Abstract
Pseudomonas aeruginosa ExoS is a bi-functional type III cytotoxin that possesses Rho GTPase activating protein (RhoGAP) and ADP-ribosyltransferase (ADPr) activities. In the current study, the RhoGAP- and ADPr- activities of ExoS were tested for the ability to disrupt mammalian epithelial cell physiology. RhoGAP, but not ADPr, inhibited internalization/phagocytosis of bacteria, while ADPr, but not RhoGAP, inhibited vesicle trafficking, both general fluid phase uptake and epidermal growth factor (EGF)-activated EGF receptor (EGFR) degradation. In ADPr-intoxicated cells, upon EGF activation, EGFR co-localized with clathrin coated vesicles (CCV) which did not mature into Rab5 positive early endosomes. Constitutively active Rab5 recruited EGFR from CCV to early endosomes. Consistent with the inhibition of Rab5 function by ADPr, several Rab proteins including Rab5 and 9, but not Rab4, were ADP ribosylated by ExoS. Thus, the two enzymatic activities of ExoS have different effects on epithelial cells with RhoGAP inhibiting bacterial internalization and ADPr interfering with CCV maturation. The ability ADP-ribosylation to inhibit mammalian vesicle trafficking provides a new mechanism for bacterial toxin-mediated virulence.
Keywords: ExoS, Rab 5, endocytosis, epidermal growth factor receptor, fluid phase uptake
Introduction
Pseudomonas aeruginosa is a Gram negative, opportunistic pathogen that infects compromised patients, including those with severe burn wounds or cystic fibrosis, causing both acute and chronic infections(1). P. aeruginosa type III secretion system (T3SS) delivers four exotoxins into host cells(2): ExoS, ExoT, ExoU(3) and ExoY (4), which create an environment that favors bacterial survival and dissemination. ExoS and ExoT are bi-functional toxins comprising a Rho GTPase Activating Protein (RhoGAP) domain and an ADP-ribosyltransferase (ADPr) domain(5). ExoS- and ExoT- RhoGAPs inactivate small Rho GTPases, including RhoA, Rac1, and Cdc42, disrupting the host cell cytoskeleton(5). ExoS ADP-ribosylates numerous substrates, including vimentin(6), Ras small GTPases (7), ERM(8), and cyclophilin A(9), eliciting a cytotoxic phenotype in cultured cells, while ExoT ADP-ribosylates a more restricted group of substrates, including CrkI and CrkII(10), uncoupling intergrin mediated signaling(11).
Rab proteins are a family of small G proteins (Rab GTPases) that regulate vesicular budding and fusion reactions by oscillating between active, GTP and inactive, GDP, bound forms(12). Rab GTPases play an essential role in host cell vesicle trafficking including endocytosis, receptor recycling, vesicle maturation and trafficking to Golgi or lysosomes(13). The coincidence of ExoS localization to Rab positive vesicles and identification that Rab5, Rab7, Rab8 and Rab11 are ADP-ribosylated by ExoS in vitro and in vivo(15) promoted the hypothesis that ExoS ADP-ribosylates Rab GTPases to inhibit host cell vesicle trafficking pathways. In vitro, ExoS uncoupled Rab5-early endosome antigen 1 interaction and inhibited fluid phase uptake (14). While an informative study, Vidal et al (14) conducted experiments with full-length ExoS and did not resolve the molecular basis for the observed phenotypes as being due to RhoGAP or ADP-r activities. Furthermore, the ability of ExoS ADPr to disrupt receptor-mediated trafficking in cultured cells has not been described.
Human epidermal growth factor receptor (EGFR) is a 180-kDa cell surface receptor tyrosine kinase that functions in the growth and development of differentiated cells. EGFR exists as a monomeric, single membrane spanning protein in the resting state(16). Upon binding of EGF to the extracellular domain, the EGFR dimerizes, which initiates autophosphorylation and signaling events that lead to cell proliferation, migration, and differentiation(17). EGF stimulation also triggers the internalization of the EGF-EGFR complex via the canonical clathrin-mediated endocytic pathway(18), through early and late endosomes for degradation in lysosomes(19). Endocytic trafficking of the EGFR is necessary for the proper temporal and spatial regulation of EGF signaling. Several G proteins have been implicated in EGFR endocytic trafficking. While Dynamin coordinates EGFR endocytosis (20), Rab5 mediates entry of the EGFR into early endosomes(21, 22). Rab7 is required for the degradation of the EGF·EGFR complex by the lysosomes(23) and Rab11 facilitates EGFR recycling to the plasma membrane(24).
In this study, the ability of ExoS RhoGAP- and ADPr-activities to modulate bacterial internalization and vesicle trafficking in host cells were investigated.
Results
ExoS RhoGAP inhibits the internalization of P. aeruginosa by HeLa cells
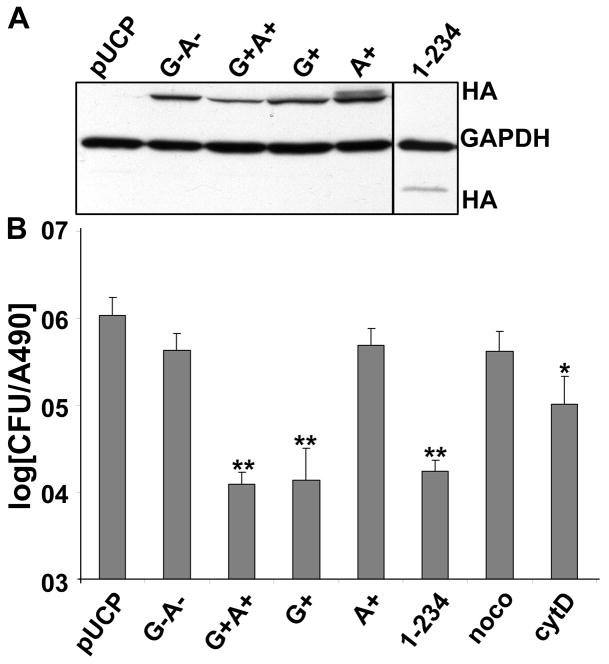
To dissect the role of ExoS RhoGAP and ADPr activities on host cell function, P. aeruginosa expressing ExoS RhoGAP (E381D, mutation within the active site for ADPr domain, termed G+) or ADPr (R146K, mutation in the active site for RhoGAP domain, termed A+) were constructed. Internalization of bacteria by HeLa cells was used to determine the role of ExoS in the anti-internalization activity of P. aeruginosa. Wild type ExoS (G+A+) reduced bacteria internalized by ~2 logs compared with P. aeruginosa expressing a vector control (pUCP), while catalytically null ExoS (R146K, E379/381D, termed G−A−) did not inhibit P. aeruginosa internalization (Figure 1B). This linked the anti-internalization function to ExoS enzymatic activities. ExoS G+ inhibited bacteria internalization to a similar extent as ExoS G+A+, while ExoS A+ showed a similar phenotype as ExoS G−A−, indicating that RhoGAP activity alone contributed to the ExoS anti-internalization activity. In addition, a truncated form of ExoS, lacking the C-terminal ADPr domain (ExoS 1–234), inhibited P. aeruginosa internalization to a similar level as ExoS G+ (Figure 1B), indicating that the ADPr domain was dispensable for anti-internalization function. Nocodazole (noco), a microtubule destabilizing reagent and cytochalasin D (cytoD), an actin inhibitor were used as negative or positive controls, respectively, where cytoD showed ~10 fold reduction of P. aeruginosa (pUCP) internalization by HeLa cells, consistent with the role of actin in phagocytosis. Immunoblot showed the amount of ExoS derivatives delivered into HeLa cells where GAPDH showed comparable loading (Figure 1A). Thus, ExoS anti-internalization activity is dependent on ExoS RhoGAP, but not ADPr activity.
Figure 1. P. aeruginosa ExoS inhibition of bacterial internalization by HeLa cells is RhoGAP dependent.
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs. A) Three hr post intoxication (pi), cells were collected, lysed in SDS-PAGE sample buffer, and subjected to SDS-PAGE and immunoblotted for HA, using Super Signal detection. GAPDH was also probed as a loading control. B) Three hr pi, cells were incubated with 300 μg/ml of Gentamycin for 1 hr to kill extracellular bacteria. Cells were then collected, washed and lysed in 1% Triton X-100 to release intracellular bacteria. Lysates were diluted serially, plated on LB agar to quantify the CFU of internalized bacteria. HeLa cells were pretreated with nocodazole (noco) or cytochalasin D (cytoD) for 0.5 hr, then infected with catalytic null pUCP-ExoS(R146K, E381/379D)-HA (G−A−) as negative or positive controls, respectively. Results were normalized with total cell number by LDH activity and reported as the average of three independent experiments. Constructs used: pUCP vector control (pUCP), catalytic null pUCP-ExoS(R146K, E381/379D)-HA (G−A−), wild type pUCP-ExoS-HA (G+A+), GAP only pUCP-ExoS-E381D-HA (G+), ADPr only pUCP-ExoS-R146K-HA (A+), truncated ExoS with GAP only pUCP-ExoS-1-234-HA, *, p<0.05, **, p<0.01.
P. aeruginosa ExoS ADPr activity inhibits fluid phase uptake of HeLa cells
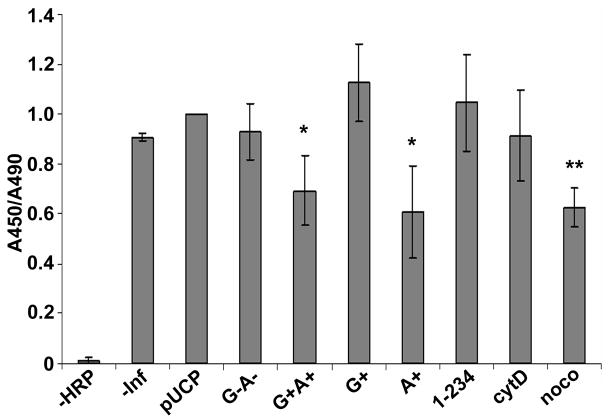
Horse radish peroxidase (HPR) uptake was used to measure early endosome function in HeLa cells intoxicated with ExoS derivatives. HeLa cells intoxicated with P. aeruginosa expressing pUCP or ExoS G−A− took up HRP at levels similar to uninfected cells (-inf), while HeLa cells intoxicated with ExoS G+A+ showed ~30% reduction in HRP uptake (Figure 2), suggesting ExoS inhibition of HRP uptake was activity dependent. ExoS A+ inhibited HRP uptake by 40% (Figure 2), but neither ExoS G+ nor ExoS(1–234) inhibited HRP uptake significantly, although ExoS G+ and ExoS(1–234) intoxicated cells showed a rounding phenotype (data not shown). This indicated that ExoS ADPr activity was responsible for fluid phase uptake inhibition. Noco and cytoD were used as positive and negative controls, respectively, to implicate a role for microtubule, but not actin, in the fluid phase uptake in HeLa cells.
Figure 2. P. aeruginosa ExoS ADPr inhibits HRP uptake.
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization or left uninfected (-inf). One hr pi, cells were incubated with 5mg/ml of HRP for 40 min. Cells were washed and lysed in 1% triton X-100 to release intracellular HRP. HPR activity was measured using Ultra-TMB substrates in ELISA plates. HeLa cells were pretreated with nocodazole (noco) or cytochalasin D (cytoD) for 0.5 hr, then infected with catalytic null pUCP-ExoS(R146K, E379/381D)-HA (KDD) as negative or positive controls. Results were normalized with total cell number by LDH activity. Value in KDD sample was normalized to one in each experiment. Constructs used were as described in Figure 1 *, p<0.01, ** p<0.001.
P. aeruginosa ExoS ADPr activity inhibits EGF-activated EGFR degradation in HeLa cells
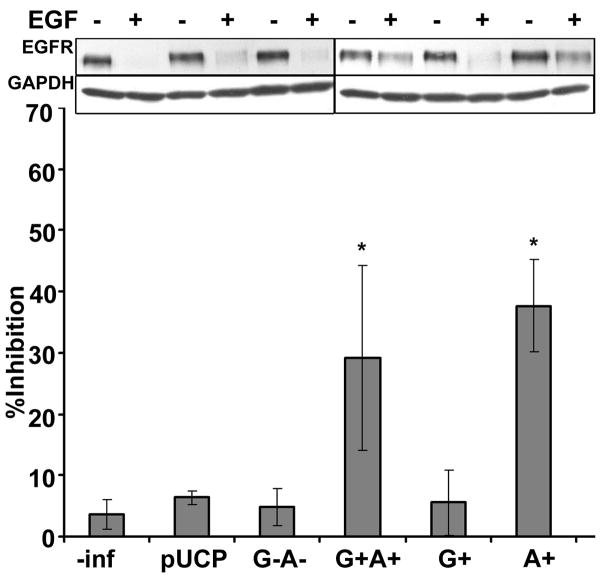
The impact of ExoS ADPr activity on host vesicle trafficking was further tested by measuring EGF-activated EGF receptor (EGFR) degradation, a pathway involving clathrin mediated uptake of activated EGFR dimer, maturation to early and late endosomes and final degradation of the EGFR in lysosomes. Initial experiments observed that the steady state level of EGFR was reduced with the addition of EGF (Figure 3, immunoblot). EGF-activated EGFR degradation was inhibited by lysosome inhibitors, bafilomycin A1 and chloroquine, but not the proteasome inhibitor, clasto-lactacyctin β-lactone (data not shown), supporting a lysosomal pathway for EGFR degradation(25). HeLa cells intoxicated with ExoS G+A+ or ExoS A+ inhibited EGF-activated EGFR degradation by ~35%. This inhibition was not observed in HeLa cells intoxicated with P. aeruginosa expressing pUCP vector control, ExoS G−A− or ExoS G+, indicating that the inhibition of EGFR degradation was dependent on the expression of ADPr activity (Figure 3). Furthermore, EGFR was phosphorylated at Tyrosine 1068 in HeLa cells intoxicated with ExoS A+ upon EGF activation (Supplemental Figure 1). This indicated that the inhibition of receptor degradation was not due to failure of EGF to bind to the receptor and stimulate EGFR phosphorylation to initiate the degradation signal.
Figure 3. P. aeruginosa ExoS ADPr inhibits EGF-activated EGFR degradation.
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization or left uninfected (-inf). One hr pi, cells were stimulated with 100 ng/ml of EGF for 1 hr. Cells were then collected, lysed in SDS-PAGE sample buffer, and subjected to 8% SDS-PAGE and immunoblotted for EGF receptor (EGFR) using Super Signal. GAPDH (GDH) was also probed as a loading control. HeLa cells were pretreated with nocodazole (noco) or cytochalasin D (cytoD) for 0.5 hr, then infected with catalytic null pUCP-ExoS(R146K, E381/379D)-HA (KDD) as negative or positive controls. A representative immunoblot is shown in the upper panel. Percentage of inhibition was quantified by densitometry of immunoblot. Results were normalized with total cell protein by GAPDH. Shown are averages of three independent experiments. Constructs used were as described in Figure 1. *, p<0.01.
P. aeruginosa ExoS ADPr inhibits EGF-activated EGFR trafficking to lysosomes
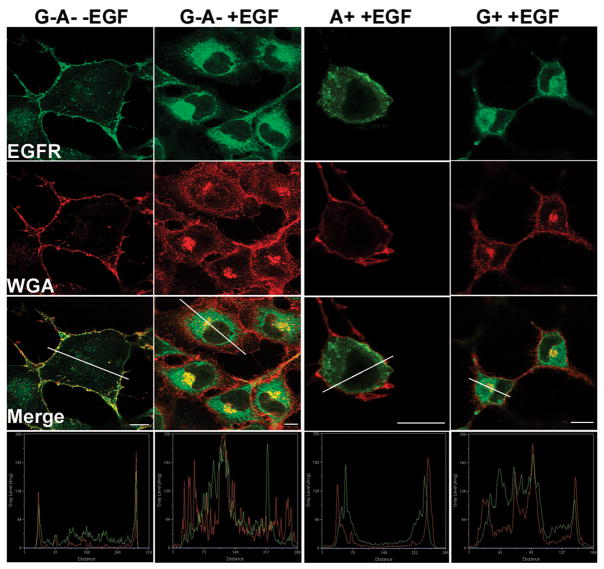
To determine how ADP-ribosylation inhibited EGFR degradation, EGF-activated EGFR localization was measured in HeLa cells intoxicated with P. aeruginosa expressing ExoS derivatives. Wheat germ agglutinin (WGA) was used as a plasma membrane marker (Figure 4) and Lamp 1 was used as lysosome marker (Figure 5). In unstimulated cells, EGFR localized on the plasma membrane with a small intracellular pool, whereas upon EGF-activation, plasma membrane localized EGFR was not detected (Figure 4). In HeLa cells intoxicated with ExoS G+ or ExoS A+, EGF activated the internalization of the EGFR, indicating movement of the EGFR off the plasma membrane and into the endocytic pathway.
Figure 4. P. aeruginosa ExoS ADPr does not inhibit EGF-activated EGFR internalization.
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization. One hr pi, cells were stimulated with 100 ng/ml of EGF for 1 hr. Cells were washed, fixed and endogenous EGF receptor (EGFR) was visualized by immunostaining using sheep anti-EFGR as primary antibody followed by alexa488 rabbit anti-sheep secondary antibody. Alexa 594-Wheat germ agglutinin (WGA) was added 20 min prior to fixation to label plasma membrane. Images were taken under a Leica confocal scanning microscope with a 100× oil objective. Fluorescence intensity along the line in each merge image was shown at the bottom. Constructs used were as described in Figure 1. Bar: 10nm.
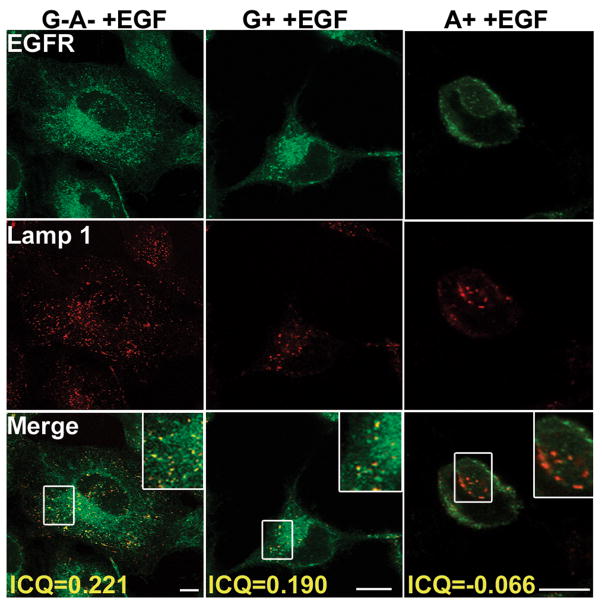
Figure 5. P. aeruginosa ExoS ADPr inhibits EGF-activated EGFR trafficking to the lysosome.
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs and treated with EGF as described in Figure 4. Cells were washed, fixed, and endogenous EGFR was visualized by immunostaining using sheep anti-EFGR as the primary antibody followed by alexa488 rabbit anti-sheep secondary antibody. Endogenous Lamp 1 was visualized by immunostaining using mouse anti-Lamp 1 as the primary antibody followed by alexa568 mouse anti-rabbit secondary antibody. Images were taken under a Leica confocal scanning microscope with a 100× oil objective. Bar: 10nm. Correlation of green and red signals was displayed as ICQ value calculated as indicated in Materials and Methods.
In cells intoxicated with ExoS G−A− or ExoS G+, EGF stimulated the EGFR to traffic to lysosomes (ICQs=~0.2, indicating partial co-localization), while in cells intoxicated with ExoS A+, upon EGF stimulation EGFR was excluded from lysosomes (ICQ=~-0.1, indicating segmentation) (Figure 5). In these experiments rounded cells (stars) were scored for EGFR localization, since ExoS intoxication induces cell rounding through either RhoGAP- or ADPr-activities(26, 27). Taken together, ExoS ADPr blocks EGF-activated EGFR trafficking to lysosomes, rather than blocking EGFR internalization.
P. aeruginosa ExoS ADPr activity inhibits EGF-activated WGA uptake of HeLa cells
WGA, which labels plasma membrane glycol-proteins and glycol-lipids, were also internalized upon EGF activation (Figure 4). In contrast to EGFR whose internalization was independent of ExoS ADPr activity, WGA internalization was blocked by ADPr activity, with 15% of ExoS A+ intoxicated HeLa cell having intracellular WGA, compared to 76% and 93% of cells intoxicated with ExoS G−A− or ExoS G+. Similar to fluid phase uptake which is enhanced with EGF activation, WGA uptake was also unregulated by EGF, possibly through the activation of Ras protein which induced membrane ruffles and enhanced fluid phase uptake(28).
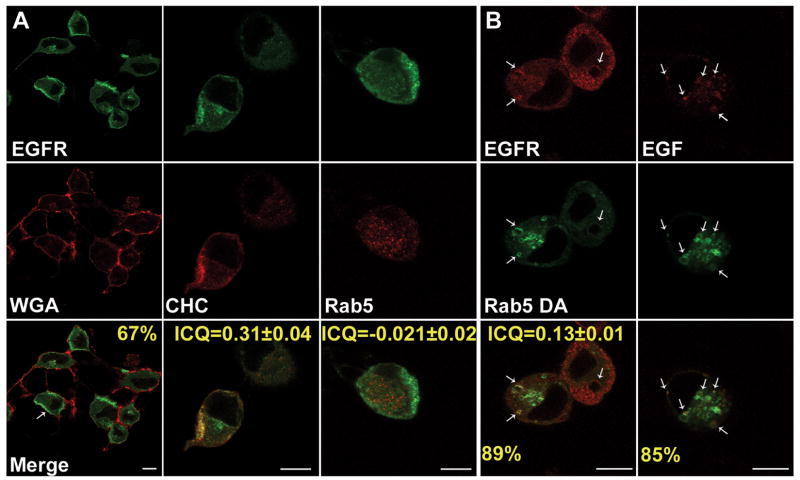
ExoS ADPr inhibits EGFR trafficking by inhibiting Rab5 function. To determine how ADPr inhibited EGFR traffics to the lysosome, the intracellular localization of EGFR after EGF-activation in ExoS A+ intoxicated cells was determined. Sixty seven percent of the intoxicated cells accumulated EGFR in the cell periphery adjacent to the plasma membrane (Figure 6A), suggesting that ADPr inhibited an early step in EGFR trafficking. Co-localization experiments observed that EGFR co-localized with clathrin heavy chain (ICQ=0.31±0.04), but not with Rab5 (ICQ=0.021±0.02), indicating EGFR trafficking from clathrin coated vesicles (CCV) to Rab5 positive vesicles was blocked. In contrast, in ExoS G-A- or ExoS G+ intoxicated cells, EGFR colocalized with clathrin to a lesser extent (Supplemental Figure 2), This may be due to flat clathrin coats that are proposed to be present on endosome microdomains to mediate protein sorting to degradative pathways(29).
Figure 6. DA-Rab5 rescues P. aeruginosa ExoS ADPr inhibition of EGF-activated EGFR trafficking in the early endosome.
A) HeLa cells were intoxicated with P. aeruginosa PA103, ΔexoU, exoT::Tc carrying pUCP-ExoS-R146K-HA (A+) with synchronization. One and ½ hr pi, cells were stimulated with 100 ng/ml EGF for 30 min. Cells were washed, fixed and endogenous EGF receptor (EGFR) was visualized by immunostaining using sheep anti-EFGR as primary antibody followed by alexa488 rabbit anti-sheep secondary antibody. Endogenous clathrin heavy chain (CHC), and Rab 5 was visualized by immunostaining using mouse anti-CHC or Rab 5 as primary antibody followed by alexa568 conjugated secondary antibody. Arrows: cells with EGF receptor accumulated in cell periphery. B) HeLa cells were transfected with constitutively active Rab 5 fused with green fluorescent protein (GFP-Rab5-DA) and infected with P. aeruginosa PA103, ΔexoU, exoT::Tc carrying pUCP-ExoS-R146K-HA (A+). One and ½ hr pi, (left) cells were treated with EGF for 30 min. Cells were then washed, fixed and endogenous EGF receptor (EGFR) was visualized as in A. (right) Alexa 568-EGF (5 mg/ml) was added to cells, fixed, and mounted. Images were taken under a Leica confocal scanning microscope with a 100× oil objective. Arrows: Rab 5 vesicles positive for EGFR staining or alexa-EGF. Bar: 10nm.
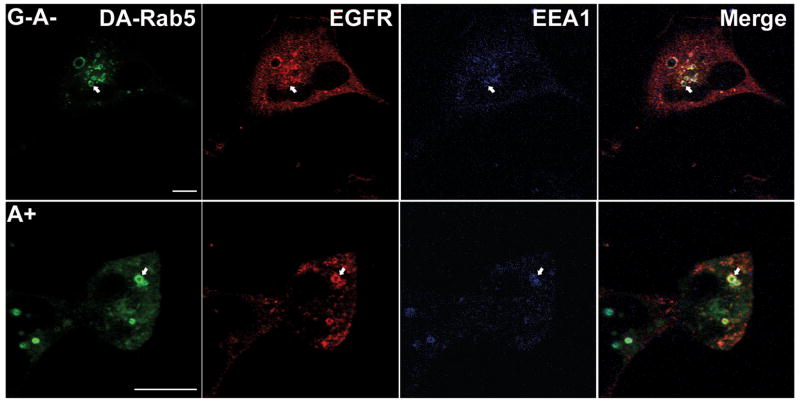
To test if the inhibition of Rab5 function was the mechanism of ExoS ADPr action on vesicle trafficking, a GFP-fused constitutively active Rab5 protein (GFP-Rab5-Q79L, termed DA-Rab5) was transiently expressed in HeLa cells and the ability of DA-Rab5 to recruit EGFR was measured. Expression of DA-Rab5 leads to enlarged Rab5 positive early endosomes due to continuous vesicle fusion(30). In ExoS A+ intoxicated cells, 89% of the enlarged early endosomes recruited EGFR upon EGF activation (Figure 6B) and the co-localization efficiency of Rab5 and EGFR increased to ICQ=0.13±0.01. In an independent experiment, Rhodamine-conjugated EGF was used to monitor the localization of EGFR initiated from the plasma membrane, rather than from an intracellular pool. Eighty five % of DA-Rab5 positive vesicles recruited Rhodamine-conjugated EGF in cell intoxicated with ExoS A+ (Figure 6B). Together, these data suggest that ExoS ADPr activity traps EGFR in CCVs by inhibiting Rab5 activity. Furthermore, 68% of DA-Rab5 vesicles positive for EGFR also accumulated detectable amounts of early endosome antigen 1 (EEA1), a Rab5 effector protein in ExoS A+ intoxicated cells (Figure 7), which suggested a functional DA-Rab5 activity.
Figure 7. DA-Rab5 recruits EEA1 in ExoS A+ intoxicated cells.
HeLa cells transfected with Constitutively active Rab 5 fused with green fluorescent protein (GFP-Rab5-DA) were co-cultured with P. aeruginosa PA103, ΔexoU, exoT::Tc carrying pUCP-ExoS-KDD-HA (G−A−) or pUCP-ExoS-R146K-HA (G+) with synchronization. One and ½ hr pi, cells were stimulated with 100 ng/ml EGF for 30 min. Cells were washed, fixed and endogenous EGF receptor (EGFR) was visualized by immunostaining, using sheep anti-EFGR as primary antibody followed by alexa568 rabbit anti-sheep secondary antibody. Endogenous early endosome antigen 1 (EEA1) was visualized by immunostaining using mouse anti-EEA1 as primary antibody followed by alexa633 conjugated secondary antibody. Images were taken with a Leica confocal scanning microscope using a 100× oil objective. Arrows: Rab 5 vesicles positive for both EGFR and EEA 1 staining. Bar =10nm.
ExoS ADP-ribosylates Rab GTPase in P. aeruginosa intoxicated HeLa cells
The ability of ExoS to ADP-ribosylate Rab GTPase in HeLa cells was confirmed by immunoblotting endogenous Rab GTPases in cells intoxicated with ExoS. Consistent with previous reports (15), Rab5 and Rab9, but not Rab4 were shifted to a slower migrating band in cells intoxicated with ExoS G+A+ or ExoS A+, but not with vector control, ExoS G−A− or ExoS G+ (Supplemental Figure 3), indicating ExoS ADP-ribosylates specific Rabs in cultured HeLa cells.
Discussion
ExoS RhoGAP
Both ExoS and ExoT have conserved RhoGAP domains which act on the three major classes of Rho GTPases, among which, Rac 1 and Cdc42 are regulators of actin dynamics linked to phagocytosis. Thus, RhoGAPs act as potent anti-internalization reagents(31). Bacterial encoded RhoGAPs mimic mammalian RhoGAPs to facilitate bacterial survival in the host environment. Similar to ExoS, the RhoGAP activity of YopE is required for the extracellular pathogen, Y. pseudotuberculosis to elicit anti-phagocytic function and virulence(32), while in the intracellular pathogen Salmonella type III effector, SopE, a Rho GTP exchange factor, activates RhoA and Cdc42 to stimulate local actin polymerization and promote the uptake of the bacteria into the cells and SptP, a RhoGAP, reverses SopE activation to maintain Salmonella in a replication competent vesicle (33).
ExoS ADPr
Although the anti-internalization activity for ExoS GAP is well documented (34, 35), whether or not ExoS ADPr plays any role in bacterial internalization is not known. ExoT ADPr domain has an anti-internalization activity(31), via the ADP-ribosylation of Crk family proteins(10). Crk I and Crk II are adaptor proteins required for recruiting P130CAS and Paxillin to activated focal adhesion sites(11), which lead to Rac 1 activation and thus phagocytosis. In contrast, ExoS ADP-ribosylates multiple substrates, including Ras and Ezrin/Radixin/Moesin (ERM) family proteins (8), which are regulators of actin network. However, ExoS ADP-r activity did not inhibit P. aeruginosa internalization, suggesting that ERM-regulated actin organization plays a limited role in regulating phagocytosis. Since most clinical isolates contain either ExoU or ExoS(36), in addition to ExoT, ExoS ADPr domain may play a distinct role, other than anti-internalization, to elicit virulence. In this study, ExoS A+ inhibited receptor-mediated endocytosis and general fluid phase uptake, which presents a new mechanism for ExoS virulence.
Role of Rab5 proteins in ExoS inhibition of host vesicle trafficking
In addition to the well characterized role of Rab5 in targeting plasma-membrane-derived vesicles to endosomes and in homotypic fusion between endosomes, Rab5 contributes to protein sorting, regulation of motility of early endosomes, signal transduction, receptor tyrosine kinase induced actin remodeling and clathrin mediated endocytosis(13). Rab proteins are ADP-ribosylated by ExoS in vitro and in vivo(15). Earlier study observed that ExoS diminished the interaction of Rab5 with EEA1 and reduced endosome-endosome fusions (14). The observation that ExoS ADP-ribosylated Rab5, together with the ability of constitutively active Rab5 protein to reverse ExoS inhibition of EGF-activated EGFR trafficking out of CCVs, suggests that ExoS ADPr inhibits Rab5 activity in cells.
ExoS inhibits CCV-EE fusion
In this study, in cells intoxicated with ExoS A+, EGFR was trapped within CCVs. Galperin and Sorkin showed that in Porcine aortic endothelial cells overexpressing dominant negative Rab5(S34N), EGFR was retained in clathrin-coated pits(37), similar to the phenotype in ExoS A+ intoxicated cells, which supports the determination that ExoS A+ inactivates Rab5. The exact role of Rab5 in clathrin-mediated endocytosis is still debated. Over-expression of constitutively active Rab5-Q79L or dominant-negative Rab5-S34N (21) and Rab5-regulating proteins, such as Rab-GDI(38) and Rab5 GTPase-activating protein(39), reduced the rate and total amount of EGFR and Transferrin receptor (TfnR) internalization, suggesting a role of Rab5 activity in clathrin-dependent endocytosis. In contrast, over-expression of Rab5Q79L did not alter trafficking of the TfnR and dominant negative Rab5 did not inhibit EGFR endocytosis, but that Rab5 determined if internalized EGF/EGFR and Rab5 co-localize(40), implicating a role for Rab5 subsequent to the movement of EGFR from the plasma membrane. Rubini et al showed that Rab5 and EEA1 are required for CCV and EE fusion in vitro(41). The observation that ExoS A+ inhibited the fusion of EGFR-containing vesicles to Rab5 positive early endosomes, rather than receptor endocytosis, is consistent with the role of Rab5 in CCV and EE fusion. However, a role for Rab5 in clathrin mediated endocytosis can not be completely ruled out since ExoS ADP-r may only disrupt a subgroup of Rab5 involved activities.
The current study is the first to observe that DA-Rab5 rescues the ExoS ADPr inhibition of EGFR trafficking in cultured cells. The observation that DA-Rab5 recruited EEA1, rescuing EGFR from CCVs to early endosomes (Figure 7) is consistent with the observation that ExoS inhibited Rab5 interaction with EEA1 in vitro(14), although the molecular mechanism of this inhibition needs further characterization.
ExoS inhibits fluid phase endocytosis
Rab5 is also involved in the regulation of EGF induced fluid-phase endocytosis(21, 42). Role of Rab5 in regulating endocytosis is supported by a presence of Rab5 exchange factors on the plasma membrane of Caenorhabditis elegans (43). While an earlier study implicated a role for ExoS in mediating an inhibition of HRP marked fluid-phase endocytosis(14), the current study showed that the ADP-r activity of ExoS was responsible for this inhibition.
ExoS A+ also inhibited EGF-activated internalization of WGA, which labels glycosylated cell surface proteins and lipids. While expression of DA-Rab5 stimulated HRP uptake(14), DA-Rab5 did not restore EGF-activated internalization of WGA in ExoS A+ intoxicated cells (data not shown), indicating that ExoS may inhibit another substrate for ADPr, such as Ras(28) to impair EGF-activated WGA uptake. EGF activated Ras induced formation of macropinosomes and increased the rate of fluid phase uptake (29). Our data indirectly imply a role for both Ras and Rab5 for EGF induced fluid phase uptake.
Summary
P. aeruginosa translocates ExoS through the type III secretion apparatus where ExoS transiently associates with plasma membrane. ExoS GAP inactivates RhoGTPase to impair local actin reorganization to inhibit bacterial internalization, while ExoS ADPr inhibits Rab5 function. Upon EGF activation, EGFR was internalized to CCVs, which did not mature to Rab5 positive early endosomes. ADPr-also inhibited fluid phase uptake and EGF-activated endocytosis of WGA labeled proteins and lipids at the plasma membrane. Taken together, ExoS inhibits CCV maturation to an early endosome in receptor mediated endocytosis and inhibits endocytosis at the plasma membrane during fluid phase uptake. Wiley and Cunningham compared the EGF dose dependency and time course of EGF-activated fluid phase endocytosis and endocytosis of the EGFR and found these two processes were not directly coupled(44). Thus, ExoS may inhibit these two endocytic processes by different mechanisms.
Maturation of the phagolysosome is orchestrated by Rab GTPases where Rab5 controls the early phases of phagosome maturation and Rab7 regulates fusion events between the late endosome and lysosome(45). Recently a Listeria GAPDH protein was reported to ADP ribosylate Rab5 and inhibit Rab5a-mediated phagosome–endosome fusion(46). Thus, Rab5 may be a common target for both Gram negative or Gram positive bacteria to inhibit phagosomal functions. For intracellular pathogens, this modification favors bacterial survival, while for extracellular pathogens, this modification may reduce antigen presentation of bacterial proteins, or maintain an intracellular supply of toxin at specific intracellular locations for regulated activities.
Materials and Methods
Materials
P. aeruginosa strain PA103 (ΔexoU, exoT::Tc) with the indicated ExoS derivative was maintained and cultured as described previously (5). Rabbit antibody against clathrin heavy chain and phospho-EGFR (P-Tyr1068) were from Cell Signaling Technology, Inc. Sheep polyclonal antibody against EGFR was from Upstate Cell Signaling Solutions. Mouse antibody against Rab9 was from Calbiochem. Mouse antibody against Rab4 and Rab5 were from BD Transduction Laboratories. Mouse antibody against LAMP 1(CD107a) was from BD biosciences. EGF and Rhodamine conjugated EGF was from Invitrogen and chemicals were from Sigma.
Cell culture
HeLa cells (CCL-2) were from the ATCC. HeLa cells were cultured in Minimum Essential Medium (Invitrogen) supplemented with 10% fetal calf serum, non-essential amino acids, sodium pyruvate, sodium bicarbonate and penicillin/streptomycin and maintained humidified at 37°C in 10% CO2 (v:v).
Transfection and Type III delivery of ExoS into HeLa cells
HeLa cells were seeded in 6 or 12 well dishes with 1.5–3×105 cells the day before use. Cells (~70% confluent) were transfected with the Lipofectamine/Plus-transfer system (Invitrogen) as described by the manufacturer. After 18–24 h, cells were intoxicated with the indicated strain of P. aeruginosa PA103 (ΔexoU, exoT::Tc). Intoxication experiments were performed at a multiplicity of 8:1 (bacteria:HeLa cells). Dishes were centrifuged at 400 ×g for 10 min at RT to synchronize the infection and incubated at 37°C for indicated time of intoxication.
HeLa cell P. aeruginosa internalization assay
This assay was modified from(31). HeLa cells, in 6 well plates, were co-cultured with P. aeruginosa expressing ExoS derived constructs at 37°C for 3 h. Cells were then washed and cultured in medium containing 300 μg/ml Gentamycin for 1 h. To ensure that invasion assays were not affected by loss of rounded, non-adherent cells, the supernatant of each well was collected in an Eppendorf tube. Adherent cells were trypsinized and combined with cells recovered from the supernatant. To release the internalized bacteria, cells were washed with PBS and suspended in 0.5 ml of PBS with 0.1% Triton X-100 (Sigma). After 15 min of incubation at RT, serial dilutions of the lysates were performed and 100 μl of each dilution were plated on LB agar and incubated at 37°C for 18 h to quantify the CFU of internalized bacteria. The invasion assays were normalized to the number of cells in each well, as determined by measuring the total amount of lactate dehydrogenase (using the CytoTox 96 Nonradioactive Cytotoxicity Assay kit [Promega] according to the manufacturer’s specification). The results were average of three independent experiments performed in duplicate.
Horse Radish Peroxidase (HRP) assay
This assay was modified from(14). HeLa cells, in 6 well plates, were intoxicated with P. aeruginosa expressing ExoS derived constructs for 1 h at 37°C when cells were washed once with serum-free MEM, followed by the addition of 1 ml of pre-warmed serum-free MEM containing 2 mg/ml of HRP (Sigma) and 1% bovine serum albumin (BSA) to each well. HRP uptake was conducted at 37°C for 40 min. The uptake was stopped by washing the cell monolayers five times with ice-cold PBS containing 1% BSA. After the final wash, cells were scraped into 1 ml of ice-cold PBS and pelleted at 800 × g for 3 min. The cell pellet was washed one more time by suspension in 1 ml of ice-cold PBS followed by centrifugation. The final cell pellet was suspended in 0.5 ml of cold PBS and divided into two equal vials. One vial was lysed with 0.1% Triton X-100 to release intracellular HRP, while the other vial was not lysed and used as a background control for the detection of residual extra cellular HRP. This was subtracted from the corresponding sample lysed with Triton. The lysate was assayed for HRP activity in a 96-well microplate (Costar Co.) using ULTRA-TMB (Thermo Scientific) as substrate. The reaction was conducted at RT for 5–30 min and stopped by adding 100 μl of 0.1 N H2SO4. The products were quantified by measuring absorption at 450 nm in a Bio-Rad microplate reader (Victor 3). The invasion assays were normalized to the number of cells in each well, as determined by measuring the total amount of lactate dehydrogenase, using the CytoTox 96 Nonradioactive Cytotoxicity Assay kit [Promega]. Results were normalized to GAPDH and were reported as the average of at least three independent experiments performed in duplicate.
EGF-activated EGFR degradation
HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization for 1 h, and incubated with or without 100 ng/ml of EGF in serum free MEM for 1 hr. Cells were then washed, lysed and analyzed by SDS-PAGE and Western blotting with sheep anti-EGFR antibody (Santa Cruz Biotech). The amount of EGFR degraded was expressed as a percentage of total EGFR present before induction. GAPDH was used as a negative control. The mean and SD from three independent experiments were shown.
Immunofluorescence microscopy
P. aeruginosa intoxicated HeLa cells were permeablized with 0.1% Triton X-100 in 4% formaldehyde for 15 min at RT, followed by 3 washes with PBS. Cells were blocked with 1 % BSA in PBS for 20 min at RT then incubated with individual primary antibodies. ExoS contained a C-terminal HA epitope that was visualized with mouse α-HA IgG (1:1,000, 1 hr at RT), cells were washed three times with PBS and incubation with goat α-mouse IgG-alexa-568 (1:500). Cells were washed again, mounted and observed by Leica confocal scanning microscopy (Leica, 100 X oil objective). Plasma membrane was visualized with Image-iT™ LIVE Plasma Membrane and Nuclear Labeling Kit (Molecular Probes) according to the manufacturer, using Alexa594 WGA.
Image quantification
Fluorescent signal was analyzed by “line scan” in MetaMorph software (Molecular Devices). Correlation efficiency (Intensity Correlation quotient, ICQ) of fluorescence was determined using ImageJ Plugin intensity correlation analysis as described in(47, 48) and are the average of scores from > 20 cells. Scale of ICQ is: −0.5~0.5, positive values indicate colocalization; 0 value indicate independent interactions; and negative values indicate segmentation of events.
Supplementary Material
Steady state level of EGF-activated phospho-EGFR remains elevated in HeLa cells intoxicated with ExoS A+. A) HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization or treated with the lysosomotropic drug chloroquine at 0.1mM. One hr pi, cells were stimulated with 100 ng/ml of EGF and then collected at the indicated time points (min, m). Cells were lysed in SDS-PAGE sample buffer, subjected to 8% SDS-PAGE, and immunoblotted for pY1068 specific EGFR (pY1068) antibody, using Super Signal. The blot was stripped and probed for total EGFR as described in Figure 3. Actin was also probed as a loading control. B) Phospho-EGFR (pY1068) was quantified by densitometry of the immunoblot. Results were normalized with EGFR with the values of phosphorylation normalized to the observed amount of phosphorylation at 10 min post EGF-activation as 1. C) EGFR was quantified by densitometry of the immunoblot. Results were normalized with total cell protein by actin and value at 0 time point was normalized to 1. Constructs used: closed circle, catalytic null pUCP-ExoS(R146K, E381/379D)-HA (G−A−), open circle, GAP only pUCP-ExoS-E381D-HA (G+), and closed triangle, ADPr only pUCP-ExoS-R146K-HA (A+), open triangle, chloroquine treated.
P. aeruginosa ExoS ADPr traps EGF-activated EGFR trafficking to the clathrin coated vesicles. A) HeLa cells were intoxicated with P. aeruginosa PA103, ΔexoU, exoT::Tc carrying ExoS derived constructs with synchronization. One and ½ hr pi, cells were stimulated with 100 ng/ml EGF for 30 min. Cells were washed, fixed and endogenous EGF receptor (EGFR) and clathrin heavy chain (CHC) were visualized as described in Figure 6. Colocalization of EGFR and CHC is quantified as ICQ as described in materials and methods.
Type III delivered P. aeruginosa ExoS ADP-ribosylates Rab GTPases. HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs as in Figure 3. Two hr pi, cells were collected, lysed in SDS-PAGE sample buffer, and subjected to SDS-PAGE and immunoblotted for type III delivered ExoS with HA epitope, or endogenous Rab 5 or Rab 4 using Super Signal. Rab 5 blot was striped and probed with Rab 9. (*residual Rab5 signal due to incomplete stripping.) GAPDH was also probed as a loading control.
Acknowledgments
The authors thank the members of the Barbieri laboratory for helpful discussion. This study was supported by a grant from the NIH-NIAID-AI-03162 to JTB.
Abbreviations and nomenclature
- P. aeruginosa
Pseudomonas aeruginosa
- G
RhoGAP, Rho GTPase Activating Protein
- A
ADPr, ADP-ribosyltransferase
- HRP
Horseradish peroxidase
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- CCV
clathrin coated vesicles
- EE
early endosomes
- TfnR
transferrin receptor
- EEA1
early endosome antigen 1
References
- 1.Mendelson MH, Gurtman A, Szabo S, Neibart E, Meyers BR, Policar M, Cheung TW, Lillienfeld D, Hammer G, Reddy S, et al. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin Infect Dis. 1994;18(6):886–895. doi: 10.1093/clinids/18.6.886. [DOI] [PubMed] [Google Scholar]
- 2.Yahr TL, Mende-Mueller LM, Friese MB, Frank DW. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J Bacteriol. 1997;179(22):7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. Embo J. 2003;22(12):2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95(23):13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbieri JT. Pseudomonas aeruginosa exoenzyme S, a bifunctional type-III secreted cytotoxin. Int J Med Microbiol. 2000;290(4–5):381–387. doi: 10.1016/S1438-4221(00)80047-8. [DOI] [PubMed] [Google Scholar]
- 6.Coburn J, Dillon ST, Iglewski BH, Gill DM. Exoenzyme S of Pseudomonas aeruginosa ADP-ribosylates the intermediate filament protein vimentin. Infect Immun. 1989;57(3):996–998. doi: 10.1128/iai.57.3.996-998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coburn J, Gill DM. ADP-ribosylation of p21ras and related proteins by Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1991;59(11):4259–4262. doi: 10.1128/iai.59.11.4259-4262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maresso AW, Baldwin MR, Barbieri JT. Ezrin/radixin/moesin proteins are high affinity targets for ADP-ribosylation by Pseudomonas aeruginosa ExoS. J Biol Chem. 2004;279(37):38402–38408. doi: 10.1074/jbc.M405707200. [DOI] [PubMed] [Google Scholar]
- 9.DiNovo AA, Schey KL, Vachon WS, McGuffie EM, Olson JC, Vincent TS. ADP-ribosylation of cyclophilin A by Pseudomonas aeruginosa exoenzyme S. Biochemistry. 2006;45(14):4664–4673. doi: 10.1021/bi0513554. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Barbieri JT. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J Biol Chem. 2003;278(35):32794–32800. doi: 10.1074/jbc.M304290200. [DOI] [PubMed] [Google Scholar]
- 11.Deng Q, Sun J, Barbieri JT. Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. J Biol Chem. 2005;280(43):35953–35960. doi: 10.1074/jbc.M504901200. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J Biol Chem. 1993;268(32):24475–24480. [PubMed] [Google Scholar]
- 13.van der Bliek AM. A sixth sense for Rab5. Nat Cell Biol. 2005;7(6):548–550. doi: 10.1038/ncb0605-548. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri AM, Sha Q, Bette-Bobillo P, Stahl PD, Vidal M. ADP-ribosylation of Rab5 by ExoS of Pseudomonas aeruginosa affects endocytosis. Infect Immun. 2001;69(9):5329–5334. doi: 10.1128/IAI.69.9.5329-5334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraylick JE, Rucks EA, Greene DM, Vincent TS, Olson JC. Eukaryotic cell determination of ExoS ADP-ribosyltransferase substrate specificity. Biochem Biophys Res Commun. 2002;291(1):91–100. doi: 10.1006/bbrc.2002.6402. [DOI] [PubMed] [Google Scholar]
- 16.Reaves BJ, Banting G, Luzio JP. Lumenal and transmembrane domains play a role in sorting type I membrane proteins on endocytic pathways. Mol Biol Cell. 1998;9(5):1107–1122. doi: 10.1091/mbc.9.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 18.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4(9):691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 19.Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15(6):375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 20.Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127(4):915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151(3):539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Wang Z. Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2001;2(1):68–74. doi: 10.1093/embo-reports/kve005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceresa BP, Bahr SJ. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem. 2006;281(2):1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 24.Cullis DN, Philip B, Baleja JD, Feig LA. Rab11-FIP2, an adaptor protein connecting cellular components involved in internalization and recycling of epidermal growth factor receptors. J Biol Chem. 2002;277(51):49158–49166. doi: 10.1074/jbc.M206316200. [DOI] [PubMed] [Google Scholar]
- 25.Kornilova E, Sorkina T, Beguinot L, Sorkin A. Lysosomal targeting of epidermal growth factor receptors via a kinase-dependent pathway is mediated by the receptor carboxyl-terminal residues 1022–1123. J Biol Chem. 1996;271(48):30340–30346. doi: 10.1074/jbc.271.48.30340. [DOI] [PubMed] [Google Scholar]
- 26.Pederson KJ, Vallis AJ, Aktories K, Frank DW, Barbieri JT. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol Microbiol. 1999;32(2):393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 27.Pederson KJ, Barbieri JT. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas exoenzyme S is cytotoxic to eukaryotic cells. Mol Microbiol. 1998;30(4):751–759. doi: 10.1046/j.1365-2958.1998.01106.x. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233(4768):1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 29.Raiborg C, Wesche J, Malerod L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci. 2006;119(Pt 12):2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- 30.Roberts RL, Barbieri MA, Ullrich J, Stahl PD. Dynamics of rab5 activation in endocytosis and phagocytosis. J Leukoc Biol. 2000;68(5):627–632. [PubMed] [Google Scholar]
- 31.Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel JN. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68(12):7100–7113. doi: 10.1128/iai.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black DS, Bliska JB. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol. 2000;37(3):515–527. doi: 10.1046/j.1365-2958.2000.02021.x. [DOI] [PubMed] [Google Scholar]
- 33.Fu Y, Galan JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401(6750):293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 34.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25(6):1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 35.Rocha CL, Coburn J, Rucks EA, Olson JC. Characterization of Pseudomonas aeruginosa exoenzyme S as a bifunctional enzyme in J774A.1 macrophages. Infect Immun. 2003;71(9):5296–5305. doi: 10.1128/IAI.71.9.5296-5305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147(Pt 10):2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 37.Galperin E, Sorkin A. Visualization of Rab5 activity in living cells by FRET microscopy and influence of plasma-membrane-targeted Rab5 on clathrin-dependent endocytosis. J Cell Sci. 2003;116(Pt 23):4799–4810. doi: 10.1242/jcs.00801. [DOI] [PubMed] [Google Scholar]
- 38.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8(1):34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 39.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408(6810):374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 40.Dinneen JL, Ceresa BP. Expression of dominant negative rab5 in HeLa cells regulates endocytic trafficking distal from the plasma membrane. Exp Cell Res. 2004;294(2):509–522. doi: 10.1016/j.yexcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Rubino M, Miaczynska M, Lippe R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem. 2000;275(6):3745–3748. doi: 10.1074/jbc.275.6.3745. [DOI] [PubMed] [Google Scholar]
- 42.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70(5):715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Sato K, Fonarev P, Huang CJ, Liou W, Grant BD. Caenorhabditis elegans RME-6 is a novel regulator of RAB-5 at the clathrin-coated pit. Nat Cell Biol. 2005;7(6):559–569. doi: 10.1038/ncb1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiley HS, Cunningham DD. Epidermal growth factor stimulates fluid phase endocytosis in human fibroblasts through a signal generated at the cell surface. J Cell Biochem. 1982;19(4):383–394. doi: 10.1002/jcb.240190407. [DOI] [PubMed] [Google Scholar]
- 45.Leiva N, Pavarotti M, Colombo MI, Damiani MT. Reconstitution of recycling from the phagosomal compartment in streptolysin O-permeabilized macrophages: role of Rab11. Exp Cell Res. 2006;312(10):1843–1855. doi: 10.1016/j.yexcr.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Dominguez C, Madrazo-Toca F, Fernandez-Prieto L, Vandekerckhove J, Pareja E, Tobes R, Gomez-Lopez MT, Del Cerro-Vadillo E, Fresno M, Leyva-Cobian F, Carrasco-Marin E. Characterization of a Listeria monocytogenes protein interfering with Rab5a. Traffic. 2008;9(3):325–337. doi: 10.1111/j.1600-0854.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Deng Q, Barbieri JT. Intracellular localization of type III-delivered Pseudomonas ExoS with endosome vesicles. J Biol Chem. 2007;282(17):13022–13032. doi: 10.1074/jbc.M606305200. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci. 2004;24(16):4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng Q, Zhang Y, Barbieri JT. Intracellular trafficking of Pseudomonas ExoS, a type III cytotoxin. Traffic. 2007;8(10):1331–1345. doi: 10.1111/j.1600-0854.2007.00626.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Steady state level of EGF-activated phospho-EGFR remains elevated in HeLa cells intoxicated with ExoS A+. A) HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs with synchronization or treated with the lysosomotropic drug chloroquine at 0.1mM. One hr pi, cells were stimulated with 100 ng/ml of EGF and then collected at the indicated time points (min, m). Cells were lysed in SDS-PAGE sample buffer, subjected to 8% SDS-PAGE, and immunoblotted for pY1068 specific EGFR (pY1068) antibody, using Super Signal. The blot was stripped and probed for total EGFR as described in Figure 3. Actin was also probed as a loading control. B) Phospho-EGFR (pY1068) was quantified by densitometry of the immunoblot. Results were normalized with EGFR with the values of phosphorylation normalized to the observed amount of phosphorylation at 10 min post EGF-activation as 1. C) EGFR was quantified by densitometry of the immunoblot. Results were normalized with total cell protein by actin and value at 0 time point was normalized to 1. Constructs used: closed circle, catalytic null pUCP-ExoS(R146K, E381/379D)-HA (G−A−), open circle, GAP only pUCP-ExoS-E381D-HA (G+), and closed triangle, ADPr only pUCP-ExoS-R146K-HA (A+), open triangle, chloroquine treated.
P. aeruginosa ExoS ADPr traps EGF-activated EGFR trafficking to the clathrin coated vesicles. A) HeLa cells were intoxicated with P. aeruginosa PA103, ΔexoU, exoT::Tc carrying ExoS derived constructs with synchronization. One and ½ hr pi, cells were stimulated with 100 ng/ml EGF for 30 min. Cells were washed, fixed and endogenous EGF receptor (EGFR) and clathrin heavy chain (CHC) were visualized as described in Figure 6. Colocalization of EGFR and CHC is quantified as ICQ as described in materials and methods.
Type III delivered P. aeruginosa ExoS ADP-ribosylates Rab GTPases. HeLa cells were intoxicated with P. aeruginosa expressing ExoS derived constructs as in Figure 3. Two hr pi, cells were collected, lysed in SDS-PAGE sample buffer, and subjected to SDS-PAGE and immunoblotted for type III delivered ExoS with HA epitope, or endogenous Rab 5 or Rab 4 using Super Signal. Rab 5 blot was striped and probed with Rab 9. (*residual Rab5 signal due to incomplete stripping.) GAPDH was also probed as a loading control.