Abstract
The increased palatability of modern diet contributes to eating beyond homeostatic need and in turn to the growing prevalence of obesity. How palatability is coded in taste-evoked neural activity and whether this activity differs between obese and lean remains unknown. To investigate this, we used extracellular single-unit recording in the second central gustatory relay, the pontine parabrachial nucleus while stimulating the tongue with various concentrations of sucrose (0.01–1.5 M) in Otsuka Long Evans Tokushima Fatty (OLETF) rats, lacking CCK-1R. The analyses included a total of 179 taste-responsive neurons in age-matched prediabetic, obese OLETF and lean Long Evans Tokushima Otsuka (LETO) controls. Compared with LETO, we found more NaCl-, and fewer sucrose-responsive neurons (67 vs. 47% and 14 vs. 32%), and an overall reduced response magnitude to sucrose in the OLETF rats. Further, in the obese rats there was a rightward shift in sucrose concentration-response functions relative to lean controls with a higher response-threshold (0.37 ± 0.05 vs. 0.23 ± 0.2 M, P < 0.05) and maximal neural response to higher sucrose concentrations (0.96 ± 0.07 vs. 0.56 ± 0.5 M, P < 0.001). These findings demonstrate altered central gustatory processing for sucrose in obese OLETF rat and further support the notion that palatability is encoded in the across neuron pattern.
INTRODUCTION
The prevalence of obesity in the United States has increased steadily over the past 30 years (Ogden et al. 2006). Although the etiology of obesity is complex, the high palatability of the modern diet in general and individual differences in responsivity to orosensory stimulation and food reward in particular may contribute to development of dietary obesity both in human and animal models (Hill et al. 2000; Kant 2000; Lucas and Sclafani 1990; Sclafani 2004; Sorensen et al. 2003; Yeomans et al. 2004). Less is known, however, about the opposite relationship that is whether taste functions (sensory and perceptional) are different in obese than in lean individuals.
A common view is that the obese individuals show enhanced liking for palatable foods. In fact, the data on this are controversial. Most studies found no effects of body weight on the perception of sweetness (Drewnowski et al. 1991; Frijters and Rasmussen-Conrad 1982; Grinker et al. 1972; Malcolm et al. 1980), whereas others did. For example, in Pima Indians, heightened hedonic response for sweet solutions was found to be associated with weight gain (Salbe et al. 2004). Multiple studies have shown increased preference for sweet in both African American children and adults (Bacon et al. 1994; Desor et al. 1975; Schiffman et al. 2000) in which population obesity is ∼40% more prevalent than in white Americans (Kumanyika 1993). Recently Bartoshuk et al. (2006) used an improved methodology for testing sensory-hedonic experiences and demonstrated that liking for sweet increases as BMI increases potentially due to reduced taste sensitivity. Despite the availability of animal models of obesity with feasibility of using invasive methods, there is no report in the literature directly investigating central gustatory processes relevant for sweet taste coding.
Recent research in our laboratory has focused on taste preference and sucrose reward functions in a rat model of obesity, the Otsuka Long Evans Tokushima Fatty (OLETF) rat. The OLETF rat has a congenital cholecystokinin (CCK)-1 receptor deficiency, resulting from a 6,847-base-pair deletion spanning the promoter region and the first and second exons of the CCK-1 receptor gene (Takiguchi et al. 1997). Consistent with the role of CCK in mediation of satiety (Moran 2000), OLETF rats have deficits in the control of meal size. The OLETF rats chronically overeat, gradually become obese, and develop noninsulin-dependent diabetes mellitus (Bi and Moran 2002; Kawano et al. 1992). In addition to diminished sensitivity to postingestive satiation signals (Covasa and Ritter 2001; Moran and Bi 2006) and vagal responses (Covasa and Ritter 2005), OLETF rats demonstrate increased avidity to sweet. Specifically, compared with lean Long Evans Tokushima Otsuka (LETO) controls, the OLETF rats exhibit increased real and sham intake of normally preferred sucrose solutions with a heightened preference for higher over lower concentrations (1 vs. 0.3 M), even before manifest diabetes occurs (De Jonghe et al. 2005). The OLETF rat also expresses increased lick responses in brief (10-s) access tests to sucrose as well as various agents that taste sweet to human including the noncaloric sweetener saccharin and the amino acid alanine (Hajnal et al. 2005). Moreover, these effects display a progressive increase with the development of obesity and diabetes (Hajnal et al. 2005). Recent studies from our laboratory have demonstrated a direct association between increased sweet preference and heightened reward sensitivity in the prediabetic obese OLETF rats. Compared with lean controls, these rats work harder for sucrose solutions on a progressive ratio reinforcement schedule (Hajnal et al. 2007) and also form conditioned taste preference based on orosensory rather than postingestive effects of sucrose (De Jonghe et al. 2007).
Despite the strong indication for altered orosensory processing, there are no reports of studies examining central taste responses in this strain except an abstract of a pilot study from our lab (Lundy and Hajnal 2006). Therefore the present study investigated whether taste processing in the pontine parabrachial nucleus (PBN) differs between prediabetic obese OLETF and age-matched lean LETO rats. The PBN is the second central gustatory relay in the rat (Norgren 1974, 1976; Norgren and Pfaffmann 1975). We chose to record from PBN because there is strong evidence indicating its role in motivational-hedonic integration of taste functions (Hajnal and Norgren 2005; Norgren et al. 2003, 2006; Scalera et al. 1995).
METHODS
Subjects
Male OLETF (n = 4) and LETO (n = 3) rats were obtained as a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. Rats were 4 wk old at their arrival, and recordings were conducted at 25–27 wk. All rats were housed individually and maintained in a temperature-controlled vivarium on a constant 12:12-h light-dark cycle (lights on at 0700). Filtered tap water and standard pelleted rat chow (Harlan Teklad 2018 rodent diet) were available ad libitum throughout the whole experiment except during the recording sessions and before the oral glucose tolerance tests.
All protocols used were conducted in accordance with the National Institute of Health Guide for the Use of Laboratory Animals (National Institutes of Health Publications No. 80–23) and approved by The Pennsylvania State University Institutional Animal Care and Use Committee. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Surgeries and semi-chronic preparation
We used a semi-chronic preparation that has been proven successful in previous studies (Lundy and Norgren 2001, 2004a). This procedure is essentially a modified chronic recording (Hajnal et al. 1999) in which a rat is operated to localize the taste area and set up with an acrylic head piece with a reclosable opening above the target brain area. This allows repetitive recording with a minimal and noninvasive preparation in a slightly anesthetized condition (see next section).
Briefly, after being anesthetized [pentobarbital sodium (Nembutal), 50 mg/kg ip, Abbott Laboratories, North Chicago, IL], the rat was mounted in a stereotaxic apparatus (Stoelting, Wood Dale, IL) using blunt ear bars with the skull leveled between bregma and lamda. The dorsal surface of the head was shaved and swabbed with iodine (Betadine) solution. The skull was exposed with a midline longitudinal incision and cleaned of periosteum. Just caudal to the interparietal suture, a 3 × 5-mm oval area of the bone was drilled away, the exposed dura was excised, and a cap of dental acrylic was anchored to the skull using stainless steel screws (1–72 × 1/8 in; Small Parts). Stainless steel wire was soldered onto two screws to serve as a ground. An antibiotic ointment (Erythromycin, Bausch and Lomb, Tampa, FL) was placed on the exposed brain and then covered with a nontoxic silicone adhesive (Kwik-Cast, World Precision Instruments, Sarasota, FL). The acrylic was built up on the skull and molded around the conical ends of two sets of stainless steel rods that were attached rigidly to the ear bars. During subsequent recording sessions, these rods were reattached to the ear bars more medially and fitted back into the acrylic impressions, thus painlessly fixing the rat's head in the stereotaxic plane.
Electrophysiological recordings and sapid stimuli
Following 1 wk of recovery from the first surgery, the rats were re-anesthetized with a lower dose of Nembutal (35 mg/kg ip, supplemented with 0.1 ml every hour) and mounted by the steel rods in the stereotaxic apparatus, and the silicon cork was removed from the hole on the acrylic headpiece and the underlying brain surface was cleaned with sterile physiological saline. The PBN gustatory area was located electrophysiologically using an electrode tilted 20° off perpendicular (tip anterior) to avoid damage to the transverse sinus. For extracellular single-unit recordings, tungsten microelectrodes were used (Z = 3–6 MΩ at 1 kHz; FHC, Bowdoinham, ME).
For taste stimulation, the tongue was gently pulled out from the mouth and tied down in an ∼45–45° side-down angle using a 5–0 silk surgical suture in the midline. The anterior 2/3 of the tongue was stimulated using a computer-controlled 16-channel fluid delivery system (Octaflow, ALA Scientific Instruments, Westbury, NY). The taste stimuli were the following: 0.1 M NaCl (Fisher Scientific, Fair Lawn, NJ), 0.01 M citric acid (J. T. Baker, Phillipsburg, NJ), 0.003 M quinine-HCl (Sigma-Aldrich, St. Louis, MO), 0.03 M monosodium glutamate (MSG, Sigma-Aldrich), and 0.01, 0.03, 0.1, 0.3, 1, and 1.5 M sucrose (Sigma-Aldrich). All solutions were prepared in distilled water, delivered at flow rate of 100 μl/s, and at room temperature (22–25°C). Each taste stimulus was presented for 10 s and was followed by a 10-s water rinse with no pause between to keep a continuous flow on the tongue avoiding on-off artifacts from mechanical stimulation. Separate stimulation sequences were used for basic taste characterization of a neuron (rinse, water, rinse, 0.3 M sucrose, rinse, NaCl, rinse, citric acid, rinse, quinine-HCl, rinse, MSG, rinse) and for consecutive concentration-response tests for sucrose (6 sucrose concentrations in ascending order, each bracketed with water rinses).
Extracellular action potentials of PBN taste neurons were recorded with a fully digital data-acquisition system (Cheetah, Neuralynx, Tucson, AZ). A recording session typically lasted 3–5 h but occurred not more than once every 4 days in a single rat.
Data analysis and classification of taste neurons
Single-unit analysis was performed off-line using the Spike 2 software (Cambridge Electronic Design, Cambridge, UK). After performing standard spike sorting routines, single-unit frequency histograms were built and pre- and poststimulus 5-s data were exported into text files and further analyzed with Excel 2003 (MicroSoft, Seattle, WA) and Statistica 6.1 (StatSoft. Tulsa, OK) softwares for PC computers. Two-tailed, independent t-test were used to determine whether taste responses were statistically different (α = 0.05) to the prestimulus 5-s water baseline activity. Nonsignificant responses were excluded from further analyses. Corrected neural responses to a taste stimulus were calculated by subtracting the 5-s discharge rate (spike/s or Hz) to each stimulus from its preceding 5-s discharge rate to water. In some comparisons, in addition to the corrected responses, the 5-s normalized responses were also calculated (baseline = 1) to control for a potential effect from differential baseline activity between strains with respect to neuronal categories. For strain and concentration effects, two-way ANOVAs were performed. When appropriate, post hoc t-test were used to determine the source of statistically significant differences. For nonparametric comparisons, χ2 tests (df = 1) were applied by using 2 × 2 frequency tables. All data were expressed as means ± SE. Differences were considered statistically significant if P < 0.05. Statistical analyses were computed with Statistica software for PC (version 6.1; StatSoft).
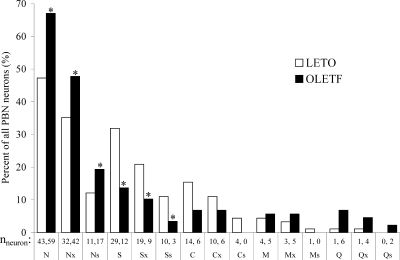
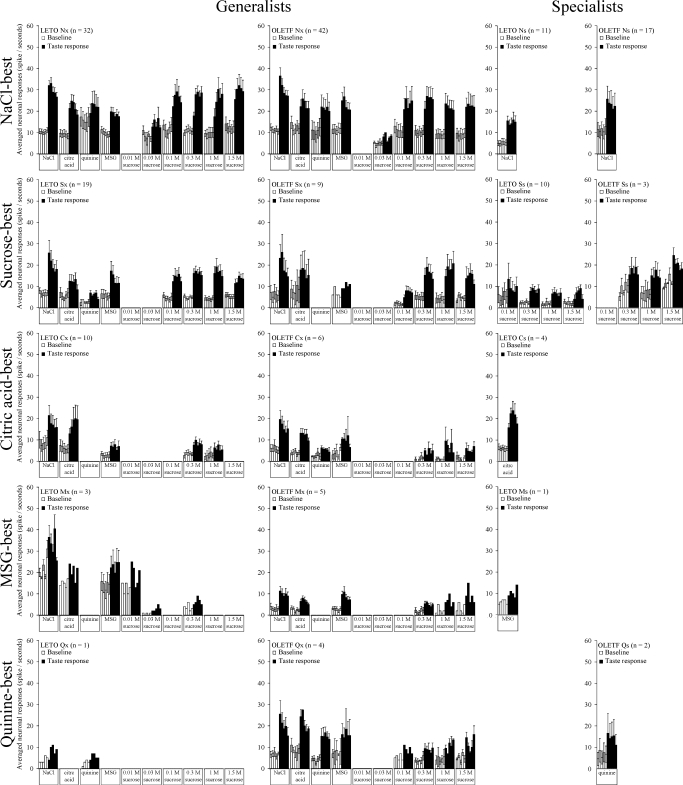
Based on the largest response to the standard stimuli (best stimulus) determined by the poststimulus 5-s response magnitudes compared with the prestimulus 5-s baseline activity (during continuous water stimulation of the tongue), five major classes [NaCl (N)-best, sucrose (S)-best, citric acid (C)-best, quinine-HCl (Q)-best, or MSG (M)-best] were characterized and further divided in subclasses. A neuron responding exclusively to one taste stimulus (e.g., Ns, Ss, Cs, Qs, and Ms neurons) was classified as “taste-specialist” (Ts), whereas neurons that responded to more than one taste stimulus (e.g., Nx, Sx, Cx, Qx, and Mx neurons) were labeled as “taste-generalist” (Tx). Accordingly, the total number of neurons responding ‘best’ to one particular type of taste is the sum of the two main subclasses: T = Ts + Tx (Fig. 1). In some analyses (see Fig. 7) within the Nx category, the more “narrowly tuned” neurons that responded best to NaCl and second best to sucrose were considered separately from the rest of the generalists neurons and labeled as NS units.
FIG. 1.
Percent distribution and number of recorded taste neurons across neuron categories. N, NaCl-best; Nx, NaCl-generalist; Ns, NaCl-specialist; S, sucrose-best; Sx, sucrose-generalist; Ss, sucrose-specialist; C, citric acid-best; Cx, citric acid-generalist; Cs, citric acid-specialist; M, MSG-best; Mx, MSG-generalist; Ms, MSG-specialist; Q, quinine-best; Qx, quinine-generalist; Qs, quinine-specialist. *P < 0.05
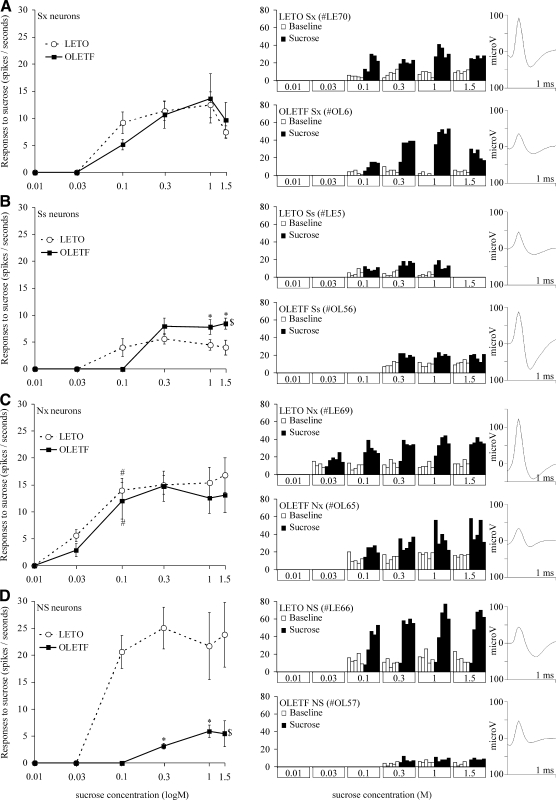
FIG. 7.
Mean corrected neuronal response magnitudes to various concentrations of sucrose (left) representative individual neuronal responses (middle) and corresponding action potential waveform (right). A: broadly tuned main band (Sx); B: narrowly tuned main band (Ss); C: broadly tuned side band (Nx); D: narrowly tuned side band (NS) sucrose-sensitive neurons. $, statistical differences between strains based on overall ANOVA; *, statistical differences between strains for a particular concentration; #, statistical difference between the actual and the former sucrose response magnitude within the same strain. For the actual values and comparisons, see results.
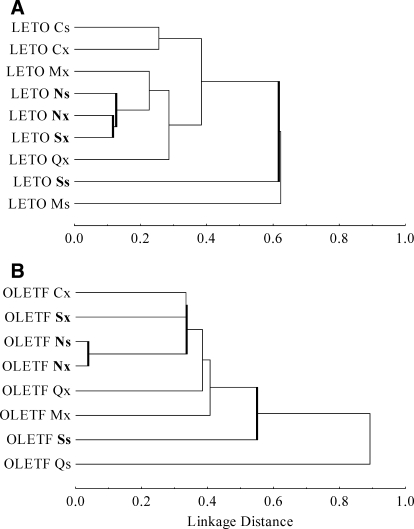
After the classification of the different taste neuron subclasses, we compared the basic taste coding similarities of these groups within the OLETF or LETO strain using hierarchical cluster analysis (Euclidian model and the average linkage method based on the Pearson's product–moment correlation coefficients) on the mean corrected taste responses of each group (Fig. 4).
FIG. 4.
Dendrograms of the main taste neuron subclasses in lean LETO (A) and obese OLETF (B) rats. Abscissa: linkage distance (1-Pearson r) between response categories.
To asses representation of a specific taste within the entire population of PBN neurons, the proportional response or power (pi) for a particular taste stimulus was computed by dividing the 5-s corrected responses to that particular stimulus by the sum of responses to all five tastants tested on the same neuron (pn, NaCl-proportional response; ps, sucrose-proportional response; pc, citric-acid-proportional response; pq, quinine-proportional response; pm, MSG-proportional response). After computing the taste power for each taste-responsive neuron, the mean values derived from all neurons were compared across stimuli for the entire population. Because this measure provides critical information about how a particular taste is coded in a larger population of neurons, we used this analysis to compare the power of sucrose-evoked neural activity in obese and lean rats. An additional population-wide measure, derived from power, is the “breadth of responsiveness.” It is determined based on the entropy (H) of the taste neurons (Smith and Travers 1979) and calculated from the proportional responses (pi) of the four main tastants (excluding MSG) using the formula
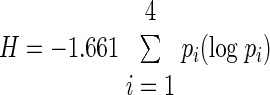 |
According to this formula, neurons that respond with equal proportional response to all four tastants have maximal entropy H = 1, whereas units specialized to some tastes express smaller H values. In contrast to power that deals with the individual tastes' relative signal strength, entropy provides general information on the tuning of the tested neuronal population.
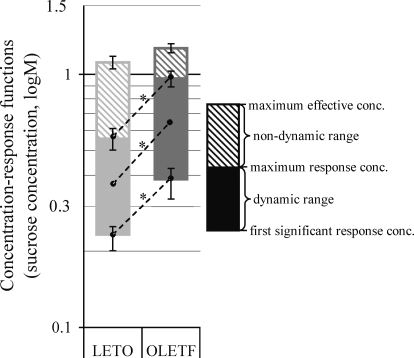
For evaluation of the sucrose-concentration response functions of the individual PBN taste neurons, the following parameters were defined. First significant response concentration: the lowest sucrose concentration that results in a significant neuronal taste response determined by significant t-test (P < 0.05) compared with the 5-s prestimulus water baseline. Maximum response concentration: the stimulus (sucrose) concentration that causes the highest magnitude taste response in the neuronal activity (one response magnitude is higher than the other if there is at least a 10% increase in the normalized firing rate). Maximum effective concentration: the highest applied sucrose concentration that results in significant taste response. It is usually a higher concentration value than the maximum response concentration because in this case the neuronal response magnitude is not required to be larger than the former response. Dynamic sucrose concentration range: a particular range within the tested sucrose concentrations (0.01, 0.03, 0.1, 0.3, 1, and 1.5 M), in which the consecutive higher concentrations are potent to cause ≥10% increase in the normalized neuronal firing rate over the effect of the one lower concentration. This range may also be defined by the difference between the maximum response concentration and the first significant response concentration. Nondynamic sucrose concentration range or “plateau”: a particular range within the tested increasing sucrose concentrations, in which the consecutive higher concentrations do not cause increase in the normalized neuronal firing rate. This range may also be defined by the difference between the maximum effective concentration and the maximum response concentration.
Oral glucose tolerance tests
To establish staging of altered glucose control as a result of the diabetic trait of the OLETF rats, an oral glucose tolerance test (OGTT) was carried out 3 days before the recording sessions. After a 16-h fasting period, an oral glucose load (2 g/kg) was delivered to each rat orally via a latex gavage. Blood glucose was measured before and at 30, 60, 90, and 120 min postglucose loading using a standard glucometer (LifeScan, One-Touch Basic). Data from OGTT tests were analyzed using one-way ANOVA, and the area under the curve (AUC) was calculated for each group and compared between strains at each time point using unpaired Student's t-test. Animals were classified as diabetic if the peak level of plasma glucose was ≥300 mg/dl (16.7 mmol/l) and >200 mg/dl at 120 min (Kawano et al. 1992). We considered the OLETF rats prediabetic if their peak plasma glucose levels were significantly elevated compared with LETOs but did not exceed the above values (Yagi et al. 1997).
Quantitative body composition analysis
Visceral and subcutaneus adiposity as well as lean body mass and body water content were determined in a separate set of animals (4 OLETF, 4 LETO) from the same colony at 26 wk using NMR technology (Bruker Minispec LF90 TD-NMR analyzer).
Histology
After the experiments, the rats were given a lethal dose of Nembutal (100 mg/kg ip) and perfused intracardially with 0.9% saline and 10% Formalin. The brain was removed, and 40-μm frozen slices were cut. Sections were stained with cresyl violet and studied under a light microscope to verify placement of the electrode tracks.
RESULTS
Age, body weight, and adiposity
At the time of first recording session (OLETF: 26.3 ± 1.1 wk, LETO: 25 ± 0.6 wk), OLETF rats were significantly heavier than age-matched LETO cohorts [580 ± 31 vs. 481 ± 21 g; F(1,5) = 8.288, P < 0.05]. Body composition analysis in a separate set of age-matched litter-mates demonstrated significantly higher body fat content in OLETF compared with LETO rats (31.13 ± 0.59 vs. 25.48 ± 0.62%, P < 0.01).
Oral glucose tolerance tests
Fasting blood glucose levels and responses to intragastric glucose load were tested in each rat ≥1 wk following the surgeries and 3 days prior to the first recording sessions. Whereas fasting blood glucose levels were normal and did not differ between strains [OLETF: 89.75 ± 2.29 mg/dl, LETO: 90.00 ± 2.01 mg/dl; F(1,5) = 0.006, P = 0.940, n = 7], glucose tolerance was significantly impaired in OLETF rats compared with age-matched LETO. The OGTTs showed significantly higher glucose levels in OLETF relative to LETO rats at 30 and 60 min (30 min: 197 ± 11 vs. 147 ± 4 mg/dl, P < 0.05; 60 min: 179 ± 12 vs. 131 ± 13 mg/dl, P < 0.05). AUC analysis revealed that OLETF rats expressed 210% increased blood glucose responses [F(1,5) = 7.187, P < 0.05]. This finding indicates that despite increased glucose intolerance, OLETF rats did not meet the criteria for clinical diabetes at the time of the taste recording sessions (Kawano et al. 1992). This stage is commonly referred to as “prediabetes” (Yagi et al. 1997), and its occurrence in this sample matches with previous studies using OLETF rats at a similar age (De Jonghe et al. 2005, 2007; Hajnal et al. 2005, 2007).
Histology
A total of 211 penetrations were made into the pons in seven rats, and in all rats, both the left and right sides were explored. Single- or multiunit electrophysiological responses to sapid stimuli occurred in 141 of these penetrations. Taste testing of neurons resulted in significant taste responses in 103 recordings (51 in OLETF, 52 in LETO) yielding a total of 179 distinct single-neuron units. To control for possible neuronal damage caused by multiple penetrations, we compared response magnitudes across recording sessions. We found no significant correlation between the normalized responses to NaCl, citric acid, quinine, MSG, or 0.3 M sucrose and repeated penetrations, either in LETO (correlation coefficients: −0.12, −0.05, 0.28, −0.08, −0.20, respectively) or in OLETF rats (correlation coefficients: −0.15, −0.14, 0.04, 0.06, −0.09, respectively).
Based on the stereotaxic coordinates of the penetrations and the residual track marks from the histology, the taste-responsive neurons were located in the caudomedial quadrant of the PBN extending from near the dorsal surface of the pons through the brachium conjunctivum and into the compact layer of cells between the brachium conjunctivum and the mesencephalic trigeminal nucleus. This approximate location is consistent with previous samples from chronic and semi-chronic studies (Hajnal et al. 1999; Lundy and Norgren 2001, 2004a) and with acute experiments in which localization was an objective (Norgren and Pfaffmann 1975). Because the penetrations were made over the course of a month, and assuming an accuracy of at best 100 μm for such measurement in chronic studies, more precise localization of individual recording sites is impractical.
Basic characteristics of taste neurons
We recorded and analyzed altogether 844 taste responses elicited by 179 PBN neurons in four obese OLETF and three lean LETO rats (88 and 91 neurons, respectively). Based on the largest response to the standard stimuli, the neurons were classified as follows: 59/43 NaCl-best (N), 12/29 sucrose-best (S), 6/14 citric acid-best (C), 6/1 QHCl-best (Q), and MSG-best (M) 4/5 in OLETF and LETO rats, respectively.
Figure 1 depicts the percent distribution of recorded taste neurons with respect to subgroups. Further analysis demonstrated an overall strain difference in distribution of neuron types [F(1,14) = 117.256, P < 0.001]. In the sample obtained from OLETF rats, there were significantly more N-best and less S-best neurons (66 vs. 48% and 14 vs. 32%, respectively; χ2 = 10.85, df = 1, P < 0.01). A similar difference was observed in the number of specialist neurons: more N-specific and less S-specific units in the OLETF rats compared with LETO cohorts (19 vs. 12% and 3 vs. 11%, respectively; χ2 = 5.35, df = 1, P < 0.05). Due to small sample size, the statistical power of χ2-tests for the C-, M-, or Q-best neurons was not sufficient.
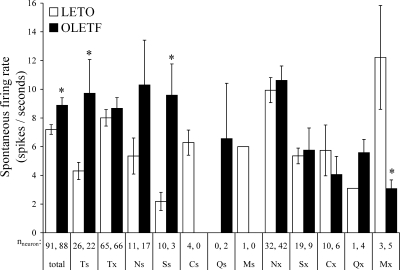
Spontaneous firing rate
The mean spontaneous firing rate of 179 taste neurons was 8.19 + 3.8 spike/s. Compared with LETO, taste neurons in the OLETF rats exhibited significantly higher baseline activity [8.88 ± 0.53 vs. 7.19 ± 0.34 Hz; P < 0.01; F(1,787) = 8.368, P = 0.004]. A breakdown of spontaneous firing rate across neuronal categories is shown in Fig. 2. This analysis revealed that the overall increased spontaneous firing rate in OLETF rats derived from the specialists neurons (Ts: 9.71 ± 2.36 vs. 4.31 ± 0.60 Hz, OLETF and LETO, respectively; P < 0.001) and that this effect was the most pronounced in the S-specialist neurons (Ss: 9.59 ± 2.18 vs. 2.19 ± 0.63 Hz, OLETF and LETO, respectively; P < 0.001). In contrast, M-best neurons (LETO: 11.33 ± 3.18 vs. OLETF: 3.08 ± 0.60 Hz; P < 0.01), including MSG-generalists (LETO: 12.22 ± 3.62 vs. OLETF: 3.08 ± 0.60 Hz; P < 0.005) had lower spontaneous firing rate in the OLETF rats. Notwithstanding, there was no strain difference in N-units' basal activity.
FIG. 2.
Comparison of average spontaneous firing rates between lean LETO, □ and obese OLETF, ▪ across main neuron categories. Ts, taste-specialist neurons; Tx, taste-generalist neurons. *P < 0.05.
Breadth of responsiveness
Of the total of 179 neurons recorded, 22 (26%) and 25 (29%) neurons in OLETF and LETO rats, respectively, were specific to one of the sapid stimuli. Despite a differential distribution with respect to the number of neurons responding specifically to sucrose and NaCl (i.e., less Ss and more Ns units occurring in OLETF vs. LETO), there was no strain difference in the overall response categories. Similarly, the mean entropy (H) measures for the generalist neurons in any subcategories were not significantly different between the two strains (HNx: 0.57 and 0.56; HSx: 0.53 and 0.54; HCx: 0.57 vs. 0.61; HQx: 0.49 vs. 0.72; HMx: 0.41 vs. 0.42, in LETO and OLETF, respectively). These values correspond well to previous findings (Hajnal et al. 1999). Interestingly, in that study-intestinal satiation had no effect on the net entropy of the PBN taste neurons. This together with the present data suggests that an analysis of response power (see below) for sucrose is more sensitive to hedonic shifts coded in the across neuron pattern than the measure of entropy.
Overall response profiles and hierarchial cluster analysis
The mean response profiles of PBN taste-responsive neurons based on raw firing rate change to oral taste stimulation are displayed in Fig. 3.
FIG. 3.
Mean taste response profiles of OLETF and LETO PBN neurons. Each bar represents 1-s time interval of mean taste responses derived from the 5-s peristimulus intervals.
To compare correlations across major taste categories between strains, hierarchial cluster analysis (Pearson product-moment correlations and the average linkage method) was used based on mean responses corrected for water baseline and computed for each major neuron type. These findings appear in Fig. 4. In the dendrograms, the level at which two neurons, or two clusters of neurons, join indicates their shared correlation coefficient. Thus a linkage distance (1 - Pearson r) of 0.0 means an identical response profile, i.e., the Pearson correlation coefficient = 1.00.
Although this analysis demonstrated an overall similar separation of the major neuron categories in obese and lean cohorts, it also revealed within-strain differences with respect to generalist and specialist S and N units. Specifically, whereas in LETO rats Nx and Ns classes form closely related but distinct clusters (linkage distance: 0.129), in the OLETF rats, these generalist and specific N groups' response profiles are almost identical (linkage distance: 0.041). The cluster of S specialist units (Ss) separated farther from the other groups in both lean and obese rats (linkage distance: 0.619 and 0.553, respectively). In contrast, the clusters of N and S generalist groups (Nx and Sx) joined at a relatively higher linkage distance in the OLETF than in LETO rats (0.441 vs. 0.119). A similar difference between lean and obese rats was apparent in the mean response profiles of the individual neurons (Fig. 3). Specifically, in OLETF rats, Nx and Sx units responded to various taste stimuli more uniquely than in LETO rats. For example, whereas in lean rats Nx and Sx neurons were uniformly responsive also to quinine and MSG stimulation, in OLETF, only the Nx neurons were sensitive to these stimuli, whereas only one Sx neuron responded to quinine and one to MSG.
Population responses to specific tastes
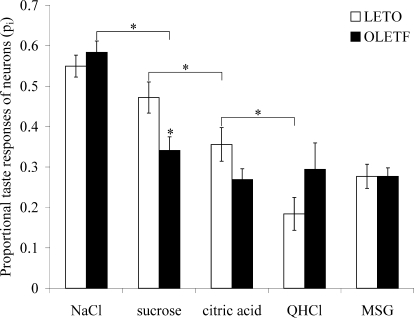
The proportion of total responses or power (pi) to a particular sapid stimulus exhibited by all taste units is displayed in Fig. 5. ANOVA revealed an overall strain difference [F(5,21) = 5.630, P = 0.002, n = 429] and significant within-group differences for taste in both OLETF and LETO rats [F(4,213 = 22.237, P < 0.001, n = 218; F(4,206 = 11.367, P < 0.001, n = 211, respectively]. Post hoc comparison showed that the sampled PBN neuron population in the OLETF rats was 27.7% less responsive to sucrose than in the LETO controls (ps: 0.34 ± 0.03 vs. 0.47 ± 0.04, P < 0.05). In addition, whereas in LETO rats, the response power to NaCl and sucrose did not differ from each other but both were significantly higher than responses to other tastes (pn = ps > pc > pq = pm, P < 0.05 for all comparisons), in OLETF rats, only NaCl produced significantly distinct effects while responses to sucrose was reduced to the power of other sapid stimuli (pn > ps = pc = pq = pm, P < 0.05 for all comparisons).
FIG. 5.
Proportion of the total neuronal responses of all PBN neurons tested with respect to oral stimuli. *P < 0.05. For further explanation, see the text.
Sucrose concentration-response function
For comparisons of sucrose concentration-effects on neuronal responses in the gustatory PBN of OLETF and LETO rats, we used parameters that have been introduced in methods. The results of these analyzes are summarized and depicted in Fig. 6. Compared with the LETO sample, sucrose-responsive neurons in the OLETF rats demonstrated a 60.1% increased first significant response concentration [0.37 ± 0.05 vs. 0.23 ± 0.2 M, ANOVA: F(1,99) = 6.723, P = 0.02, n = 101], which demonstrates an elevation in the lowest oral sucrose concentration that PBN neurons can detect. Neurons in obese rats also showed a 71.4% higher maximum response concentration [0.96 ± 0.07 vs. 0.56 ± 0.5 M; ANOVA: F(1,99) = 22.611, P < 0.001, n = 102], indicating that PBN sweet sensing neurons in obese reach their maximum response magnitudes at higher sucrose concentrations. The difference between these two values (maximum response concentration and the first significant response concentration) determine the dynamic sucrose concentration range, which is 78.8% broader in obese compared with lean rats [0.59 ± 0.07 vs. 0.33 ± 0.6 M; ANOVA: F(1,59) = 7.458, P < 0.01, n = 61]. This effect suggests that PBN neurons in obese rats may differentiate better and in wider range of concentrations than neurons in lean rats. There was, however, no significant difference between strains at the nondynamic sucrose concentration range or at the maximum effective concentration. Collectively, these effects resulted in an overall 1/3 log rightward shift in the sweet taste coding of PBN neurons in obese compared with lean cohorts, based on changes in response magnitude to increasing sucrose concentrations (0.01–1.5 M, Fig. 6).
FIG. 6.
Concentration effects on mean response magnitude in all sucrose-responsive PBN neurons. For definitions of measures, see methods. *P < 0.05.
Across neuron pattern code for sucrose concentration effects
To further analyze how concentration responses were coded across neurons as a proportion of neural activity carried by the narrowly tuned main band (Ss), narrowly tuned side band (NS), broadly tuned main band (Sx) or broadly tuned side band (Nx) sucrose-sensitive neurons, we repeated the analysis in separate subpopulations of NaCl- and sucrose-responsive neurons that together constitute the largest portion of our sample. Results from this analysis appear in Fig. 7.
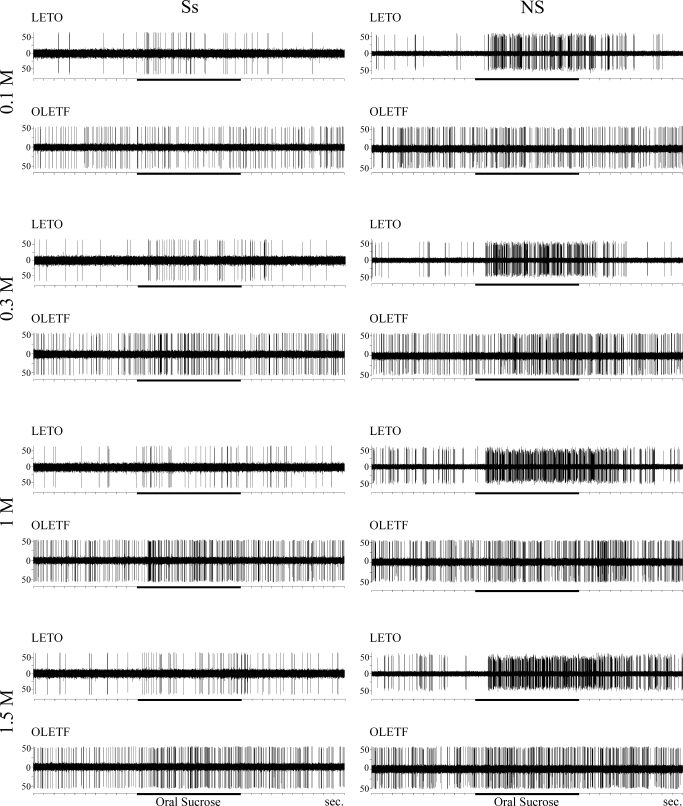
Overall both S- and N-best units responded to increasing concentrations of sucrose in a dynamic fashion in both strains (Fig. 7, left). However, there was no significant difference in the NaCl-response magnitudes between the two strains. In contrast, there was significant strain effect in neuronal response magnitudes to sucrose for the narrowly tuned sucrose neurons [Ss; Fig. 7B; F(1,38) = 7.511, P < 0.01, n = 40] and the narrowly tuned N-units that responded only to NaCl and sucrose but no other taste stimulus [NS; Fig. 7D; F(1,23) = 41.776, P < 0.001, n = 25]. Post hoc analysis revealed that whereas the strain effect in the NS neurons was not specific to a particular concentration resulting in overall suppressed responsiveness of OLETF neurons to sucrose across all concentrations tested, Ss neurons of the OLETF rats exhibited an opposite and a more specific effect. Particularly, in obese rats, Ss neurons responded with increased magnitude to 1 and 1.5 M (P < 0.05 for both comparisons; Fig. 7B), showed no difference to 0.3 M relative to lean rats, and did not respond to any concentrations tested <0.3 M. This rightward shift in the concentration coding by the sucrose-specific neurons was similar to the analysis for the total population of our sample (see previous section and Fig. 6). This strongly suggests that the altered concentration effects in the obese rats are carried by the narrowly tuned sucrose responsive neurons (Ss and NS). Figure 8 summarizes these observations showing actual recording traces of individual neurons.
FIG. 8.
Representative recording traces of a narrowly tuned sucrose main band (Ss, left) and a narrowly tuned sucrose side band (NS, right) neuron before, during (horizontal black bars), and after oral infusion of sucrose at various concentrations (0.1–1.5 M). Notice that the typical LETO NS neuron respond at higher frequency to all sucrose concentrations compared with the OLETF NS neurons; only LETO Ss and NS neurons respond significantly to low concentration (0.1 M) sucrose, while OLETF neurons do not; the typical OLETF Ss neuron respond more vigorously to higher sucrose concentrations (1–1.5 M) compared with the typical LETO Ss neuron. This together with elevated threshold results in a rightward shift in the concentration code for sapid sucrose in the obese rats with augmented responses to the higher concentrations.
DISCUSSION
Using extracellular single-unit recording, the present study demonstrates that neuronal activity in the gustatory PBN evoked by oral sucrose stimulation is altered in prediabetic obese OLETF rats compared with age-matched, lean LETO controls. This is the first electrophysiological evidence indicating that central taste processing is altered in an animal model of obesity caused by overconsumption.
PBN neural coding in obese rat
Specifically, we found that the pattern of activation of different neuron types by sucrose differed between OLETF and LETO. In addition, sucrose-responsive neurons of the obese rats demonstrated altered concentration responses: decreased sensitivity to lower concentrations and augmented responsivity to higher concentrations compared with lean rats. Further analysis revealed that whereas this effect (i.e., the rightward-shifted concentration-response function in obese) resulted from the reduced activity of the few sucrose-specific neurons (main band) to lower concentrations (<0.3 M) combined with an increased responsiveness to the higher ones (>1.0 M), sucrose responses carried by N-units (side band) were diminished across all concentrations in the OLETF rats compared with LETO controls. Because neuronal responses to NaCl either by N or S units (i.e., on the main, and side band, respectively) were not different between strains, the proportion of sucrose responses carried by the broadly tuned (generalist) neurons was further reduced in the OLETF rats. A corresponding effect was apparent in the population analyses revealing an overall reduced response-power to sucrose and a reduced correlation in response characteristics between sucrose and sodium generalist clusters in the OLETF sample. These effects together with an increased occurrence of N units and reduced number of S units in the obese cohorts result in an overall reduced sucrose responsiveness with accentuated responses to higher concentrations of sucrose.
Whereas OLETF M-best and M-generalist neurons showed significantly lower spontaneous firing rate, taste responses were not significant. Similarly, spontaneous firing rate and responses by C- and Q-best neurons were not significantly different between obese and lean cohorts. The lack of significant differences in the taste responses of these groups, however, may be due to the relatively small sample size compared with S- or N-best groups. Such distributions are commonly observed in the PBN (Hajnal et al. 1999; Nishijo et al. 1991).
This finding is in concert with previous behavioral observations in this strain demonstrating an increased preference for and intake of highly concentrated sucrose solutions (De Jonghe et al. 2005; Hajnal et al. 2005). This coding scheme is consistent also with the opposite shift in the PBN across-neuron pattern evoked by sucrose observed following intraduodenal lipid infusion (Hajnal et al. 1999), which reduces intake of sucrose (Foster et al. 1996). Similarly, gastric distension, an inhibitory feedback signal that enhances satiation and diminishes taste reactivity (Cabanac and Lafrance 1992; Gyetvai and Bardos 1999), also has been shown to reduce sucrose-evoked neuronal activity in the PBN while NaCl-responses remain unaltered (Baird et al. 2001). All this suggests that the PBN may take part in neural processes underlying hedonic evaluation of gustatory information and may contribute to the regulation of meal size by increasing or reducing the oro-sensory positive feedback. The present data demonstrate for the first time that this function is perturbed in an obese animal. It remains to be determined, however, whether these observed parabrachial changes in gustatory responses lead or follow the changes in ingestive behavior.
Peripheral modulation
Neural coding in gustation begins in the oral cavity with the interaction between a soluble chemical stimulus and the taste receptor cells (TRCs). It has been proposed that gustatory transduction mechanisms may be subject to functional modulation and plasticity during development and adulthood (Hill 2004). In fact, TRCs express a number of proteins that impart sensitivity to underlying nutritional and physiological changes (Herness and Gilbertson 1999). One such factor that may modulate electrical excitability of TRCs is CCK acting via CCK-1 receptors (Herness et al. 2002). Therefore altered neural processing of sugars in OLETF rats might be mediated through changes at the level of the taste bud. Despite this relationship, to the best of our knowledge, no study has yet examined sugars' effect on TRCs in the OLETF rats. The closest approach was a study by Tsunoda et al. (1998) that examined taste responsiveness of the whole chorda tympani nerve that provides innervation of taste buds located on the anterior 2/3 of the tongue. This study found that the relative response magnitude to concentrations of sucrose >1 M was greater in OLETF rats compared with LETO. This finding is consistent with our present results and also with the enhanced sensitivity of the whole chorda tympani nerve to sucrose in another murine genetic model of obesity and type-2 diabetes, the db/db mouse (Ninomiya et al. 1995).
Although the role of CCK cannot be completely excluded, it is unlikely that CCK-dependent mechanisms at the TRCs were responsible for the altered PBN code for sucrose because the population of anterior tongue receptor cells innervated by the chorda tympani nerve does not appear to express CCK-1 receptors (Herness et al. 2002). Accordingly, this nerve's response to sucrose is unaffected by CCK treatment (Simon et al. 2003). Nevertheless other peripheral peptides such as leptin that has been shown to act on the TRCs may also contribute to altered taste functions in obese animals or individuals (Kawai et al. 2000; Shigemura et al. 2004). For example, in the db/db mouse the sucrose responses of the TRCs may be subject to reduced inhibition in the absence of direct action of leptin. Because leptin levels are significantly elevated in OLETF rats (Kawano et al. 1992), an opposite effect from leptin is expected unless leptin resistance develops on the TRCs; a scenario that has not been demonstrated yet.
Irrespective of whether peripheral factors contributed to the observed effects, it is obvious that central modulation played a critical role. This is because the direction of effect on the whole chorda tympani nerve response to 1.0 M sucrose two synapses further along in the PBN was reflected only in a subset of neurons (the most narrowly tuned sucrose units), whereas other neurons (NS units) and the population as a whole responded in the opposite way.
Central modulation
For the forebrain to participate in assigning hedonic value to afferent activity, the sensory systems involved must interact with the neural mechanisms that elaborate reward and aversion and those that monitor actual homeostatic variables (Rolls 2005). Both the NTS and the PBN possess bidirectional connections with various forebrain areas (Lundy and Norgren 2004b). Activation of descending projections from reciprocally connected areas such as the lateral hypothalamus, central nucleus of the amygdala, and the gustatory cortex has been shown to alter taste responses in the PBN (Li et al. 2005; Lundy and Norgren 2001, 2004a). For example, electrical stimulation of the central nucleus of the amygdala was shown to produce a dramatic shift in the across-neuron pattern for sucrose, increasing the proportion of the total sucrose response carried by sucrose-best neurons from 62 to 91%. Based on this, one may assume that the observed effects of the present study could also be related to a top-down modulation by the forebrain on the PBN gustatory neurons. One potential mechanism is a change in descending inhibitory tone from the forebrain on the PBN neurons (Jia et al. 2005; Li and Cho 2006) that, in turn, could alter input resistance and the signal-to-noise ratio in neuron subtypes receiving differential proportion of the afferent stimulation from the TRCs via the NTS (Kobashi and Bradley 1998). In fact, the present study demonstrated a significantly higher spontaneous firing rate in the sucrose-specific units of the OLETF rats, whereas the basal activity of generalist neurons was unaltered relative to lean cohorts.
Pontine taste code and altered homeostatic conditions
According to the “alliesthesia” theory proposed by Cabanac (1971), perceived pleasantness of a relevant stimulus (e.g., sweet taste) is modified by a change of internal state (hunger and satiety). Thus taste functions in some form of obesity may be altered due to a persistent and exaggerated motivational state to eat. Relevant to this are data showing that gustatory responses in the hindbrain are sensitive to the animal's actual feeding and metabolic state. Specifically, in addition to homeostatic imbalance due to salt-deprivation and following sodium repletion (Nakamura and Norgren 1995; Tamura and Norgren 1997), administration of satiety factors (glucose, insulin, pancreatic glucagon, and cholecystokinin) have been shown to affect taste activity in the NTS (Giza et al. 1992). The present data further support this notion by showing an opposite effect in ad libitum-fed OLETF rats to that of satiation from intraduodenal nutrients in normal Sprague-Dawley rats (Hajnal et al. 1999) on responses exhibited by sucrose-specific neurons to oral sucrose. This observation together with the known satiety deficit (Covasa and Ritter 2001, 2005; De Jonghe et al. 2005; Hajnal et al. 2005; Moran and Bi 2006) and increased sweet preference of the OLETF rats suggests that taste information carried by the subset of sucrose-specific neurons has particular relevance to the motivational control of meal-size.
In addition to acute homeostatic deficits, chronic regulatory or metabolic associates of obesity may also contribute to altered central taste functions. To investigate this possibility, in a separate set of animals, we used a similar procedure to record from the PBN of OLETF rats multiple times over an extended period during development of obesity and diabetes. Although the sample size in that study was rather limited (a total of 44 neurons from 2 OLETF and 1 LETO rats), we did see significantly reduced sucrose sensitivity in the OLETF rats' N-best neuronal subgroup and an augmentation of this effect with the progression of reduced oral glucose tolerance (Lundy and Hajnal 2006). Although this observation together with the findings of the present paper strongly suggest that the observed effects were related to obesity more so than to the CCK-1 receptor deficient phenotype of the OLETF rats, future studies targeting central taste functions in dietary obese animals are necessary.
Conclusions
The present study confirmed and extended prior data suggesting altered gustatory processing in OLETF rats. Specifically we demonstrated that central taste processing of sucrose in general and concentration-response functions in particular are different in this obese strain compared with lean controls. Notably, the effects were specific to sucrose, while responses to other tastes were unaffected. The generally reduced sucrose sensitivity appeared to result from a lower proportion of the total neural information being carried in an across-neuron pattern for sucrose, whereas a rightward shift in the sucrose concentration responses was specific to the altered functioning of the S-specific units, which are highly responsive to stimuli that have a positive hedonic value and normally produce ingestion. Thus the heightened behavioral preference for sugars in OLETF rats might be due to an altered population code for sweet taste that is modulated by descending input from forebrain areas involved in regulation of metabolism and food reward.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-065709 and by The Pennsylvania Tobacco Settlement Fund Research Grant SAP 4100031293.
Acknowledgments
The authors are thankful to Dr. Robert F. Lundy Jr. for collecting an exploratory set of data in the OLETF rats (not included in the present analysis). We also thank him and Dr. Mihai Covasa for useful comments on an earlier version of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bacon et al. 1994.Bacon AW, Miles JS, Schiffman SS. Effect of race on perception of fat alone and in combination with sugar. Physiol Behav 55: 603–606, 1994. [DOI] [PubMed] [Google Scholar]
- Baird et al. 2001.Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regulatory Integrative Comp Physiol 281: R1581–1593, 2001. [DOI] [PubMed] [Google Scholar]
- Bartoshuk et al. 2006.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci 361: 1137–1148, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi and Moran 2002.Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides 36: 171–181, 2002. [DOI] [PubMed] [Google Scholar]
- Cabanac 1971.Cabanac M Physiological role of pleasure. Science 173: 1103–1107, 1971. [DOI] [PubMed] [Google Scholar]
- Cabanac and Lafrance 1992.Cabanac M, Lafrance L. Ingestive/aversive response of rats to sweet stimuli. Influence of glucose, oil, and casein hydrolyzate gastric loads. Physiol Behav 51: 139–143, 1992. [DOI] [PubMed] [Google Scholar]
- Covasa and Ritter 2001.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides 22: 1339–1348, 2001. [DOI] [PubMed] [Google Scholar]
- Covasa and Ritter 2005.Covasa M, Ritter RC. Reduced CCK-induced Fos expression in the hindbrain, nodose ganglia, and enteric neurons of rats lacking CCK-1 receptors. Brain Res 1051: 155–163, 2005. [DOI] [PubMed] [Google Scholar]
- De Jonghe et al. 2005.De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regulatory Integrative Comp Physiol 288: R292–300, 2005. [DOI] [PubMed] [Google Scholar]
- De Jonghe et al. 2007.De Jonghe BC, Hajnal A, Covasa M. Conditioned preference for sweet stimuli in OLETF rat: effects of food deprivation. Am J Physiol Regulatory Integrative Comp Physiol 292: R1819–1827, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desor et al. 1975.Desor JA, Greene LS, Maller O. Preferences for sweet and salty in 9- to 15-year-old and adult humans. Science 190: 686–687, 1975. [DOI] [PubMed] [Google Scholar]
- Drewnowski et al. 1991.Drewnowski A, Kurth CL, Rahaim JE. Taste preferences in human obesity: environmental and familial factors. Am J Clin Nutr 54: 635–641, 1991. [DOI] [PubMed] [Google Scholar]
- Foster et al. 1996.Foster LA, Nakamura K, Greenberg D, Norgren R. Intestinal fat differentially suppresses sham feeding of different gustatory stimuli. Am J Physiol Regulatory Integrative Comp Physiol 270: R1122–1125, 1996. [DOI] [PubMed] [Google Scholar]
- Frijters and Rasmussen-Conrad 1982.Frijters JE, Rasmussen-Conrad EL. Sensory discrimination, intensity perception, and affective judgment of sucrose-sweetness in the overweight. J Gen Psychol 107: 233–247, 1982. [DOI] [PubMed] [Google Scholar]
- Giza et al. 1992.Giza BK, Scott TR, Vanderweele DA. Administration of satiety factors and gustatory responsiveness in the nucleus tractus solitarius of the rat. Brain Res Bull 28: 637–639, 1992. [DOI] [PubMed] [Google Scholar]
- Grinker et al. 1972.Grinker J, Hirsch J, Smith DV. Taste sensitivity and susceptibility to external influence in obese and normal weight subjects. J Pers Soc Psychol 22: 320–325, 1972. [DOI] [PubMed] [Google Scholar]
- Gyetvai and Bardos 1999.Gyetvai B, Bardos G. Modulation of taste reactivity by intestinal distension in rats. Physiol Behav 66: 529–535, 1999. [DOI] [PubMed] [Google Scholar]
- Hajnal et al. 2007.Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regulatory Integrative Comp Physiol 293: R1846–1854, 2007. [DOI] [PubMed] [Google Scholar]
- Hajnal et al. 2005.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regulatory Integrative Comp Physiol 289: R1675–1686, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal and Norgren 2005.Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005. [DOI] [PubMed] [Google Scholar]
- Hajnal et al. 1999.Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182–7190, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herness and Gilbertson 1999.Herness MS, Gilbertson TA. Cellular mechanisms of taste transduction. Annu Rev Physiol 61: 873–900, 1999. [DOI] [PubMed] [Google Scholar]
- Herness et al. 2002.Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci 22: 10018–10029, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill 2004.Hill DL Neural plasticity in the gustatory system. Nutr Rev 62: S208–217, discussion S224–241, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill et al. 2000.Hill JO, Melanson EL, Wyatt HT. Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr 130: 284S–288S, 2000. [PubMed] [Google Scholar]
- Jia et al. 2005.Jia HG, Zhang GY, Wan Q. A GABAergic projection from the central nucleus of the amygdala to the parabrachial nucleus: an ultrastructural study of anterograde tracing in combination with post-embedding immunocytochemistry in the rat. Neurosci Lett 382: 153–157, 2005. [DOI] [PubMed] [Google Scholar]
- Kant 2000.Kant AK Consumption of energy-dense, nutrient-poor foods by adult Americans: nutritional and health implications. The third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 72: 929–936, 2000. [DOI] [PubMed] [Google Scholar]
- Kawai et al. 2000.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA 97: 11044–11049, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano et al. 1992.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422–1428, 1992. [DOI] [PubMed] [Google Scholar]
- Kobashi and Bradley 1998.Kobashi M, Bradley RM. Effects of GABA on neurons of the gustatory and visceral zones of the parabrachial nucleus in rats. Brain Res 799: 323–328, 1998. [DOI] [PubMed] [Google Scholar]
- Kumanyika 1993.Kumanyika SK Special issues regarding obesity in minority populations. Ann Intern Med 119: 650–654, 1993. [DOI] [PubMed] [Google Scholar]
- Li and Cho 2006.Li CS, Cho YK. Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regulatory Integrative Comp Physiol 291: R914–926, 2006. [DOI] [PubMed] [Google Scholar]
- Li et al. 2005.Li CS, Cho YK, Smith DV. Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93: 1183–1196, 2005. [DOI] [PubMed] [Google Scholar]
- Lucas and Sclafani 1990.Lucas F, Sclafani A. Hyperphagia in rats produced by a mixture of fat and sugar. Physiol Behav 47: 51–55, 1990. [DOI] [PubMed] [Google Scholar]
- Lundy and Hajnal 2006.Lundy RF, Hajnal A. Altered parabrachial taste processing in obese OLETF rats. Chem Senses 31: A114–A114, 2006. [Google Scholar]
- Lundy and Norgren 2001.Lundy RF, Norgren R. Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol 85: 770–783, 2001. [DOI] [PubMed] [Google Scholar]
- Lundy and Norgren 2004a.Lundy RF, Norgren R. Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91: 1143–1157, 2004a. [DOI] [PubMed] [Google Scholar]
- Lundy and Norgren 2004b.Lundy RF, Norgren R. Gustatory system. In: The Rat Nervous System (3rd ed.), edited by Paxinos G. San Diego, CA: Elsevier Academic, 2004b, p. 891–921.
- Malcolm et al. 1980.Malcolm R, O'Neil PM, Hirsch AA, Currey HS, Moskowitz G. Taste hedonics and thresholds in obesity. Int J Obes 4: 203–212, 1980. [PubMed] [Google Scholar]
- Moran 2000.Moran TH Cholecystokinin and satiety: current perspectives. Nutrition 16: 858–865, 2000. [DOI] [PubMed] [Google Scholar]
- Moran and Bi 2006.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360–367, 2006. [DOI] [PubMed] [Google Scholar]
- Nakamura and Norgren 1995.Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am J Physiol Regulatory Integrative Comp Physiol 269: R647–661, 1995. [DOI] [PubMed] [Google Scholar]
- Ninomiya et al. 1995.Ninomiya Y, Sako N, Imai Y. Enhanced gustatory neural responses to sugars in the diabetic db/db mouse. Am J Physiol Regulatory Integrative Comp Physiol 269: R930–937, 1995. [DOI] [PubMed] [Google Scholar]
- Nishijo et al. 1991.Nishijo H, Ono T, Norgren R. Parabrachial gustatory neural responses to monosodium glutamate ingested by awake rats. Physiol Behav 49: 965–971, 1991. [DOI] [PubMed] [Google Scholar]
- Norgren 1974.Norgren R Gustatory afferents to ventral forebrain. Brain Res 81: 285–295, 1974. [DOI] [PubMed] [Google Scholar]
- Norgren 1976.Norgren R Taste pathways to hypothalamus and amygdala. J Comp Neurol 166: 17–30, 1976. [DOI] [PubMed] [Google Scholar]
- Norgren et al. 2003.Norgren R, Grigson PS, Hajnal A, and Lundy RF Jr. Motivational modulation of taste. In: Cognition and Emotion in the Brain, edited by T. Ono GM, R. Llinas, A. Berthoz, R. Norgren, H. Nishijo, R. Tamura. New York: Elsevier, 2003, p. 319–334.
- Norgren et al. 2006.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren and Pfaffmann 1975.Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res 91: 99–117, 1975. [DOI] [PubMed] [Google Scholar]
- Ogden et al. 2006.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc 295: 1549–1555, 2006. [DOI] [PubMed] [Google Scholar]
- Rolls 2005.Rolls ET Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 85: 45–56, 2005. [DOI] [PubMed] [Google Scholar]
- Salbe et al. 2004.Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr 79: 372–378, 2004. [DOI] [PubMed] [Google Scholar]
- Scalera et al. 1995.Scalera G, Spector AC, Norgren R. Excitotoxic lesions of the parabrachial nuclei prevent conditioned taste aversions and sodium appetite in rats. Behav Neurosci 109: 997–1008, 1995. [PubMed] [Google Scholar]
- Schiffman et al. 2000.Schiffman SS, Graham BG, Sattely-Miller EA, Peterson-Dancy M. Elevated and sustained desire for sweet taste in african-americans: a potential factor in the development of obesity. Nutrition 16: 886–893, 2000. [DOI] [PubMed] [Google Scholar]
- Sclafani 2004.Sclafani A Oral and postoral determinants of food reward. Physiol Behav 81: 773–779, 2004. [DOI] [PubMed] [Google Scholar]
- Shigemura et al. 2004.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology 145: 839–847, 2004. [DOI] [PubMed] [Google Scholar]
- Simon et al. 2003.Simon SA, Liu L, Erickson RP. Neuropeptides modulate rat chorda tympani responses. Am J Physiol Regulatory Integrative Comp Physiol 284: R1494–1505, 2003. [DOI] [PubMed] [Google Scholar]
- Smith and Travers 1979.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses 4: 215–229, 1979. [Google Scholar]
- Sorensen et al. 2003.Sorensen LB, Moller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord 27: 1152–1166, 2003. [DOI] [PubMed] [Google Scholar]
- Takiguchi et al. 1997.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene 197: 169–175, 1997. [DOI] [PubMed] [Google Scholar]
- Tamura and Norgren 1997.Tamura R, Norgren R. Repeated sodium depletion affects gustatory neural responses in the nucleus of the solitary tract of rats. Am J Physiol Regulatory Integrative Comp Physiol 273: R1381–1391, 1997. [DOI] [PubMed] [Google Scholar]
- Tsunoda et al. 1998.Tsunoda K, Osada K, Komai M, Zhang H, Morimoto K, Suzuki H, Furukawa Y. Effects of dietary biotin on enhanced sucrose intake and enhanced gustatory nerve responses to sucrose seen in diabetic OLETF rat. J Nutr Sci Vitaminol 44: 207–216, 1998. [DOI] [PubMed] [Google Scholar]
- Yagi et al. 1997.Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H. Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima Fatty rats. Hypertension 29: 728–735, 1997. [DOI] [PubMed] [Google Scholar]
- Yeomans et al. 2004.Yeomans MR, Blundell JE, Leshem M. Palatability: response to nutritional need or need-free stimulation of appetite? Br J Nutr 92 Suppl 1: S3–14, 2004. [DOI] [PubMed] [Google Scholar]