mouse models. However, the ‘gold standard’ of drug metabolism studies required by the Food and Drug Administration (FDA) in the United States still remains the use of primary cultures of human hepatocytes. We therefore sought to determine the extent to which the expression of the CYP3A4 gene was altered by administration of tian xian. Figure 5 reveals that treatment of primary cultures of human hepatocytes with increasing concentrations of tian xian produced a concentration-dependent increase in the expression of the important drug metabolizing enzyme CYP3A4.
Figure 5. The Expression of CYP3A4 is Induced Tian Xian in Hepatocytes Isolated from the Transgenic Humanized PXR Mice.
Primary cultures of hepatocytes were obtained from XenoTech, LLC. Cultures were treated with vehicle (Veh, 0.1% Ethanol), or with three different dilutions of tian xian (1:64,000; 1:8,000, and 1:1,000). All compounds were delivered as 1000X (1 μl/ml) and all wells were treated for 24 hr. All cells were treated for 24 h before RNA isolation. Total RNA was isolated and used in real time quantitative PCR analysis. The data are normalized to 18S levels and are expressed as average values (n=3) ± SD. * = Statistically different from vehicle control group.
Discussion
It has been nearly twenty years since the identification of drug-inducible members of the CYP3A subfamily of drug metabolizing enzymes (Aoyama et al., 1989; Bork et al., 1989; Schuetz et al., 1989). It is now well known that induction of CYP3A4 gene expression in liver and intestine at the level of transcription by nuclear receptor proteins produces clinically relevant elevations in enzymatic activity of this extremely important drug-metabolizing enzyme (Kliewer et al., 2002). It is also well known that both drug-drug and herb-drug interactions can affect the clinical outcome in cancer patients on combination therapy (Harmsen et al., 2007). The purpose of this study was to determine the extent to which treatment with tian xian has the potential to produce alterations in the expression and activity of CYP3A4 in human patients. Moreover, we present here a novel humanized PXR mouse model that will undoubtedly be useful for future studies involved in the pre-clinical testing of candidate drug molecules and additional herbal remedies.
As of publication of this manuscript, there are two previously described transgenic ‘humanized’ PXR mouse models. The first transgenic mouse model created utilized the albumin promoter to drive expression of the human PXR cDNA selectively in liver, but this model subsequently lacked any expression of the transgene in intestine (Xie et al., 2000). To compensate for this lack of intestinal expression, another transgene was engineered using the fatty acid-binding protein promoter, thus generating a bi-transgenic mouse model with expression in both liver and intestine (Gong et al., 2006). Recently, another group took a different approach that utilized a bacterial artificial chromosome containing the entire human PXR gene, including the relevant promoter regulatory sequences, to drive expression of potentially all PXR splice variants in a manner that more closely recapitulates PXR expression in humans (Ma et al., 2007). The model we present here has utilized the transthyretin promoter that expresses a FLAG-tagged human PXR cDNA selectively in liver and choroid plexus in brain (data not shown) in our PXR-KO mouse model, and has been backcrossed to produce a congenic line of mice containing the C57Bl6 genetic background.
Humanized mouse models are becoming extremely important in the pre-clinical testing of novel drug candidate molecules. By knocking-out the rodent pxr gene and replacing it with the human receptor, ‘humanized’ PXR mouse models have been established as unique tools to dissect the drug-induced xenobiotic response, and are aiding the development of safer drugs at an earlier stage of pre-clinical drug development. These unique mouse models all have the advantage of providing reliable, cost-effective, plentiful, convenient, and genetically uniform systems that can be used to test for potential drug-drug and herb-drug interactions. This is of particular importance in the herbal remedy industry, as it is not currently required by the FDA in this country to determine the extent to which their products are safe for co-administration with concurrently used prescription medications.
Tian xian represents only one example of potential herb-drug interactions, but we feel that the experiments presented here are particularly important because this herbal remedy is marketed on the world-wide web and is available without a prescription as an anti-cancer therapy to be used in conjunction with ‘western’ chemotherapeutic agents. This product line purports to validate its efficacy as an anti-cancer herbal remedy using on-line ‘testimonials’. These testimonials tend to be cancer patients that are not responding to their conventional chemotherapy, but testify that tian xian co-administration eased their side-effects and increased the effectiveness of their chemotherapy. Cancer patients can not test the validity of these on-line claims before using tian xian, and the experiments presented here do not specifically refute these claims. However, our data strongly suggest that co-administration of tian xian together with conventional chemotherapeutic agents, many of which are indeed substrates of the CYP3A4 enzyme, would likely increase their biotransformation. The potential danger highlighted in this study is that co-administration of tian xian and conventional chemotherapeutics would tend to decrease the efficacy of anti-cancer agents that are metabolized and excreted by PXR-dependent mechanisms. Conversely, co-administration of tian xian with a pro-drug that requires bioactivation would likely increase the rate of such a conversion. As is the case with cyclophosphamide and ifosfamide, induction of CYP3A4 activity would likely promote accumulation and possible toxicity due to their narrow therapeutic index. Finally, since the tian xian extract is a complex mixture of compounds, it is a formal possibility that tian xian could simultaneously activate PXR and inhibit CYP enzymatic activity. Future studies should address this issue by examining alterations in cytochrome P450 activity following administration of this herbal remedy.
It is well known that activation of PXR coordinately regulates the expression and activity of multiple drug transporter proteins, as well as numerous other drug metabolizing enzymes. In addition to CYP3A4, PXR is involved in regulating numerous members of the UDP-glucuronosyltransferase family (Chen et al., 2003; Xie et al., 2003), sulfotransferases (Sonoda et al., 2002), drug transporter proteins and many other enzymes involved in handling oxidative stress in cells (Geick et al., 2001; Maglich et al., 2002; Wei et al., 2002; Staudinger et al., 2003). Future studies will involve determining the extent to which this Chinese herbal remedy modulates the expression and activity of these enzymes in liver using primary cultures of human hepatocytes and the line of transgenic mice described here.
Acknowledgments
a) Research Support: Research Supported by an NIH grant NIDDK 068443.
Non-Standard Abbreviations
- PXR
pregnane X receptor
- PXR-KO
PXR Knockout
- SRC1
Steroid Receptor Coactivator 1
- NCoR
Nuclear Receptor Corepressor
- CYP
cytochrome P450
- PCN
pregnenolone 16α-carbonitrile
References
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV, et al. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989;264:10388–10395. [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork RW, Muto T, Beaune PH, Srivastava PK, Lloyd RS, Guengerich FP. Characterization of mRNA species related to human liver cytochrome P-450 nifedipine oxidase and the regulation of catalytic activity. J Biol Chem. 1989;264:910–919. [PubMed] [Google Scholar]
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther. 2004;310:528–535. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregname X receptor, is required for induction of UDP-glucuronosyltranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug Metab Dispos. 2003;31:908–915. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- Ding X, Lichti K, Staudinger JL. The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor. Toxicol Sci. 2006;91:448–455. doi: 10.1093/toxsci/kfj163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005a;312:849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. The ratio of constitutive androstane receptor to pregnane X receptor determines the activity of guggulsterone against the Cyp2b10 promoter. J Pharmacol Exp Ther. 2005b;314:120–127. doi: 10.1124/jpet.105.085225. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005c;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, Ren S, Kagan VE, Day BW, Zimniak P, Xie W. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. CYP3A4 and pregnane X receptor humanized mice. J Biochem Mol Toxicol. 2007;21:158–162. doi: 10.1002/jbt.20173. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane × receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007;33:369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane × receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P, Vilarem MJ. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115:177–186. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- Schuetz JD, Molowa DT, Guzelian PS. Characterization of a cDNA encoding a new member of the glucocorticoid-responsive cytochromes P450 in human liver. Arch Biochem Biophys. 1989;274:355–365. doi: 10.1016/0003-9861(89)90449-9. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci U S A. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001a;29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Ding X, Lichti K. Pregnane X receptor and natural products: beyond drug-drug interactions. Expert Opin Drug Metab Toxicol. 2006;2:847–857. doi: 10.1517/17425255.2.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001b;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31:523–527. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- Sun A, Chia JS, Chiang CP, Hsuen SP, Du JL, Wu CW, Wang WB. The chinese herbal medicine Tien-Hsien liquid inhibits cell growth and induces apoptosis in a wide variety of human cancer cells. J Altern Complement Med. 2005a;11:245–256. doi: 10.1089/acm.2005.11.245. [DOI] [PubMed] [Google Scholar]
- Sun A, Chia JS, Wang WB, Chiang CP. Immunomodulating effects of “tien-hsien liquid” on peripheral blood mononuclear cells and T-lymphocytes from patients with recurrent aphthous ulcerations. Am J Chin Med. 2004;32:221–234. doi: 10.1142/S0192415X04001886. [DOI] [PubMed] [Google Scholar]
- Sun A, Chia JS, Wang WB, Chiang CP. “Tien-Hsien liquid” can modulate antigen-stimulated cytokine production by T-cells isolated from patients with recurrent aphthous ulcerations. Am J Chin Med. 2005b;33:559–571. doi: 10.1142/S0192415X05003168. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, Evans RM. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci U S A. 2003;100:4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999;19:8570–8580. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]