Abstract
Tyrosine sulphation is a common modification of many proteins, and the ability to phosphorylate tyrosine residues is an intrinsic property of many growth factor receptors. We have utilized the peptide hormone cholecystokinin (CCK8), which occurs naturally in both sulphated and unsulphated forms, as a model to investigate the effect of tyrosine modification on metal ion binding. The changes in absorbance and fluorescence emission on Fe3+ ion binding indicated that tyrosine sulphation or phosphorylation increased the stoichiometry from 1 to 2, without greatly affecting the affinity (0.6–2.8 μM at pH 6.5). Measurement of calcium binding with a calcium-selective electrode revealed that phosphorylated CCK8 bound two Ca2+ ions. CCK8 and sulphated CCK8 each bound only one Ca2+ ion with lower affinity. Binding of Ca2+, Zn2+ or Bi3+ ions to phosphorylated CCK8 did not cause any change in absorbance, but substantially increased the change in absorbance on subsequent addition of Fe3+ ions. Our results demonstrate that tyrosine modification may increase the affinity of metal ion binding to peptides, and imply that metal ions may directly regulate many signaling pathways.
Keywords: calcium, cholecystokinin, ferric, iron, phosphotyrosine, sulphotyrosine
INTRODUCTION
Modification of tyrosine residues is a critical post-translational modification of many proteins. Tyrosine sulphation occurs in the trans-Golgi network, and is thought to be important in protein secretion [1, 2]. A more specific role for tyrosine sulphation in viral entry into cells has been revealed by the observation that sulphation of tyrosines 10 and 14 of the chemokine receptor CCR5 facilitates interaction with the envelope glycoprotein of the human immunodeficiency virus type 1 [3]. Tyrosine phosphorylation is a common feature of many intracellular signaling pathways [4]. For example ligand-dependent activation of the tyrosine kinase activity of growth factor receptors results in autophosphorylation of their cytoplasmic domains. The phosphotyrosine residues are essential elements in the docking sites for proteins that trigger the signaling cascades that are ultimately responsible for cell proliferation. Although ferric ions are known to bind avidly to the phosphoserine residues of phosvitin and casein [5], and to induce aggregation of the hyperphosphorylated tau protein of Alzheimer’s disease [6], the binding of ferric ions to tyrosine-modified peptides or proteins has not been reported previously.
The octapeptide of the hormone cholecystokinin (CCK8, DYMGWMDFamide) provides a convenient model system in which to study the effects of tyrosine modification on metal ion binding. CCK, which was originally isolated as a 33-residue peptide from the mucosa of the gastrointestinal tract, is responsible for gallbladder contraction and pancreatic enzyme secretion, and also functions as a neurotransmitter in the central nervous system [7]. Truncation of the N-terminal end of CCK33 to CCK8 occurs naturally, and has no effect on immunoreactivity or bioactivity, but sulphation on the sole tyrosine residue greatly increases receptor binding and biological potency [8]. CCK is structurally and functionally related to the gastric peptide hormone gastrin (ZGPWLEEEEEAYGWMDFamide), with which it shares a common amidated C-terminal pentapeptide. The first reported biological activity of gastrin was the stimulation of gastric acid secretion, but gastrin is now also recognized as an important growth factor for the gastric mucosa [9]. Like CCK, gastrins occur in sulphated and unsulphated forms [9]. Although gastrin can also be phosphorylated by the EGF receptor tyrosine kinase in vitro [10], there are no reports of phosphorylated gastrin or CCK occurring naturally.
Gastrins bind two ferric ions [11], the first to Glu7 and the second to Glu8 and Glu9 [12]. Ferric ions are essential for the biological activity of non-amidated forms of the peptide as a stimulant of cell proliferation and migration. Thus, either the substitution Glu7Ala, or treatment with the iron chelator desferrioxamine, completely blocked the biological activity of glycine-extended gastrin [12]. In contrast, ferric ions were not required for the biological activity of amidated gastrin [13]. In the present study we anticipated that the high affinity of gastrin for ferric ions might be disadvantageous, as the contribution from phosphorylation or sulphation of the tyrosine would be less apparent. Because the binding of ferric ions to CCK8 is much weaker than to gastrin, and since CCK8SO4 is more readily obtainable than sulphated gastrin, we chose to study the effects of tyrosine modification on metal ion binding using CCK8 as a model system. Although phosphorylated CCK8 does not occur naturally we also examined the binding of metal ions to CCK8PO4 to allow direct comparison with CCK8SO4.
EXPERIMENTAL
Peptides
CCK8 and sulphated CCK8 (89 and 93% pure, respectively) were purchased from Research Plus Inc. (Manasquan, NJ). Phosphorylated CCK8 (81% pure) was from Peptide Solutions (Bundoora, Australia). All peptides were C-terminally amidated, and the impurities consisted of water and salts.
Absorption spectroscopy
Absorption spectra of peptides (40 μM in 10 mM Na acetate (pH 4.0) or 10 mM Na PIPES (pH 6.5) containing 100 mM NaCl and 0.005% Tween 20) in the presence of increasing concentrations of ferric ions were measured against a buffer blank, in 1 ml quartz cuvettes thermostatted at 298 K, with a Cary 5 spectrophotometer (Varian, Mulgrave, Australia).
Fluorescence spectroscopy
The tryptophan fluorescence of peptides (10 μM in the above buffers) in the presence of increasing concentrations of ferric ions was measured in 3 ml quartz cuvettes thermostatted at 298 K, with a Spex Fluorolog-τ2 spectrofluorimeter (Spex Industries, Edison, NJ), with the excitation and emission wavelengths set at 290 and 345 nm, respectively.
NMR spectroscopy
CCK8SO4 was dissolved in 90%H2O/10% 2H2O. CCK8 required the presence of 2H6-DMSO (80% H2O/10% 2H2O/10% 2H6-DMSO) to achieve solubility at 0.23 mM. The pH was adjusted to 4.0 or 6.5 with NaO2H/2HCl, and pH readings are uncorrected for the presence of 2H2O. 1H NMR spectra were recorded at 298 K on Bruker Avance 500 or 600 spectrometers, and referenced to 2,2-dimethyl-2-silapentane-5-sulphonate at 0 ppm via the chemical shift of the H2O resonance at 4.77 ppm, as described previously [12]. Sequence-specific 1H NMR resonance assignments were made from two-dimensional nuclear Overhauser enhancement spectroscopy (NOESY), total correlation spectroscopy (TOCSY) and double quantum filtered COSY (DQF-COSY) spectra. Two-dimensional spectra were analyzed using Sparky 3 (T.D. Goddard and D.G. Kneller, University of California, San Francisco).
Expression of CCK1 and CCK2 receptors in COS-7 cells
COS-7 cells were cultured at 37°C in 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Melbourne, Australia) supplemented with 5% FBS in 75 cm2 flasks (Nunc, Roskilde, Denmark) until 95% confluent. On day 1 the cells were dislodged with 0.25% trypsin/0.02% EDTA and seeded into 100mm Petri dishes at 7.5×105 cells/10ml per dish. Cells were transfected on day 2 by the DEAE-dextran method with 2.5 μg pRFNEO plasmids encoding the human CCK1 receptor or the human CCK2 receptor as described previously [14]. After overnight incubation the cells were collected from the Petri dish with trypsin/EDTA, seeded in the wells of a 24-well plate (20000 cells/well) and incubated in standard conditions for 72 h before the binding assay was performed.
Receptor binding assay
Dilutions of ligands were prepared in binding buffer (DMEM with BSA 0.1%, PMSF 0.15M, Bacitracin 0.05%). Transfected COS-7 cells were washed twice with PBS and incubated in binding buffer (150 μl/well) containing the ligands under investigation and sulphated [125I]-Bolton and Hunter labelled-CCK8 (50,000 cpm/well, Amersham Biosciences, Castle Hill, Australia) for 90 min at 37°C on a slowly rotating platform. The binding solution was then aspirated, and the cells were washed once with PBS and dissolved in 0.25 M NaOH (300 μl/well). Radioactivity in the resulting solution was measured in a gamma-counter (LKB Wallac, Turku, Finland).
Measurement of calcium binding
The change in free [Ca2+] during addition of aliquots of calcium chloride to peptides (15 – 40 μM) in 10 mM Na+ PIPES, pH 6.5, 100 mM NaCl, 0.005% Tween 20, 0.4% DMSO was measured at 293 K with a uniPROBE calcium-selective electrode (TPS, Springwood, Australia) connected to a Hanna 8521 pH meter (Hanna Instruments, Tullamarine, Australia), as described by Park and coworkers [15]. The electrode was first calibrated with solutions of known [Ca2+] in the range 1–1000 μM in the same buffer. The concentration of Ca2+ ion bound to each peptide was calculated by subtraction of the free [Ca2+] from the total added [Ca2+].
Curve fitting and statistics
Data (expressed as means ± S.E.M.) for the independent binding of ferric or calcium ions to CCK8PO4 were fitted to one-site or two-site ordered models with the program BioEqs [16, 17]. Because of the large number of parameters and the limited number of data points, estimates of the equilibrium constants and absorbance ratios for the interaction of ferric ions with CCK8PO4 in the presence of calcium ions were obtained by simulation with the program Sigmaplot with the competitive two-site ordered model shown in Figure 6, and the experimentally determined equilibrium constants and absorbance ratios given in Table 1 for the interaction of CCK8PO4 with ferric or calcium ions alone.
Receptor binding data were analyzed by one-way analysis of variance, followed by Bonferroni’s t-test. Differences with P values < 0.05 were considered significant.
RESULTS AND DISCUSSION
Binding of ferric ions to tyrosine-modified CCK
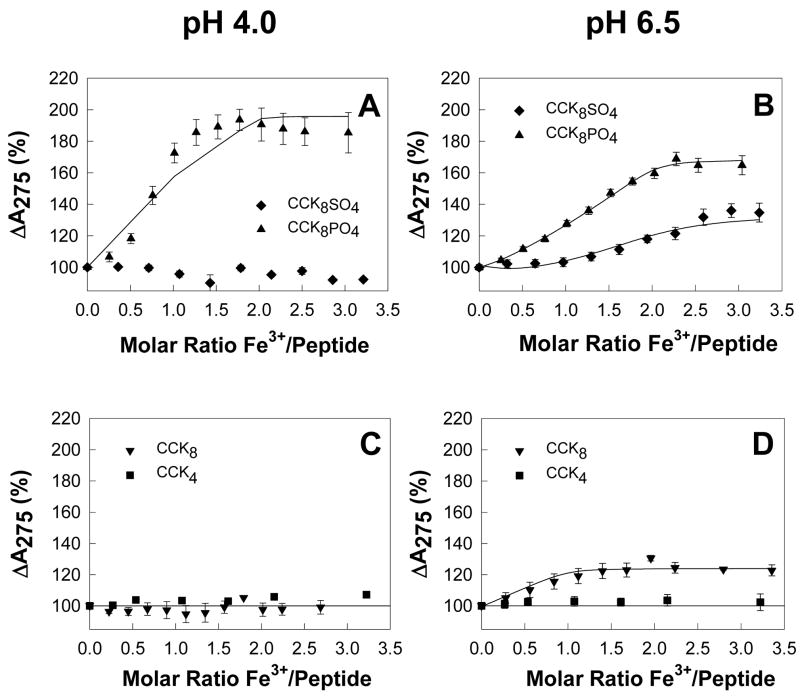
The effect of addition of Fe3+ ions on the absorption spectrum of CCK4 (the C-terminal tetrapeptide of CCK4), CCK8, CCK8SO4 and CCK8PO4 was first investigated at pH 4.0. This pH value was chosen in order to avoid any problems with precipitation of ferric hydroxides. Although no change in the absorbance of CCK4, CCK8 or CCK8SO4 was observed at 275 nm, the absorbance of CCK8PO4 increased to a maximum of 194% after the addition of 1.77 mol ferric chloride/mol peptide (Figure 1A). Because a two-site model, with dissociation constants of 0.22 pM and 0.13 μM, did not adequately fit the non-linearity of the absorbance data (solid line, Figure 1A), the possibility of dimerisation was investigated. Measurement of the molecular mass of CCK8PO4 by analytical ultracentrifugation (Supplementary Figure 1) gave very similar values in the presence of EDTA (1326 Da) or Fe3+ ions (1257 Da), and both values were similar to the theoretical value for the monomer of 1142.7. Dimerisation of Fe3+ ions to form the species Fe2(OH)24+ has also been reported [18], but speciation plots indicate that no significant amount of the dimeric species would exist at the pH values and [Fe3+] used in our experiments. Since there was no evidence for peptide or iron dimerisation, CCK8PO4 appears to belong to the growing class of proteins which demonstrate allosteric effects as monomers [19].
Figure 1. Ferric ions enhance CCK8PO4 absorbance.
At pH 4.0 (A,C), addition of aliquots of ferric chloride to 40 μM CCK8PO4 (▲) in 10 mM Na+ acetate, 100 mM NaCl, 0.005% Tween 20, 0.4% DMSO, 298 K resulted in an increase in the absorption at 275 nm up to a molar ratio of 1.8. Addition of aliquots of FeCl3 to 40 μM CCK4 (■), CCK8 (▼), or CCK8SO4 (◆) did not cause any change in absorption. At pH 6.5 (B,D) in 10 mM Na+ PIPES, 100mM NaCl, 0.005% Tween 20, 0.4% DMSO, an increase in absorption at 275 nm was observed on addition of ferric chloride to CCK8, CCK8SO4, or CCK8PO4, but not CCK4. Data are expressed as a percentage of the absorbance of that peptide without ferric ions. Points are means of at least three separate experiments; bars represent the SEM. Lines represent the best fit to one (CCK8) or two (CCK8SO4, CCK8PO4) site models with the program BioEqs; the appropriate Kd values are given in Table 1.
The titration experiments were then repeated at pH 6.5, to assess the contribution to ferric ion binding of ionization of the modified tyrosine residue. The pKa of the phosphoryl group of phosphotyrosine is 5.9 [20]), and although a value for the pKa of the sulphate group of sulphotyrosine has not been published, electrophoretic data indicate that the pKa is slightly greater than 4.5 [21]. At pH 6.5, no precipitation of iron hydroxides was observed by centrifugation of the sample at the end of the experiment, provided the total ferric ion concentration did not exceed 100 μM. The absorbance of CCK8PO4 increased on addition of ferric chloride to a maximum of 169% after the addition of 2.28 mol ferric chloride/mol peptide (Figure 1B). An ordered two-site model, with dissociation constants of 0.68 μM and 0.77 μM, gave a good fit to the absorbance data. The absorbance of CCK8 and CCK8SO4 also increased on addition of ferric chloride, to maxima of 131 and 136%, respectively (Figure 1B, D). A two-site model, with dissociation constants of 2.80 μM and 4.69 μM, again gave a good fit to the absorbance data for CCK8SO4. In the case of CCK8 a one-site model, with dissociation constant 0.60 μM, gave a better fit to the absorbance data. No evidence of binding of ferric ions to CCK4 was observed (Figure 1D). Thus an increase in pH from 4.0 to 6.5 enhanced the binding of ferric ions to both CCK8 and CCK8SO4, consistent with the direct involvement of the deprotonated sulphate or phosphate group in metal ion binding. At pH 6.5 sulphation or phosphorylation of CCK8 increased the stoichiometry of iron binding from 1 to 2, without greatly affecting the affinity (Table 1).
Table 1.
Binding of ferric or calcium ions by CCK8 and its derivatives
| Ferric ions pH4.0 | Ferric ions pH6.5 | Calcium ions pH 6.5 | |||
|---|---|---|---|---|---|
| Kd (μM) | A275 (%) | Kd (μM) | A275 (%) | Kd (μM) | |
| CCK8 | Not Detected | - | 0.60 | 124 | 83 |
| CCK8SO4 Kd1 | Not Detected | - | 2.80 | 86 | 870 |
| Kd2 | 4.69 | 135 | |||
| CCK8PO4 Kd1 | Co-operative | 194 | 0.68 | 115 | 46 |
| Kd2 | 0.77 | 169 | 580 | ||
The affinity of, and the percentage absorbance change at 275 nm on, ferric ion binding to CCK8, CCK8SO4 or CCK8PO4 were determined by fitting the mean data obtained in the absorbance experiments described in the Figure 1 legend with the program BioEqs. The affinity of calcium ion binding to CCK8, CCK8SO4 or CCK8PO4 was determined by fitting the mean data obtained with a calcium-selective electrode as described in the Figure 5 legend with the program BioEqs.
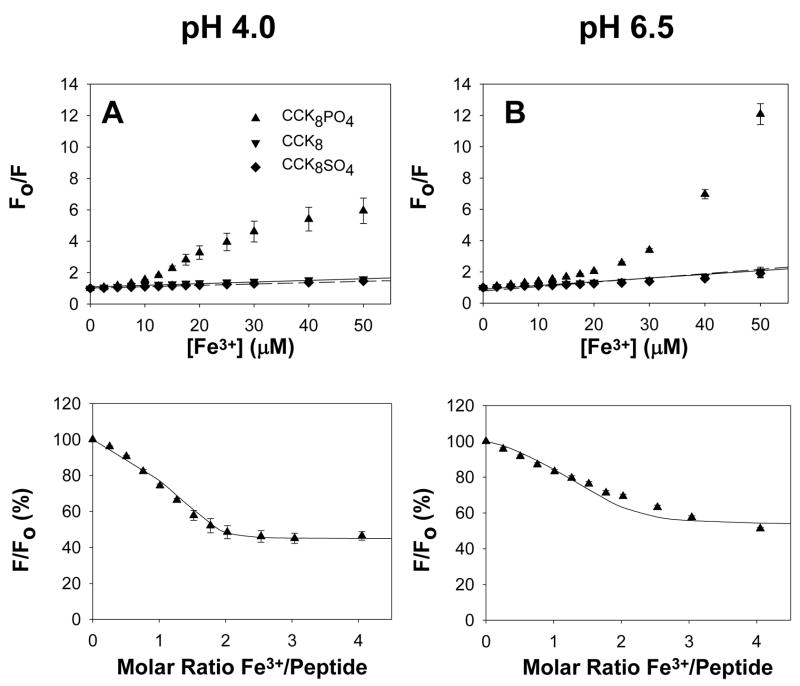
The effect of addition of Fe3+ ions on the tryptophan fluorescence of CCK8, CCK8SO4 and CCK8PO4 was also monitored. Analysis of the quenching of tryptophan fluorescence on addition of ferric chloride at pH 4.0 had previously revealed that glycine-extended gastrin bound two ferric ions with an apparent dissociation constant of 0.6 μM [11]. Although the fluorescence of CCK8 or CCK8SO4 was quenched by Fe3+ ions, the quenching fitted the Stern-Volmer relationship (Figure 2A, B), and is therefore likely to the result of random collisions rather than complex formation [22]. In contrast, quenching of the fluorescence of CCK8PO4 by Fe3+ ions deviated from the Stern-Volmer relationship (Figure 2A, B). After allowance for the collisional component of quenching the fluorescence data for CCK8PO4 were reasonably well fitted (Figure 2C, D) by the two-site model with the affinity constants determined from the absorbance data as described in the previous paragraph and given in Table 1. Thus, fluorescence data are also consistent with the conclusion that CCK8PO4 binds two ferric ions with μM affinity.
Figure 2. Ferric ions reduce CCK8PO4 fluorescence.
At pH 4.0 (A) or pH 6.5 (B) addition of aliquots of ferric chloride to 10 μM CCK8 (▼, solid line), or CCK8SO4 (◆, dashed line) in the buffers described in the Figure 1 legend resulted in a decrease in fluorescence that was well fitted by the Stern-Volmer equation. In contrast on addition of aliquots of FeCl3 to 10 μM CCK8PO4 (▲) the decrease in fluorescence was greater than predicted by the Stern-Volmer equation. The points in (C) and (D) were obtained by correction of the experimental data from (A) and (B), respectively, for the collisional component of quenching by subtraction. The lines were constructed by fitting the values for the reduction in fluorescence on occupation of the first (pH 4.0, 0.773; pH 6.5, 0.949) and second (pH 4.0, 0.448; pH 6.5, 0.528) sites with the program BioEqs, using the dissociation constants presented in Table 1 which had been obtained from fitting the absorbance data at the appropriate pH. Data are expressed as a percentage of the absorbance of that peptide without ferric ions. Points are means of at least three separate experiments; bars represent the SEM.
The possibility must be considered that the observed effects of Fe3+ ions on the absorbance and fluorescence of CCK peptides may be an artefact caused by precipitation of ferric hydroxides from solution, followed by passive adsorption to the peptide. The following arguments strongly suggest that such artefacts were avoided. Firstly a series of absorbance experiments was performed with 40 μM CCK peptides at pH 4.0, at which pH a 1 mM FeCl3 solution is stable indefinitely. Secondly no precipitation was observed in the absorbance experiments at pH 6.5 when the concentration of Fe3+ ions was less than 100 μM. These experimental observations were confirmed at both pH values by speciation plots which did not indicate any of the polymeric species [Fe2(OH)2]4+or [Fe3(OH)4]5+, formation of which precedes precipitation [18]. Thirdly the experiments were internally controlled. Thus at pH 6.5 no increase in absorbance was seen with CCK4, and the observed increase differed between CCK8, CCK8SO4 and CCK8PO4. It seems improbable that passive adsorption should differ so significantly between four such closely related peptides. At pH 4.0 an increase in absorbance was seen only with CCK8PO4, and not with CCK4, CCK8, or CCK8SO4. If passive adsorption were the explanation of the observed increases, then CCK8 and CCK8SO4 would have to adsorb iron differently at the two pH values. Finally attempts were made to eliminate the possibility of precipitation by the addition of Fe3+ ions as ferric citrate rather than FeCl3. However, citrate binds Fe3+ ions more tightly than any of the CCK peptides, as shown by the relative association constants (Table 1), and consequently no increase in absorbance was observed for CCK8, CCK8SO4 or CCK8PO4 during titration with ferric citrate. We conclude that the most likely explanation for the observed changes in absorbance and fluorescence of CCK peptides on addition of Fe3+ ions is the formation of the Fe-CCK complexes described above.
Ferric ion ligands
In order to define the ligands involved in ferric ion binding, the effect of ferric ions on the NMR spectra of CCK8, CCK8SO4 and CCK8PO4 was investigated. Assignments of the spectrum of CCK8SO4 at pH 6.5 and 298 K (Supplementary Table 1) were in general agreement with the data of Fournié-Zaluski and coworkers [23]. Assignments of the spectrum of CCK8PO4 at pH 6.5 and 298 K (Supplementary Table 2) have not been reported previously. At pH 4.0, addition of 1 mol/mol FeCl3 to CCK8 (data not shown) or CCK8PO4 (Figure 3A) resulted in a significant reduction of peptide signal. A precipitate was observed in the NMR tube, and no changes in chemical shifts of the peptide remaining in solution were observed. These results indicated the formation of an insoluble Fe3+-peptide complex. Addition of 1 mol/mol of FeCl3 to CCK8SO4 resulted in a loss of approximately 50% of the signal intensity (Figure 3C). However, in this case the chemical shifts of the peptide remaining in solution after addition of FeCl3 showed small but significant downfield movements, in particular for the CHβ resonances of both Asp1 and Asp7, consistent with interaction between these residues and Fe3+ ions. The largest changes observed were 0.04 ppm for the Asp7 CHβ and CHβ′ resonances, twice that of the Asp1 peaks.
Figure 3. Site of ferric ion binding to CCK8SO4.
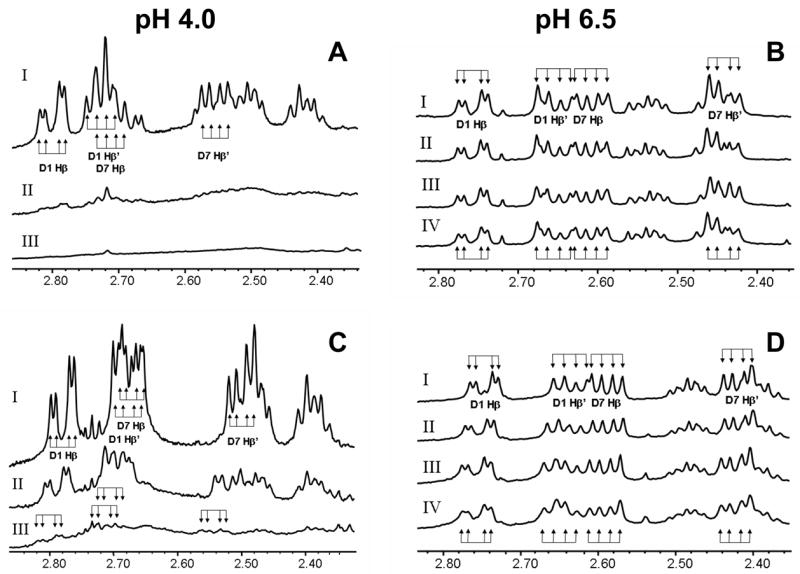
At pH 4.0 and 298 K addition to 50 μM CCK8PO4 (A) or CCK8SO4 (C) of 1 (spectrum II) or 3 mol/mol (spectrum III) of FeCl3 resulted in a substantial loss of signal intensity compared to the spectrum without ferric ions (spectrum I). These results indicate the formation of insoluble Fe3+-peptide complexes over the duration of the NMR experiment. For CCK8SO4 remaining in solution, shifts were observed in several resonances on addition of ferric ions. The largest change was 0.04 ppm for the Asp7 CHβ and CHβ′ resonances, while the change in the corresponding Asp1 peaks was only half as great. The Asp1 and Asp7 CHβ and CHβ′ resonances each appear as a doublet of doublets because of geminal coupling of each proton to its twin and vicinal coupling to the CHα proton. No such shifts were observed for CCK8PO4 remaining in solution. At pH 6.5 and 298 K addition to 140 μM CCK8PO4 (B) of 0, 1, 2 or 4 mol/mol of ferric ions (spectra I-IV, respectively) resulted in a slight broadening of all peaks in the spectrum. As the same amounts of ferric citrate were added to CCK8SO4 (D) there was a downfield shift in the Asp1 CHβ and CHβ′ resonances to a maximum of 0.02 ppm. Parallel but smaller changes in chemical shifts to a maximum of 0.01 ppm were observed for the Asp7 CHβ and CHβ′ resonances. The chemical shifts of other resonances were unaffected by addition of ferric citrate.
The effect of Fe3+ ions on the NMR spectra of CCK8, CCK8SO4 and CCK8PO4 at pH 6.5 was also investigated. To avoid precipitation of ferric hydroxides at the higher iron concentrations required for NMR titration experiments, ferric ions were added as ferric citrate. Although there was no change in the spectrum of CCK8 on addition of ferric citrate, with CCK8PO4 there was a general broadening of all peaks in the spectrum (Figure 3B). In the case of CCK8SO4, in addition to the broadening, there were also small incremental downfield shifts in the Asp1 CHβ and CHβ′ resonances (Figure 3D), to a maximum of 0.02 ppm at 10 mol/mol of added Fe3+ ion. Parallel changes in chemical shifts to a maximum of 0.01 ppm were observed for the Asp7 CHβ and CHβ′ resonances, but the chemical shifts of other resonances were unaffected by addition of ferric citrate, consistent with selective interaction between Asp1 and Asp7 and Fe3+ ions. The magnitude of the NMR shifts was more consistent with a distant interaction with the metal ion than with direct ligation. Interestingly, the absence of any effect of Fe3+ ions on specific resonances of CCK8PO4 suggested that, in contrast to CCK8SO4, the metal ion does not interact with the sidechains of the two aspartate residues, and hence presumably interacts directly with the phosphate group.
Effect of ferric ions on CCK receptor binding
The role of Fe3+ ions in the binding of CCK8 and its modified derivatives to either human CCK1 or CCK2 receptors was then examined. Although sulphation of CCK8 increased its affinity for both the human CCK1 (Figure 4A) and CCK2 (Figure 4B) receptors, phosphorylation of CCK8 had the opposite effect. The IC50 values for the binding of CCK8, CCK8SO4 and CCK8PO4 to the CCK1 receptor were 35 ± 17 nM, 7.1 ± 0.7 nM and >1 μM, respectively, and the corresponding values for binding to the CCK2 receptor were 6.9 ± 2.0 nM, 2.1 ± 0.7 nM and 43 ± 7 nM, respectively. The IC50 values for the binding of CCK8 and CCK8SO4 were similar to the values previously reported for the CCK1 and CCK2 receptors. The observation that the iron chelator desferrioxamine had no significant effect on the binding of [125I]-Bolton and Hunter labeled-CCK8SO4 to either the human CCK1 receptor or the CCK2 receptor (Figure 4C) indicated that Fe3+ ions were not essential for binding of sulphated CCK8 to either receptor. This observation is in agreement with the previous reports that the sulphate group of CCK8SO4 interacts directly with Met195 and Arg197 of the CCK1 receptor [24, 25], and with either Arg57 and Tyr61 [26], or His 207 and Arg 208 [27], of the CCK2 receptor. We conclude that enhancement by metal binding is not the explanation for the previous observation that sulphation of CCK8 increases CCK1 receptor binding and biological activity [8].
Figure 4. Effect of tyrosine modification on receptor binding.
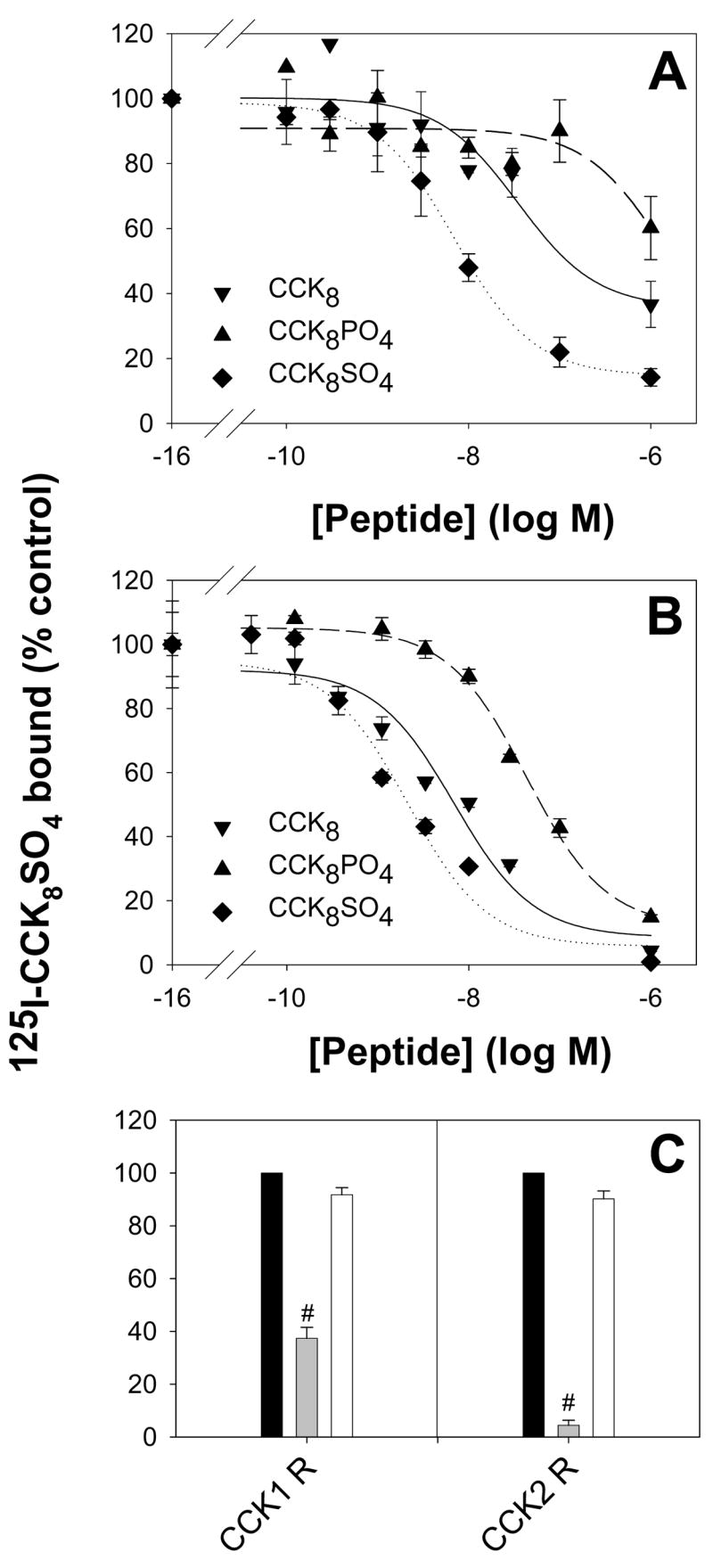
The ability of CCK8 (▼), CCK8SO4 (◆), or CCK8PO4 (▲), to compete with [125I]-Bolton and Hunter labelled-CCK8SO4 (150 pM, 100,000 cpm) for binding to the human CCK1 (A) or CCK2 (B) receptor on transiently transfected COS-7 cells was measured as described in Experimental. Points represent the mean data from at least three experiments, each in triplicate, and lines represent the best fit to a one site model. The IC50 values for the binding of CCK8, CCK8SO4 and CCK8PO4 to the CCK1 receptor were 35 ± 17 nM, 7.1 ± 0.7 nM and >1 μM, respectively, and the corresponding values for binding to the CCK2 receptor were 6.9 ± 2.0 nM, 2.1 ± 0.7 nM and 43 ± 7 nM, respectively. In contrast to the previously reported enhancement of binding on sulphation of CCK8, phosphorylation reduced the peptide’s affinity for both receptors. (C) The inability of the chelator desferrioxamine (DFO, 1 μM, white bars) to compete with [125I]-Bolton and Hunter labelled-CCK8SO4 for binding indicated that ferric ions were not essential for binding of CCK8 to the human CCK1 or CCK2 receptors. Unlabelled 1 μM CCK8SO4 (grey bars) served as a positive control. Values are the means ± S.E. of triplicates, expressed as a percentage of the value obtained in the absence of peptide competitor (black bars). Statistical significance was assessed by ANOVA (#, P < 0.001).
Binding of other metal ions to tyrosine-modified CCK
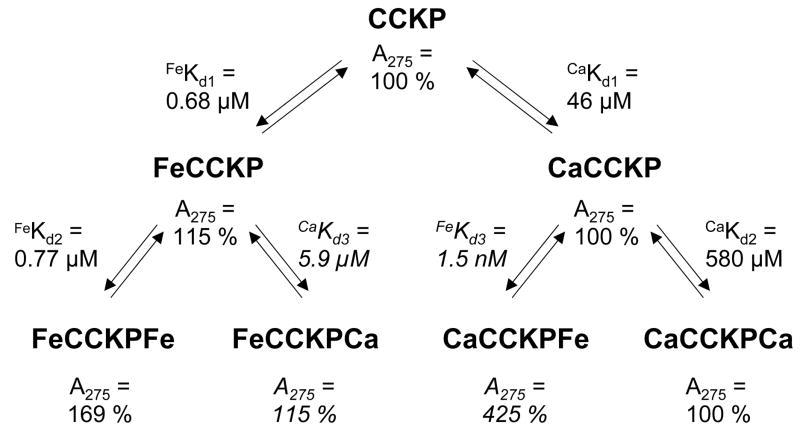
To determine whether or not CCK8 and related peptides bound calcium ions as well as ferric ions, the changes in free [Ca2+] during addition of aliquots of calcium chloride to CCK8, CCK8PO4 or CCK8SO4 were measured with a calcium-selective electrode. A pH of 6.5 was chosen to maximize the chance of detecting an interaction. The binding curves (Figure 5A) indicate that CCK8 and CCK8SO4 each bound 1 mol calcium/mol peptide, with dissociation constants of 83 and 870 μM, respectively. For CCK8PO4 the data were better fitted by a two-site (Kd1 46 μM, Kd2 580 μM) than a one-site model (Kd 24 μM). With either model the affinity of CCK8PO4 for calcium was greater than the affinity of CCK8, and both peptides had higher affinity for calcium than CCK8SO4.
Figure 5. Binding of calcium or zinc ions to phosphorylated CCK8 enhances subsequent ferric ion binding.
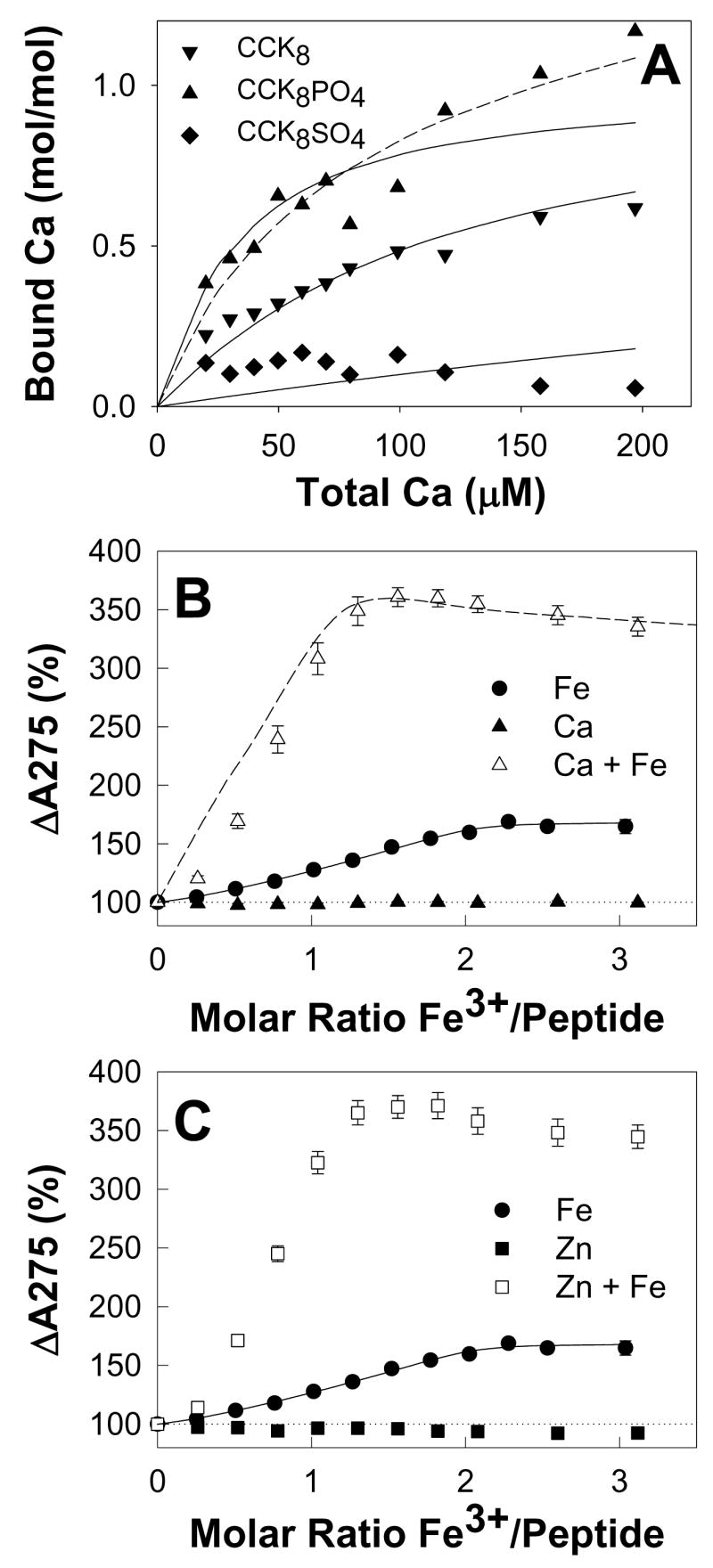
(A) The change in free [Ca2+] during addition of aliquots of calcium chloride to 44.6 μM CCK8 (▼),15.2 μM CCK8PO4 (▲) or 35.6 μM CCK8SO4 (◆) in 10 mM Na+ PIPES, pH 6.5, 100mM NaCl, 0.005% Tween 20, 0.4% DMSO, 293 K was measured with a calcium-selective electrode as described in Experimental. Points are means of at least three separate experiments; solid and dashed lines represent the best fits with the program BioEqs to one-site and two-site models, respectively. The CCK8PO4 data was better fitted by a two-site than a one-site model. Binding of Ca2+ to the first CCK8PO4 site (Kd = 46 μM) was tighter than binding to CCK8 (Kd = 83 μM), which in turn was at least 10-fold tighter than binding to CCK8SO4 (Kd = 870 μM). (B,C) Addition of aliquots of calcium chloride (B, ▲) or zinc sulphate (C,■) to 40 μM CCK8PO4 in the same buffer did not result in any increase in the absorption at 275 nm up to a molar ratio of 5. Subsequent addition of aliquots of ferric chloride to the solutions containing calcium (△) or zinc (□) resulted in a greater increase in absorption than that seen when ferric ions were added to CCK8PO4 in the absence of calcium or zinc ions (●). Points are means of at least three separate experiments; bars represent the SEM. The solid lines represent the best fit to a two-site ordered model with the program BioEqs, with the Kd and A275 values for ferric ions only given in Table 1. The data for ferric ions in the presence of calcium ions was simulated (dashed line) as described in Experimental with the equation for a competitive two-site ordered model and the Kd and A275 values given in Figure 6.
To determine whether or not the binding of other metal ions to CCK8PO4 enhanced the binding of ferric ions, the effects of prior addition of Ca2+, Zn2+ or Bi3+ ions on the changes in absorption spectrum of CCK8PO4 in response to Fe3+ ions were investigated. None of the above metal ions caused any increase in the absorption maximum at 275 nm for CCK8PO4 (Figure 5B, C) or CCK8SO4 (data not shown) at pH 6.5. For CCK8PO4 with added Ca2+ (Figure 5B), Zn2+ (Figure 5C), or Bi3+ (data not shown), subsequent addition of ferric ions caused an increase in absorption significantly greater (3.5 fold) than the 1.5 fold increase observed in the presence of ferric ions alone. Although further work will be required to define the precise mechanism of this effect, the data was fitted with reasonable accuracy by the competitive two-site ordered mechanism shown in Figure 6. The data is consistent with the conclusion that the two ferric ion binding sites can also bind Ca2+, Zn2+ or Bi3+ ions without any effect on peptide absorption, but that binding of a Ca2+, Zn2+ or Bi3+ ion to the first site enhances not only the changes in absorption on binding of a ferric ion to the second site, but also the affinity of ferric ions for the second site. In the case of Ca2+ ions the enhancements of absorption and affinity for the subsequent binding of ferric ions are 2.5-fold and 500-fold, respectively. In contrast, for CCK8SO4 with added Ca2+ or Zn2+, subsequent addition of ferric ions caused an increase in absorption less than that observed in their absence.
Biological implications
Our observation that phosphorylation or sulphation of the sole tyrosine of CCK8 creates an additional binding site(s) for Fe3+ ions is the first report that tyrosine modification enhances metal ion binding to a peptide. Phosphorylation of CCK8 also creates an additional binding site for Ca2+ ions. Although the Ca2+ concentration in living cells rarely exceeds 10 μM, while the affinity for binding of Ca2+ to CCK8PO4 is 46 μM, once a ferric ion is bound this value reduces to 5.9 μM (Figure 6). Hence in the presence of other metal ions the calcium binding site on CCK8PO4 could be greater than 50% occupied. It should also be borne in mind that binding of calcium to other phosphorylated peptides may well be tighter than to CCK8. Similarly, although there is no change in absorbance on binding of Ca2+, Zn2+ or Bi3+ ions to CCK8PO4, their presence enhances the interaction of the peptide with Fe3+ ions. Finally, while metal binding does not appear to influence binding of CCK8SO4 to either the human CCK1 or CCK2 receptors, elucidation of the general biological significance of the observation that tyrosine modification enhances metal ion binding will require further investigation of other peptides and proteins.
Figure 6. Constants for the binding of ferric or calcium ions to phosphorylated CCK8.
Equilibrium constants for the binding of ferric or calcium ions to CCK8PO4 (CCKP) were obtained by fitting the data in Figures 1B and 5A, respectively, to a two-site ordered model with the program BioEqs. Absorbance ratios are given relative to the absorbance of CCK8PO4 at 275 nm in the absence of added metal ions. Values in italics were the best estimates obtained by simulation of the data in Figure 5B for ferric ion binding in the presence of calcium ions as described in Experimental. Binding of a Ca2+ ion to the first site enhances not only the changes in absorption on binding of a ferric ion to the second site by 2.5-fold, but also the affinity of ferric ions for the second site by 500-fold.
Our discoveries may have significant biological implications in several different fields. For example binding of a metal ion to a phosphotyrosine residue in the cytosolic domain of a growth factor receptor might interfere with the binding of a SH2 domain of a downstream target molecule. Such interference might permit direct regulation of intracellular signaling pathways in response to alteration of the intracellular concentration of a particular metal ion such as Ca2+. Although ferric ions are unlikely to be present in the reducing environment within cells, binding of a ferric or other metal ion to a sulphotyrosine residue in the extracellular domain of a viral receptor might interfere with the binding of the viral envelope glycoproteins. Hence competitive binding by metal ions might be a useful strategy for prevention of viral infection of target cells.
Supplementary Material
Sedimentation equilibrium analysis of the interaction of CCK8PO4 with ferric ions, and the chemical shifts of CCK8SO4 and CCK8PO4 is provided online as supplementary data.
Acknowledgments
This work was supported in part by grants from the National Health and Medical Research Council of Australia (400062, 454322; GB) and the National Institutes of Health (5RO1GM065926-05; GB, RN).
Abbreviations
- CCK
cholecystokinin
- DMEM
Dulbecco’s Modified Eagle Medium
References
- 1.Niehrs C, Beisswanger R, Huttner WB. Protein tyrosine sulfation, 1993--an update. Chem Biol Interact. 1994;92:257–271. doi: 10.1016/0009-2797(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 2.Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J Biol Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 3.Huang CC, Lam SN, Acharya P, Tang M, Xiang SH, Hussan SS, Stanfield RL, Robinson J, Sodroski J, Wilson IA, Wyatt R, Bewley CA, Kwong PD. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317:1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawson T. Regulation and targets of receptor tyrosine kinases. Eur J Cancer. 2002;38(Suppl 5):S3–10. doi: 10.1016/s0959-8049(02)80597-4. [DOI] [PubMed] [Google Scholar]
- 5.Hegenauer J, Saltman P, Nace G. Iron(III)--phosphoprotein chelates: stoichiometric equilibrium constant for interation of iron(III) and phosphorylserine residues of phosvitin and casein. Biochemistry. 1979;18:3865–3879. doi: 10.1021/bi00585a006. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem. 2002;82:1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka K, Funakoshi A. Cholecystokinin and cholecystokinin receptors. J Gastroenterol. 2003;38:1–13. doi: 10.1007/s005350300000. [DOI] [PubMed] [Google Scholar]
- 8.Jensen SL, Holst JJ, Nielsen OV, Rehfeld JF. Effect of sulfation of CCK-8 on its stimulation of the endocrine and exocrine secretion from the isolated perfused porcine pancreas. Digestion. 1981;22:305–309. doi: 10.1159/000198675. [DOI] [PubMed] [Google Scholar]
- 9.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin GS, Knesel J, Monckton JM. Phosphorylation of gastrin-17 by epidermal growth factor-stimulated tyrosine kinase. Nature. 1983;301:435–437. doi: 10.1038/301435a0. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin GS, Curtain CC, Sawyer WH. Selective, high-affinity binding of ferric ions by glycine-extended gastrin(17) Biochemistry. 2001;40:10741–10746. doi: 10.1021/bi010016h. [DOI] [PubMed] [Google Scholar]
- 12.Pannequin J, Barnham KJ, Hollande F, Shulkes A, Norton RS, Baldwin GS. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602–48609. doi: 10.1074/jbc.M208440200. [DOI] [PubMed] [Google Scholar]
- 13.Pannequin J, Tantiongco JP, Kovac S, Shulkes A, Baldwin GS. Divergent roles for ferric ions in the biological activity of amidated and non-amidated gastrins. J Endocrinol. 2004;181:315–325. doi: 10.1677/joe.0.1810315. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin GS, Hollande F, Yang Z, Karelina Y, Paterson A, Strang R, Fourmy D, Neumann G, Shulkes A. Biologically active recombinant human progastrin(6–80) contains a tightly bound calcium ion. J Biol Chem. 2001;276:7791–7796. doi: 10.1074/jbc.M009985200. [DOI] [PubMed] [Google Scholar]
- 15.Park O, Swaisgood HE, Allen JC. Calcium binding of phosphopeptides derived from hydrolysis of alpha s-casein or beta-casein using immobilized trypsin. J Dairy Sci. 1998;81:2850–2857. doi: 10.3168/jds.s0022-0302(98)75844-8. [DOI] [PubMed] [Google Scholar]
- 16.Royer CA, Smith WR, Beechem JM. Analysis of binding in macromolecular complexes: a generalized numerical approach. Anal Biochem. 1990;191:287–294. doi: 10.1016/0003-2697(90)90221-t. [DOI] [PubMed] [Google Scholar]
- 17.Royer CA. Improvements in the numerical analysis of thermodynamic data from biomolecular complexes. Anal Biochem. 1993;210:91–97. doi: 10.1006/abio.1993.1155. [DOI] [PubMed] [Google Scholar]
- 18.Flynn CM. Hydrolysis of inorganic iron(III) salts. Chem Rev. 1984;84:31–41. [Google Scholar]
- 19.Gunasekaran K, Ma B, Nussinov R. Is allostery an intrinsic property of all dynamic proteins? Proteins. 2004;57:433–443. doi: 10.1002/prot.20232. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann R, Reichert I, Wachs WO, Zeppezauer M, Kalbitzer HR. 1H and 31P NMR spectroscopy of phosphorylated model peptides. Int J Pept Protein Res. 1994;44:193–198. doi: 10.1111/j.1399-3011.1994.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones JG, Dodgson KS. Biosynthesis of L-Tyrosine O-Sulphate from the Methyl and Ethyl Esters of L-Tyrosine. Biochem J. 1965;94:331–336. doi: 10.1042/bj0940331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer SS. Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry. 1971;10:3254–3263. doi: 10.1021/bi00793a015. [DOI] [PubMed] [Google Scholar]
- 23.Fournie-Zaluski MC, Belleney J, Lux B, Durieux C, Gerard D, Gacel G, Maigret B, Roques BP. Conformational analysis of cholecystokinin CCK26-33 and related fragments by 1H NMR spectroscopy, fluorescence-transfer measurements, and calculations. Biochemistry. 1986;25:3778–3787. doi: 10.1021/bi00361a008. [DOI] [PubMed] [Google Scholar]
- 24.Gigoux V, Escrieut C, Silvente-Poirot S, Maigret B, Gouilleux L, Fehrentz JA, Gully D, Moroder L, Vaysse N, Fourmy D. Met-195 of the cholecystokinin-A receptor interacts with the sulfated tyrosine of cholecystokinin and is crucial for receptor transition to high affinity state. J Biol Chem. 1998;273:14380–14386. doi: 10.1074/jbc.273.23.14380. [DOI] [PubMed] [Google Scholar]
- 25.Gigoux V, Maigret B, Escrieut C, Silvente-Poirot S, Bouisson M, Fehrentz JA, Moroder L, Gully D, Martinez J, Vaysse N, Fourmy AD. Arginine 197 of the cholecystokinin-A receptor binding site interacts with the sulfate of the peptide agonist cholecystokinin. Protein Sci. 1999;8:2347–2354. doi: 10.1110/ps.8.11.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer I, Tikhonova IG, Travers MA, Archer-Lahlou E, Escrieut C, Maigret B, Fourmy D. Evidence that interspecies polymorphism in the human and rat cholecystokinin receptor-2 affects structure of the binding site for the endogenous agonist cholecystokinin. J Biol Chem. 2005;280:22198–22204. doi: 10.1074/jbc.M501786200. [DOI] [PubMed] [Google Scholar]
- 27.Anders J, Bluggel M, Meyer HE, Kuhne R, ter Laak AM, Kojro E, Fahrenholz F. Direct identification of the agonist binding site in the human brain cholecystokininB receptor. Biochemistry. 1999;38:6043–6055. doi: 10.1021/bi990269z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sedimentation equilibrium analysis of the interaction of CCK8PO4 with ferric ions, and the chemical shifts of CCK8SO4 and CCK8PO4 is provided online as supplementary data.