Abstract
Background
The World Health Organization (WHO) recently recommended that the time to first malaria episode serve as the primary endpoint in phase III malaria vaccine trials—the first of which will be held in Africa. Although common red blood cell (RBC) polymorphisms such as sickle hemoglobin (Hb) S are known to protect against malaria in Africa, their impact on this endpoint has not been investigated.
Methods
A longitudinal study of 225 individuals aged 2-25 years was conducted in Mali. The association between common RBC polymorphisms and the time to first malaria episode was evaluated.
Results
Among children aged 2-10 years, sickle cell trait (HbAS) was associated with a 34-day delay in the median time to first malaria episode (p=0.017). Cox regression analysis showed that age (hazard ratio [HR] 0.87 [95% CI, 0.80-0.94]; p=0.001), HbAS (HR 0.48 [95% CI, 0.26-0.91]; p=0.024), and asymptomatic parasitemia at enrollment (HR 0.35 [95% CI, 0.14-0.85]; p=0.021) were associated with decreased malaria risk.
Conclusion
Given the delay in the time to first malaria episode associated with HbAS, it would be advisable for clinical trials and observational studies that use this endpoint to include Hb typing in the study design where HbAS is prevalent.
Keywords: Plasmodium falciparum malaria, red blood cell polymorphisms, sickle cell trait, vaccines, survival analysis, Mali, longitudinal study, observational study, alpha-thalassemia, G6PD deficiency
Introduction
The World Health Organization (WHO) recently recommended that the time to first malaria episode serve as the primary endpoint in phase III malaria vaccine trials [1]. This endpoint and the statistical methods used to analyze it, namely time to event or survival analysis, are also used in clinical trials of antimalarial drugs [2] and increasingly in observational studies of malaria immunity [3-5]. Red blood cell (RBC) polymorphisms that produce sickle hemoglobin (Hb) S, HbC, alpha-thalassemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency, and blood group O are common in regions of Africa where such studies are conducted. The prevalence of HbAS, for example, exceeds 25% in some areas [6]. Although these RBC polymorphisms are associated with decreased risk of malaria, as measured by odds [7-11] or incidence rate ratios [12], their impact on the time to first malaria episode is not known. Evidence of a significant effect would provide a rationale for routine typing of RBC polymorphisms in clinical trials and observational studies of malaria that use this endpoint.
To this end, we investigated the impact of common RBC polymorphisms on the time to first malaria episode in the context of a longitudinal study in which individuals were enrolled during a period of little or no Plasmodium falciparum transmission, and then followed clinically through an 8-month malaria season. Here we describe this observational cohort study designed to investigate the acquisition and maintenance of malaria immunity. We found that HbAS is associated with a 34-day delay in the median time to first malaria episode, an association that remained statistically significant in multivariate Cox regression analysis. HbAS was unique in this regard as we found no association of HbAC, alpha-thalassemia, G6PD deficiency, or blood group O with a delayed time to first malaria episode.
Methods
Study site
The study was carried out in Kambila, a small (~1 km2), well-circumscribed, rural village with a population of 1500, situated 20 kilometers north of Bamako, the capital of Mali (Fig. 1). Transmission of P. falciparum is seasonal and intense, peaking in September through November (Fig. 2). The entomological inoculation rate (EIR) measured in a nearby village was near zero during the dry season, and approximately 50-60 infective bites/person/month in October 2000 [13].
Figure 1.
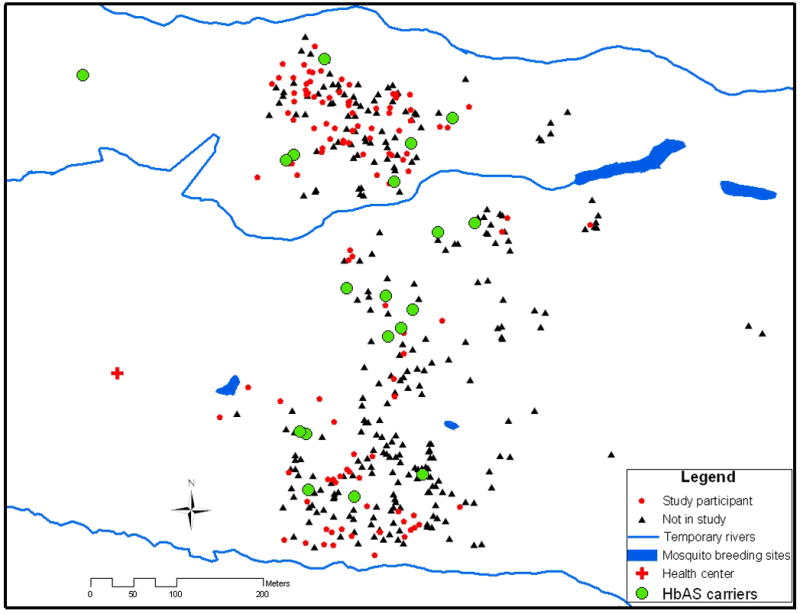
Distribution of study participants and HbAS carriers within the entire Kambila village population. Three houses included more than one HbAS carrier and are superimposed on one another (total number of HbAS carriers = 22).
Figure 2.
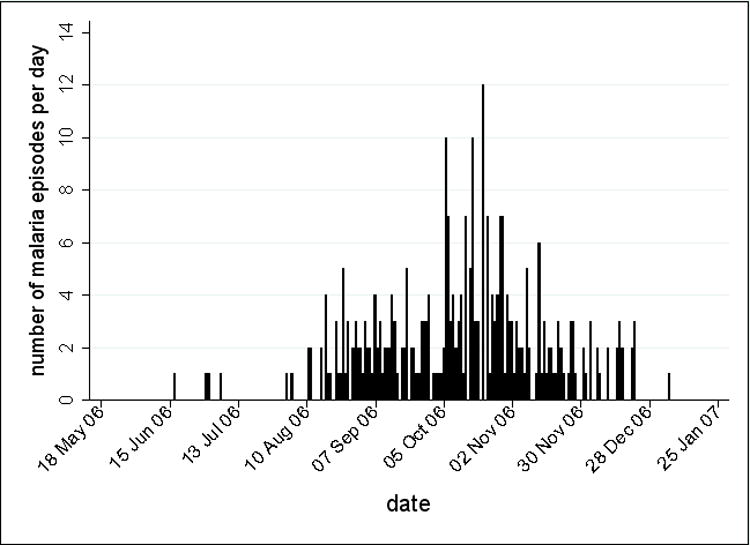
The number of clinical malaria episodes per day from May 2006 to January 2007. Within the cohort of 225 subjects, there were 298 episodes of clinical malaria defined as axillary temperature ≥37.5°C and P. falciparum asexual parasitemia ≥5000/μl.
Sampling strategy, study participants, and malaria case definition
Approval for this study was obtained from the Faculty of Medicine, Pharmacy and Odonto-Stomatology Ethics Committee; and the Institutional Review Board at the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Written, informed consent was obtained from adult participants, and from the parents or guardians of participating children. The study was externally monitored for protocol compliance, data integrity, and human subjects protections.
This study is part of an ongoing observational study of the acquisition and maintenance of malaria immunity that began in May 2006. Individuals were invited to be screened for the study after being randomly selected from an age-stratified census of the entire village population. Enrollment exclusion criteria were hemoglobin <7 g/dL, fever ≥37.5°C, acute systemic illness, use of anti-malarial or immunosuppressive medications in the past 30 days, or pregnancy. Venous blood samples and blood smears were collected before the malaria season (May 2006), at cross-sectional time points every 2 months during the malaria season (July, October, and December 2006), prior to the second malaria season (May 2007), and 14 days after the first episode of malaria. Stool and urine were examined at enrollment for the presence of helminth infections. Participants were encouraged to report symptoms of malaria at the village health center, staffed 24 hours per day by a study physician. From those with signs or symptoms of malaria, blood smears were prepared and examined for the presence of P. falciparum. Slide positive patients were treated with a standard 3-day course of artesunate plus amodiaquine, following the guidelines of the Mali National Malaria Control Program. Children with severe malaria were referred to Kati District Hospital after an initial parenteral dose of quinine. The research definition of malaria was an axillary temperature ≥37.5°C, P. falciparum asexual parasitemia ≥5000/μl, and a non-focal physical exam by the study physician. Severe malaria, as defined by the WHO [14], was included in this definition. At the end of the malaria season, participants (or their parents or guardians in the case of children) were asked whether or not they had used a bednet nightly during the rainy season.
Blood samples
Blood samples were drawn by venipuncture into sodium citrate-containing cell preparation tubes (BD, Franklin Lakes, NJ) and transported to the laboratory (20 km) for processing within 2 hours. Following centrifugation (1800 RCF, 20 min), plasma was collected and stored at -80°C for future studies. Peripheral blood mononuclear cells were collected, washed twice with sterile phosphate buffered saline (PBS; KD Medical, Columbia, MD), resuspended in 90% heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY) and 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), kept at -80°C for 24 hours, and transferred to liquid nitrogen for future studies. Two hundred microliters of whole blood was used to identify RBC polymorphisms.
Identification of RBC polymorphisms
Hemoglobin was typed by high performance liquid chromatography (HPLC; D-10 instrument; Bio-Rad, Hercules, CA). The 3.7-kb deletional determinant of α-thalassemia (−α3.7) was detected by a nested PCR protocol (Appendix) to identify hetero- (−α/αα) and homozygotes (−α/−α). The mutation responsible for G6PD deficiency in Mali (G6PD*A-) was identified by restriction fragment length polymorphism analysis of PCR-amplified DNA samples, as previously described [10]. ABO blood groups were determined by a monoclonal antibody-based kit (Linear Chemicals, Montgat, Spain).
Malaria slides
Thick blood smears were stained with Giemsa and counted against 300 leukocytes. P. falciparum densities were recorded as the number of asexual parasites/μl of whole blood, based on an average leukocyte count of 7500/μl. Each smear was evaluated separately by two expert microscopists blinded to the clinical status of study participants. Any discrepancies were resolved by a third expert microscopist.
Stool and urine exam for helminth infection
At enrollment, duplicate stool samples were examined for Schistosoma mansoni eggs and other intestinal helminths using the semi-quantitative Kato-Katz method. To detect Schistosoma haematobium eggs, 10 ml of urine were poured over Whatman filter paper. One or two drops of ninhydrine were placed on the filter and left to air dry. After drying, the filter was dampened with tap water and helminths eggs detected by microscopy.
Geographic information system (GIS) data collection
Latitude and longitude coordinates and the altitude of households were measured by a handheld global positioning system (GPS) receiver (Trimble® GeoXM™).
Data management and analysis
Data were doubly entered and verified in a Microsoft Access database (Microsoft, 2003) and analyzed using STATA software (StataCorp LP, 2007, Release 10.0). Fisher’s exact and Kruskall-Wallis tests were used to compare binary outcomes and continuous variables between groups, respectively. The malaria-free probability over an 8-month period was estimated by the Kaplan-Meier method, and time to event curves of different groups were compared by the log rank test. The Cox proportional hazards model was used to assess the effect of the following factors on malaria risk: age, gender, weight, ethnicity, distance lived from clinic, bednet use, baseline P. falciparum parasitemia, helminth infection, HbAS, HbAC, G6PD*A- (hemi-, hetero-, and homozygosity), −α/αα, and ABO blood group. The same list of variables was included in a Poisson regression model to determine their impact on malaria incidence. The covariates included in the final Cox and Poisson regression models were determined by applying the stepwise model selection procedure, where p values < 0.1 and p values > 0.2 were set as criteria for covariate entry and removal. For all tests, two-tailed p values were considered significant if ≤0.05.
RESULTS
Baseline characteristics of study cohort
Of the 237 individuals screened, 225 were enrolled during a 2-week period in May 2006, approximately 1 month before the malaria transmission season began (Fig. 2). The study cohort consisted of four predefined age groups: 2-4 years (n = 73), 5-7 years (n = 52), 8-10 years (n = 51), and 18-25 years (n = 49). Follow up at scheduled cross-sectional visits was >99% for children and 82% for adults during the 8-month study. Table 1 shows baseline characteristics by age group. Overall, 52% were female, and the predominant ethnic groups were Bambara (60%) and Sarakole (34%). The prevalence of asymptomatic parasitemia at enrollment was 7.1% and did not vary significantly with age (p=0.83). Of note, asymptomatic parasitemia during the dry season is commonly observed in Mali [15, 16]. Nine percent of stool and 6% of urine samples showed intestinal helminths or S. haematobium, respectively; these prevalences were lower than expected likely due to community-wide albendazole treatment prior to this study. The average distance between study clinic and subject residence was 382 meters (range, 127–881 meters) and 27% of participants self-reported nightly bednet use during the rainy season.
Table 1.
Baseline Characteristics by Age Group
| Age group, years | |||||
|---|---|---|---|---|---|
| 2-4 (n=73) | 5-7 (n=52) | 8-10 (n=51) | 18-25 (n=49) | All (n=225) | |
| Gender, % female (no.) | 57.5 (42) | 48.1 (25) | 37.3 (19) | 61.2 (30) | 51.6 (115) |
| Ethnicity, % (no.) | |||||
| Bambara | 65.8 (48) | 50.0 (26) | 53.0 (27) | 67.4 (33) | 59.6 (134) |
| Sarakole | 28.8 (21) | 44.2 (23) | 39.2 (20) | 26.5 (13) | 34.2 (77) |
| Fulani | 4.1 (3) | 5.8 (3) | 5.9 (3) | 4.1 (2) | 4.9 (11) |
| Malinke | 1.4 (1) | 0.0 (0) | 2.0 (1) | 2.0 (1) | 1.3 (3) |
| P. falciparum smear positive at enrollment, % (no.)a | 5.5 (4) | 7.7 (4) | 9.8 (5) | 6.1 (3) | 7.1 (16) |
| Parasitemia if smear positive at enrollment, parasites/microliter [geometric mean (95% CI)] | 1438 (159–12973) | 3616 (1500–8715) | 415 (134–1287) | 953 (39–23382) | 1137 (579–2232) |
| GI helminth, % positive at enrollment (no.)b | 13.0 (9) | 8.3 (4) | 9.3 (4) | 0 (0) | 9.0 (17) |
| Urine schistosomiasis, % positive at enrollment (no.)c | 0 (0) | 0 (0) | 4.3 (2) | 28.1 (9) | 6.0 (11) |
| Hemoglobin at enrollment, g/dL (mean ±SD) | 11.2 (±1.2) | 11.8(±1.0) | 12.3(±1.1) | 13.6(±1.4) | 12.1(±1.5) |
| Distance lived from clinic, meters (mean ±SD) | 393 (±109) | 393 (±135) | 378 (±105) | 360 (±92) | 382 (±112) |
| Bed net use, % (no.)d | 28.8 (21) | 30.8 (16) | 17.6 (9) | 30.6 (15) | 27.1 (61) |
All subjects were asymptomatic at enrollment.
Data available for 190 subjects; GI=gastrointestinal.
Data available for 184 subjects.
Nightly bednet use self-reported at the end of the malaria season.
Prevalence of RBC polymorphisms
HbAS and HbAC phenotypes were present in10.4% and 13.7% of individuals, respectively (Table 2). The prevalence of G6PD*A- was 17% among females (hetero-and homozygotes) and 14% among males (hemizygotes). −α/αα and −α/−α genotypes were found in 39.8% and 1% of individuals, respectively. Due to the low frequency of −α/−α, these individuals were excluded from further analyses. Blood groups O, B, A, and AB were identified in 33.2%, 30.5%, 26.3%, and 10.0% of individuals, respectively.
Table 2.
Red Blood Cell Polymorphism Frequencies by Age Group
| Age group, years | |||||
|---|---|---|---|---|---|
| 2-4 (n=73) | 5-7(n=52) | 8-10 (n=51) | 18-25 (n=49) | All (n=225) | |
| Hemoglobin type, % (no.)a | |||||
| AA | 72.5 (50) | 86 (43) | 68.6 (35) | 76.2 (32) | 75.5 (160) |
| AS | 11.6 (8) | 8.0 (4) | 9.8 (5) | 11.9 (5) | 10.4 (22) |
| AC | 14.5 (10) | 6.0 (3) | 21.6 (11) | 11.9 (5) | 13.7 (29) |
| CC | 1.5 (1) | 0 (0) | 0 (0) | 0 (0) | 0.5 (1) |
| G6PD*A-, % (no.)b | |||||
| Female | |||||
| Normal | 76.2 (32) | 92.0 (23) | 79.0 (15) | 88.5 (23) | 83.0 (93) |
| Heterozygous | 14.3 (6) | 8.0 (2) | 15.8 (3) | 11.5 (3) | 12.5 (14) |
| Homozygous | 9.5 (4) | 0 (0) | 5.3 (1) | 0 (0) | 4.5 (5) |
| Male | |||||
| Normal | 89.7 (26) | 75.0 (18) | 90.0 (27) | 87.5 (14) | 85.9 (85) |
| Hemizygous | 10.3 (3) | 25.0 (6) | 10.0 (3) | 12.5 (2) | 14.1 (14) |
| α-thalassemia, % (no.)c | |||||
| Normal | 52.2 (35) | 66.7 (34) | 51.0 (26) | 71.4 (30) | 59.2 (125) |
| Heterozygous | 47.8 (32) | 29.4 (15) | 49.0 (25) | 28.6 (12) | 39.8 (84) |
| Homozygous | 0 (0) | 3.9 (2) | 0 (0) | 0 (0) | 1.0 (2) |
| ABO blood group, % (no.)d | |||||
| O | 29.9 (20) | 30.6 (15) | 33.3 (16) | 46.2 (12) | 33.2 (63) |
| A | 40.3 (27) | 26.5 (13) | 22.9 (11) | 26.9 (7) | 30.5 (58) |
| B | 23.9 (16) | 30.6 (15) | 29.2 (14) | 19.2 (5) | 26.3 (50) |
| AB | 6.0 (4) | 12.2 (6) | 14.6 (7) | 7.7 (2) | 10.0 (19) |
Data available for 212 subjects.
Data available for 212 subjects.
Data available for 211 subjects.
Data available for 190 subjects.
Malaria outcomes by age group
The number of malaria cases per day from May 2006 to January 2007 (Fig. 2) illustrates the intense, seasonal malaria transmission at this site. From 495 clinic visits during the study period, 298 episodes of malaria were diagnosed. Table 3 summarizes malaria outcomes by age group. As expected, malaria incidence decreased with age (p<0.001) and the five cases of severe malaria were confined to children <5 years old. Among those who presented with malaria, the median time to first malaria episode (as measured in days from study enrollment) increased with age (101 days, 2–4 years old vs. 153 days, 18–25 years old; p=0.016). The geometric mean parasite density at the first malaria episode decreased with age (p=0.036).
Table 3.
Malaria Outcomes by Age Category
| Age group, Years | ||||||
|---|---|---|---|---|---|---|
| 2-4 (n=73) | 5-7 (n=52) | 8-10 (n=51) | 18-25 (n=49) | All (n=225) | p value | |
| Malaria incidence, mean (±SD)a | 1.99 (±1.25) | 1.94 (±1.21) | 0.98 (±1.05) | 0.08 (±0.28) | 1.33 (±1.30) | <0.001 |
| Severe malaria incidence, no.b | 4 | 1 | 0 | 0 | 5 | |
| At least one malaria episode, % (no.) | 86.3 (63) | 86.5 (45) | 60.8 (31) | 8.2 (4) | 63.6 (143) | <0.001 |
| Time to first malaria episode, days (median)c | 101 | 114 | 130 | 153 | 115 | 0.016 |
| Parasitemia at first malaria episode, parasites/microliter [geometric mean (95% CI)] | 34374 (24955 – 47348) | 15687 (9623 – 25574) | 10433 (5079 – 21427) | 8816 (4082 – 19037) | 19625 (15004 – 25668) | 0.036 |
Malaria episode defined as T ≥37.5°C, asexual parasitemia ≥ 5000/microliter, and non-focal physical examination.
WHO definition of severe malaria [14].
Days since study enrollment.
Impact of RBC polymorphisms on malaria outcomes among children aged 2-10 years
Since adults had few episodes of malaria (Table 3), the analysis of RBC polymorphisms and malaria outcomes focused on 2–10 year-old children. Time to event analysis (Fig. 3A) showed that HbAS was associated with a significant delay in the time to first malaria episode (p=0.038 by log rank test). Among children who presented with malaria, HbAS was associated with a median 34-day delay to the first episode compared to the non-HbAS group (HbAS, 145 days vs. non-HbAS, 111 days; p=0.017). HbAS was also associated with a 53% reduction in malaria incidence [HbAS, 0.82 (95% CI, 0.48–1.17) vs. non-HbAS, 1.76 (95% CI, 1.56–1.96); p=0.003]. Although geometric mean parasite densities at the first malaria episode were lower in HbAS children, this was not statistically significant [HbAS, 9033 parasites/μl (95% CI, 1364–59825) vs. non-HbAS, 21257 parasites/μl (95% CI, 16312–27701); p=0.83]. The five cases of severe malaria occurred in non-HbAS children (3 HbAA, 2 HbAC). HbAC, G6PD*A- hemi-, hetero-and homozygosity, −α/αα, and blood group O were associated with neither a delayed time to first malaria episode (Fig. 3B–D) nor a decreased incidence of malaria (not shown).
Figure 3.
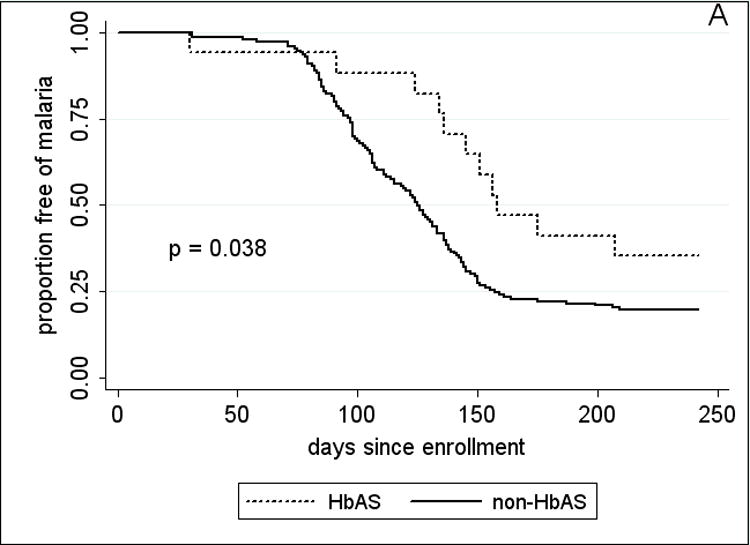
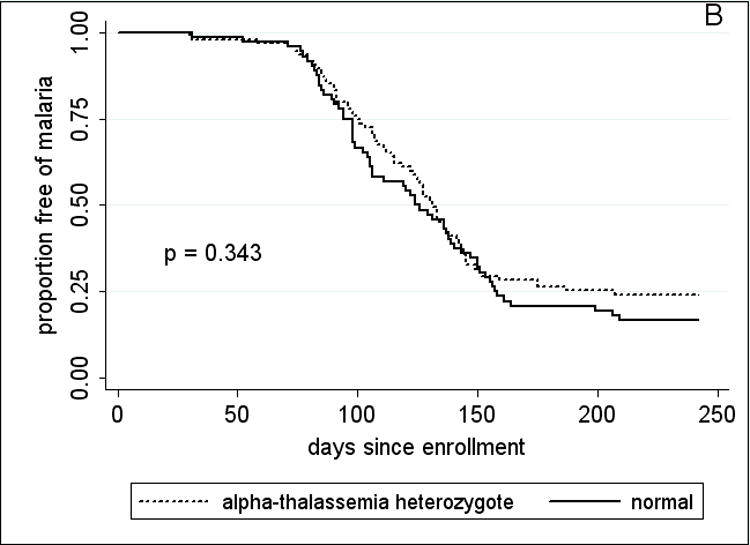
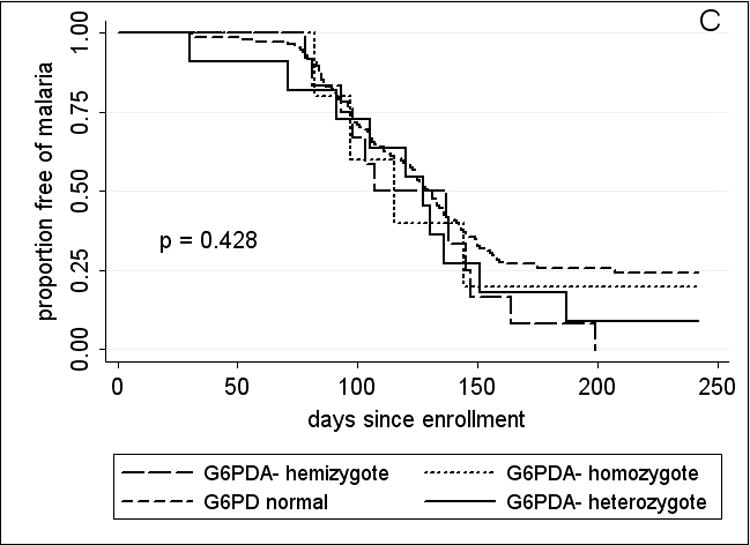
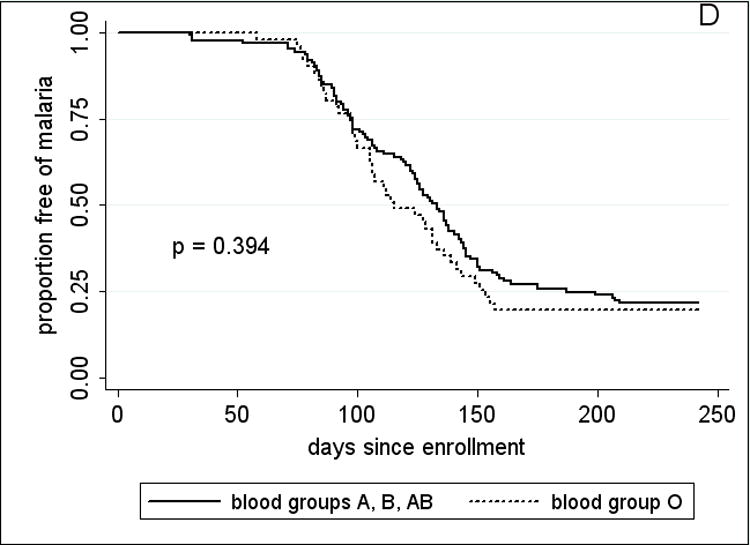
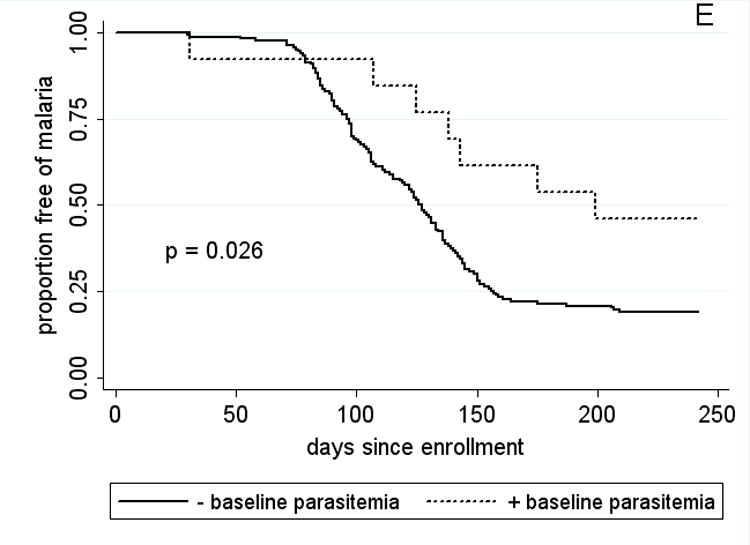
Kaplan-Meier estimates of the time from study enrollment to the first episode of malaria in children 2 – 10 years old by A, HbAS vs. non-HbAS; B, alpha thalassemia heterozygotes vs. normal alpha hemoglobin; C, G6PD*A- hemi- vs. heterozygotes vs. normal G6PD; D, blood group O vs. non-blood group O; and E, P. falciparum parasitemia status at enrollment. P values were obtained using the log rank test.
Baseline characteristics of children aged 2-10 years, stratified by hemoglobin type
To assess for potential confounding of the protective effect of HbAS, factors that might influence malaria risk were stratified by Hb type (Table 4). While there was a higher proportion of females in the HbAS group (p=0.022), HbAS children did not differ significantly from non-HbAS children with regards to age, weight, ethnicity, distance lived from the clinic, or frequency of G6PD*A- and −α/αα. The prevalence of asymptomatic parasitemia at enrollment was 9.4% among HbAA children, and 0% among HbAC and HbAS children. However, asymptomatic parasitemia at enrollment was associated with a delayed time to first malaria episode (Fig. 3E; p=0.026 by log rank test) and a trend toward decreased malaria incidence. Thus, HbAS children were not delayed in their time to first malaria episode due to the effect of asymptomatic parasitemia. There were too few HbAS children to make meaningful comparisons among the remaining variables.
Table 4.
Univariate Analysis of Baseline Characteristics by Hemoglobin Type Among 2-10 Year-Old Children
| Hemoglobin type | ||||
|---|---|---|---|---|
| Characteristic | HbAA (n=128) | HbAC (n=24) | HbAS (n=17) | p valuea |
| Age, years, mean (±SD) | 5.6 (±2.3) | 6.0 (±2.8) | 5.1 (±2.2) | 0.382 |
| Gender, % female (no.) | 46.1 (59) | 50.0 (12) | 76.5 (13) | 0.022 |
| Weight, kg, mean (±SD) | 17.9 (±5.4) | 18.6 (±6.8) | 18.1 (±8.1) | 0.718 |
| Ethnicity, % of Hb type (no.) | ||||
| Bambara | 56.3 (72) | 58.3 (14) | 47.1(8) | 0.453 |
| Sarakole | 37.5 (48) | 33.3 (8) | 47.1 (8) | 0.435 |
| Fulani | 4.7 (6) | 8.3 (2) | 5.9 (1) | |
| Malinke | 1.6 (2) | 0 (0) | 0 (0) | |
| Distance lived from clinic, meters, mean (±SD) | 386 (±111) | 395 (±59) | 381 (±177) | 0.163 |
| Bednet use, % (no.)b | 29.7 (38) | 20.8 (5) | 11.8 (2) | |
| P. falciparum smear positive at enrollment, % (no.) | 9.4 (12) | 0 (0) | 0 (0) | |
| GI helminth, % (no.) | 8.6 (10) | 13.6 (3) | 12.5 (2) | |
| Urine schistosomiasis, % (no.) | 0.9 (1) | 4.4 (1) | 0 (0) | |
| G6PD*A-, % (no.)c | 17.9 (22) | 12.5 (3) | 17.6 (3) | 1.000 |
| α-thalassemia, heterozygotes, % (no.) | 41.3 (50) | 62.5 (15) | 37.5 (6) | 0.792 |
| Blood group O, % (no.) | 31.3 (40) | 37.5 (9) | 11.8 (2) | |
For the comparison of HbAS versus non-HbAS children (HbAA and HbAC); statistical test not done if <3 in subgroup.
Nightly bednet use self-reported at the end of the malaria season.
Includes hetero-, hemi-, and homozygotes.
Predictors of malaria outcomes, multivariate analysis of 2 to 10 year-old children
Cox regression analysis revealed that age (hazard ratio [HR] 0.87 [95% CI, 0.80-0.94]; p =0.001), HbAS (HR 0.48 [95% CI, 0.26-0.91]; p=0.024), and asymptomatic parasitemia at enrollment (HR 0.35 [95% CI, 0.14-0.85]; p=0.021) were associated with decreased malaria risk. Poisson regression analysis showed that age (incidence rate ratio [IRR] 0.90 [95% CI, 0.85-0.95]; p<0.001), and HbAS (IRR 0.46 [95% CI, 0.27-0.79]; p=0.005) were significant predictors of decreased malaria incidence, whereas asymptomatic parasitemia at enrollment was not (p>0.100). Removal of −α/αα from the analysis decreased the HR (0.44; p=0.020) and IRR (0.44; p=0.004) for HbAS, indicating negative epistasis between these polymorphisms. Factors that did not independently predict either measure of malaria risk (HR or IRR) were gender, weight, distance from clinic, bednet use, helminth infection, HbAC, G6PD*A- hemi-, hetero- or homozygosity, −α/αα, and blood group O.
Discussion
In this study we found that HbAS was associated with a delayed time to first malaria episode, an association that remained statistically significant in multivariate Cox regression analysis. Numerous case-control [7, 17-20] and longitudinal [12, 21] studies have established that HbAS decreases the risk of malaria, as measured by odds and incidence rate ratios, respectively. However, the effect of HbAS on the time to first malaria episode had not been established using time to event analysis. Aidoo et al. showed by time to event analysis that HbAS is associated with a reduction in all-cause mortality, but malaria-specific outcomes, including severe malarial anemia and high-density parasitemia, were reported as incidence rates [21]. In Gabon, investigators found no association between HbAS and time to malaria episodes [19], but the results of this study are difficult to interpret for several reasons: 1) individuals were initially enrolled in a hospital-based, case-control study of severe malaria, 2) age and other covariates were not taken into account when HbAS and non-HbAS individuals were compared longitudinally, and 3) statistical methods were chosen that were inappropriate for the analysis of correlated sojourn times between consecutive malaria episodes of the same subject. This approach is further complicated by the problem of defining when a malaria episode ends, and when an individual becomes susceptible to subsequent episodes after treatment.
Several features of our study made it well-suited to assess the impact of RBC polymorphisms on the time to first malaria episode. The study population was an age-stratified random sample representing 15% of all individuals living in a rural, well-circumscribed, non-migratory community where anti-malarial drugs were provided exclusively by the study investigators. Enrollment occurred over a short period of time approximately 1 month prior to the abrupt onset of malaria transmission (Fig. 2). Ninety-three percent of individuals had negative blood smears for P. falciparum at the time of enrollment, obviating the need for a treatment-reinfection study design [22], an approach that may alter the subsequent risk of malaria [23]. Moreover, other aspects of our study favored an unbiased detection of malaria episodes: 1) follow-up at regular cross-sectional visits was >99% among 2–10 year-old children, indicating a high degree of study awareness and participation, 2) the average distance of individuals’ homes to the study clinic was 382 meters, minimizing geographic and logistic barriers to study participation, and 3) a study physician was available at all times at the only easily accessible health care facility in the area.
The use of time to first malaria episode as a study endpoint is increasingly common in clinical trials of malaria vaccines [24] and anti-malarial drugs [2], and in observational studies of malaria immunity [3-5]. Indeed, the WHO recently recommended that the time to first malaria episode serve as the primary endpoint in phase III clinical trials of candidate malaria vaccines [1]. An imbalance in the distribution of HbAS between comparator groups, whether in randomized trials or observational studies, may occur by chance and lead to invalid conclusions. The clear impact of HbAS on this endpoint, and the possibility of a biological interaction between HbAS and certain interventions (e.g. candidate blood stage vaccines), provides a rationale for routine Hb typing in such studies that are often conducted in areas where the prevalence of HbAS exceeds 25% [6]. To date, Hb typing has not been consistently reported in such studies [5, 24, 25]. Hb typing not only ensures a balanced distribution of HbAS in randomized trials, but incorporating the information into data analyses can increase the statistical power for detecting the effect of an intervention and hence reduce the required sample size. This is particularly important in malaria vaccine trials in which current vaccine candidates are anticipated to have relatively low efficacy (e.g.≤50%) [26].
Several mechanisms have been proposed to explain how HbAS confers protection against malaria, including decreased RBC invasion or poor growth under low-oxygen tension [27, 28], enhanced removal of parasitized RBC [29, 30], accelerated acquisition of antibodies specific for PfEMP-1 and other variant surface antigens [31, 32], and more recently, reduced cytoadherence of parasitized RBC [33]. Anti-PfEMP-1 antibodies could thus work cooperatively with HbS to further reduce cytoadherence [33], which may explain the apparent effects of age-associated acquired immunity on malaria protection by sickle trait [34]. It is conceivable that any one or a combination of these processes underlies the association between HbAS and the delayed time to first malaria episode observed in this study, either by prolonging the time it takes to achieve symptomatic parasite densities or increasing the probability that asymptomatic infections will be aborted. Since we did not actively survey for asymptomatic parasitemia we could not distinguish between these two possibilities. However, the former is supported by the results of a study in Senegal in which weekly blood smears after quinine treatment showed a delay in the reappearance of asymptomatic parasitemia in HbAS individuals [22]. However, the relationship between asymptomatic parasitemia and the subsequent risk of malaria is complex and not fully understood [35, 36].
It is unlikely that differential mosquito exposure confounded the association between HbAS and the time to first malaria episode, since HbAS individuals appeared to be randomly distributed within the small, well-circumscribed village that lacks a dominant body of water (Fig. 1). Moreover, the frequency of bednet use was evenly distributed between HbAS and non-HbAS individuals. It is also unlikely that access to the study clinic played a significant role, since distance to the clinic from individuals’ homes did not differ by hemoglobin type.
G6PD*A-, −α/αα, and blood group O have been associated with protection from severe malaria [9-11], but their role in protection against uncomplicated malaria is less clear. Consistent with this, we did not observe an association between these polymorphisms and the time to first malaria episode, or malaria incidence, although more subtle effects may have been detected with a larger sample size. Notably, we did not detect an increase in the incidence of uncomplicated malaria among G6PD*A heterozygous females that has been observed by others [19, 37, 38]. Consistent with a recent study [20], we observed negative epistasis between HbAS and −α/αα. However, the frequency of −α/−α in this cohort was too low to confirm the negative epistatic interaction with HbAS reported previously [39].
Interestingly, asymptomatic parasitemia at enrollment (end of the dry season) was associated with a decreased risk of subsequent malaria in multivariate analysis. This finding is consistent with those of a recent longitudinal study in Senegal where malaria is also highly seasonal [36]. In Uganda, however, where malaria is meso-endemic, asymptomatic parasitemia was reported as a risk factor for malaria [35], suggesting that the clinical outcome of asymptomatic parasitemia might vary with the epidemiological setting. In neither of these studies, however, were HbAS and other malaria-protective polymorphisms investigated as potentially contributing factors. In-depth studies of both host and parasite factors may reveal important mechanisms by which host immune responses and parasite immune evasion are balanced.
The delayed presentation of malaria associated with HbAS in this study adds to the measures by which the protective effect of this remarkable polymorphism has been documented. Clinical studies of malaria that use the time to first malaria episode as an endpoint should incorporate routine Hb typing in the study design to account for its effect. It appears that other common RBC polymorphisms do not significantly impact this endpoint, though this may vary in different epidemiological settings. Although this study was designed to investigate the acquisition and maintenance of malaria immunity, the observed effects of HbAS validate this study’s ability to detect factors that influence malaria morbidity.
Acknowledgments
We sincerely thank the residents of Kambila, Mali for participating in this study. We also thank Cheick Tidiane Dabo for clinical laboratory support, Boubacar Guindo and Dansine Diarra for collecting GIS data, Seydou Dia and Tonkoro Diarra for assisting at the study clinic, Bakary Coulibaly and Daouda Kane for helping to prepare the study site, and Richard Sakai and Julie Kim for logistic support.
Financial support: This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Appendix
PCR protocol for detection of the 3.7-kb deletional determinant of α-thalassemia
The 3.7-kb deletional determinant of −α-thalassemia (−α 3.7) was identified by a nested PCR protocol. Approximately 5 ng of extracted genomic DNA (Qiagen, Valencia, CA) was amplified in a 25 μL reaction volume consisting of 20 mmol/L Tris-HCl pH 8.5, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 1 M betaine (Sigma, St. Louis, MO), 0.3 μmoles/L of each primer, 0.2 mmol/L each dNTP and 1.25 units Platinum Taq polymerase (Invitrogen, Carlsbad, CA). In the first round (multiplexed), forward 5’-CCCCTCGCCA AGTCCACC C-3’ [40] and reverse 5’- AAAGCACTCTAGGGTCCAG CG-3’ [40] primers were used to generate a product that would only amplify if the 3.7-kb region was deleted. A different reverse primer 5’-AGACCAGGAAGGGCCGGTG-3’ [40] was used in the same reaction mixture to generate a product that would only amplify if the 3.7-kb region was present. Denaturation at 95°C for 5 min was followed by 35 cycles of denaturation at 97°C for 45 s, annealing at 60°C for 75 s, and extension at 72° for 2.5 min with a final extension at 72° for 5 min. Separate nested amplifications of the first round products (1 μL of 1:20 dilution) were performed in the same reaction buffer with forward primer 5’-CTTTCCCTACCCAGAGCCAGGTT -3’ [41] and reverse primer 5’-AGGAG GGCCCGTTGGGAGGC-3’ (to generate a 1.8-kb product that amplifies in the absence of α-3.7) or forward primer 5’-CTTTCCCTACCCAGAGCCAGGTT-3’ [41] and reverse primer 5’-CCACTTTCCCTCCTCCATCCC-3’ (to generate a 2.0-kb product that amplifies in the presence of α-3.7). Thermal cycling steps were the same as for the first round. Amplified products were separated and visualized on 1.2% agarose gels (Lonza, Basel, Switzerland). The sole presence of the 1.8-kb band indicated no deletion; the sole presence of the 2-kb band indicated α-3.7 homozygosity (−α/−α); the presence of both the 1.8-kb and 2-kb bands indicated α-3.7 heterozygosity (−α/αα).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 56th Annual Meeting of the American Society of Tropical Medicine and Hygiene, November 2007, Philadelphia, PA.
References
- 1.Moorthy V, Reed Z, Smith PG. Measurement of malaria vaccine efficacy in phase III trials: report of a WHO consultation. Vaccine. 2007;25:5115–23. doi: 10.1016/j.vaccine.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 2.Stepniewska K, White NJ. Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria. Malar J. 2006;5:127. doi: 10.1186/1475-2875-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perraut R, Marrama L, Diouf B, et al. Distinct surrogate markers for protection against Plasmodium falciparum infection and clinical malaria identified in a Senegalese community after radical drug cure. J Infect Dis. 2003;188:1940–50. doi: 10.1086/379838. [DOI] [PubMed] [Google Scholar]
- 4.Meraldi V, Nebie I, Tiono AB, et al. Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 2004;26:265–72. doi: 10.1111/j.0141-9838.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- 5.John CC, Tande AJ, Moormann AM, et al. Antibodies to Pre-erythrocytic Plasmodium falciparum Antigens and Risk of Clinical Malaria in Kenyan Children. J Infect Dis. 2008;197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moormann AM, Embury PE, Opondo J, et al. Frequencies of sickle cell trait and glucose-6-phosphate dehydrogenase deficiency differ in highland and nearby lowland malaria-endemic areas of Kenya. Trans R Soc Trop Med Hyg. 2003;97:513–4. doi: 10.1016/s0035-9203(03)80010-x. [DOI] [PubMed] [Google Scholar]
- 7.Hill AV, Allsopp CE, Kwiatkowski D, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 8.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–8. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 9.Williams TN, Wambua S, Uyoga S, et al. Both heterozygous and homozygous alpha+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–71. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 10.Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLoS Med. 2007;4:e66. doi: 10.1371/journal.pmed.0040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe JA, Handel IG, Thera MA, et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc Natl Acad Sci U S A. 2007;104:17471–6. doi: 10.1073/pnas.0705390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–86. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dicko A, Klion AD, Thera MA, et al. The etiology of severe anemia in a village and a periurban area in Mali. Blood. 2004;104:1198–200. doi: 10.1182/blood-2003-11-3884. [DOI] [PubMed] [Google Scholar]
- 14.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 15.Delley V, Bouvier P, Breslow N, et al. What does a single determination of malaria parasite density mean? A longitudinal survey in Mali. Trop Med Int Health. 2000;5:404–12. doi: 10.1046/j.1365-3156.2000.00566.x. [DOI] [PubMed] [Google Scholar]
- 16.Dicko A, Mantel C, Kouriba B, et al. Season, fever prevalence and pyrogenic threshold for malaria disease definition in an endemic area of Mali. Trop Med Int Health. 2005;10:550–6. doi: 10.1111/j.1365-3156.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 17.Raper AB. Sickling in relation to morbidity from malaria and other diseases. Br Med J. 1956;1:965–6. doi: 10.1136/bmj.1.4973.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilles HM, Fletcher KA, Hendrickse RG, Lindner R, Reddy S, Allan N. Glucose-6-phosphate-dehydrogenase deficiency, sickling, and malaria in African children in South Western Nigeria. Lancet. 1967;1:138–40. doi: 10.1016/s0140-6736(67)91037-9. [DOI] [PubMed] [Google Scholar]
- 19.Lell B, May J, Schmidt-Ott RJ, et al. The role of red blood cell polymorphisms in resistance and susceptibility to malaria. Clin Infect Dis. 1999;28:794–9. doi: 10.1086/515193. [DOI] [PubMed] [Google Scholar]
- 20.May J, Evans JA, Timmann C, et al. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA. 2007;297:2220–6. doi: 10.1001/jama.297.20.2220. [DOI] [PubMed] [Google Scholar]
- 21.Aidoo M, Terlouw DJ, Kolczak MS, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–2. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 22.Sokhna CS, Rogier C, Dieye A, Trape JF. Host factors affecting the delay of reappearance of Plasmodium falciparum after radical treatment among a semi-immune population exposed to intense perennial transmission. Am J Trop Med Hyg. 2000;62:266–70. doi: 10.4269/ajtmh.2000.62.266. [DOI] [PubMed] [Google Scholar]
- 23.Coulibaly D, Diallo DA, Thera MA, et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg. 2002;67:604–10. doi: 10.4269/ajtmh.2002.67.604. [DOI] [PubMed] [Google Scholar]
- 24.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–8. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 25.Bejon P, Ogada E, Mwangi T, et al. Extended follow-up following a phase 2b randomized trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS ONE. 2007;2:e707. doi: 10.1371/journal.pone.0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vekemans J, Ballou WR. Plasmodium falciparum malaria vaccines in development. Expert Rev Vaccines. 2008;7:223–40. doi: 10.1586/14760584.7.2.223. [DOI] [PubMed] [Google Scholar]
- 27.Friedman MJ. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci U S A. 1978;75:1994–7. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–3. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 29.Luzzatto L, Nwachuku-Jarrett ES, Reddy S. Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet. 1970;1:319–21. doi: 10.1016/s0140-6736(70)90700-2. [DOI] [PubMed] [Google Scholar]
- 30.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–71. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 31.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191:1631–8. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 32.Marsh K, Otoo L, Hayes RJ, Carson DC, Greenwood BM. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 33.Cholera R, Brittain NJ, Gillrie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–6. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams TN, Mwangi TW, Roberts DJ, et al. An immune basis for malaria protection by the sickle cell trait. PLoS Med. 2005;2:e128. doi: 10.1371/journal.pmed.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njama-Meya D, Kamya MR, Dorsey G. Asymptomatic parasitaemia as a risk factor for symptomatic malaria in a cohort of Ugandan children. Trop Med Int Health. 2004;9:862–8. doi: 10.1111/j.1365-3156.2004.01277.x. [DOI] [PubMed] [Google Scholar]
- 36.Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis. 2008;46:516–22. doi: 10.1086/526529. [DOI] [PubMed] [Google Scholar]
- 37.Migot-Nabias F, Mombo LE, Luty AJ, et al. Human genetic factors related to susceptibility to mild malaria in Gabon. Genes Immun. 2000;1:435–41. doi: 10.1038/sj.gene.6363703. [DOI] [PubMed] [Google Scholar]
- 38.Parikh S, Dorsey G, Rosenthal PJ. Host polymorphisms and the incidence of malaria in Ugandan children. Am J Trop Med Hyg. 2004;71:750–3. [PubMed] [Google Scholar]
- 39.Williams TN, Mwangi TW, Wambua S, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–7. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chong SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood. 2000;95:360–2. [PubMed] [Google Scholar]
- 41.Baysal E, Huisman TH. Detection of common deletional alpha-thalassemia-2 determinants by PCR. Am J Hematol. 1994;46:208–13. doi: 10.1002/ajh.2830460309. [DOI] [PubMed] [Google Scholar]


