Abstract
Brain derived neurotrophic factor (BDNF) mediates survival and neuroplasticity through the activation of phosphoinositide 3-kinase (PI3K)-Akt pathway. Although previous studies suggested the roles of MAPK, PLC-γ-mediated intra-cellular calcium ([Ca2+]i) increase, and extra-cellular calcium influx in regulating Akt activation, the cellular mechanisms are largely unknown. We demonstrated that sub-nanomolar BDNF significantly induced Akt activation in developing cortical neurons. The TrkB-dependent Akt phosphorylation at S473 and T308 required only PI3K, but not PLC and MAPK activity. Blocking NMDA receptors, L-type voltage-gated calcium channels, and chelating extra-cellular calcium by EGTA failed to block BDNF-induced Akt phosphorylation. In contrast, chelating [Ca2+]i by BAPTA-AM abolished Akt phosphorylation. Interestingly, sub-nanomolar BDNF did not stimulate [Ca2+]i increase under our culture conditions. Together with that NMDA- and membrane depolarization-induced [Ca2+]i increase did not activate Akt, we conclude that the basal level of [Ca2+]i gates BDNF function. Furthermore, inhibiting calmodulin by W13 suppressed Akt phosphorylation. On the other hand, inhibition of protein phosphatase 1 by okadaic acid and tautomycin rescued Akt phosphorylation in BAPTA- and W13-treated neurons. We further demonstrated that the phosphorylation of PDK1 did not correlate with Akt phosphorylation at T308. Our results suggested novel roles of basal [Ca2+]i, rather than activity-induced calcium elevation, in BDNF-Akt signaling.
Keywords: BDNF, calcium, PI3 kinase, Akt, MAPK, synaptic plasticity
Introduction
The function of brain-derived neurotrophic factor (BDNF) was initially demonstrated in neuronal development, survival and differentiation (Huang and Reichardt 2001). For example, application of BDNF inhibited neural apoptosis induced by high concentrations of glutamate and serum withdrawal in vitro (Poser et al. 2003; Almeida et al. 2005). Consistently, BDNF rescues cells from neuronal death during ischemia in live animals (Beck et al. 1994; Miyata et al. 2001). Furthermore, mice lacking BDNF show loss of neurons in the peripheral nervous sensory system and pre-mature cell death leading to deficits in movement and balance (Ernfors et al. 1995; Bianchi et al. 1996). Another important aspect of BDNF function was recently revealed in regulating activity-dependent synaptic plasticity (Patterson et al. 1996; Akaneya et al. 1997; Ji et al. 2005; Lu et al. 2005). For example, during early development, application of exogenous BDNF converted short-term potentiation (STP) to long-term potentiation (LTP) (Figurov et al. 1996). Conversely, LTP was significantly impaired in the hippocampus of homozygous BDNF mutant mice (Korte et al. 1995). Furthermore, mice with a reduced level of BDNF (heterozygous mutants) were defective in several forms of hippocampus-dependent memory formation (Linnarsson et al. 1997). Based on the function of BDNF in stimulating the expression of plasticity-related gene expression and promoting neurotransmitter release, both post-synaptic and pre-synaptic mechanisms were suggested (Lohof et al. 1993; Finkbeiner et al. 1997; Jovanovic et al. 2000; Huang and Reichardt 2001).
Molecular investigations have revealed that, upon binding and activating the receptor tyrosine kinase TrkB, BDNF stimulates three major signaling pathways including PLC-γ, Ras-Raf-MAPK and PI3K-Akt (Segal and Greenberg 1996; Reichardt 2006). Interestingly, the role of PI3K-Akt was also initially implicated in neuronal survival with recent evidence for its function in regulating synaptic plasticity such as LTP and memory formation (Krasilnikov 2000; van der Heide et al. 2005; Karpova et al. 2006). PI3K was first found phosphorylated by a virus transforming gene product and involved in the transformation process (Sugimoto et al. 1984). Further investigations demonstrated its broad function in pro-survival and anti-apoptosis for many cell types and tissues (Scheid et al. 1995; Franke et al. 1997; Kauffmann-Zeh et al. 1997; Cheng et al. 2003). For example, inhibition of PI3K-Akt abolished neuro-protective effects of BDNF in neurons challenged with a high concentration of glutamate (Almeida et al. 2005). Additionally, the activation of the PI3K pathway was also required for rescuing neuronal cell death by a preconditioning dose of NMDA (Soriano et al. 2006), nerve growth factor (Gerling et al. 2004), insulin-like growth factor (Leinninger et al. 2004) and dopamine D2 agonists (Kihara et al. 2002). The anti-apoptosis effects of PI3K are mediated by its down-stream target protein kinase B (PKB, also known as Akt), which can regulate the expression of several apoptosis-related genes, such as Bad/BCL2, caspases and GSK3. Interestingly, the function of PI3K/Akt pathway is also implicated in regulating synaptic plasticity. For example, it was demonstrated that inhibition of Akt abolished the expression and maintenance of LTP in the hippocampus (Horwood et al. 2006; Karpova et al. 2006). At the cellular level, blockage of PI3K/Akt attenuated calcium influx through NMDA receptor (Sanchez-Perez et al. 2006). It was also reported that PI3K/Akt enhanced voltage-dependent calcium channel currents by promoting Ca2+ channel trafficking (Viard et al. 2004). In addition, PI3K activity is both sufficient and necessary for synaptic potentiation induced by neurotrophin-3 (Yang et al. 2001). Furthermore, Akt phosphorylation was up-regulated in vivo after memory acquisition and retrieval. Consistently, inhibition of PI3K-Akt abolished the retrieval of contextual memory (Chen et al. 2005).
Despite the broad effects of BDNF and Akt in neuronal functions, it is not clear how BDNF-induced Akt activation is regulated. In particular, the molecular mechanism of Akt activation is largely unknown in situations unrelated to apoptosis.
In this study, we demonstrate that a sub-nanomolar concentration of BDNF, which is not sufficient to protect neuronal apoptosis, significantly induced Akt phosphorylation in cultured cortical neurons. We investigated the potential cross-talk among MAPK, PLC-γ and PI3K/Akt in BDNF-stimulated neurons. We found that neither MAPK nor PLC-γ was required to support BDNF-induced Akt phosphorylation. Surprisingly, we identified that the basal level of [Ca2+]i and calmodulin (CaM) activity, rather than activity-induced elevation of [Ca2+]i, were essential to gate the PI3K-Akt signaling in BDNF-stimulated neurons. Furthermore, protein phosphatase 1 (PP1) was identified as an important negative regulator, and the balance between PP1 and PI3K determines the [Ca2+]i-gated Akt activation. Our results provided novel regulating mechanisms for PI3K-Akt signaling.
Materials and methods
Cell culture and treatments
Primary cortical neuronal cultures were prepared from post-natal day 0 Sprague Dawley rats as described (Chan et al. 1998). DIV (days in vitro) 5 to 7 cultures were stimulated with KCl (50mM), NMDA (50uM, Sigma)/glycine (1uM) or recombinant human BDNF (at 5ng/ml, or specified) (Calbiochem).
We used well-documented inhibitors to block the activity of TrkB (by 0.2uM K252a, or 0.4ug/ml TrkB-IgG), NMDA receptors (by 100uM APV), L-VGCC (by 10uM nifedipine), MAPK (by 50uM PD98059 or 10uM U0126), PI3K (by 30uM LY294002), CaM KII/IV (by 5uM KN93 or 10uM KN62), PLC (by 5, 10 or 25uM U73122), extracellular calcium (by 2.5mM 6 EGTA), intracellular calcium (by 33uM BAPTA-AM), calmodulin (by 70uM W13), PP1 (by 1uM okadaic acid or 3nM tautomycin), PP2A (by 10nM okadaic acid or 0.1uM cantharidin), and PP2B (by 1uM FK506). The inhibitors were added to neuronal cultures 30min before BDNF stimulation. The samples were harvested 15min after BDNF, KCl, or NMDA treatment, and analyzed by either Western blots or immuno-cytochemistry. For some experiments, neurons were harvested 30min after the treatments with different inhibitors, and compared with untreated neurons by Western blots.
Examination of neuronal apoptosis
Neuronal apoptosis of DIV 5 to 7 cultures was induced by 15min incubation with 125uM glutamate in Neurobasal A (supplemented with B27). To test the effect of BDNF, the glutamate-treated neurons were pre-treated with BDNF for 16hr before glutamate. After glutamate incubation, the treated cultures were further incubated with the original medium containing or lacking BDNF for 8hr. Control cells were treated with fresh glutamate-free Neurobasal A (supplemented with B27) for 15min (without BDNF application), then incubated with the original medium for 8 hr. Cell viability was measured by staining with propidium iodide as described (Bosel et al. 2005). Briefly, neurons were fixed with 70% ethanol for 15min, and stained with 5 ug/ml PI (in PBS) at 37°C for 30min. The stained neurons were examined by fluorescent microscopy. Glutamate-induced neuronal death was marked by shrunken nuclei and nuclear condensation.
Western blotting
Control and stimulated neurons were harvested in SDS loading buffer (10mM Tris-HCl buffer pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate, 0.01% bromophenol blue and 5% β-mercaptoethanol). Samples were boiled for 10min, separated by 10% SDS-PAGE, and transferred to nitrocellulose membranes. The blots were incubated with polyclonal antibodies against phosphorylated-Akt at Ser473 (p-Akt473, 1:1 000, Cell Signaling) or Thr308 (p-Akt308, 1:1 000, Cell Signaling), p-ERK1/2 (1:1 000, Cell Signaling), p-PDK1 at S241 (1:1 000, Cell Signaling), p-Trk (1:500, Cell Signaling), and p-CaM KII (1:1 000, Cell Signaling). Overnight incubation at 4°C in PBS with 0.1% triton X-100 and 3% non-fat milk was carried out for primary antibodies. After extensive washing with PBS and 0.1% triton X-100, HRP-conjugated secondary antibodies (1:5000, Pierce) were used for signal detection with the ECL system (SuperSignal® West Pico, Pierce, Rockford, IL). The level of protein loading was determined by antibodies against total Akt (T-Akt, 1:2 000, Cell Signaling), total ERK1/2 (T-ERK, 1:1 000, Cell Signaling) and beta-Actin (1:5 000, Sigma). Several exposure times were used to obtain signals in the linear range. The bands were quantified using Scion Image software (Scion Corp. Frederick, Maryland).
Immunocytochemistry
Control and stimulated neurons were fixed by 6% PFA in PBS at room temperature for 20min, washed 3X with PBS, and permeablized in PBS with 0.5% Triton X-100. Neurons were double-labeled with polyclonal antibody against p-Akt and monoclonal antibody against NeuN, a neuronal marker protein. Alexa-488-conjugated goat-anti-rabbit and Alexa-594-conjugated goat-anti-mouse antibodies (1:300, Invitrogen) were used for detection. The stained samples were examined by a Nikon fluorescent microscope, and images were captured by the Q-capture program. The surface plots were obtained by using the Image J software. All antibody incubations were done in PBS with 0.1% triton X-100, 3% BSA and 3% goat serum.
Calcium imaging
The cell permeable fluorescent indicator fura-2 acetoxymethyl ester (fura-2-AM) was used to measure intracellular calcium as described (Nunez et al. 2005). Cells were loaded with fura-2-AM (3uM) in DMSO (<0.5%) and incubated for 30 min. Neurons were treated with PSS (137 mM NaCl, 20mM KCl, 4mM MgCl2, 12mM CaCl2, 40 mM HEPES, 90 mM 20% Glucose) (to obtain baseline measurements of resting calcium), KCl (50mM), or NMDA (50uM) for 5 min or with BDNF (5ng/ml) for 15min followed by 5-min KCl treatment. Intracellular calcium concentrations were calculated from the ratio of background corrected fura-2 emission (520 nm) at two excitation wavelengths (340nm/380nm).
Data analysis
The results were analyzed among groups using the post hoc Student–Newman–Keuls procedure for multiple comparisons. Student's paired t-test was used to assess significance for data with two groups. All quantification data are expressed as the average ± SEM. Differences with p-values less than 0.05 were considered significant.
Results
Significant stimulation of Akt phosphorylation at both S473 and T308 by sub-nanomolar BDNF through the receptor tyrosine kinase TrkB
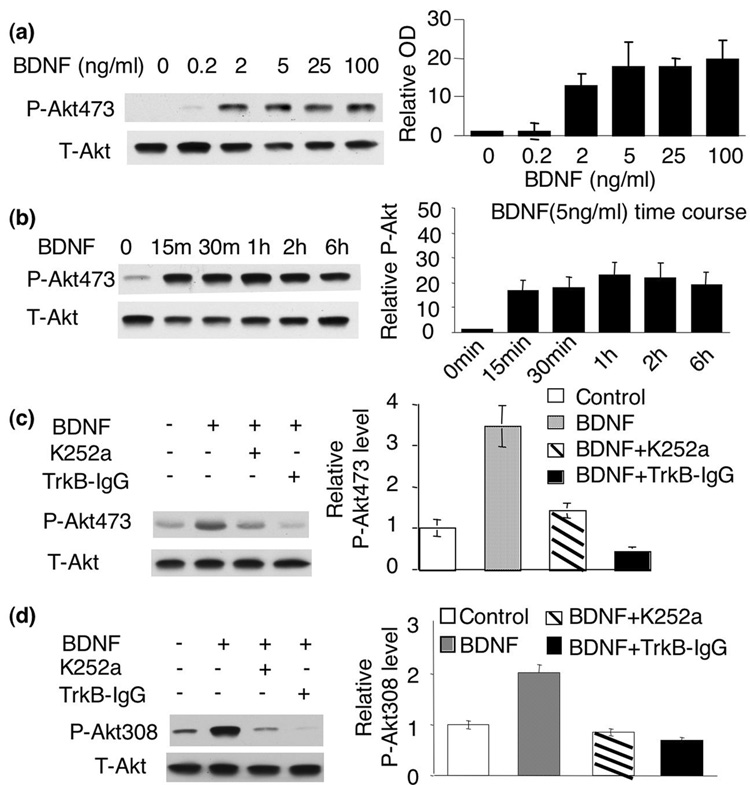
Two phosphorylation sites of Akt, Ser473 and Thr308 were both required for its full activation (Hill et al. 2001). It was demonstrated that Akt phosphorylation at both sites was induced in cortical neurons by BDNF at 100ng/ml (about 3nM for BDNF dimer), which was sufficient to rescue glutamate-induced neuronal cell death (Almeida et al. 2005). Here, we found that significant p-Akt induction was stimulated by BDNF at a much lower concentration (Fig. 1a and d). The dose-response curve indicated that treatment of BDNF, at as low as 2ng/ml (or about 0.06nM), resulted in a robust increase (p<0.01) of p-Akt at S473 in cultured cortical neurons. The maximal induction was achieved by 5ng/ml BDNF (p<0.05). No significant further induction of p-Akt473 was observed at 100ng/ml (Fig. 1a). These results suggested that 5ng/ml of BDNF was sufficient to fully induce the Akt phosphorylation at S473. Moreover, we found that 5ng/ml BDNF induced a persistent activation of p-Akt at S473. The elevation of p-Akt lasted for at least 6hr (Fig. 1b). Significant up-regulation of phosphorylation at T308 was also stimulated by 5ng/ml BDNF (Fig. 1d).
Fig. 1.
Activation of Akt by BDNF depends on TrkB. a) Cortical neurons were treated with 0.1, 0.2, 2, 5, or 100ng/ml BDNF. The activation of PI3K-Akt signaling was measured by the level of phosphorylated Akt (p-Akt) at S473 with Western blot analysis. b) 5ng/ml BDNF induced robust and long-lasting activation of Akt. The treated neurons were assayed for the level of p-Akt at S473 15min, 30min, 1hr, 2hr and 6hr after BDNF treatment. c and d) BDNF (5ng/ml)-induced Akt phosphorylation at S473 and T308 depends on TrkB. The level of p-Akt473 and p-Akt308 was analyzed by Western blots. Representative images are shown in the left panels, and quantification are shown in the right panels (n=3 from separate experiments). The relative intensity of p-Akt473 or p-Akt308 was normalized to T-Akt.
To test whether the phosphorylation of Akt at S473 and T308 is caused by binding of BDNF to TrkB receptors, we pre-treated neurons with the tyrosine kinase inhibitor K252a. Also, we utilized TrkB-IgG as a BDNF scavenger that mimics the extra-cellular domain of TrkB and competitively binds BDNF (Shelton et al. 1995). Both K252a and TrkB-IgG significantly inhibited BDNF-induced Akt phosphorylation at both S473 and T308 (Fig. 1c and 1d, p<0.01), indicating that Akt phosphorylation is dependent on the TrkB receptor activity.
Although 5ng/ml of BDNF induced significant activation of Akt, such dosage did not block glutamate-induced neuronal death (Supplemental Fig. 1). Rather, 100ng/ml of BDNF was required to reduce glutamate-induced cell death. The % of viability was 20.2+/−2.8% with glutamate-treated neurons, 22.4+/−6.5% with neurons pre-treated with 5ng/ml BDNF (followed by glutamate challenge) (P>0.05), and 34.3+/−7.4 % (P<0.05) with neurons pre-treated with 100ng/ml BDNF (followed by glutamate challenge) (Supplemental Fig. 1). These data indicated that low dose and high dose of BDNF-induced Akt activation might have different physiological effects.
BDNF-induced Akt phosphorylation does not depends on PLC-γ and MAPK activity
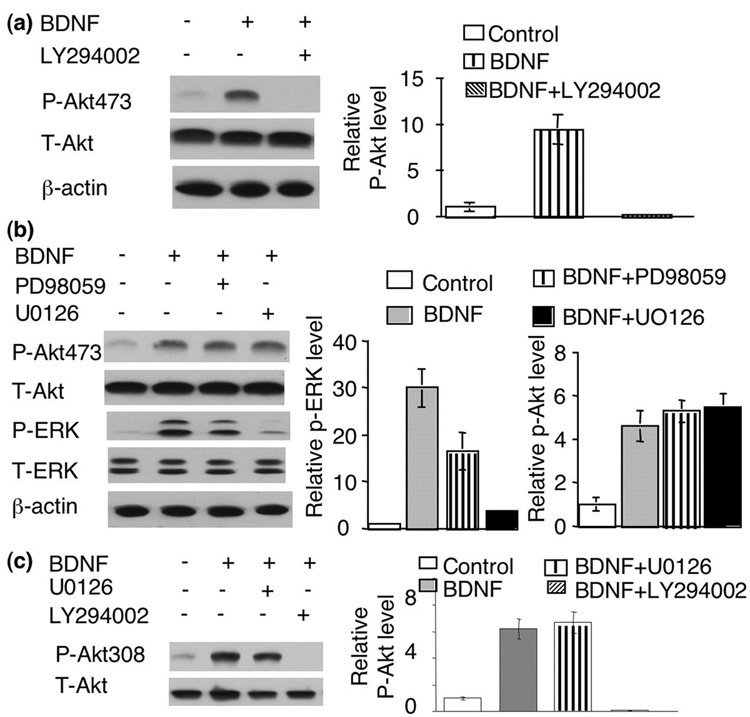
It is well documented that BDNF activates TrkB, and leads to the activation of three major pathways including PLC-γ, Ras-Raf-MAPK and PI3K-Akt signaling. Indeed, cross-talk among these pathways was documented (Opazo et al. 2003). For example, inhibition of MEK led to the reduction of p-Akt in striatal medium-sized spiny neurons (Stroppolo et al. 2001). Blocking MEK or PI3K inhibited the neuro-protective effects of BDNF.
To address the role of MAPK in BDNF-induced Akt phosphorylation, MEK activity was blocked by pre-treatment of PD98059 and U0126. As shown in Fig 2b, treatment with PD98059 or U0126 significantly blocked BDNF stimulation on p-ERK. However, the induction of p-Akt at S473 was not affected by these two MEK inhibitors (Fig 2b). U0126 also failed to block the induction of p-Akt at T308 (Fig 2c). Taken together, these results indicated that activation of the PI3K-Akt pathway by BDNF is independent of MAPK activity. In contrast, blocking PI3K by LY294002 totally abolished BDNF-induced Akt phosphorylation at S473 and T308 (Fig. 2a and c), supporting that Akt phosphorylation at S473 and T308 both depends on PI3K activity.
Fig. 2.
Activation of Akt by BDNF does not depend on MAPK activity. a) Inhibition of PI3K blocked BDNF-induced Akt phosphorylation at S473. b) Inhibition of MEK did not block Akt phosphorylation at S473. c) Akt phosphorylation at T308 were not affected by U0126 but abolished by LY294002. Representative images are shown in the left panels and quantifications (normalized to T-Akt, n=3 for each group) in the right panels.
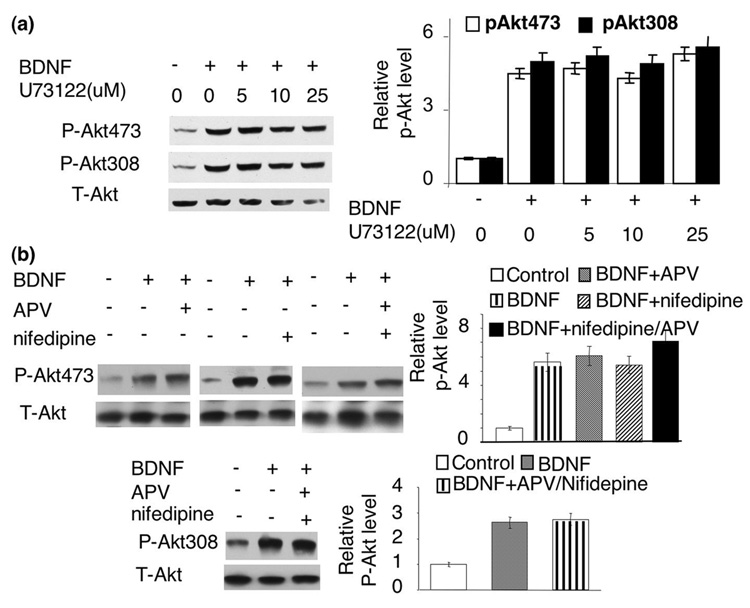
We next used U73122 to block PLC activity. As shown in Fig 3a, blocking PLC had no effect on BDNF stimulation of p-Akt at S473 or T308. We chose different U73122 concentrations, all of which had been shown to significantly block PLC activity. These data suggest that PLC-γ mediated calcium release may not be required.
Fig. 3.
The BDNF-induced Akt phosphorylation at S473 and T308 was not mediated by PLC activity, NMDA receptors or L-VGCC. a) PLC activity is not required for BDNF-induced Akt phosphorylation. b) BDNF-induced phosphorylation at S473 and T308 was not blocked by NMDA receptor and L-VGCC antagonists. The level of Akt phosphorylation at S473 and T308 was analyzed by Western blot using phospho-specific antibodies. Representative Western blots are shown in left panels, and quantifications (n=3 for each treatment group) are shown in right panels. The intensity of p-Akt signal was normalized to T-Akt.
Calcium influx through NMDA receptors, L-VGCC, and extracellular calcium are not required for Akt phosphorylation
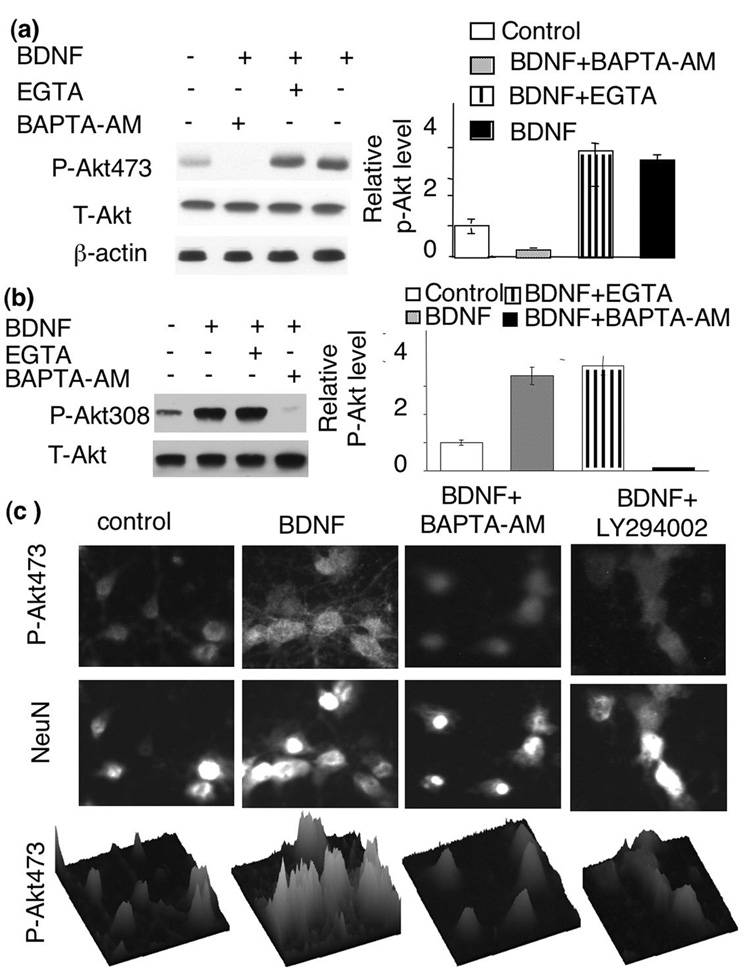
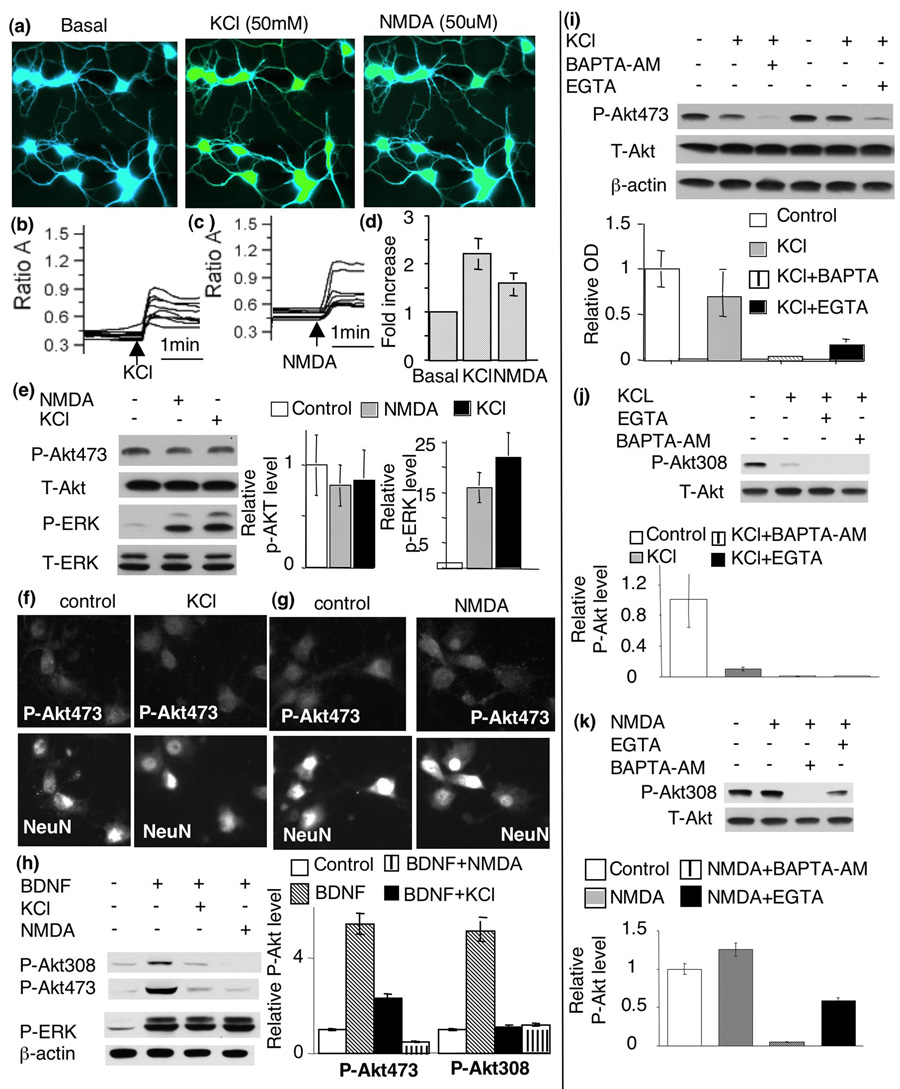
Calcium plays essential roles in regulating many signaling pathways associated with both apoptosis and neuroplasticity. In deed, BDNF may promote calcium influx by enhancing glutamate release, directly potentiating NMDA receptor activity, and evoking Ca2+ influx through NMDA receptors and voltage-gated calcium channels (VGCC) by TrkB-dependent opening of sodium channels (Nav 1.9) (Rose et al. 2004). To test the role of NMDA receptors, we pre-treated neurons with APV (an antagonist of NMDA receptor). Blocking NMDA receptors with APV did not affect BDNF-induced Akt phosphorylation at S473 (Fig. 3b). Similarly, blocking L-VGCC by nifedipine did not suppress Akt phosphorylation at S473 either (Fig. 3b). Furthermore, co-application of both APV and nifedipine did not block BDNF-induced Akt phosphorylation at S473 or T308 (Fig. 3b). In contrast, APV and nifedipine significantly blocked NMDA- and depolarization-induced ERK phosphorylation and c-fos transcription (data not shown). To determine the overall role of extra-cellular calcium, we treated the neurons with EGTA. EGTA abolished ERK phosphorylation induced by NMDA receptor and membrane depolarization (data not shown), but did not affect BDNF-induced Akt phosphorylation at S473 or T308 (Fig. 4a and b). These data demonstrated that extra-cellular calcium was not required for PI3K-Akt activation in BDNF-stimulated neurons.
Fig. 4.
The level of intra-cellular, but not extra-cellular, calcium is required for BDNF-induced Akt phosphorylation. BDNF-induced Akt phosphorylation at S473 (a) and T308 (b) was blocked by BAPTA-AM but not by EGTA. Representative Western blot images are shown on the left and quantifications (n=3 for each group) were shown on the right. c) Immuno-fluorescent staining of BDNF-stimulated neurons was performed to double-label p-Akt (upper panels) and NeuN (middle panels). BDNF-stimulated Akt phosphorylation at S473 was significantly blocked by BAPTA-AM. PI3K inhibitor, LY294002 also blocked BDNF-stimulated p-Akt. Surface plots (lower panels) of p-Akt signal were used to further demonstrate the inhibiting effects of BAPTA-AM and LY294002.
The basal level of intracellular calcium gates Akt phosphorylation at S473 and T308 in BDNF-stimulated neurons
BDNF may induce calcium release from intracellular storage through the activation of PLC-γ. Because U73122 did not block p-Akt induction (Fig. 3a), we conclude that a PLC-γ-dependent increase of [Ca2+]i may not be required for p-Akt elevation. To examine the effects of overall reduction in intracellular calcium, we treated neurons with BAPTA-AM to chelate intracellular calcium. Interestingly, pre-treatment with BAPTA-AM abolished BDNF-induced p-Akt at S473 and T308, as indicated by both Western blot (Fig. 4a and b) and immuno-cytochemistry determinations (Fig. 4c). These results suggest that intracellular calcium is required for Akt activation.
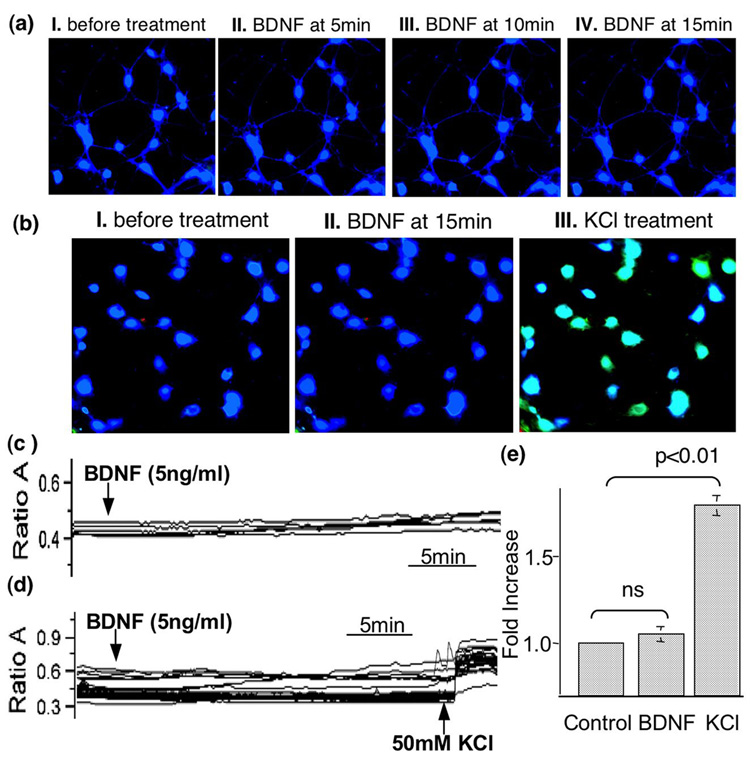
Although it is generally accepted that neurotrophins increase [Ca2+]i, BDNF-stimulated calcium elevation may depend on different culture conditions and cell types (Finkbeiner et al. 1997; Pizzorusso et al. 2000; Numakawa et al. 2002; Yang and Gu 2005). To examine whether BDNF induces [Ca2+]i elevation in cortical neurons, we used calcium imaging to measure the level of [Ca2+]i in BDNF (5ng/ml)-stimulated cortical neurons (Fig. 5). The fluorescent intensity of fura-2-loaded neurons was not significantly altered after BDNF application (Fig. 5a). The traces of ratio value (A340/A380) (Fig. 5c) and quantification (Fig. 5e) also demonstrated that [Ca2+]i remained constant after BDNF stimulation. In contrast, when KCl was applied to BDNF-treated neurons, significant elevation of [Ca2+]i was observed (Fig. 5b, d, and e). These data indicated 5ng/ml BDNF was not sufficient to elevate [Ca2+]i under our culture condition.
Fig. 5.
Sub-nanomolar BDNF is not sufficient to elevate intracellular calcium. The concentration of intracellular calcium [Ca2+]i was determined by calcium imaging using fura-2-based method. a). Fluorescent images were obtained at different time points from Fura-2-filled neurons before (a I and b I) and after BDNF (5ng/ml) (a II to a IV, and b II). To address the responsiveness of BDNF-treated neurons, KCl was applied after the 15min BDNF treatment (b III). Traces of the Fura-2 ratio (340nm/380nm) changes in randomly chosen neurons demonstrated that BDNF did not induce significant elevation of [Ca2+]i (c and e). In contrast, KCl (50mM) application caused significant increase in [Ca2+]i (d and e). The arrows indicate when BDNF (5ng/ml) or KCl (50mM) is applied. e). Quantification of fold changes in [Ca2+]i. Data were collected from 45 neurons in two independent experiments.
To determine whether elevation of intracellular calcium is sufficient for Akt activation, we examined p-Akt at S473 and T308 in KCl- and NMDA-stimulated neurons. As shown in Fig. 6a–d, both KCl and NMDA treatment caused significant elevation of intracellular calcium. Compared to control neurons, KCl-induced membrane depolarization led to a 2.2 fold increase of [Ca2+]i. NMDA treatment resulted in a 1.7 fold increase of [Ca2+]i (Fig. 6d). Interestingly, neither KCl nor NMDA stimulated any significant up-regulation of p-Akt at S473 (Fig. 6e, f, and g). In contrast, the phosphorylation of ERK was greatly enhanced by both KCl and NMDA treatment (Fig. 6e). Surprisingly, co-application of NMDA or KCl with BDNF (5ng/ml) suppressed BDNF-induced Akt phosphorylation (Fig. 6h). Although an increase of [Ca2+]i failed to elevate p-Akt at S473, removal of either extra-cellular (by EGTA) or intra-cellular (by BAPTA-AM) calcium caused down-regulation of p-Akt in KCl-treated neurons (Fig. 6i). The phosphorylation at T308 showed similar activation and deactivation profile to that of S473 (Fig. 6j and k). Taken together, these data demonstrated that the basal level of calcium was necessary, but the elevation of intracellular calcium was not sufficient for Akt activation. Furthermore, elevation of intracellular calcium did not facilitate, but rather suppressed, BDNF-induced Akt phosphorylation.
Fig. 6.
An increase in intra-cellular calcium is not sufficient to stimulate Akt phosphorylation. To increase the level of intracellular calcium, cortical neurons were depolarized by KCl (50mM) or stimulated by NMDA (50uM). The concentration of intracellular calcium was examined by the Fura-2-based fluorescent method. a). Fluorescent images were obtained from Fura-2-filled neurons before (left panel) and after KCl (middle panel) or NMDA (right panel) treatments. b) and c). Traces of the Fura-2 ratio (340nm/380nm) changes in randomly-chosen neurons demonstrated a significant increase (p<0.05) of intracellular calcium after KCl (b) and NMDA (c) stimulation. The arrows indicate when KCl or NMDA is applied. d) Quantification plot shows fold increase of intracellular calcium after KCl and NMDA treatment. e) KCl and NMDA resulted in significant phosphorylation of ERK but not Akt. Quantification (n=5) shows no induction of p-Akt at S473 (middle panels). In contrast p-ERK was significantly induced by both KCl and NMDA (p<0.05). f) and g). Immuno-fluorescent staining also showed that KCl (f) and NMDA (g) failed to induce Akt phosphorylation. Cortical neurons were double-stained for p-Akt and the neuronal marker NeuN. h) increase in intracellular calcium by NMDA and KCl suppressed BDNF-induced Akt phosphorylation. i) Both intra-cellular and extra-cellular calcium are required for the maintenance of p-Akt level of S473. j and k) Akt phosphorylation at T308 was not induced by either KCl (j) or NMDA (k), but decreased by EGTA or BAPTA-AM application. Representative Western blots are shown in the left panels. Quantification (n=4) is shown on the right.
Calmodulin activity is required for BDNF-induced Akt phosphorylation
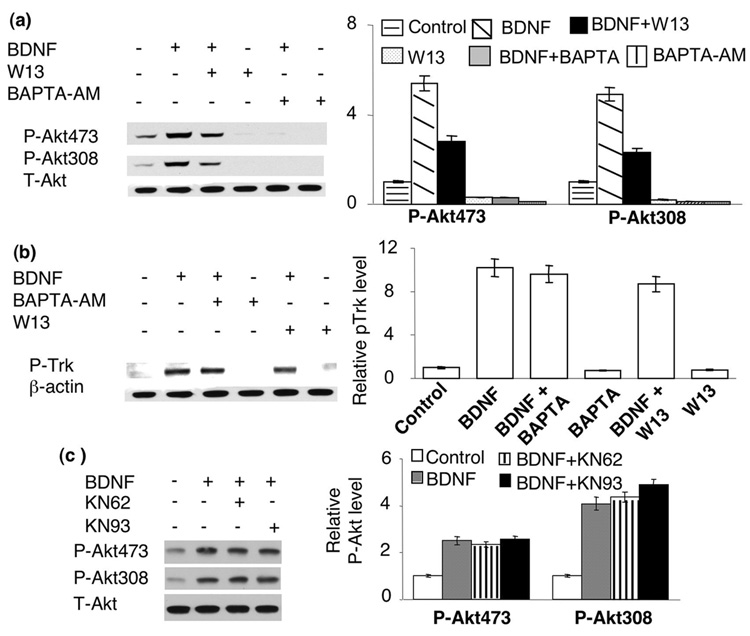
Because many aspects of activity-dependent neuroplasticity are regulated by calcium through calmodulin (CaM) (Soderling 2000), we examined the function of CaM activity. As shown in Fig. 7a, pre-treatment of neurons with a CaM antagonist W13 significantly inhibited the BDNF (5ng/ml)-induced Akt phosphorylation at both S473 and T308. Treatment with BAPTA-AM and W13 also significantly suppressed the basal level of p-Akt in un-stimulated neurons (Fig. 7a). These data suggested that the basal level [Ca2+]i might regulate PI3K-Akt signaling through CaM. To examine whether the basal level [Ca2+]i affect BDNF signaling upstream of Akt, we determined the level of Trk receptor phosphorylation (Fig. 7b). We found that BDNF-induced Trk phosphorylation was not inhibited by either BAPTA-AM or W13, indicating that the initial signaling was intact. Because the phosphorylation site of Trk A, B, and C shared the same epitope, the specific receptor type could not be discriminated. Nevertheless, it was established that BDNF specifically activates TrkB. We further examined whether CaM functioned through calmodulin-dependent kinase II and IV (CaM KII and CaM KIV), the 2 major CaM-dependent kinases involved in neuroplasticity (Anderson and Kane 1998; Soderling 2000; Griffith 2004). We pre-treated neurons with KN62 and KN93 (both are CaM KII and CaM KIV inhibitors) before BDNF application. We found no inhibition effects on S473 and T308 phosphorylation (Fig. 7c). These data suggest that the regulatory function of CaM is independent of the activity of CaM KII and IV.
Fig. 7.
Calmodulin (CaM) activity is required for BDNF-induced Akt phosphorylation. a). CaM inhibitor W-13 suppressed both basal and BDNF-induced Akt phosphorylation. b). BDNF-induced Trk receptor phosphorylation was not affected by BAPTA-AM and W13. c). Blocking CaM kinase II and IV had no effects on BDNF-induced Akt phosphorylation. Representative Western blots are shown in the left panels, and quantification in the right panels (n=3 for each treatment group). The signal of p-Akt or p-Trk was normalized to T-Akt or beta actin.
PDK1 phosphorylation profile does not correlate with that of Akt/T308 in cortical neurons
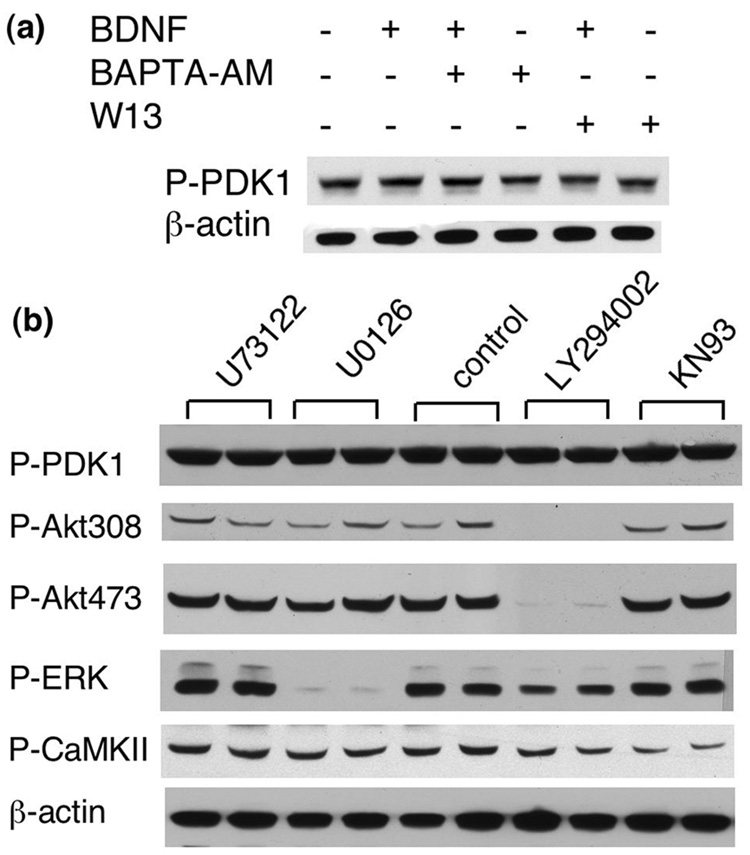
It was demonstrated that PI3K regulated Akt through phosphoinositide-dependent kinase-1 and 2 (PDK1 and PDK2) in non-neuronal cells. Because the molecular identity of PDK2 is unknown, we examined whether calcium/CaM regulation of Akt was mediated through PDK1 downstream of PI3K by using antibodies against phosphorylated PDK1 at S241. The phosphorylation within the activation loop at S241 is necessary for PDK1 activity. Although the level of phosphorylation at S241 in HEK 293 cells was not changes by insulin-like growth factor-1 (indicating that PDK 1 activity may not be stimulated by agonists) (Casamayor et al. 1999; Mora et al. 2004), PDK1 phosphorylation at S241 was up-regulated by high frequency stimulation in hippocampal neurons (Tsokas et al. 2007). Here, we found that the level of p-PDK1 at S241 was not induced by 5ng/ml BDNF (Fig. 8a). Blocking TrkB by K252a and TrkB-IgG did not change the level of p-PDK1 (data not shown). In contrast to the case of Akt, the phosphorylation of PDK1 at S241 was not affected by either BAPTA-AM or W13 (Fig. 8a). We further compared p-PDK1 and p-Akt in neurons treated with different protein kinase inhibitors. As shown in Fig 8b, PI3K inhibitor LY294002 (but not U73122, U0126, and KN93) significantly suppressed Akt phosphorylation at both S473 and T308. In contrast, the phosphorylation of p-PDK1 is not regulated by any of the inhibitors. As controls, p-ERK was significantly suppressed by U0126. Mild inhibition was observed for p-ERK by LY294002 (Fig. 8b). KN93 significantly suppressed the level of CaM KII phosphorylation (Fig. 8b). Therefore, the phosphorylation profile of PDK1 did not correlate with that of Akt phosphorylation at T308.
Fig. 8.
The level of p-PDK1 dose not correlate with that of p-Akt. a). The level of p-PDK1 at S241 was not regulated by BDNF, BAPTA-AM and W13. b). The level of p-PDK1 at S241 was not regulated by PI3K.
Protein phosphatase 1 is a negative regulator of Akt activation
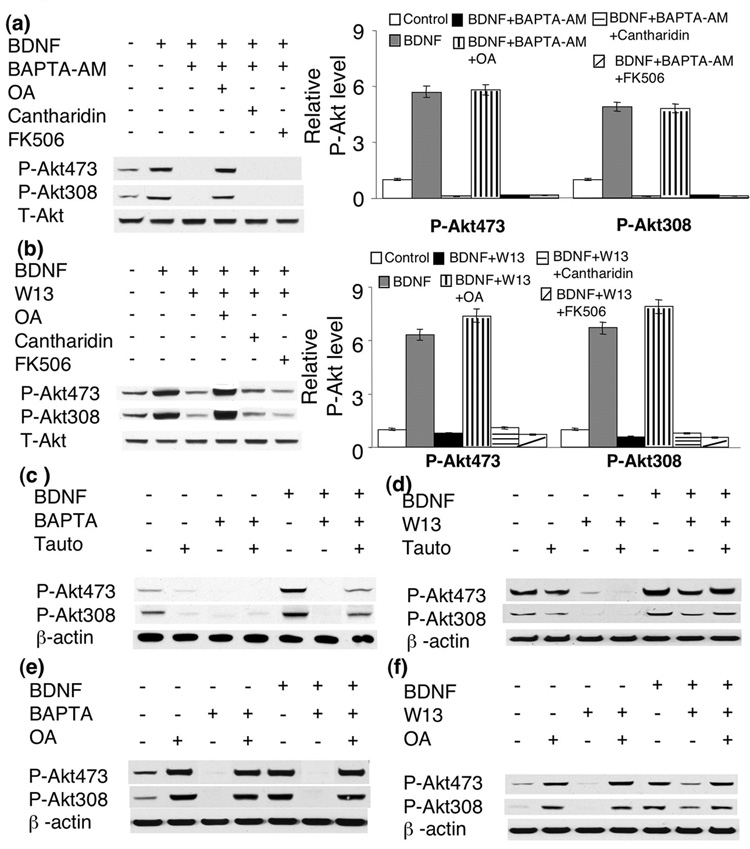
Because protein phosphatases (PPs) were suggested as important negative regulators on many protein kinases and to regulate numerous neuronal events [for reviews, please see (Hemmings et al. 1989; Winder and Sweatt 2001)], we thus examined the roles of the major neuronal PPs, including PP1, PP2A and PP2B, in BDNF-induced Akt activation. We found that inhibition of PP1 by okadaic acid (OA) (1uM) antagonized the abolishment of Akt phosphorylation by BAPTA-AM. In contrast, inhibition of PP2B by FK506 (1uM) has no effect (Fig. 9a). Although inhibition of PP2A by cantharidin (0.1uM) enhanced p-ERK and p-CaM KII (see supplementary Fig. 2), it did not rescue the suppression of p-AKT by BAPTA-AM in BDNF-stimulated neurons (Fig. 9a). Similarly, W13-attenuated Akt phosphorylation at S473 and T308 was also rescued by OA application. Neither cantharidin nor FK506 rescued Akt phosphorylation in W13-pretreated neurons (Fig. 9b). This indicates that PP1 may negatively regulate calcium-gated Akt activation in BDNF-stimulated neurons.
Fig. 9.
Protein phosphatase 1 (PP1) negatively regulates BDNF-induced Akt activation. a and b) PP1 inhibitor Okadaic Acid (OA, 1uM), but not PP2A inhibitor Cantharidin or PP2B inhibitor FK506, rescued p-Akt in BAPTA- (a) or W13- (b) treated neurons. c and d) tautomycin (3nM) attenuated the effects of BAPTA (c) or W13 (d) in BDNF-treated neurons. e) effects of OA, BAPTA and BDNF on p-Akt in cortical neurons. f) effects of OA, W13, and BDNF on p-Akt in cortical neurons. Akt phosphorylation at S473 and T308 were analyzed by Western blot. Representative images are shown on the left panels, and quantification on the right panels (n=3 for each treatment group).
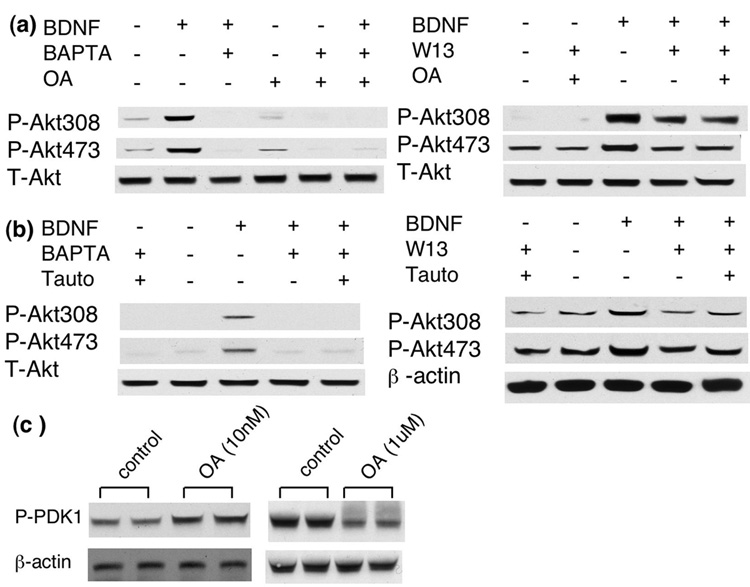
Because OA at 1uM may also inhibit PP2A, PP4, PP5, and PP6, we used another relatively selective PP1 inhibitor tautomycin. It was reported that tautomycin mainly inhibits PP1 (and marginally inhibits PP2A) at low concentration (3nM), and inhibits both PP1 and PP2A at high concentration (300nM) (MacKintosh and Klumpp 1990). We found that tautomycin (at 3nM) had minimal effects on basal p-Akt level, and did not rescue p-Akt in BAPTA-treated neurons. However, compared to BAPTA/BDNF-treated neurons, p-Akt at both S473 and T308 was significantly enhanced in BAPTA/BDNF/tautomycin-treated neurons (Fig. 9c). Similar rescuing effects of tautomycin (at 3nM) were observed for W13/BDNF-treated neurons (Fig. 9d). Interestingly, treatment with OA (at 1uM) enhanced basal p-Akt level, and also rescued p-Akt in BAPTA- and W13-treated neurons (Fig. 9e, and 9f). The level of p-AKT was comparable between BAPTA/OA- and BAPTA/BDNF/OA-treated neurons, indicating a ceiling effect of OA (at 1uM) (Fig. 9e). BDNF did not further enhance p-Akt in neurons pre-treated with both OA and W13 (Fig. 9f). These data demonstrated that inhibition of PP1 antagonized the effects of W13 and BAPTA. Difference between tautomycin and OA may be due to the different strength of these inhibitors.
Because OA and tautomycin also regulated PP2A, we tested their effects at different concentrations. As described earlier, low dose of OA (at 10nM) mainly inhibits PP2A and high dose of tautomycin (at 300nM) inhibits both PP1 and PP2A. Here, we show that OA at 10nM did not rescue the inhibiting effects of BAPTA and W13 (Fig. 10a). High dose of tautomycin (at 300nM) also failed to rescue p-Akt in BAPTA- and W13- treated neurons (Fig. 10b), suggesting the PP2A and PP1 might have opposing regulatory function on p-Akt. Surprisingly, low dose OA (10nM) enhanced p-PDK1, and high dose OA (1uM) decreased p-PDK1 (Fig. 10c). These data further demonstrated that the phosphorylation level of PDK1 at S241 and Akt at T308 was differentially regulated. In summary, these results demonstrated that PP1, but not PP2A, might be a negative regulator for Akt activation.
Fig. 10.
Low dose of OA (10nM, a) and high dose of tautomycin (300nM, b) did not rescue p-Akt in BAPTA- and W13-treated neurons. c). Okadaic acid enhanced p-PDK1 at 10nM, and suppressed p-PDK1 at 1uM.
Discussion
BDNF was initially isolated from pig brains as a neurotrophin that promotes neuronal survival (Barde et al. 1982). The protecting function of BDNF was demonstrated to inhibit apoptosis in different types of neurons (Ernfors et al. 1995; Lewin and Barde 1996). Previous investigations identified several signal transduction pathways what were activated by BDNF through TrkB (Segal and Greenberg 1996; Finkbeiner et al. 1997; Huang and Reichardt 2001; Reichardt 2006). First, BDNF-induced auto-phosphorylation of the TrkB receptor, which then activates PLC-γ, leading to elevation of IP3 and diacylglycerol (DAG), and promotes Ca2+ release from the intracellular pool and stimulates PKC. It is also hypothesized that PLC-γ-induced increase in [Ca2+]i stimulates CaM KII and IV through CaM, and consequently regulates the activity of both MAPK and Akt (Blum and Konnerth 2005). Second, BDNF activates Ras/Raf/MAPK through scaffold proteins Frs2, Shc, and Grb2. Third, BDNF-induced activation of PI3K phosphorylates Akt (Franke et al. 1997), and promotes cell survival by regulating the transcription of Bad/BCL2, caspases and GSK3. However, these molecular mechanisms were mostly investigated under pathological conditions, such as in the case of glutamate-induced neuronal death.
Consistent with the published data, we found that BDNF at 100ng/ml, but not at 5ng/ml, attenuated glutamate-induced cell death in cortical neurons. However, both 5ng/ml and 100ng/ml caused significant and comparable activation of p-Akt. Although blocking PI3K activity abolished the rescuing effects of BDNF on neuronal death (Almeida et al. 2005), our data demonstrated that the activation of PI3K-Akt alone was not sufficient to attenuate cell death. Because BDNF promotes neurotransmitter release (Paredes et al. 2007), stimulates mTOR-dependent protein translation (Takei et al. 2004), and induces the induction of several plasticity-related genes, the activation of Akt by low dose of BDNF may reflect its role in regulating long-term neuronal modifications. Although inhibition of MAPK significantly abolished p-Akt elevation in neurons treated with 100ng/ml BDNF (Almeida et al. 2005), the activation of PI3K/Akt by 5ng/ml BDNF is independent of MAPK. Additionally, at sub-nanomolar concentrations, BDNF-stimulated p-Akt elevation does not require the concomitant activation of PLC-γ. This is in contrast to the case of proapoptotic mouse epithelial cells, in which EGF-induced Akt activation and cell survival were significantly blocked by a PLC-γ inhibitor (Deb et al. 2004). We assume that the difference is due to tissue types and stimulants. .
PLC-γ, Ras-Raf-MAPK and PI3K-Akt are the major signal transduction pathways stimulated by BDNF. The cross-talk among these 3 pathways was extensively documented in many different types of cells. It was demonstrated that a dominant negative form of Ras GTPase inhibited PI3K activity (Rodriguez-Viciana et al. 1994). Potentially, PLC-γ may regulate PI3K activity through mediating IP3-dependent calcium release. Convincingly, the trkBPLC/PLC mutant mice, in which the signal transduction between TrkB and PLC-γ was blocked, were defective in Ca2+/CaM signaling and showed impairment in hippocampal LTP (Minichiello et al. 2002). However, we found that Akt activation was solely regulated by PI3K. Blocking PLC-γ and MAPK activity showed no effects on BDNF-induced Akt phosphorylation in cortical neurons.
We further demonstrated that blocking NMDA receptor, L-VGCC, and chelating extra-cellular calcium by EGTA did not affect BDNF-induced Akt phosphorylation. These results are surprising, and differ from a previous study describing the pro-survival role of Akt in the spinal cord motoneurons (MTNs) (Perez-Garcia et al. 2004). The study showed that the glial line-derived neurotrophic factor (GDNF) application (100ng/ml) induced [Ca2+]i elevation, phosphorylation of Akt, and promoted MTN survival. Although blocking L-VGCC by nifedipine showed no effects, chelating extra-cellular calcium by EGTA significantly dampened GDNF-induced phosphorylation at both S473 and T308. Therefore, the regulation of PI3K-Akt signaling by extra-cellular calcium may depend on 1) the types of neurotrophins (GDNF vs. BDNF), 2) the types of neurons (MTN vs. cortical neurons). Our results, however, are consistent with a previous study, in which nifedipine and EGTA failed to block Akt activation in cortical neurons treated with 100ng/ml BDNF (Cheng et al. 2003).
Several studies demonstrated the role of [Ca2+]i and CaM in regulating Akt phosphorylation in the context of describing neuro-protective effects of neurotrophic factors. First, NGF stimulated PI3K-Akt signaling in PC12 cells, and attenuated serum starvation-induced cell death. The protective effects of NGF were significantly reduced by inhibiting PI3K (by LY294002) and CaM (by W13). Consistently, chelating [Ca2+]i by BAPTA-AM and inhibiting CaM by W13 decreased NGF-induced Akt phosphorylation at both S473 and T308 in PC12 cells (Egea et al. 2001). Second, BNDF-stimulated Akt phosphorylation was abolished by BAPTA-AM or W13 in cortical neurons (Cheng et al. 2003) by W13 in PC12 cells and the chicken spinal cord motoneurons (Egea et al. 2001). Third, over-expression of CaM with mutated calcium binding sites abolished neuro-protective effects of BDNF and BDNF-induced Akt phosphorylation, although the phosphorylation sites (i.e. S473 or T308) were not specified (Cheng et al. 2003). However, it is not clear whether the same mechanism applies in neurons under non-pathological conditions. The role of basal level or elevated level of [Ca2+]i in stimulated neurons was not evidently demonstrated. Interestingly, we found that there was no significant increase in [Ca2+]i level after BDNF stimulation. These results implicate that the increase in intracellular calcium is not required for Akt phosphorylation. Consistently, KCl- and NMDA-induced calcium influx failed to activate Akt. Because chelation of intracellular calcium by BAPTA-AM abolished Akt phosphorylation, we suggest that the basal level of intracellular Ca2+ gate the PI3K-Akt signaling in BDNF-stimulated neurons. Although BDNF-induced elevation of [Ca2+]i was well documented (Finkbeiner et al. 1997; Li et al. 1998), it was shown that BDNF failed to increase intracellular Ca2+ in the visual cortex (Sakai et al. 1997; Pizzorusso et al. 2000). This confliction may be caused by different culture conditions, the age of neurons, and the dose of BDNF application. Our results showed that 5ng/ml BDNF failed to increase [Ca2+]i in cortical neurons on 7 DIV. We are aware that, at DIV7, the neurons are not fully matured, lacking glutamate-mediated calcium oscillation (Tanaka et al. 1996; Yoshimura et al. 2005). However, it has been demonstrated that axons and dendrites have been well established by DIV 5–7 (Dotti et al. 1988). Functional and mature synapses are also apparent in DIV 7 neurons; PSD95 and synapsin showed colocalization at this stage.
How does CaM regulate PI3K-Akt signaling? Because the involvement of CaM KII and IV was previously demonstrated in regulating numerous neuronal functions, we tested their effects by pharmacological inhibition. Neither KN62 nor KN93 blocked BDNF-induced Akt phosphorylation, consistent with previous reports (Egea et al. 2001; Cheng et al. 2003). Another possibility is that CaM may directly modulate PI3K activity. In deed, it was demonstrated that CaM physically bound the p85 regulatory, as well as the p110 catalytic subunit of PI3K in a calcium-dependent manner (Fischer et al. 1998; Perez-Garcia et al. 2004). In MTNs, treatment with W13 and BAPTA-AM significantly suppressed GDNF-stimulated PI3K activity (Perez-Garcia et al. 2004). However, inhibiting CaM by W13 in PC12 cells had no effects on NGF or BDNF-stimulated PI3K (Egea et al. 2001). The investigators concluded that CaM regulation on Akt phosphorylation might occur somewhere down stream of PI3K. Additionally, we found that the effect of BAPTA-AM was specific for PI3K-Akt signaling. Chelation of intracellular calcium by BAPTA-AM had no effects on BDNF-induced Trk phosphorylation (Fig. 7b) and ERK phosphorylation (Zheng and Wang, manuscript submitted).
Although Toker and colleagues suggested the phosphorylation of Akt at S473 may depend on T308 phosphorylation in HEK293 cells (Toker and Newton 2000), recent studies suggests these two sites may be differentially regulated by PDK1 and PDK2. For example, TNF-α induces Akt phosphorylation at S473 but not T308 (O'Toole et al. 2001). Studies with several IGF-II-overexpressing rhabdomyosarcomas cell lines demonstrated that the level of T308 phosphorylation did not always pairs with the level of S473 phosphorylation (Wan and Helman 2003). Although the cross talk between PDK1 and PDK2 in neurons is unknown, we showed that the phosphorylation at T308 and S473, however, displayed similar activation and inhibition profiles in BDNF-stimulated neurons.
Recently, the roles of protein phosphatases in synaptic plasticity (Winder and Sweatt 2001) and cell survival (Garcia et al. 2003) are strongly implicated. Our results showed that Akt phosphorylation was negatively regulated by PP1, suggesting a possible regulatory balance between PP1 and PI3K. Because PP4, PP5 and PP6 are all sensitive to PP1 and PP2A inhibitors including OA, tautomycin and cantharidin (Honkanen and Golden 2002), our experiment cannot completely exclude the effect of these PPs. However, similar to the effects of cantharidin, low dose of OA and high dose of tautomycin failed to affect p-Akt level, indicating that PP1 is, at least, a major regulator. Although several proteins, such as Inhibitor-1, DARPP-32 (dopamine-and cAMP-regulated phosphoprotein 32), and yotiao (reviewed by Winder and Sweatt 2001), have been reported to regulate PP1, it is not clear how PP1 activity is regulated in BDNF-stimulated neurons. Sala et al suggested that calcium influx from NMDA receptor may induce PP1 activity (Sala et al. 2000). It is interesting that co-application of NMDA and BDNF suppressed p-Akt (Fig. 6h). Future experiments would be designed to examine how calcium, in conjunction with BDNF, regulates molecules in the PP1 pathway.
In summary, the regulatory function of Ca2+/CaM on PI3K/Akt signaling is unknown in the situations unrelated to apoptosis. Our present work showed that intracellular Ca2+ elevation per se was not sufficient to activate PI3K-Akt signaling. Furthermore, low dose of BDNF is incapable to increase intracellular calcium concentration. Because BAPTA-AM completely abolished BDNF-induced p-Akt and OA/tautomycin rescued this abolishment, we conclude that the basal level of [Ca2+]i gates Akt activation in BDNF-stimulated neurons. We also suggest PP1 as a potential negative regulator for PI3K-Akt signaling.
Acknowledgement
We would like to thank Dr. Zachary F. Burton for his valuable comments on this manuscript. Dr. Xianju Zhou helped with the neuronal cultures. This work was supported by grants from the National Institutes of Health (MH076906 to H.W. and MH068347 to J.N.).
Abbreviations
- BAPTA-AM
1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid-Acetoxymethyl ester
- BDNF
brain derived neurotrophic factor
- [Ca2+]i
intra-cellular calcium
- DIV
days in vitro
- ERK/ MAPK
extracellular signal-regulated kinase/mitogen-activated protein kinase
- LTP
long-term potentiation
- PI3K
phosphoinositide 3-kinase
- PLC-γ
phospholipase C-gamma
- PP
protein phosphatase
- VGCC
voltage-gated calcium channels
Footnotes
Supplemental Fig. 1. Sub-nanomolar BDNF activates Akt without neural protective effects. a) 5ng/ml BDNF treatment did not rescue glutamate-induced neuronal cell death (p>0.05 compared with control). Neuronal cell death was induced by 125uM glutamate as described in materials and methods. The effects of BDNF at different doses (5ng/ml vs. 100 ng/ml) were compared (n=3 for each experiment group, p<0.05). The neurons were pre-treated with BDNF (at 5ng/ml or 100ng/ml) before glutamate challenges.
Supplemental Fig. 2. Effects of PP2A inhibitor cantharidin on a) p-CaMKII in cortical neurons and b) p-ERK in striatal preparation.
References
- Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Kane CD. Ca2+/calmodulin-dependent protein kinase IV and calcium signaling. Biometals. 1998;11:331–343. doi: 10.1023/a:1009276932076. [DOI] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Embo J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Lindholm D, Castren E, Wree A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J Cereb Blood Flow Metab. 1994;14:689–692. doi: 10.1038/jcbfm.1994.86. [DOI] [PubMed] [Google Scholar]
- Bianchi LM, Conover JC, Fritzsch B, DeChiara T, Lindsay RM, Yancopoulos GD. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Bosel J, Gandor F, Harms C, Synowitz M, Harms U, Djoufack PC, Megow D, Dirnagl U, Hortnagl H, Fink KB, Endres M. Neuroprotective effects of atorvastatin against glutamate-induced excitotoxicity in primary cortical neurones. J Neurochem. 2005;92:1386–1398. doi: 10.1111/j.1471-4159.2004.02980.x. [DOI] [PubMed] [Google Scholar]
- Casamayor A, Morrice NA, Alessi DR. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J. 1999;342(Pt 2):287–292. [PMC free article] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wang S, Yang D, Xiao R, Mattson MP. Calmodulin mediates brain-derived neurotrophic factor cell survival signaling upstream of Akt kinase in embryonic neocortical neurons. J Biol Chem. 2003;278:7591–7599. doi: 10.1074/jbc.M207232200. [DOI] [PubMed] [Google Scholar]
- Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem. 2004;279:38903–38911. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea J, Espinet C, Soler RM, Dolcet X, Yuste VJ, Encinas M, Iglesias M, Rocamora N, Comella JX. Neuronal survival induced by neurotrophins requires calmodulin. J Cell Biol. 2001;154:585–597. doi: 10.1083/jcb.200101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39:799–807. [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fischer R, Julsgart J, Berchtold MW. High affinity calmodulin target sequence in the signalling molecule PI 3-kinase. FEBS Lett. 1998;425:175–177. doi: 10.1016/s0014-5793(98)00225-7. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie. 2003;85:721–726. doi: 10.1016/j.biochi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Gerling N, Culmsee C, Klumpp S, Krieglstein J. The tyrosine phosphatase inhibitor orthovanadate mimics NGF-induced neuroprotective signaling in rat hippocampal neurons. Neurochem Int. 2004;44:505–520. doi: 10.1016/j.neuint.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Griffith LC. Calcium/calmodulin-dependent protein kinase II: an unforgettable kinase. J Neurosci. 2004;24:8391–8393. doi: 10.1523/JNEUROSCI.2888-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Nairn AC, McGuinness TL, Huganir RL, Greengard P. Role of protein phosphorylation in neuronal signal transduction. Faseb J. 1989;3:1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hill MM, Andjelkovic M, Brazil DP, Ferrari S, Fabbro D, Hemmings BA. Insulin-stimulated protein kinase B phosphorylation on Ser-473 is independent of its activity and occurs through a staurosporine-insensitive kinase. J Biol Chem. 2001;276:25643–25646. doi: 10.1074/jbc.C100174200. [DOI] [PubMed] [Google Scholar]
- Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Karpova A, Sanna PP, Behnisch T. Involvement of multiple phosphatidylinositol 3-kinase-dependent pathways in the persistence of late-phase long term potentiation expression. Neuroscience. 2006;137:833–841. doi: 10.1016/j.neuroscience.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Kanki R, Yamashita H, Akaike A. Protective effect of dopamine D2 agonists in cortical neurons via the phosphatidylinositol 3 kinase cascade. J Neurosci Res. 2002;70:274–282. doi: 10.1002/jnr.10426. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Krasilnikov MA. Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc) 2000;65:59–67. [PubMed] [Google Scholar]
- Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. Faseb J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Klumpp S. Tautomycin from the bacterium Streptomyces verticillatus. Another potent and specific inhibitor of protein phosphatases 1 and 2A. FEBS Lett. 1990;277:137–140. doi: 10.1016/0014-5793(90)80828-7. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36:121–137. doi: 10.1016/s0896-6273(02)00942-x. [DOI] [PubMed] [Google Scholar]
- Miyata K, Omori N, Uchino H, Yamaguchi T, Isshiki A, Shibasaki F. Involvement of the brain-derived neurotrophic factor/TrkB pathway in neuroprotecive effect of cyclosporin A in forebrain ischemia. Neuroscience. 2001;105:571–578. doi: 10.1016/s0306-4522(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yamagishi S, Adachi N, Matsumoto T, Yokomaku D, Yamada M, Hatanaka H. Brain-derived neurotrophic factor-induced potentiation of Ca(2+) oscillations in developing cortical neurons. J Biol Chem. 2002;277:6520–6529. doi: 10.1074/jbc.M109139200. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- O'Toole A, Moule SK, Lockyer PJ, Halestrap AP. Tumour necrosis factor-alpha activation of protein kinase B in WEHI-164 cells is accompanied by increased phosphorylation of Ser473, but not Thr308. Biochem J. 2001;359:119–127. doi: 10.1042/0264-6021:3590119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Watabe AM, Grant SG, O'Dell TJ. Phosphatidylinositol 3-kinase regulates the induction of long-term potentiation through extracellular signal-related kinase-independent mechanisms. J Neurosci. 2003;23:3679–3688. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes D, Granholm AC, Bickford PC. Effects of NGF and BDNF on baseline glutamate and dopamine release in the hippocampal formation of the adult rat. Brain Res. 2007;1141:56–64. doi: 10.1016/j.brainres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MJ, Cena V, de Pablo Y, Llovera M, Comella JX, Soler RM. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Ratto GM, Putignano E, Maffei L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J Neurosci. 2000;20:2809–2816. doi: 10.1523/JNEUROSCI.20-08-02809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser S, Impey S, Xia Z, Storm DR. Brain-Derived Neurotrophic Factor Protection of Cortical Neurons from Serum Withdrawal-Induced Apoptosis Is Inhibited by cAMP. J. Neurosci. 2003;23:4420–4427. doi: 10.1523/JNEUROSCI.23-11-04420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rose CR, Blum R, Kafitz KW, Kovalchuk Y, Konnerth A. From modulator to mediator: rapid effects of BDNF on ion channels. Bioessays. 2004;26:1185–1194. doi: 10.1002/bies.20118. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamada M, Numakawa T, Ogura A, Hatanaka H. BDNF potentiates spontaneous Ca2+ oscillations in cultured hippocampal neurons. Brain Res. 1997;778:318–328. doi: 10.1016/s0006-8993(97)01052-4. [DOI] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez AM, Llansola M, Felipo V. Modulation of NMDA receptors by AKT kinase. Neurochem Int. 2006;49:351–358. doi: 10.1016/j.neuint.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Lauener RW, Duronio V. Role of phosphatidylinositol 3-OH-kinase activity in the inhibition of apoptosis in haemopoietic cells: phosphatidylinositol 3-OH-kinase inhibitors reveal a difference in signalling between interleukin-3 and granulocyte-macrophage colony stimulating factor. Biochem J. 1995;312(Pt 1):159–162. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning Doses of NMDA Promote Neuroprotection by Enhancing Neuronal Excitability. J. Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroppolo A, Guinea B, Tian C, Sommer J, Ehrlich ME. Role of phosphatidylinositide 3-kinase in brain-derived neurotrophic factor-induced DARPP-32 expression in medium size spiny neurons in vitro. J Neurochem. 2001;79:1027–1032. doi: 10.1046/j.1471-4159.2001.00651.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Whitman M, Cantley LC, Erikson RL. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984;81:2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Intracellular calcium oscillation in cultured rat hippocampal neurons: a model for glutamatergic neurotransmission. Jpn J Pharmacol. 1996;70:89–93. doi: 10.1254/jjp.70.89. [DOI] [PubMed] [Google Scholar]
- Toker A, Newton AC. Akt/protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J Biol Chem. 2000;275:8271–8274. doi: 10.1074/jbc.275.12.8271. [DOI] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- Wan X, Helman LJ. Levels of PTEN protein modulate Akt phosphorylation on serine 473, but not on threonine 308, in IGF-II-overexpressing rhabdomyosarcomas cells. Oncogene. 2003;22:8205–8211. doi: 10.1038/sj.onc.1206878. [DOI] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Yang B, Gu Q. Contribution of glutamate receptors to brain-derived neurotrophic factor-induced elevation of intracellular Ca2+levels. Neuroreport. 2005;16:977–980. doi: 10.1097/00001756-200506210-00019. [DOI] [PubMed] [Google Scholar]
- Yang F, He X, Feng L, Mizuno K, Liu XW, Russell J, Xiong WC, Lu B. PI-3 kinase and IP3 are both necessary and sufficient to mediate NT3-induced synaptic potentiation. Nat Neurosci. 2001;4:19–28. doi: 10.1038/82858. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Sugai T, Honjo M, Segami N, Onoda N. NMDA receptor-dependent oscillatory signal outputs from the retrosplenial cortex triggered by a non-NMDA receptor-dependent signal input from the visual cortex. Brain Res. 2005;1045:12–21. doi: 10.1016/j.brainres.2005.02.084. [DOI] [PubMed] [Google Scholar]