Abstract
Abnormal amplification of centrosomes, which occurs frequently in cancers, leads to high frequencies of mitotic defect and chromosome segregation error, profoundly affecting the rate of tumor progression. Centrosome amplification results primarily from overduplication of centrosomes, and p53 is involved in the regulation of centrosome duplication partly through controlling the activity of cyclin-dependent kinase (CDK) 2-cyclin E, a kinase complex critical for the initiation of centrosome duplication. Thus, loss or mutational inactivation of p53 leads to an increased frequency of centrosome amplification. Moreover, the status of cyclin E greatly influences the frequency of centrosome amplification in cells lacking functional p53. Here, we dissected the roles of CDK2-associating cyclins, namely cyclins E and A, in centrosome amplification in the p53-negative cells. We found that loss of cyclin E was readily compensated by cyclin A for triggering the initiation of centrosome duplication, and thus the centrosome duplication kinetics was not significantly altered in cyclin E-deficient cells. It has been shown that cells lacking functional p53, when arrested in either early S-phase or late G2 phase, continue to reduplicate centrosomes, resulting in centrosome amplification. In cells arrested in early S phase, cyclin E, but not cyclin A, is important in centrosome amplification, whereas in the absence of cyclin E, cyclin A is important for centrosome amplification. In late G2-arrested cells, cyclin A is important in centrosome amplification irrespective of the cyclin E status. These findings advance our understandings of the mechanisms underlying the numeral abnormality of centrosomes and consequential genomic instability associated with loss of p53 function and aberrant expression of cyclins E and A in cancer cells.
Keywords: centrosome, cyclin A, cyclin E, CDK2, p53, centrosome amplification
Introduction
The centrosome, a major microtubule-organizing center of an animal cell, directs the bipolar mitotic spindle formation during mitosis, which is essential for accurate chromosome transmission into daughter cells (for reviews, see Fukasawa, 2005, 2007). Centrosomes duplicate once per cell cycle in coordination with the DNA duplication cycle: centrosomes initiate duplication at the time of S-phase entry by physical separation of the paired centrioles, the duplication units of the centrosome, and duplication is completed in late G2. Coupling of the centrosome and DNA duplication cycles is mediated in part by cyclin-dependent kinase (CDK) 2-cyclin E (Hinchcliffe et al., 1999; Lacey et al., 1999). CDK2-cyclin E, which is activated in late G1 phase primarily by the temporal increase of cyclin E expression, triggers initiation of both DNA synthesis and centrosome duplication. Uncoupling of the centrosome and DNA duplication cycles by genetic mutations or adverse growth conditions lays a favorable ground for centrosomes to duplicate more than once, resulting in the presence of more than two centrosomes (centrosome amplification). Centrosome amplification leads to increased frequencies of defective mitoses, resulting in chromosome loss and/or gain, which promote tumor progression by simultaneously introducing multiple genetic alterations responsible for acquisition of the malignant phenotypes. Indeed, centrosome amplification is commonly found in various types of cancers, and is believed to contribute to chromosome instability in cancer cells (Pihan et al., 1998; Carroll et al., 1999).
When cell cycling is halted, centrosome duplication is also blocked in part by upregulation of the p53 tumor suppressor protein, hence the numeral integrity of centrosomes is maintained (Tarapore et al., 2001). p53 is stabilized and functionally upregulated in response to various physiological stresses, including deoxyribonucleotide triphosphate (dNTP) deprivation, inhibition of DNA polymerases and DNA damage. p53 then transactivates many proteins, including p21Waf1/Cip1 CDK inhibitor, which efficiently inhibits the activity of CDK2 (for a review, see Horn and Vousden, 2007), and consequently centrosome duplication (Tarapore et al., 2001). Thus, when p53 is lost or inactivated in the cell-cycle-arrested cells, centrosomes continue to reduplicate, resulting in centrosome amplification. It has further been shown that the cyclin E status (that is, expression level of cyclin E) and consequential changes in the CDK2-cyclin E activity affect the frequency of centrosome amplification associated with loss or inactivation of p53 (Mussman et al., 2000; Kawamura et al., 2004).
In this study, we dissected the roles of cyclins A and E, two major cyclins that associate with CDK2, for centrosome duplication during the cell cycle and centrosome reduplication/centrosome amplification in the cell-cycle-arrested cells using mouse embryonic fibroblasts (MEFs), lacking both E-type cyclins (E1−/−E2−/−) and expressing the dominant-negative (DN) p53 as an experimental tool. We found that cyclin A is not required for initiation of centrosome duplication during the normal cell cycle as long as cyclin E is present. However, in the absence of cyclin E, cyclin A is important in initiating centrosome duplication. In cells arrested in early S phase by exposure to a DNA polymerase inhibitor, cyclin E, but not cyclin A, is important in induction of centrosome amplification. However, in the absence of cyclin E, cyclin A functionally replaces cyclin E to induce centrosome amplification. In contrast, in cells arrested in late G2 by the G2 checkpoint response to DNA damage, cyclin A is important in centrosome amplification irrespective of the cyclin E status.
Results
Analysis of the centrosome duplication kinetics in cyclin E−/− cells lacking functional p53
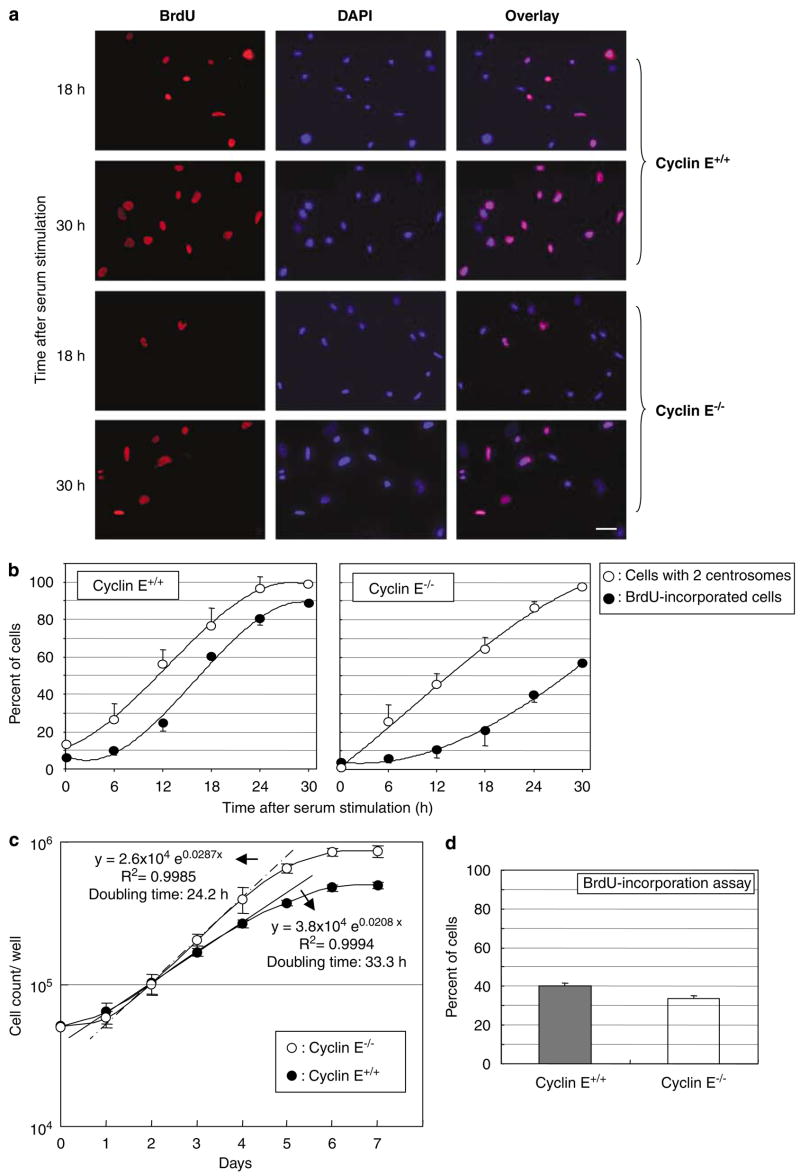
p53 is frequently mutated in cancer cells, and the status of cyclin E is known to influence the generation of amplified centrosomes and consequential chromosome destabilization in cells lacking functional p53 (Mussman et al., 2000; Kawamura et al., 2004). We thus further dissected the roles of CDK2-cyclin E in the initiation of centrosome duplication and coupling of the centrosome and DNA duplication cycles under the p53-negative condition. We used MEFs derived from mice deficient for cyclin E (both cyclins E1 and 2) and wild-type litter mates (Geng et al., 2003), both of which were transfected with DN p53 mutant (DNp53/GSE56). DNp53 encodes the amino acids 275–368 of p53, and suppresses p53 activity presumably by interfering with oligomerization of the protein (Ossovskaya et al., 1996). These cyclins E+/+and E−/− cells were synchronized by serum starvation followed by serum stimulation, and cell-cycle progression and centrosome duplication were determined by 5-bromo-2-deoxyuridine (BrdU) incorporation and immunostaining of γ-tubulin, a major centrosomal component (Joshi, 1994). Unlike primary cyclin E-deficient MEFs, which failed to reenter cell cycle from quiescence (Geng et al., 2003), the cells immortalized by DN p53 reentered the cell cycle, although with a significant delay (Figure 1b, images of the BrdU-incorporation assay are shown in Figure 1a). For instance, at 18 h after serum stimulation, >50% of immortalized, p53-negative cyclin E+/+ cells entered S phase compared with only ~20% of immortalized, p53-negative cyclin E−/− cells. In contrast, initiation of centrosome duplication was only slightly delayed in cyclin E−/− cells compared with cyclin E+/+ cells: at 18 h after serum stimulation, ~80% of cyclin E+/+ cells initiated centrosome duplication compared with ~70% of cyclin E−/− cells. Thus, in the absence of functional p53, the timely initiation of DNA synthesis heavily depends on the presence of cyclin E, whereas that of centrosome duplication is less dependent on cyclin E. The fact that all of the synchronized p53-negative cyclin E−/− cells eventually entered S phase and completed the cell cycle, in contrast to primary MEFs, indicating that inactivation of p53 partially rescues the inability of cyclin E−/− cells to reenter the cell cycle from the G0 state, leading to a delayed cell-cycle reentrance. When unsynchronized, cyclins E+/+ and E−/− cells in the p53- negative background were compared under the condition of continuous growth; the doubling time of cyclin E−/− cells was not longer than that of cyclin E+/+ cells, but interestingly faster (~24.2 h) than that of cyclin E+/+ cells (~33.3 h; Figure 1c). The short-term BrdU-incorporation assay of these cells under a continuous growth showed the noticeably less S-phase population in cyclin E−/− cells than in cyclin E+/+ cells (Figure 1d), suggesting that the faster doubling time of cyclin E−/− cells may be owing to the shortened S phase. Taken together, upon p53 inactivation, the presence of cyclin E is critical for the timely initiation of DNA synthesis specifically for cells that enter the cell cycle from the quiescent state, but not for the continuously cycling cells. In contrast, cyclin E may be dispensable for the timely initiation of centrosome duplication.
Figure 1.
Analysis of the DNA and centrosome duplication kinetics in cyclins E+/+ and E−/− mouse embryonic fibroblasts (MEFs) expressing DNp53. Cyclins E+/+ and E−/− MEFs expressing DNp53 were serum-starved for 48 h, and then serum-stimulated with the medium containing 20% fetal bovine serum (FBS) and bromodeoxyuridine (BrdU). At indicated time points, cells were fixed and immunostained for incorporated BrdU and γ-tubulin. Representative images of immunofluorescence assay for BrdU incorporation are shown in (a). Scale bar: 40 μm. The rates of BrdU incorporation and centrosome duplication in cyclins E+/+ and E−/− cells were plotted in the graph as average±standard error from three independent experiments (b). For the analysis of centrosomes, >200 γ-tubulin immunostained cells were examined for each time point. In this analysis, cells with amplified (⩾3) centrosomes were excluded from the analysis; it is technically difficult to differentiate unduplicated or duplicated centrosomes in the cells with amplified centrosomes. Determining the rate of centrosome duplication by examining only the cells with one or two centrosomes in the synchronized cells is possible, because the newly duplicated centrosomes do not reduplicate until acquiring the duplication competency. The time course period after serum stimulation in this experiment, there is not sufficient time for the duplicated centrosomes to reestablish the duplication competency. The percentages of the number of cells with two centrosomes among the total number of cells with either one or two centrosomes are plotted in the graph (b) as average±standard error from three independent experiments. Similar results were obtained with the procedure to immunostain centrioles (see Materials and methods section) (data not shown). (c) Determination of doubling times of cyclins E+/+ and E−/− MEFs. Cyclins E+/+ and E−/− cells were plated in the cell culture wells, and the number of cells in the well was counted at every 24 h for 7 days, and plotted in the graph as average±standard error from three independent experiments. (d) Cyclins E+/+ and E−/− MEFs under an optimal growth condition were incubated with BrdU for 1 h, and the percent of cells that had incorporated BrdU was determined, and shown in the graph as average±standard error from three experiments.
Role of cyclin A in the initiation of centrosome duplication in the absence of cyclin E
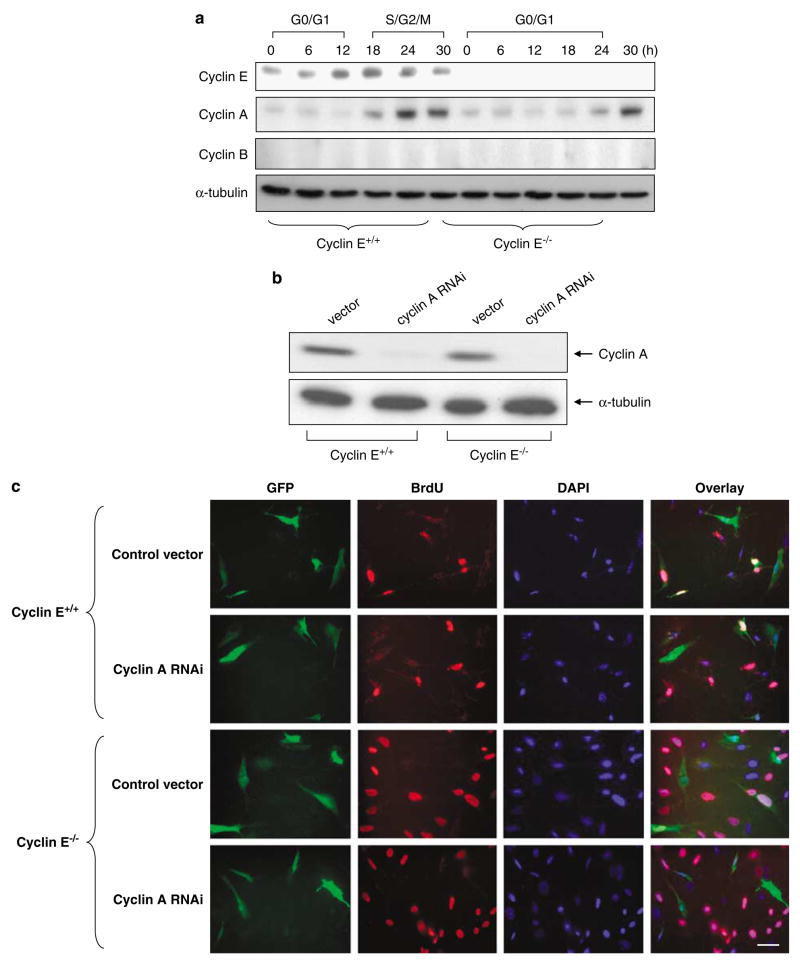
Because the initiation of centrosome duplication was not significantly affected in cyclin E-deficient cells, we hypothesized that loss of cyclin E in respect to centrosome duplication may be functionally compensated by cyclin A, which also associates with CDK2. To test this, we first examined the changes in the cyclin A levels during the cell-cycle progression of synchronized cyclins E+/+ and E−/− MEFs (Figure 2a). There are two A-type cyclins, cyclin A1 that is restricted to germ cells, and cyclin A2 that is expressed in virtually all tissues and the main A-type cyclin present in somatic cells (Sweeney et al., 1996). Thus, we focused on cyclin A2 (referred to as cyclin A hereafter). We found that, in the synchronized cells, there was a slight delay in the increase of cyclin A expression in cyclin E−/− cells compared with cyclin E+/+ cells, which is consistent with the previous finding that cyclin A expression is indirectly and partially controlled by cyclin E (DeGregori et al., 1995).
Figure 2.
Silencing of cyclin A and its effect on centrosome duplication in cyclins E+/+ and E−/− mouse embryonic fibroblasts (MEFs). (a) Cyclin A expression patterns during the cell-cycle progression in cyclins E+/+ and E−/− MEFs. Cyclins E+/+ and E−/− MEFs expressing DNp53 were serum-starved for 48 h, and then serum-stimulated. At indicated time points for a period of 30 h, the lysates were prepared and immunoblotted for cyclins E, A and B and α-tubulin (loading control). Cells were also analysed for cell-cycle phase distributions by flow cytometry, and the respective cell-cycle phases are indicated, in which the majority (>60%) of cells are in the indicated cell-cycle phases (cf. Figure 1b). (b) Cyclins E+/+ and E−/− MEFs were transfected with either a pSUPER plasmid harboring small-interfering RNA (siRNA) sequence specific for cyclin A (pSUPER/cyclin A) or a pSUPER RNA interference (RNAi) vector containing a randomized sequence (pSUPER-vec), along with a puromycin resistance gene as a selection marker. The cells selected in the growth media containing puromycin for 6 days were examined for cyclin A expression by immunoblot analysis. As a loading control, the lysates were probed with anti-α-tubulin antibody. (c–e) Cyclins E+/+ and E−/− MEFs were transfected with either pSUPER/cyclin A or pSUPER-vec harboring the randomized sequences along with a plasmid encoding green fluorescent protein (GFP) as a transfection marker. The successfully transfected cells identified by GFP (CycE+/+/CycA RNAi, CycE−/−/CycA RNAi, CycE+/+/vec and CycE−/−/vec cells) were serum-starved for 48 h, and then serum-stimulated in the media containing bromodeoxyuridine (BrdU). At indicated time points, cells were fixed and immunostained with either anti-BrdU or anti-γ-tubulin antibody. Representative images of the BrdU-incorporation assay are shown in (c). Scale bar: 40 μm. Representative images of the γ-tubulin immunostaining are shown in (d). Scale bar: 20 μm. In the magnified images, each centrosome is pointed by an arrow. The rates of BrdU incorporation and centrosome duplication in GFP-positive CycE+/+/CycA RNAi, CycE−/−/CycA RNAi, CycE+/+/vec and CycE−/−/vec cells were plotted in the graph (e). For the analysis of the γ-tubulin immunostained cells,>200 cells for each time point were examined. The rate of centrosome duplication was determined as described in the legend to Figure 1b. The results are shown as the average±standard error from three experiments.
We next silenced the cyclin A expression in cyclins E+/+ and E−/− MEFs by transfecting the small-interfering RNA (siRNA) sequence specific for cyclin A using a pSUPER siRNA vector (pSUPER/cyclin A). In both cell types, cyclin A was successfully silenced (>95%; Figure 2b). These cells are denoted as CycE+/+/CycARNAi and CycE−/−/CycARNAi, respectively. CycE+/+/CycARNAi and CycE−/−/CycARNAi were synchronized by serum starvation, followed by serum stimulation, and cell-cycle progression and centrosome duplication kinetics were determined by BrdU incorporation and γ-tubulin immunostaining, respectively (Figures 2c–e). There was a noticeable delay in S-phase entry in both CycE+/+/CycARNAi and CycE−/−/CycARNAi cells compared with the control cells (CycE+/+/vec and CycE−/−/vec cells), suggesting that cyclin A may be important in the S-phase entry. Between CycE+/+/CycARNAi and CycE+/+/vec control cells, there was no significant difference in the centrosome duplication kinetics, indicating that cyclin A is not important in the initiation of centrosome duplication when cyclin E is present. In contrast, there was a substantial delay in the initiation of centrosome duplication in CycE−/−/CycARNAi cells compared with CycE−/−/vec control cells, indicating that cyclin A is important in the initiation of centrosome duplication when cyclin E is absent, likely through functionally replacing cyclin E.
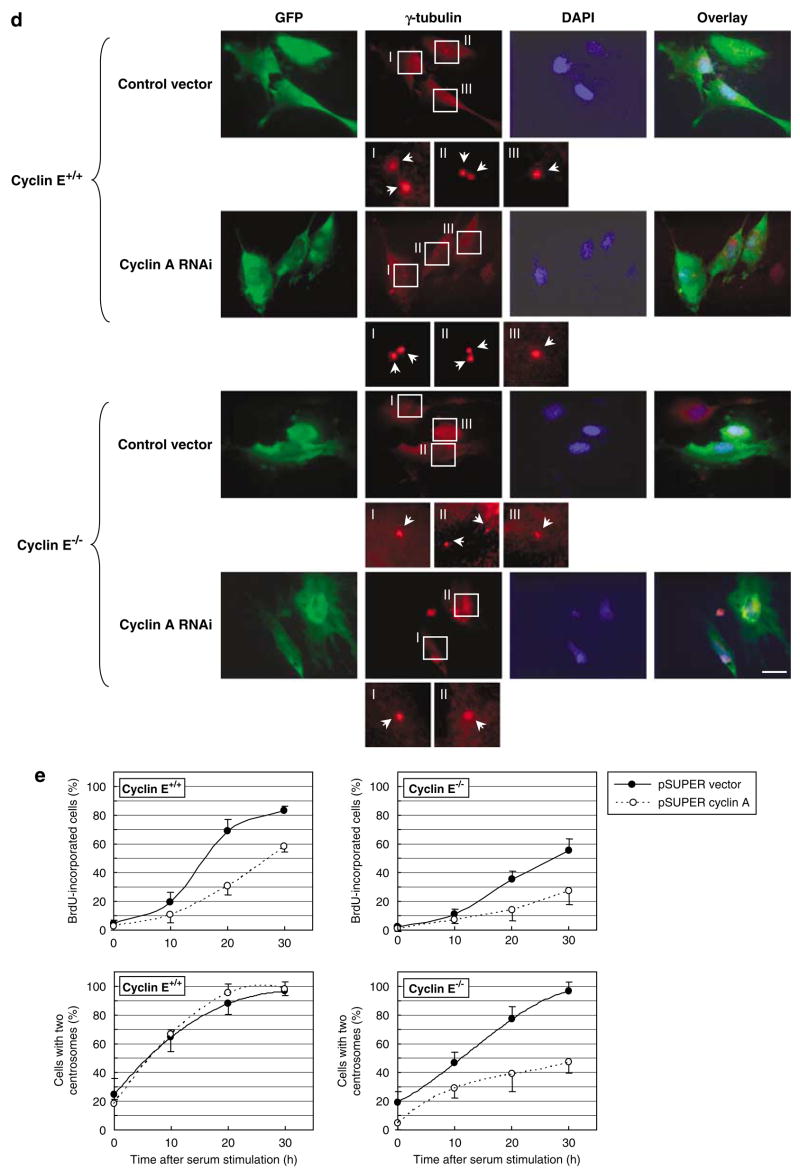
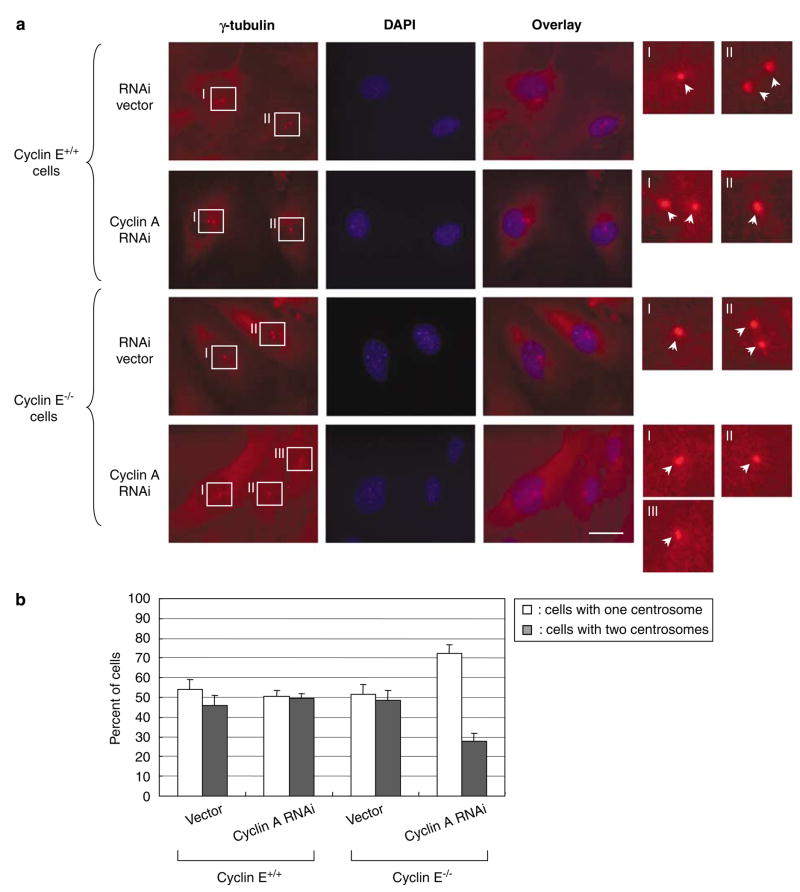
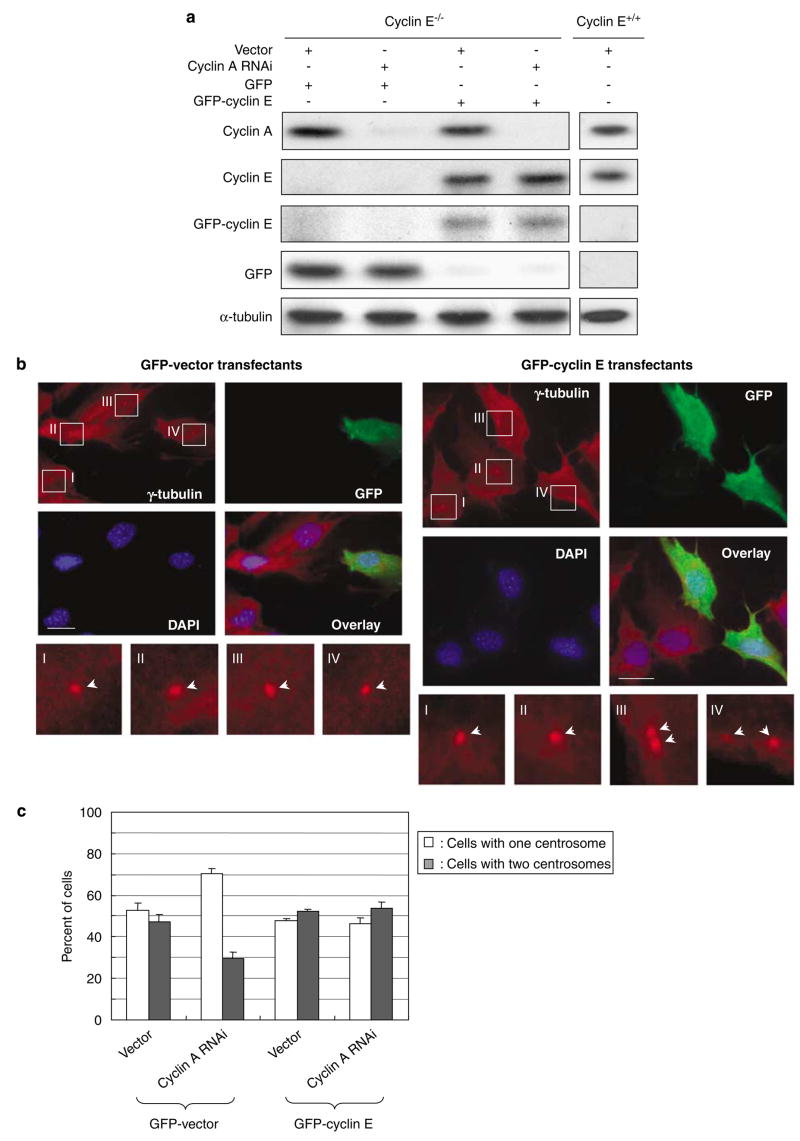
We also examined the centrosome numbers in CycE+/+/CycARNAi and CycE−/−/CycARNAi cells under a continuous growth (Figure 3b, representative images are shown in Figure 3a). There was no significant difference in the ratio of the number of cells with one and two centrosomes between CycE+/+/CycARNAi and CycE+/+/vec cells, further indicating that cyclin A is not critically involved in the initiation of centrosome duplication in the presence of cyclin E. In contrast, the number of cells with two centrosomes was significantly reduced in CycE−/−/CycARNAi cells (~30%) compared with the control CycE−/−/vec cells (~50%). To exclude the possibility that the silencing of cyclin A suppresses centrosome duplication in cyclin E−/− cells in the manner different from its activity to compensate for cyclin E loss, we cotransfected pSUPER/cyclin A and a plasmid encoding green fluorescent protein (GFP)-tagged cyclin E into cyclin E−/− cells. The expression levels of cyclins E and A in those transfectants are shown in Figure 4a. The cells cotransfected with GFP-cyclin E expressed cyclin E at a comparable level with the cyclin E+/+ cells, and the cells cotransfected with pSUPER/cyclin A were successfully silenced for cyclin A expression. These transfectants were examined for the centrosome numbers under a continuous growth (Figure 4c, γ-tubulin immunostaining images of cyclin E−/− cells are shown in Figure 4b). Cotransfection of GFP-cyclin E almost completely reverted the suppressive effect of cyclin A depletion on centrosome duplication, indicating that cyclin A functionally replaces cyclin E in cyclin E−/− cells for the initiation of centrosome duplication, and thus is important in the initiation of centrosome duplication when cyclin E is absent.
Figure 3.
Analysis of centrosomes in cyclins E+/+ and E−/− mouse embryonic fibroblasts (MEFs) silenced for cyclin A expression under a continual growth condition. CycE+/+/CycA RNAi and CycE−/−/CycA RNAi cells as well as the control CycE+/+/vec and CycE−/−/vec cells were cultured in the complete media, and immunostained with anti-γ-tubulin antibody. The representative immunostaining images are shown in (a). In the magnified images, each centrosome is indicated by an arrow. Scale bar: 20 μm. The ratio of the number of cells with one and two centrosomes in each cell line was determined, and plotted in the graph (b). For each cell line,>200 cells were examined. In this analysis, cells with ⩾3 centrosomes were excluded. The results are shown as the average±standard error from three experiments.
Figure 4.
Reintroduction of cyclin E1 abrogates the effect of cyclin A silencing on centrosome duplication. (a) Cyclin E−/− cells were cotransfected with pSUPER/cyclin A and either a pEGFP-C1 vector or a plasmid encoding green fluorescent protein (GFP)-tagged cyclin E1 along with a plasmid encoding a puromycin resistant gene as a rapid selection marker. The lysates prepared from the transfectants were immunoblotted with anti-cyclins A and E1, anti-GFP and anti-α-tubulin antibodies. In parallel, the lysates prepared from cyclin E+/+ mouse embryonic fibroblasts (MEFs) were immunoblotted as a reference for the endogenous level of cyclin E1. (b, c) The transfectants described above were subjected to coimmunostaining with anti-γ-tubulin and anti-GFP antibodies. The representative images of GFP-vector- and GFP-cyclin E1-transfected cells are shown in (b). In the magnified images, each centrosome is indicated by an arrow. Scale bar: 20 μm. The centrosome profiles of the GFP-positive cells were determined, and shown in the graph as the average±standard error from three experiments (c). In this analysis, cells with ⩾3 centrosomes were excluded. For each experiment,>200 cells were examined.
Role of cyclin A in centrosome amplification in cells arrested by aphidicolin in the absence of cyclin E
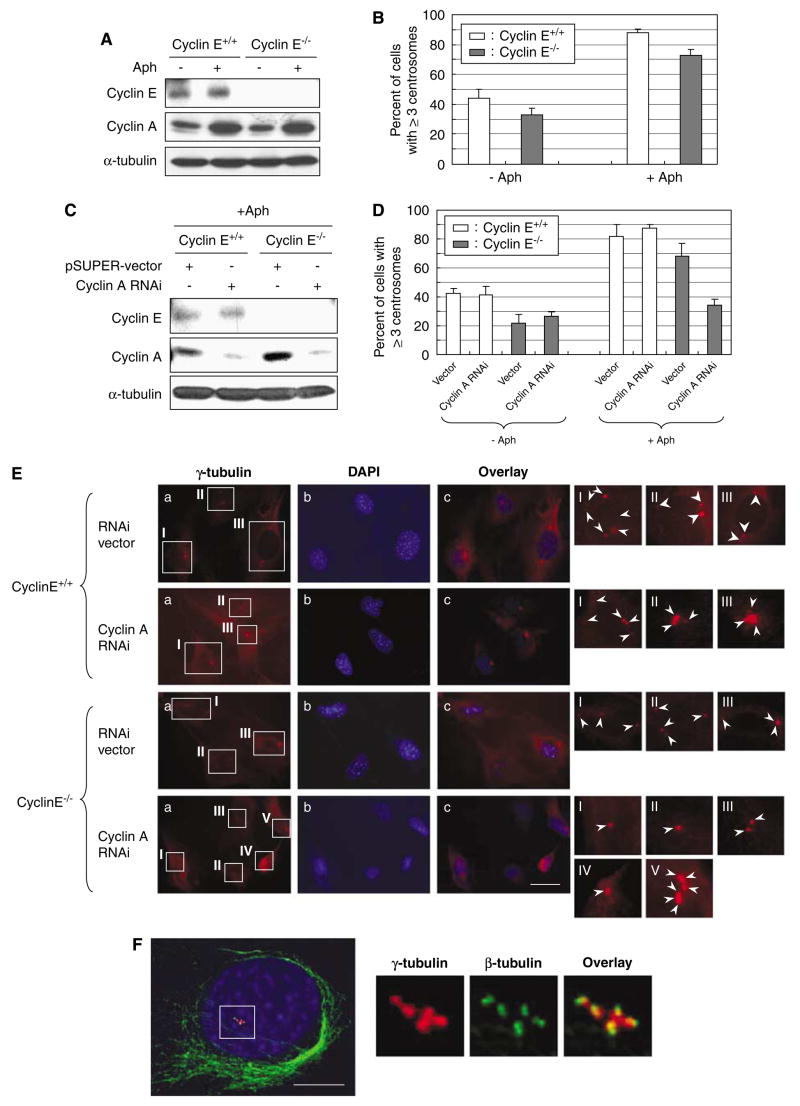
It has been shown that when cells are arrested at early S phase by exposure to aphidicolin (Aph), an inhibitor of DNA replication, centrosomes continue to reduplicate without DNA synthesis, resulting in centrosome amplification (Balczon et al., 1995; Tarapore et al., 2001). The cell-cycle arrest induced by Aph is believed to allow newly duplicated centrosomes to regain the duplication competency and to reduplicate depending on the availability of active CDK2 (Fukasawa, 2007). However, centrosome reduplication during the cell-cycle arrest occurs preferentially in cells compromised for p53 function. In normal cells, p53 is upregulated in response to the physiological stress associated with the prolonged Aph exposure, leading to an increased level of p21, which in turn efficiently inhibits CDK2, and thus blocking reduplication of centrosomes. In contrast, functional loss of p53 allows activation of CDK2, resulting in centrosome reduplication (Tarapore et al., 2001). We thus examined whether centrosome would reduplicate in the p53-inactivated cyclin E−/− MEFs upon exposure to Aph. Cyclins E+/+ and E−/− MEFs expressing DNp53 were treated with Aph for 96 h. As expected (Hoffman et al., 1991), the majority of cells exposed to Aph were arrested at the G1/S boundary and S phase. We also found that endogenous cyclin A was upregulated in both cyclins E+/+ and E−/− upon Aph treatment (Figure 5A). Similarly, endogenous cyclin E was upregulated in the Aph-treated cyclin E+/+ cells (Figure 5A). The cells were also immunostained for γ-tubulin, and the frequencies of centrosome amplification (⩾3 centrosomes) were determined (Figure 5B). Because functional loss of p53 alone is known to induce centrosome amplification (Fukasawa et al., 1996; Wang et al., 1998), ~40% of cyclin E+/+ cells have already contained amplified centrosomes without Aph treatment. In contrast, we detected a noticeable decrease in the frequency of centrosome amplification in cyclin E−/− cells (~30%) without Aph treatment, although the difference is within the standard error. This observation is, however, in agreement with the previous reports (Mussman et al., 2000; Kawamura et al., 2004), in which the presence of cyclin E favors centrosome amplification associated with p53 inactivation. It also suggests that cyclin E is not required for the occurrence of centrosome amplification per se. After the Aph treatment, cyclin E+/+ cells underwent efficient centrosome reduplication, resulting in an increased frequency of centrosome amplification (>80%). Similarly, cyclin E−/− cells underwent centrosome amplification (>70%), suggesting that centrosome reduplication in the Aph-arrested cells does not require cyclin E.
Figure 5.
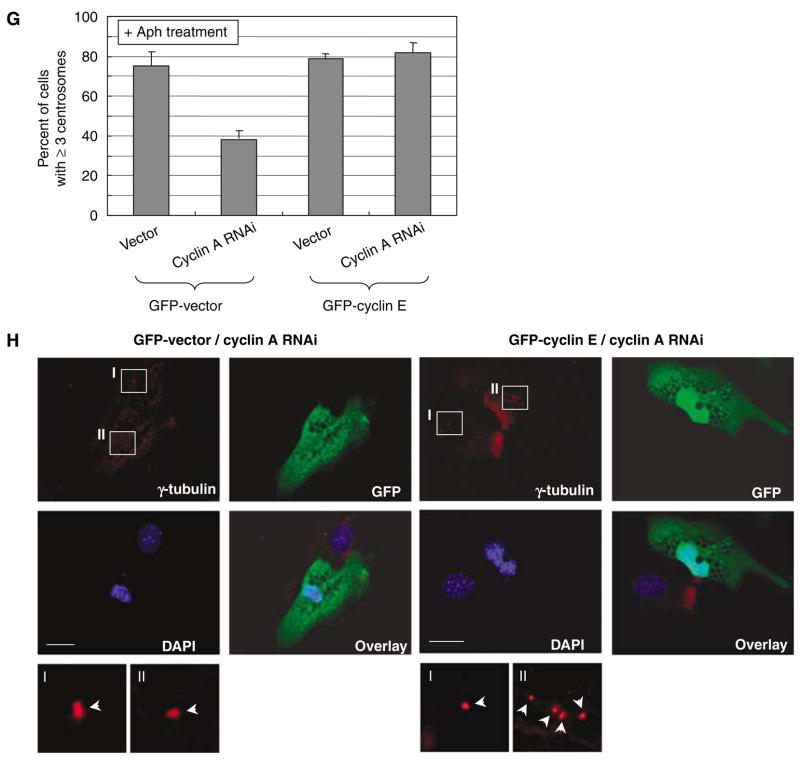
Roles of cyclins A and E in centrosome reduplication in cells arrested by exposure to aphidicolin. (A) Cyclins E+/+ and E−/− mouse embryonic fibroblasts (MEFs) were exposed to Aph (1 μg/ml) for 72 h. The lysates prepared from Aph-treated cells and untreated control cells were immunoblotted with anti-cyclins E and A, and anti-α-tubulin (loading control) antibodies. (B) The Aphtreated and untreated control cells were immunostained for γ-tubulin, and the number of cells with amplified centrosomes were scored. The results are shown in the graph as the average±standard error from three experiments. For each experiment,>200 cells were examined. (C) CycE+/+/CycA RNAi and CycE−/−/CycA RNAi cells as well as the control CycE+/+/vec and CycE−/−/vec cells were treated with Aph for 72 h, and the lysates prepared from Aph-treated cells and untreated control cells were immunoblotted with anticyclins E and A and anti-α-tubulin (loading control) antibodies. (D) The Aph-treated and untreated CycE+/+/CycA RNAi, CycE−/−/CycA RNAi, CycE+/+/vec and CycE−/−/vec cells were immunostained for γ-tubulin, and the number of cells with amplified centrosomes were determined. For each experiment,>200 cells were examined. The results are shown as the average±standard error from three experiments. Representative γ-tubulin immunostaining images are show in (E). In the magnified images, each centrosome is indicated by an arrow. Scale bar: 20 μm. (F)We also examined the centrosomes in the Aph-treated cells by coimmunostaining of γ- and β-tubulins after cold-treatment prior to fixation (see Materials and methods section). By this procedure, centrioles within the centrosome can be visualized. The representative immunostaining images of CycE+/+/CycA RNAi cells after Aph treatment are shown. Scale bar: 5 μm. As described previously, centrosome amplification occurring in cells arrested by Aph is mostly due to centrosome reduplication, and not due to centrosome fragmentation (Tarapore et al., 2001). In addition, by this method, the results for the frequency of centrosome amplification were similar to those by γ-tubulin immunostaining. (G, H) CycE−/−/CycA RNAi and CycE−/−/vec cells were transfected with either a pEGFP-C1 vector or a plasmid encoding green fluorescent protein (GFP)-tagged cyclin E1 (see Figure 4a). The transfectants were then exposed to Aph for 72 h. The Aph-treated and untreated control cells were immunostained for γ-tubulin, and the number of cells with amplified centrosomes were scored. The results are shown in the graph as the average±standard error from three experiments (G). For each experiment, >200 cells were examined. The representative immunostaining images are shown in (H). Scale bar: 20 μm.
To test whether cyclin A compensates for cyclin E loss to induce centrosome reduplication in the Aph-arrested cyclin E−/− cells, CycE+/+/CycARNAi and CycE−/−/CycARNAi cells (also control CycE+/+/vec and CycE−/−/vec cells) were exposed to Aph for 96 h. We first confirmed that the exposure to Aph for 96 h had no effect on the efficacy of the siRNA-mediated silencing of cyclin A (Figure 5C). The cells after Aph treatment were examined for the frequencies of centrosome amplification (Figure 5D, representative images are shown in Figure 5E). The CycE+/+/CycARNAi cells underwent centrosome amplification efficiently at a level similar to CycE+/+/vec cells, indicating that cyclin A is not required for centrosome amplification during the Aphinduced arrest as long as cyclin E is present, which is consistent with the previous studies showing that CDK2-cyclin E is important in centrosome reduplication in the Aph-arrested cells (Tarapore et al., 2001). In contrast, centrosome amplification was severely blocked in CycE−/−/CycARNAi cells compared with CycE−/−/vec cells, indicating the critical involvement of cyclin A in centrosome reduplication during Aph-induced arrest when cyclin E is absent. We also examined the integrity of amplified centrosomes in the Aph-treated cells by coimmunostaining of γ- and β-tubulins after cold treatment, which allows visualization of individual centrioles within the centrosome (Figure 5F). As previously shown (Tarapore et al., 2001), centrosome amplification that occurs in Aph-arrested cells results mostly from ‘true’ duplication rather than structural fragmentation of centrosomes; the majority of γ-tubulin-positive spots colocalized to the paired dots of centriole staining (for representative images, see Figure 5F). The frequencies of centrosome amplification determined by coimmunostaining of γ- and β-tubulins were similar to those determined by γ-tubulin immunostaining (data not shown).
We also tested whether cyclin A functionally replaced cyclin E in centrosome amplification in the Aph-arrested cells by reintroducing cyclin E in CycE−/−/CycARNAi cells. GFP-vector and -cyclin E were transfected into CycE−/−/vec and CycE−/−/CycARNAi cells, and the transfectants were subjected to the Aph exposure, and the frequencies of centrosome amplification were determined (Figure 5G, representative images are shown in Figure 5H). Reintroduction of cyclin E almost completely restored the frequency of centrosome amplification in CycE−/−/CycARNAi cells, demonstrating that cyclin A compensates for the loss of cyclin E in cyclin E−/− cells for centrosome reduplication during the Aph-induced arrest. Taken together, these observations indicate that cyclin A functionally replaces cyclin E for initiation of centrosome reduplication in the Apharrested cyclin E−/− cells.
Role of cyclin A in centrosome amplification in cells arrested by doxorubicin in the absence of cyclin E
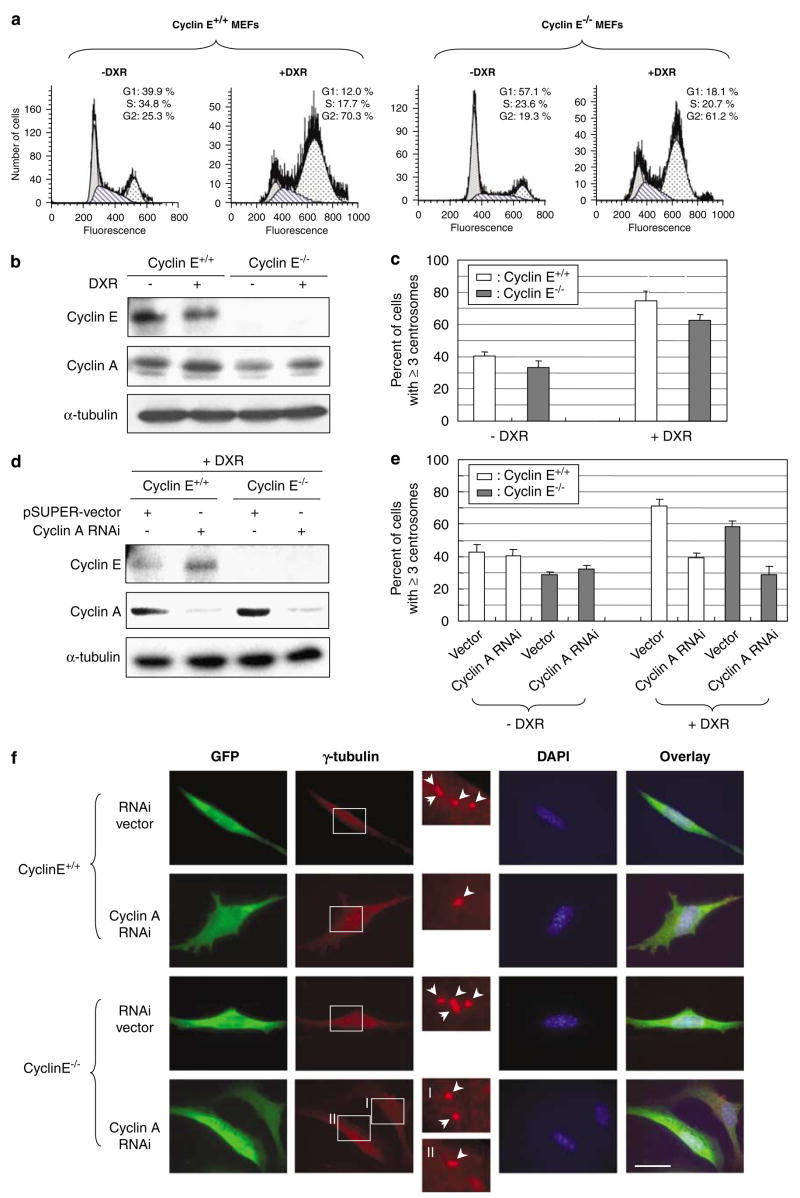
Exposure to certain genotoxic drugs such as DNA intercalating drugs and topoisomerase II inhibitors (that is, doxorubicin, DXR), the majority of cells become arrested in late G2 owing to the DNA damage responsive activation of the G2/M transition checkpoint. The G2/M checkpoint is mediated by both p53-dependent and p53-independent mechanisms (for reviews, see Taylor and Stark, 2001; Kastan and Bartek, 2004), and thus p53-deficient cells are also arrested in late G2 by exposure to such DNA damaging agents. For instance, flow cytometric analysis of cyclins E+/+ and E−/− MEFs lacking functional p53 with DXR for 48 h showed the accumulation of cells with G2 DNA contents (60–70%; Figure 6a). Similar to the Aph-mediated early S-phase arrest, the G2 arrest is believed to allow newly duplicated centrosomes to regain the duplication competency, and reduplicate upon availability of active CDK2. Indeed, the G2 arrest induced by exposure to genotoxic insults results in centrosome amplification (Dodson et al., 2004; Kawamura et al., 2006). We thus tested whether it is also the case in cells lacking cyclin E. Cyclins E+/+ and E−/− MEFs were exposed to DXR for 48 h. As the majority of cells are arrested in G2, and cyclin A is known to be expressed at high levels in S and G2/M (for a review, see Desdouets et al., 1995), a noticeable increase in cyclin A was detected in both cyclins E+/+ and E−/− cells (Figure 6b). We then examined the frequencies of centrosome amplification in the DXR-treated cells (Figure 6c). Both cyclins E+/+ and E−/− cells reduplicated centrosomes during DXR treatment, although cyclin E−/− cells reduplicated centrosomes with a slightly lower efficiency (~60%) compared with cyclin E+/+ cells (~70%). Thus, cyclin E is largely dispensable for centrosome reduplication in cells arrested in late G2 by DXR.
Figure 6.
Roles of cyclins A and E in centrosome reduplication in cells arrested by exposure to doxorubicin (DXR). (a) Cyclins E+/+ and E−/− mouse embryonic fibroblasts (MEFs) were exposed to DXR (0.05 μg/ml) for 48 h, and cell-cycle phase distributions of the cells were analysed by flow cytometry. (b) The lysates prepared from DXR-treated and untreated control cells were immunoblotted with anti-cyclins E and A, and anti-α-tubulin (loading control) antibodies. (c) The DXR-treated and untreated control cells were immunostained for γ-tubulin, and the number of cells with amplified centrosomes were scored. The results are shown in the graph as the average±standard error from three experiments. For each experiment,>200 cells were examined. (d) CycE+/+/CycA RNAi and CycE−/−/CycA RNAi cells as well as the control CycE+/+/vec and CycE−/−/vec cells were treated with DXR for 48 h, and the lysates prepared from the Aph-treated cells and control untreated cells were immunoblotted with anti-cyclins E and, A and anti-α-tubulin (loading control) antibodies. (e) The DXR-treated and untreated control CycE+/+/CycA RNAi, CycE−/−/CycA RNAi, CycE+/+/vec and CycE−/−/vec cells were immunostained for γ-tubulin, and the number of cells with amplified centrosomes were determined. For each experiment,>200 cells were examined. The results are shown in the graph as the average±standard error from three experiments. Representative γ-tubulin immunostaining images are shown in (f). In the magnified images, each centrosome is indicated by an arrow. Scale bar: 20 μm.
We next tested the role of cyclin A in centrosome reduplication during the G2 arrest in response to DNA damage. To this end, CycE+/+/CycARNAi and CycE−/−/CycARNAi cells (also control CycE+/+/vec and CycE−/−/vec cells) were exposed to DXR for 48 h. The DXR exposure did not affect the efficacy of siRNAmediated silencing of cyclin A (Figure 6d). We then examined the frequencies of centrosome amplification (Figure 6e, representative images are shown in Figure 6f). Both CycE+/+/CycARNAi and CycE−/−/CycARNAi cells failed to undergo centrosome reduplication, indicating that cyclin A is critical for centrosome reduplication in cells arrested in late G2 in response to DNA damage irrespective of the cyclin E status.
Discussion
Loss or mutational inactivation of p53 has been known to lay a favorable ground for numeral destabilization of centrosomes. In human cancers, mutational upregulation of cyclin E and consequential high activity of CDK2 have been shown to induce centrosome amplification synergistically with loss of p53 (Kawamura et al., 2004). Considering the significance of the CDK2-associating cyclins in centrosome amplification in p53-negative cells, we here dissected the roles of cyclins A and E in induction of centrosome amplification in cells with inactivated p53.
Cyclin E−/− cells expressing DN p53 (denoted simply as cyclin E−/− cells), when synchronized for cell cycling, the S-phase entry was severely delayed as compared with cyclin E+/+ cells. In contrast, there was no signifiant difference in the rates of S-phase entry between cyclins E+/+ and E−/− cells under a continuous growth condition, indicating that like in primary MEFs, in p53-negative cells, cyclin E is also required for cell-cycle reentry from quiescence, but not in continuously proliferating cells. In contrast to p53-positive primary MEFs, cyclin E−/− cells lacking the p53 function eventually enter S phase. Coverley et al. (2002) demonstrated that cyclin E is required for minichromosome maintenance (MCM) loading onto chromatin during G0–S progression. Consistently, we observed that cyclin E-null primary MEFs failed to incorporate MCM into chromatin, explaining an inability of these cells to enter the S phase (Geng et al., 2003). Our observations that cyclin E−/− p53-negative cells eventually reentered the cell cycle—albeit with a significant delay—indicate that functional deficiency of p53 can somehow bypass the requirement for cyclin E in cell-cycle reentry of MEFs from quiescence.
In contrast to the delay in S-phase entry from quiescence, initiation of centrosome duplication was not affected in the synchronized cyclin E−/− cells, indicating that the cyclin E activity is either not required or readily compensated by other proteins for initiation of centrosome duplication. When cyclin A expression was silenced in cyclin E−/− cells, initiation of centrosome duplication was significantly delayed, indicating that cyclin A is functionally replacing cyclin E for initiation of centrosome duplication in cyclin E−/− cells. It has been shown that one of the centrosomal targets of CDK2-cyclin E for the initiation of centrosome duplication is nucleophosmin, which can be phosphorylated by both CDK2-cyclins E and A on same residues in vitro (Tokuyama et al., 2001), supporting the functional replacement of cyclin E by cyclin A for triggering the initiation of centrosome duplication in cyclin E−/− cells. However, cyclin A probably does so less efficiency than cyclin E, as initiation of centrosome duplication is slightly, but noticeably, delayed in cyclin E−/− cells compared with cyclin E+/+ cells.
The newly duplicated centrosomes do not reduplicate immediately, but require regaining of duplication competency to reduplicate (Wong and Stearns, 2003). Cell-cycle arrest in S phase by Aph and in late G2 phase by the G2/M checkpoint in response to DNA damage is believed to allow centrosomes to regain the duplication competency, and initiate reduplication upon availability of the active CDK2. Reduplication of centrosomes in these cells occurs efficiently when the p53-dependent checkpoint function is compromised (Kawamura et al., 2006): in normal cells, physiological stress as well as DNA damage stabilizes and activates p53, leading to upregulation of p21, which efficiently inhibits CDK2, resulting in inhibition of centrosome reduplication (Fukasawa, 2007). We found that, in cells with the impaired p53 function, cyclins A and E participate in centrosome reduplication in a distinct manner depending on in which cell-cycle phase that cells are arrested. In cells arrested in early S phase, cyclin E is important in centrosome reduplication. For instance, Aph-arrested cyclin E+/+ cells silenced for cyclin A expression reduplicate centrosomes at a similar efficiency with cyclin E+/+ cells with normal cyclin A expression. However, if cyclin E is lost, cyclin A actively participates in induction of centrosome reduplication. Thus, Aph-arrested cyclin E−/− cells undergo centrosome amplification at a similar efficiency with cyclin E+/+ cells, but silencing of cyclin A expression in cyclin E−/− cells almost completely inhibits centrosome reduplication. In contrast to the early S-phase arrest, cyclin A is important in the induction of centrosome reduplication in cells arrested in late G2. For both cyclins E+/+ and E−/− cells arrested in late G2 by DXR, silencing of cyclin A almost completely blocked centrosome reduplication. These findings agree with the known expression kinetics of cyclins E and A as well as activation kinetics of CDK2-cyclins E and A during the cell cycle: cyclin E is highly expressed and CDK2-cyclin E is activated specifically in late G1 to early S phase, whereas cyclin A is highly expressed and CDK2-cyclin A is activated in S phase through the rest of the cell cycle.
It has been shown that the subtoxic concentrations of the commonly used cancer chemotherapeutic drugs reversibly arrest the cell cycle, and cells undergo centrosome amplification if p53 is either lost or inactivated. Upon removal of the drugs, those cells with amplified centrosomes reenter the cell cycle and suffer extensive chromosome instability (Bennett et al., 2004). Some cells within the tumors are expected to be exposed to subtoxic levels of drugs that only reversibly arrest the cell cycle, and undergo centrosome amplification. Upon cessation of chemotherapy, those cells with amplified centrosomes resume cell cycling, and suffer chromosome instability, which provides cells an opportunity to acquire further malignant phenotypes. Our present findings advance our understanding of the mechanism underlying centrosome amplification, especially in cells arrested by exposure to those chemotherapeutic agents, potentially providing critical information on the improvement of chemotherapy, including the reduction of the adverse effects of the chemotherapeutic drugs.
Materials and methods
Cells and transfection
For generation of immortalized cyclins E+/+ and E−/− (cyclin E1−/− E2−/−) MEFs that stably express DNp53, cells (passage 2) were infected with retroviruses expressing the DN p53 (amino acid 275–368 in the pBabe-hygro vector). The cells were selected with hygromycin for 2 weeks and passaged for 20 times. Cells stably expressing DNp53 were maintained in the complete medium (Dulbecco’s modified Eagle’s medium supplemented with 10% serum, penicillin and streptomycin). For the expression of the plasmid-based siRNA, we used a pSUPER vector system as described (Brummelkamp et al., 2002). The cyclin A sequence corresponding to its cDNA 389–407 (5′-AACCACTGACACCTCTTGA-3′) was used as the siRNA sequence. For the control, a randomized sequence was inserted into the vector. Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) along with a puromycin resistance gene plasmid or a GFP plasmid.
Antibodies
The primary antibodies used in this study were: anti-cyclins E (sc-481, Santa Cruz Biotechnology, Santa Cruz, CA, USA), A (sc-596, Santa Cruz Biotechnology), B (sc-752, Santa Cruz Biotechnology), anti-γ-tubulin (monoclonal, GTU-88, Sigma, St Louis, MO, USA), anti-α-tubulin (DM1A, Sigma) and anti-β-tubulin (TUB2.1, Sigma) antibodies. Anti-γ-tubulin polyclonal antibody was raised in our laboratory.
Immunoblot analysis
Cells were lysed in lysis buffer (1% SDS, 1% NP-40, 50mM Tris (pH 8.0), 150mM NaCl, 4mM Pefabloc SC, 2 μg/ml leupeptin, 2 μg/ml aprotinin). The lysates were briefly sonicated, boiled for 5 min and cleared by centrifugation at 20 000 g at 4 °C for 10 min. The lysates were denatured at 95 °C for 5 min in sample buffer (2% SDS, 10% glycerol, 60mM Tris (pH 6.8), 5% β-mercaptoethanol, 0.01% bromophenol blue), resolved by SDS–polyacrylamide gel electrophoresis and transferred to Immobilon-P sheets (Millipore Corp., Bedford, MA, USA). The blots were incubated in blocking buffer (5% (w/v) nonfat dry milk in Tris-buffered saline +0.2% Tween 20, TBS-T) for 1 h at room temperature (RT), and then incubated with primary antibody for 16 h at 4 °C, rinsed in TBS-T and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at RT. The antibody–antigen complex was visualized by enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL, USA).
Indirect immunofluorescence
Cells were fixed with 10% formalin/1% methanol for 20 min, incubated with 10% normal goat serum in phosphate-buffered saline (PBS) for 1 h at RT, probed with primary antibodies for 2 h and antibody–antigen complexes were detected with either Alexa Fluor 488- or Alexa Fluor 594-conjugated goat antibody (Molecular Probes, Eugene, OR, USA). The cells were counterstained for DNA with 4′,6-diamidino-2-phenylindole. For analysis of BrdU incorporation of synchronized cells, cells were serum-starved for 48 h, followed by serum stimulation in the presence of BrdU using the BrdU labeling kit (Roche, Indianapolis, IN, USA). Detection of the BrdU incorporation was performed according to the manufacturer’s instruction. For coimmunostaining of β- and γ-tubulins to examine centrioles, cells were first incubated on ice for 30 min to destabilize microtubules nucleated at centrosomes, followed by brief extraction (~15 s) with cold extraction buffer (0.75% Triton X-100, 5mM Pipes, 2mM EGTA, pH 6.7). Cells were washed in cold PBS, and fixed with 10% formalin/1% methanol.
Flow cytometry
Cells were trypsinized and fixed in 70% ethanol for 30 min at 4 °C. The cells were collected, washed in PBS, resuspended in Vindelov solution (10mM Tris-HCl, pH 8.0, 100 μg/ml RNase A, 0.1% (v/w) NP-40, 10mM NaCl and 50 μg/ml propidium iodide), incubated for 30 min at 37 °C and subjected to low cytometric analysis.
Acknowledgments
We thank C French, A Wilson and M Crowe for technical assistance, and Drs Z Andrysik and S Schwemberger for FACS analysis. This research is supported by the grants from National Institutes of Health, USA (CA90522 to KF and CA108950 to PS).
References
- Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RA, Izumi H, Fukasawa K. Induction of centrosome amplification and chromosome instability in p53-null cells by transient exposure to subtoxic levels of S-phase-targeting anticancer drugs. Oncogene. 2004;23:6823–6829. doi: 10.1038/sj.onc.1207561. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Carroll PE, Okuda M, Horn HF, Biddinger P, Stambrook PJ, Gleich LL, et al. Centrosome hyperamplification in human cancer: chromosome instability induced by p53 mutation and/or Mdm2 overexpression. Oncogene. 1999;18:1935–1944. doi: 10.1038/sj.onc.1202515. [DOI] [PubMed] [Google Scholar]
- Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- DeGregori J, Kowalik T, Nevins JR. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis-and G1/S-regulatory genes. Mol Cell Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouets C, Sobczak-Thépot J, Murphy M, Bréchot C. Cyclin A: function and expression during cell proliferation. Prog Cell Cycle Res. 1995;1:115–123. doi: 10.1007/978-1-4615-1809-9_9. [DOI] [PubMed] [Google Scholar]
- Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S, et al. Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J. 2004;23:3864–3873. doi: 10.1038/sj.emboj.7600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa K. Centrosome amplification, chromosome instability and cancer development. Cancer Lett. 2005;230:6–19. doi: 10.1016/j.canlet.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Fukasawa K. Oncogenes and tumour suppressors take on centrosomes. Nat Rev Cancer. 2007;7:911–924. doi: 10.1038/nrc2249. [DOI] [PubMed] [Google Scholar]
- Fukasawa K, Choi T, Kuriyama R, Rulong S, Vande Woude GF. Abnormal centrosome amplification in the absence of p53. Science. 1996;271:1744–1747. doi: 10.1126/science.271.5256.1744. [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G. Requirement of Cdk2-cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science. 1999;283:851–854. doi: 10.1126/science.283.5403.851. [DOI] [PubMed] [Google Scholar]
- Hoffman BD, Hanauske-Abel HM, Flint A, Lalande M. A new class of reversible cell cycle inhibitors. Cytometry. 1991;12:26–32. doi: 10.1002/cyto.990120105. [DOI] [PubMed] [Google Scholar]
- Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- Joshi HC. Microtubule organizing centers and gamma-tubulin. Curr Opin Cell Biol. 1994;6:54–62. doi: 10.1016/0955-0674(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Izumi H, Ma Z, Ikeda R, Moriyama M, Tanaka T, et al. Induction of centrosome amplification and chromosome instability in human bladder cancer cells by p53 mutation and cyclin E overexpression. Cancer Res. 2004;64:4800–4809. doi: 10.1158/0008-5472.CAN-03-3908. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Morita N, Domiki C, Fujikawa-Yamamoto K, Hashimoto M, Iwabuchi K, et al. Induction of centrosome amplification in p53 siRNA-treated human fibroblast cells by radiation exposure. Cancer Sci. 2006;97:252–258. doi: 10.1111/j.1349-7006.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey KR, Jackson PK, Stearns T. Cyclin-dependent kinase control of centrosome duplication. Proc Natl Acad Sci USA. 1999;96:2817–2822. doi: 10.1073/pnas.96.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussman JG, Horn HF, Carroll PE, Okuda M, Tarapore P, Donehower LA, et al. Synergistic induction of centrosome hyperamplification by loss of p53 and cyclin E overexpression. Oncogene. 2000;19:1635–1646. doi: 10.1038/sj.onc.1203460. [DOI] [PubMed] [Google Scholar]
- Ossovskaya VS, Mazo IA, Chernov MV, Chernova OB, Strezoska Z, Kondratov R, et al. Use of genetic suppressor elements to dissect distinct biological effects of separate p53 domains. Proc Natl Acad Sci USA. 1996;93:10309–10314. doi: 10.1073/pnas.93.19.10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihan GA, Purohit A, Wallace J, Knecht H, Woda B, Quesenberry P, et al. Centrosome defects and genetic instability in malignant tumors. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, Wolgemuth DJ, et al. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Tarapore P, Horn HF, Tokuyama Y, Fukasawa K. Direct regulation of the centrosome duplication cycle by the p53-p21Waf1/Cip1 pathway. Oncogene. 2001;20:3173–3184. doi: 10.1038/sj.onc.1204424. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Horn HF, Kawamura K, Tarapore P, Fukasawa K. Specific phosphorylation of nucleophosmin on Thr(199) by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. Biol Chem. 2001;276:21529–21537. doi: 10.1074/jbc.M100014200. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Jiang A, He D, Zhong L, Brinkley BR, et al. Analysis of centrosome abnormalities and angiogenesis in epidermal-targeted p53172H mutant and p53-knockout mice after chemical carcinogenesis: evidence for a gain of function. Mol Carcinog. 1998;23:185–192. doi: 10.1002/(sici)1098-2744(199811)23:3<185::aid-mc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wong C, Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat Cell Biol. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]