Summary
To initiate infection, a microbial pathogen must be able to evade innate immunity. Here we show that the Lyme disease spirochete Borrelia burgdorferi depends on its surface lipoproteins for protection against innate defenses. The deficiency for OspC, an abundantly expressed surface lipoprotein during early infection, led to quick clearance of B. burgdorferi after inoculation into the skin of SCID mice. Increasing expression of any of the four randomly chosen surface lipoproteins, OspA, OspE, VlsE or DbpA, fully protected the ospC mutant from elimination from the skin tissue of SCID mice; moreover, increased OspA, OspE, or VlsE expression allowed the mutant to cause disseminated infection and restored the ability to effectively colonize both joint and skin tissues, albeit the dissemination process was much slower than that of the mutant restored with OspC expression. When the ospC mutant was modified to express OspA under control of the ospC regulatory elements, it registered only a slight increase in the 50% infectious dose than the control in SCID mice but a dramatic increase in immunocompetent mice. Taken together, the study demonstrated that the surface lipoproteins provide B. burgdorferi with an essential protective function against host innate elimination.
Like typical Gram-negative bacteria, the Lyme disease spirochete Borrelia burgdorferi possesses inner and outer membranes, between which is a periplasmic space (Cullen et al., 2004; Steere, 2001). Gram-negative pathogens make a thick LPS coat to provide a broad array of crucial protection (Raetz and Whitfield, 2002). However, B. burgdorferi does not produce any LPS but instead abundantly expresses lipoproteins and anchors them to the outer membranous surface through lipidation (Cullen et al., 2004; Radolf et al., 1994; Takayama et al., 1987).
B. burgdorferi appears to maintain its overall surface lipoprotein expression level during the enzootic life cycle traveling between the tick vector and a mammal, and the course of mammalian infection. The pathogen abundantly expresses outer surface proteins (Osps) A and B in the unfed tick (de Silva et al., 1996; Ohnishi et al., 2001; Schwan et al., 1995; Schwan and Piesman, 2000), a fresh blood meal induces the down-regulation of OspA/B and the up-regulation of OspC and others, a process that prepares B. burgdorferi for infection of mammals (Fingerle et al., 2007; Grimm et al., 2004; Pal et al., 2004b; Stewart et al., 2006). Abundant OspC expression ultimately induces a robust early humoral response that imposes tremendous pressure on the pathogen (Fung et al., 1994; Xu et al., 2006). To evade the specific humoral response and cause persistent infection, B. burgdorferi down-regulates OspC and dramatically upregulates other surface lipoproteins, including VlsE and BBF01 (Crother et al., 2004; Liang et al., 2002a; Liang et al., 2002b; Liang et al., 2004). These changes in surface antigen expression certainly allow B. burgdorferi to better adapt to different environments, and potentially also provide a scheme for maintaining the overall surface lipoprotein expression level.
The various expression levels and diverse functions of different surface lipoproteins make investigation into their common role extremely challenging. For instance, deletion of the ospAB locus or even the ospB gene alone diminishes the ability of B. burgdorferi to persist in the tick vector but does not affect virulence in mammalian hosts (Neelakanta et al., 2007; Yang et al., 2004), while inactivation of the ospD gene does not reduce the viability either in the tick or a mammal (Li et al., 2007). The fibronectin-binding protein BBK32 and decorin-binding proteins (Dbps) A and B are not expressed during the life cycle in ticks because their expression depends on the induction of rpoS, which is silent in the tick (Caimano et al., 2007; He et al., 2007; Hubner et al., 2001), and thus should not be expected to have a role in the vector. In fact, none of the three surface lipoprotein adhesins is required for mammalian infection (Li et al., 2006; Seshu et al., 2006; Shi et al., 2006), although both DbpA and DbpB are critical for the overall virulence of B. burgdorferi (Shi et al., 2008). VlsE, the variable surface antigen identified in B. burgdorferi (Zhang et al., 1997), is required for persistent infection of immunocompetent animals but does not significantly contribute to infectivity in SCID mice (Bankhead and Chaconas, 2007; Labandeira-Rey et al., 2003; Xu et al., 2005). OspE, a member of a large surface lipoprotein family called Erp (Lam et al., 1994; Stevenson et al., 1998), is one of five surface lipoproteins that are shown binding the complement regulator factor H (Bykowski et al., 2007; Hartmann et al., 2006; Hellwage et al., 2001; Kraiczy et al., 2004; McDowell et al., 2004; Metts et al., 2003; Stevenson et al., 2002). Although remaining to be investigated, OspE is very unlikely to be critical for mammalian infection because other members of the families may compensate for its loss. To date, OspC has been the only surface lipoprotein shown to be essential for mammalian infection (Grimm et al., 2004; Stewart et al., 2006; Tilly et al., 2006; Tilly et al., 2007).
The essential role repeatedly demonstrated for OspC in mammalian infection led Rosa and colleagues to hypothesize that OspC is required for evasion of innate immunity during initial infection (Tilly et al., 2007). However, given that OspC is probably the most abundantly expressed surface lipoprotein during early infection, deletion of the ospC gene may severely compromise the integrity of the surface lipoprotein layer, which may provide B. burgdorferi with protection against innate defenses. None of the other surface lipoproteins has been shown to be essential for mammalian infection, probably because they are expressed at relatively low levels so their absence does not significantly reduce the integrity of the lipoprotein layer. To explore the hypothesis that the surface lipoproteins collectively play an essential protective role against innate defenses, OspC-deficient B. burgdorferi was modified to increase expression of well-defined surface lipoproteins, OspA, OspE, VlsE and DbpA, and then examined for dissemination, tissue colonization, infectivity and persistence in the murine model.
Results
Generation of B. burgdorferi with increased expression of OspA, OspE, VlsE or DbpA
Five constructs, namely pBBE22-ospC', pBBE22-ospA', pBBE22-ospE', pBBE22-vlsE' and pBBE22-dbpA', and their parental vector, pBBE22, were electroporated into the ospC mutant, which was generated and characterized in our previous study (Xu et al., 2007a). pBBE22-ospA', pBBE22-ospE', and pBBE22-vlsE' were constructed as illustrated in Fig. 1A; pBBE22-ospC' and pBBE22-dbpA' were generated in our previous studies (Xu et al., 2006; Xu et al., 2007b). All the five introduced genes would be expressed under control of the flaB promoter (Table 1). Because the ospC mutant had lost lp25, the plasmid that carries the gene bbe22 coding for a nicotinamidase essential for survival of B. burgdorferi in the mammalian environment, the recombinant plasmid pBBE22, which harbors a copy of bbe22, was used as the shuttle vector (Purser et al., 2003). Between 8 and 15 transformants were obtained from transformation with each construct. Plasmid analyses identified two clones receiving each construct, namely ΔospC/E22/1, ΔospC/E22/2, Δosp/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, ΔospC/vlsE'/2, ΔospC/dbpA'/1, and ΔospC/dbpA'/2. These clones shared the same plasmid content as the ospC mutant, which had lost lp25, lp5, lp21, lp56 and cp9 (Xu et al., 2007a). Increased Osp expression resulting from transformation was confirmed by immunoblot analysis. Introduction of pBBE22-ospC', pBBE22-ospE', pBBE22-vlsE', and pBBE22-dbpA' led to dramatically increased expression of respective Osps (Fig. 1B). Unfortunately, overwhelming OspA expression resulting from the native ospA copy masked the potential contribution of the introduced pBBE22-ospA'.
Fig. 1. Generation of B. burgdorferi with increased Osp expression.
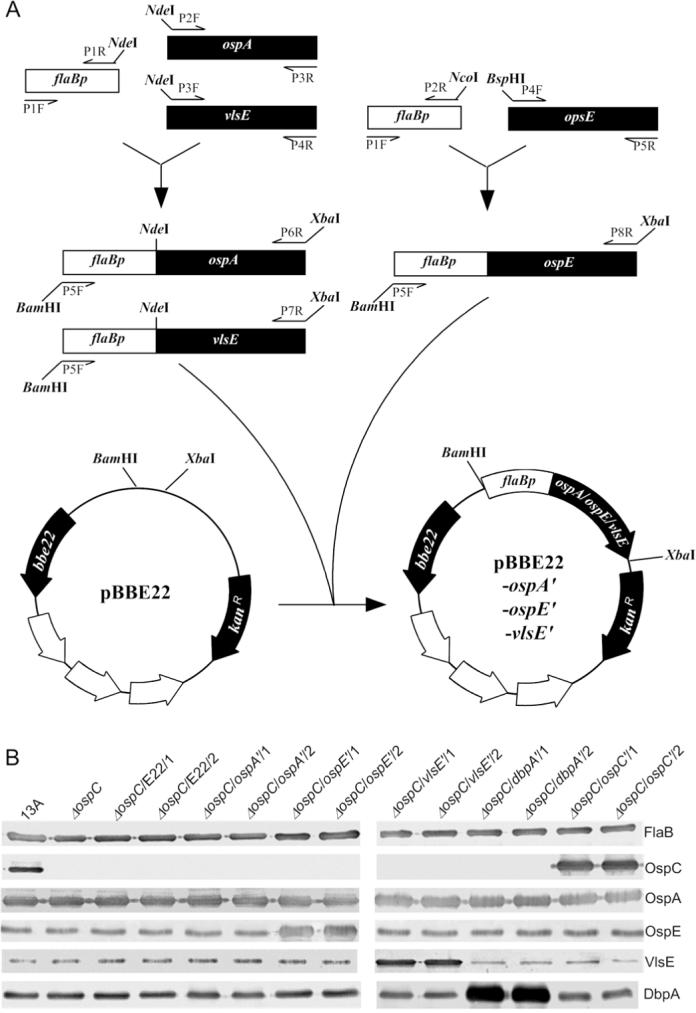
A. Construction of pBBE22-ospA', pBBE22-ospE', and pBBE22-vlsE'. The flaB promoter region (flaBp) and a promoterless ospA, ospE, or vlsE gene were PCR amplified, fused, and cloned into pBBE22.
B. Generation of OspC-deficient B. burgdorferi with increased OspC, OspA, OspE, DbpA or VlsE expression. pBBE22-ospA', pBBE22-ospA', pBBE22-ospE', pBBE22-dbpA', pBBE22-vlsE', and pBBE22 were electroporated into the ospC mutant. pBBE22-ospC' and pBBE22-dbpA' were constructed in our previous studies (Xu et al., 2006; Xu et al., 2007b). The parental clone 13A, the ospC mutant, and transformants ΔospC/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, and ΔospC/vlsE'/2 were verified for Osp expression by immunoblots probed with FlaB, OspC and OspA MAbs, and mouse antisera raised against recombinant OspE, DbpA and VlsE.
Table 1.
Constructs and clones used in the study.
| Construct or clone | Description | Source |
|---|---|---|
| pBBE22 | pBSV2 carrying a bbe22 copy | (Purser et al., 2003) |
| pBBE22-ospC′ | pBBE22 carrying promoterless ospC fused with flaB promoter | (Xu et al., 2006) |
| pBBE22-ospA′ | pBBE22 carrying promoterless ospA fused with flaB promoter | This study |
| pBBE22-ospE′ | pBBE22 carrying promoterless ospE fused with flaB promoter | This study |
| pBBE22-vlsE′ | pBBE22 carrying promoterless vlsE fused with flaB promoter | This study |
| pBBE22-dbpA′ | pBBE22 carrying promoterless dbpA fused with flaB promoter | (Xu et al., 2007b) |
| pBBE22-CpospA | pBBE22 carrying promoterless ospA fused with ospC regulatory elementsa | This study |
| ΔospC | ospC mutant | (Xu et al., 2007a) |
| ΔospA | ospA mutant | This study |
| ΔospC/E22/1 | ospC mutant without increased osp expression | This study |
| ΔospC/E22/2 | ospC mutant without increased osp expression | This study |
| ΔospC/ospC′/1 | ospC mutant expressing ospC driven by flaB promoter | This study |
| ΔospC/ospC′/2 | ospC mutant expressing ospC driven by flaB promoter | This study |
| ΔospC/ospA′/1 | ospC mutant expressing ospA driven by flaB promoter | This study |
| ΔospC/ospA′/2 | ospC mutant expressing ospA driven by flaB promoter | This study |
| ΔospC/ospE′/1 | ospC mutant expressing ospE driven by flaB promoter | This study |
| ΔospC/ospE′/2 | ospC mutant expressing ospE driven by flaB promoter | This study |
| ΔospC/vlsE′/1 | ospC mutant expressing vlsE driven by flaB promoter | This study |
| ΔospC/vlsE′/2 | ospC mutant expressing vlsE driven by flaB promoter | This study |
| ΔospC/dbpA′/1 | ospC mutant expressing dbpA driven by flaB promoter | This study |
| ΔospC/dbpA′/2 | ospC mutant expressing dbpA driven by flaB promoter | This study |
| ΔospC/FL/1 | ospC mutant expressing ospC controlled by ospC regulatory elements | (Xu et al., 2007a) |
| ΔospC/FL/2 | ospC mutant expressing ospC controlled by ospC regulatory elements | (Xu et al., 2007a) |
| ΔospC/CpospA/1 | ospC mutant expressing ospA controlled by ospC regulatory elements | This study |
| ΔospC/CpospA/2 | ospC mutant expressing ospA controlled by ospC regulatory elements | This study |
| ΔospA/CpospA/1 | ospA mutant expressing ospA controlled by ospC regulatory elements | This study |
| ΔospA/CpospA/2 | ospA mutant expressing ospA controlled by ospC regulatory elements | This study |
The ospC regulatory elements include both operator and promoter.
Increasing expression of an outer surface protein overrides the essential role of OspC in protecting B. burgdorferi from quick clearance in murine skin
Groups of six SCID mice each received two intradermal/subcutaneous inoculations of 105 spirochetes of the clone ΔospC/E22/1, ΔospC/E22/2, ΔospC/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, ΔospC/vlsE'/2, ΔospC/dbpA'/1, or ΔospC/dbpA'/2. The two inoculation sites were at least 2 cm apart. Two animals from each group were euthanized at 24, 48 or 72 h later; inoculation site skin specimens were harvested for spirochete culture. As a positive control, the ΔospC/ospC'/1 and ΔospC/ospC'/2 bacteria were consistently grown from each of the 24 inoculation sites from all 12 inoculated mice (Table 2). In contrast, the ΔospC/E22/1 and ΔospC/E22/2 spirochetes were recovered from only two of the eight sites harvested within 24 hours, and from none of the 16 specimens collected after then, confirming the essential role of OspC in protecting B. burgdorferi from quick clearance in murine skin reported by Tilly et al. (Tilly et al., 2007). Like the positive control, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, ΔospC/vlsE'/2, ΔospC/dbpA'/1, and ΔospC/dbpA'/2 spirochetes were consistently grown from each of the specimens harvested from all inoculated mice at all time points. Thus, the study also demonstrated that the essential protective role of OspC against early elimination can be overridden by increasing expression of any of the four Osps.
Table 2.
Increasing expression of an Osp protects OspC-deficient B. burgdorferi from quick elimination in murine skin.a
| Clone | No. of sites positive/Total no. of sites examined at post-inoculation hours |
||
|---|---|---|---|
| 24 | 48 | 72 | |
| ΔospC/ospC′/1 | 4/4 | 4/4 | 4/4 |
| ΔospC/ospC′/2 | 4/4 | 4/4 | 4/4 |
| ΔospC/E22/1 | 1/4 | 0/4 | 0/4 |
| ΔospC/E22/2 | 1/4 | 0/4 | 0/4 |
| ΔospC/ospA′/1 | 4/4 | 4/4 | 4/4 |
| ΔospC/ospA′/2 | 4/4 | 4/4 | 4/4 |
| ΔospC/ospE′/1 | 4/4 | 4/4 | 4/4 |
| ΔospC/ospE′/2 | 4/4 | 4/4 | 4/4 |
| ΔospC/vlsE′/1 | 4/4 | 4/4 | 4/4 |
| ΔospC/vlsE′/2 | 4/4 | 4/4 | 4/4 |
| ΔospC/dbpA′/1 | 4/4 | 4/4 | 4/4 |
| ΔospC/dbpA′/2 | 4/4 | 4/4 | 4/4 |
Groups of six BALB/c SCID mice each received two intradermal/subcutaneous injections of the clone ΔospC/ospC′/1, ΔospC/ospC′/2, ΔospC/E22/1, ΔospC/E22/2, ΔospC/ospA′/1, ΔospC/ospA′/2, ΔospC/ospE′/1, ΔospC/ospE′/2, ΔospC/vlsE′/1, ΔospC/vlsE′/2, ΔospC/dbpA′/1, or ΔospC/dbpA′/2. Approximately 105 organisms were administered in each inoculation; two inoculation sites were at least 2 cm apart. Two animals from each group were euthanized at 24, 48, and 72 h post-inoculation; skin specimens were harvested from inoculation sites and cultured for spirochetes in BSK-H complete medium.
OspC is required for efficient dissemination and this function can be substituted to varying extents by other outer surface lipoproteins
Groups of six to 12 SCID mice each received a single intradermal/subcutaneous inoculation of 105 spirochetes of the clone ΔospC/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, ΔospC/vlsE'/2, ΔospC/dbpA'/1, or ΔospC/dbpA'/2. Three animals from each group were euthanized at 1-wk intervals; inoculation site and remote site skin, ear, heart, and joint specimens were harvested for spirochete isolation. Bacteria were injected into the dermis of the chest so the skin from the back was harvested as remote sites. As a positive control, the ΔospC/ospC'/1 and ΔospC/ospC'/2 bacteria were grown from all of the skin, joint and heart specimens but from none of the ear samples at first week; all sites became culture positive at 2 wk after initial inoculation (Table 3). The ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, and ΔospC/vlsE'/2 bacteria were grown from all of the inoculation sites and joint specimens but from only one heart sample at a week post-inoculation; all hearts but only 3 ear specimens became positive at 2 wk; most ear samples were not colonized until 3 wk. Although the ΔospC/dbpA'/1 and ΔospC/dbpA'/2 spirochetes were consistently grown from all of the inoculation sites, they were not recovered from any distal tissues during the 4-wk period. These data demonstrated that OspC is required for efficient dissemination and that this function can be substituted to varying extents by other Osps.
Table 3.
OspC-deficient B. burgdorferi with increased expression of OspA, OspE or VlsE but not DbpA causes disseminated infection in SCID mice. a
| Clone | No. of specimens positive/Total specimens examined at post-inoculation weeks |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|||||||||||||||||
| I.S. | R.S. | Ear | Heart | Joint | I.S. | R.S. | Ear | Heart | Joint | I.S. | R.S. | Ear | Heart | Joint | I.S. | R.S. | Ear | Heart | Joint | |
| ΔospC/ospC′/1 | 3/3 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | NDb | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ΔospC/ospC′/2 | 3/3 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ΔospC/ospA′/1 | 3/3 | 0/3 | 0/3 | 1/3 | 3/3 | 3/3 | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/ospA′/2 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/ospE′/1 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/ospE′/2 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 1/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/vlsE′/1 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/vlsE′/2 | 3/3 | 0/3 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | ND | ND | ND | ND | ND |
| ΔospC/dbpA′/1 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| ΔospC/dbpA′/2 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 |
Groups of six --12 BALB/c SCID mice each received a single intradermal/subcutaneous injection of 105 organisms of the clone ΔospC/ospC′/1, ΔospC/ospC′/2, ΔospC/ospA′/1, ΔospC/ospA′/2, ΔospC/ospE′/1, ΔospC/ospE′/2, ΔospC/vlsE′/1, ΔospC/vlsE′/2, ΔospC/dbpA′/1, or ΔospC/dbpA′/2. Three animals from each group were euthanized at 1, 2, 3, and 4 wk post-inoculation; inoculation site (I.S.) and remote site (R.S.) skin, ear, heart, and joint specimens were harvested for spirochete isolation. The I.S. site was at the chest; therefore the R.S. site was at the back of mice.
ND, not determined.
All isolated spirochetes were grown to stationary phase and analyzed for OspC expression by immunoblotting. All recovered ΔospC/ospC'/1 and ΔospC/ospC'/2 spirochetes abundantly expressed OspC but none of the ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, ΔospC/vlsE'/2, ΔospC/dbpA'/1 or ΔospC/dbpA'/2 isolates produced the antigen (data not shown), indicating that all of the ospC mutant derivatives remained OspC-deficient.
OspC is not required for efficient colonization in the joint or skin but heart tissues of SCID mice
To examine the influence of OspC deficiency on tissue colonization, subgroups of five SCID mice each received a single intradermal/subcutaneous inoculation of 105 spirochetes of the clone ΔospC/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, or ΔospC/vlsE'/2. In 10 mice that were inoculated with the ΔospC/ospC'/1 or ΔospC/ospC'/2, joint swelling evolved around 10 d post-inoculation and developed into severe arthritis a wk later (data not shown). In the remaining mice, joint swelling did not become apparent until 3 wk post-inoculation and slowly developed after then, indicating that the OspC-deficient phenotypes with increased Osp expression cause delayed, less severe arthritis.
Ear biopsies were taken for bacterial culture at 2 and 3 wk post-inoculation. At 2 wk, all of the 10 mice that were inoculated with the ΔospC/ospC'/1 or ΔospC/ospC'/2 bacteria had a positive biopsy (data not shown). The remaining mice did not produce a positive biopsy until 3 wk post-inoculation. Again, the study demonstrated that OspC is not required for infection of immunodeficient mice but is important for efficient dissemination once the ospC mutant is modified with increased osp expression.
All the 40 mice were euthanized 1 mo post-inoculation; DNA was extracted from heart, joint and skin specimens and quantified for bacterial burden. In heart tissue, the ΔospC/ospC' spirochete burden was 84%, 89%, and 81% higher than those of the ΔospC/ospA' (P = 4.5 × 10−4), ΔospC/ospE' (P = 3.5 × 10−4), and ΔospC/vlsE' (P = 9.1 × 10−4), respectively, while the three genotypes with increased OspA, OspE or VlsE expression generated similar bacterial loads (P > 0.05) (Fig. 2). However, there was no significant difference in bacterial load among the four genotypes either in joint (P > 0.05) or skin tissues (P > 0.05). The study indicated that OspC is not required for efficient colonization in both joint and skin but in the heart tissue of SCID mice.
Fig. 2. OspC deficiency does not reduce the ability of B. burgdorferi to colonize joint or skin but heart tissues of SCID mice.

Subgroups of five SCID mice were inoculated with 105 spirochetes of the clone ΔospC/ospC'/1, ΔospC/ospC'/2, ΔospC/ospA'/1, ΔospC/ospA'/2, ΔospC/ospE'/1, ΔospC/ospE'/2, ΔospC/vlsE'/1, or ΔospC/vlsE'/2, and euthanized a month later. DNA was prepared from heart, joint and skin specimens and analyzed for spirochete flaB and murine actin DNA copies by qPCR. Data are expressed as spirochete numbers per 106 host cells and presented in four groups by combining the subgroups ΔospC/ospC'/1 and ΔospC/ospC'/2, ΔospC/ospA'/1 and ΔospC/ospA'/2, ΔospC/ospE'/1 and ΔospC/ospE'/2, and ΔospC/vlsE'/1 and ΔospC/vlsE'/2.
OspC-deficient B. burgdorferi with increased OspA expression registers a slight ID50 increase in SCID mice but a dramatic increase in immunocompetent mice
To further assess the contribution of OspC to infectivity, the ID50 value was determined. For this purpose, OspC-deficient B. burgdorferi was modified to express OspA under control of the ospC regulatory elements, including both promoter and operator (Xu et al., 2007a). Because the ospC promoter is RpoS-dependent (Hubner et al., 2001), the absence of the operator allows it to drive constitutive expression and, as a consequence, diminishes the ability of B. burgdorferi to evade humoral immunity during infection of immunocompetent mice (Xu et al., 2007a). The inclusion of the operator may allow recombinant B. burgdorferi to down-regulate the introduced ospA copy in response to the development of specific humoral responses. OspA was chosen for this purpose because OspA and OspC are so unrelated. First, unlike OspC, OspA is abundantly expressed in the tick (Schwan et al., 1995). Second, OspA is β-sheet dominant, while OspC is primarily composed of α-helices (Eicken et al., 2001; Kumaran et al., 2001; Li et al., 1997).
The construct pBBE22-CpospA was created as illustrated in Fig. S1 (Supplemental Material). To examine whether it drove phase-dependent ospA expression, an ospA mutant was generated (Supplemental Material). pBBE22-CpospA was electroporated into the ospA mutant. Twelve transformants were obtained; plasmid content analyses led to selection of two clones, namely, ΔospA/CpospA/1 and ΔospA/CpospA/2. Immunoblotting showed that both clones exhibited phase-dependent ospA expression, when grown in vitro (Fig. 3).
Fig. 3. The ospC promoter drives phase-dependent ospA expression.
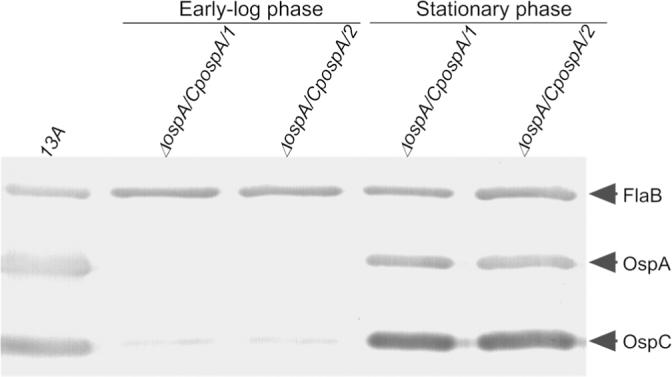
The 13A spirochetes harvested at stationary phase and the ΔospA/CpospA/1 and ΔospA/CpospA/2 bacteria grown to early-log and stationary phases were analyzed by immunoblot probed with a mixture of FlaB, OspA and OspC MAbs.
Next, pBBE22-CpospA was electroporated into the ospC mutant. Fifteen transformants were obtained; plasmid content analyses led to selection of two clones, namely, ΔospC/CpospA/1 and ΔospC/CpospA/2, which shared the same plasmid content as the ospC mutant (Xu et al., 2007a). Groups of three SCID mice each received one single inoculation of 101 to 104 spirochetes of the clone ΔospC/FL/1, ΔospC/FL/2, ΔospC/CpospA/1, or ΔospC/CpospA/2. The clones ΔospC/FL/1 and ΔospC/FL/2 were generated via introduction of a full-length ospC gene including both the promoter and operator carried by the shuttle vector pBBE22 into the ospC mutant in our previous study (Xu et al., 2007a). Ear biopsies were taken for bacterial culture at 2, 3 and 4 wk post-inoculation. At 2 wk, all of the 20 mice that were found to be infected with the ΔospC/FL/1 or ΔospC/FL/2 bacteria at the end of study had a positive biopsy (Table 4). In contrast, none of the mice inoculated with the clone ΔospC/CpospA/1 or ΔospC/CpospA/2 produced a positive biopsy at 2 wk. All the six mice that received 102 organisms of the clone ΔospC/CpospA/1 or ΔospC/CpospA/2 did not show a positive biopsy until 4 wk post-inoculation. Again, the study demonstrated that OspC deficiency severely impairs dissemination.
Table 4.
OspC-deficient B. burgdorferi with increased OspA expression registers a slight ID50 increase in SCID mice.a
| Expt, clone, and dose (no. of organisms) | No. of biopsies positive/Total ear biopsies conducted at post-inoculation weeks |
No. of cultures positive/Total specimens examined |
No. of mice infected/Total mice inoculated | ID50 (no. of organisms) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | Heart | Joint | Skin | All site | |||
| I | |||||||||
| ΔospC/FL/1 | 18 | ||||||||
| 104 | 3/3 | NDb | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 1/3 | ND | ND | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| ΔospC/FL/2 | 18 | ||||||||
| 104 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 1/3 | ND | ND | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| ΔospC/CpospA/1 | 32 | ||||||||
| 104 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/CpospA/2 | 32 | ||||||||
| 104 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| II | |||||||||
| ΔospC/FL/1 | 18 | ||||||||
| 103 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 1/3 | ND | ND | 1/3 | 1/3 | 1/3 | 1/9 | 1/3 | |
| ΔospC/FL/2 | 18 | ||||||||
| 103 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 1/3 | ND | ND | 1/3 | 1/3 | 1/3 | 3/9 | 1/3 | |
| ΔospC/CpospA/1 | 32 | ||||||||
| 103 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/CpospA/2 | 18 | ||||||||
| 103 | 0/3 | 3/3 | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 1/3 | 1/3 | 1/3 | 1/9 | 1/3 | |
The ΔospC/FL/1, ΔospC/FL/2, ΔospC/CpospA/1, and ΔospC/CpospA/2 spirochetes were grown to late-log phase (108 cells per ml) and 10-fold serially diluted with BSK-H medium. Groups of three BALB/c SCID mice each received a single intradermal/subcutaneous dose of 100 μl of bacterial suspension; ear biopsies were performed up to four weeks post-inoculation, starting at week 2. Once all three animals of a dose group became positive, biopsies were no longer performed on the group. All animals were sacrificed 1 month post-inoculation; heart, tibiotarsal joint, and skin specimens were harvested for bacterial isolation. The ID50 values were calculated by the method of Reed and Muench (Reed and Muench, 1938), and determined in two separate experiments.
ND, not determined.
All animals were euthanized 1 mo post-inoculation; heart, joint and skin specimens were cultured for spirochetes. The ID50 values of both clones ΔospC/CpospA/1 and ΔospC/CpospA/2 were 32 organisms, compared to 18 organisms determined for both clones ΔospC/FL/1 and ΔospC/FL/2 (Table 4). Similar results were obtained in a separate experiment, in which the ID50 values of the clones ΔospC/CpospA/1 and ΔospC/CpospA/2 were 32 and 18 organisms, respectively, compared to 18 organisms for both clones ΔospC/FL/1 and ΔospC/FL/2. By combining the two experiments, the study indicated that the OspC deficiency led to only a 1.6-fold ID50 increase in SCID mice (P = 0.05) after modification with increased OspA expression.
Next, the influence of OspC-deficiency on spirochetal dissemination and the ID50 value was investigated in immunocompetent mice. In two separate experiments, all mice that were found to be infected with the ΔospC/FL/1 or ΔospC/FL/2 bacteria at the end of study produced a positive biopsy either at 2 or 3 wk post-inoculation, depending on inoculation doses (Table 5). In contrast, none of the mice inoculated with the clone ΔospC/CpospA/1 or ΔospC/CpospA/2 produced a positive biopsy within the first 3 wk; most of the inoculated mice produced a positive biopsy at 4 wk, and four infected mice did not develop a positive ear biopsy until wk 5. The study demonstrated that OspC deficiency severely impairs dissemination in immunocompetent mice.
Table 5.
OspC-deficient B. burgdorferi with increased OspA expression registers a dramatic ID50 increases in immunocompetent mice.a
| Expt, clone, and dose (no. of organisms) | No. of biopsies positive/Total ear biopsies conducted at post-inoculation weeks |
No. of cultures positive/Total specimens examined |
No. of mice infected/Total mice inoculated | ID50 (no. of organisms) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | Heart | Joint | Skin | All site | |||
| I | ||||||||||
| ΔospC/FL/1 | 32 | |||||||||
| 104 | 3/3 | NDb | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 1/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/FL/2 | 32 | |||||||||
| 104 | 3/3 | ND | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 103 | 1/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/CpospA/1 | 1000 | |||||||||
| 105 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 3/3 | 3/3 | 7/9 | 3/3 | |
| 104 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 2/3 | 3/3 | 6/9 | 3/3 | |
| 103 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 1/3 | 1/9 | 1/3 | |
| 102 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 1/3 | 1/9 | 1/3 | |
| ΔospC/CpospA/2 | 1778 | |||||||||
| 105 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 2/3 | 3/3 | 6/9 | 3/3 | |
| 104 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 1/3 | 3/3 | 5/9 | 3/3 | |
| 103 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 1/3 | 1/9 | 1/3 | |
| 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| II | ||||||||||
| ΔospC/FL/1 | 32 | |||||||||
| 103 | 1/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/FL/2 | 32 | |||||||||
| 103 | 0/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 102 | 0/3 | 3/3 | ND | ND | 3/3 | 3/3 | 3/3 | 9/9 | 3/3 | |
| 101 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/CpospA/1 | 1778 | |||||||||
| 104 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 1/3 | 3/3 | 5/9 | 3/3 | |
| 103 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 1/3 | 1/9 | 1/3 | |
| 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| ΔospC/CpospA/2 | 3162 | |||||||||
| 104 | 0/3 | 0/3 | 3/3 | ND | 1/3 | 1/3 | 3/3 | 5/9 | 3/3 | |
| 103 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
| 102 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/9 | 0/3 | |
The ΔospC/FL/1, ΔospC/FL/2, ΔospC/CpospA/1, and ΔospC/CpospA/2 spirochetes were grown to late-log phase (108 cells per ml) and 10-fold serially diluted with BSK-H medium. Groups of three BALB/c mice each received a single intradermal/subcutaneous dose of 100 μl of bacterial suspension; ear biopsies were performed up to five weeks post-inoculation, starting at week 2. Once all three animals of a dose group became positive, biopsies were no longer performed on the group. All animals were sacrificed 6 week post-inoculation; heart, tibiotarsal joint, and skin specimens were harvested for bacterial isolation. The ID50 values were calculated by the method of Reed and Muench (Reed and Muench, 1938), and determined in two separate experiments.
ND, not determined.
The ID50 values for both clones ΔospC/FL/1 and ΔospC/FL/2 were 32 organisms in both experiments, in comparison to 1000, 1778, 1778 and 3162 organisms determined for the clones ΔospC/CpospA/1 and ΔospC/CpospA/2 (Table 5). Overall, OspC-deficient B. burgdorferi with OspA expression under control of the ospC regulatory elements registered a 60-fold ID50 increase than the mutant restored with wild-type OspC expression (P = 0.006).
Another defect noted from OspC-deficient B. burgdorferi with increased OspA expression was a reduced frequency in colonization of the heart and joint tissues of immunocompetent mice. The ΔospC/CpospA/1 and ΔospC/CpospA/2 spirochetes were grown only from 6 heart and 10 joint specimens of the 22 infected mice (Table 5); in contrast, the clones ΔospC/FL/1 and ΔospC/FL/2 colonized all the tissues of the 30 infected mice. The study indicated that the OspC deficiency leads to a 73% and 55% decrease in frequency of colonizing heart (P = 7.1 × 10−26) and joint tissues (P = 1.2 × 10−12), respectively, during early infection of immunocompetent mice. As shown in Table 3, the joint was the first distal tissue to be colonized, followed by the heart, in SCID mice, it is possible that spirochetes might have colonized these tissues but subsequently been cleared by the specific humoral response during infection in immunocompetent mice. Alternatively, the specific humoral response might have blocked spirochetes from disseminating to these tissues.
OspC-deficient B. burgdorferi with increased OspA expression can persist in the skin but not heart or joint tissues during chronic infection of immunocompetent mice
Next, OspC deficient spirochetes with increased OspA expression were investigated for the ability to evade adaptive immunity and cause persistent infection. Subgroups of five BALB/c mice each received one single intradermal/subcutaneous injection of 104 spirochetes of the clone ΔospC/FL/1, ΔospC/FL/2, ΔospC/CpospA/1, or ΔospC/CpospA/2. Animals were euthanized 4 mo after initial inoculation; heart, joint, and skin samples were cultured for spirochetes. B. burgdorferi was grown from each skin specimen of all 20 mice, regardless of whether they received OspC-deficient or control spirochetes (Table 6). The ΔospC/FL/1 and ΔospC/FL/2 spirochetes were successfully grown from all of the 10 hearts and 9 of the 10 joint specimens; however, OspC-deficient B. burgdorferi was not recovered from 9 of the 10 hearts and 8 of the 10 joint specimens. The study indicated that OspC-deficient B. burgdorferi with increased OspA expression has a diminished ability to colonize or persist in heart and joint tissues of immunocompetent mice during chronic infection.
Table 6.
OspC-deficient B. burgdorferi with increased OspA expression can persist in the skin but not heart or joint tissue of chronically infected immunocompetent mice.a
| Clone | No. of cultures positive/Total no. of specimens examined |
|||
|---|---|---|---|---|
| Heart | Joint | Skin | All sites | |
| ΔospC/FL/1 | 5/5 | 5/5 | 5/5 | 15/15 |
| ΔospC/FL/2 | 5/5 | 4/5 | 5/5 | 14/15 |
| ΔospC/CpospA/1 | 0/5 | 1/5 | 5/5 | 6/15 |
| ΔospC/CpospA/2 | 1/5 | 1/5 | 5/5 | 7/15 |
Groups of five BALB/c mice were inoculated with 104 spirochetes of the clone ΔospC/FL/1, ΔospC/FL/2, ΔospC/CpospA/1, or ΔospC/CpospA/2. Mice were sacrificed 4 mo post-inoculation; heart, tibiotarsal joint and skin specimens were harvested for spirochete culture in BSK-H complete medium.
The anti-OspA humoral response was analyzed in the 20 infected mice. No or weak anti-OspA response was detected in the 10 mice that were infected with the genotype ΔospC/FL (Fig. 4). In contrast, mice infected with the genotype ΔospC/CpospA produced anti-OspA responses 382-fold higher than those that were inoculated with the ΔospC/FL spirochetes (P < 0.05).
Fig. 4. Strong anti-OspA responses are induced by OspC-deficient B. burgdorferi with OspA expression under control of the ospC regulatory elements.
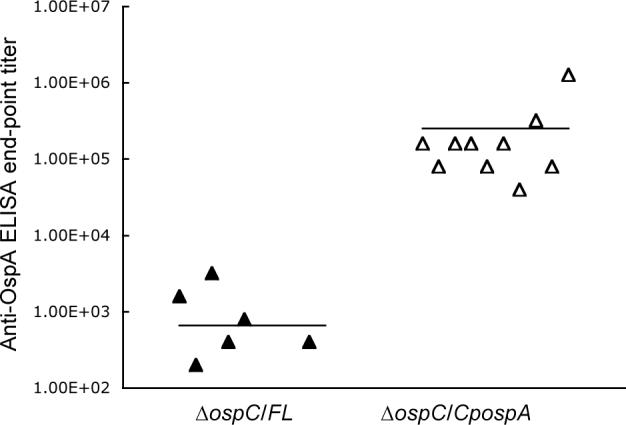
Groups of 10 mice were infected with either ΔospC/FL or ΔospC/CpospA spirochetes for 4 mo. Serum samples were collected and analyzed for anti-OspA antibody titers by an end-point ELISA. No response was detected in four of the mice infected with the genotype ΔospC/FL so that only six datum points are shown for this group. The average titers (horizontal lines) for each group are also presented.
Discussion
The current study took the advantage that inactivation of the ospC gene results in quick clearance of B. burgdorferi in the murine host, and clearly demonstrated that increasing expression of any of the four randomly chosen but well-studied surface lipoproteins can fully restore the ability of the ospC mutant to evade innate defenses. Because OspC is an abundantly expressed surface antigen and may contribute dominantly to the overall integrity of the outer lipoprotein layer during initial infection, the OspC deficiency may cause a severe compromise of the lipoprotein layer and, as a result, completely abrogate its protective function and lead to quick clearance of B. burgdorferi by the first line of host defenses. Increasing expression of OspA, OspE, VlsE or DbpA may successfully compensate for the loss of OspC and restore the integrity of the lipoprotein layer and, consequently, its protective function. Clearly, the study revealed one common function of the surface lipoproteins, which is to protect the pathogen from elimination by host innate defenses.
The current study showed different roles of individual surface lipoproteins, while demonstrating their common role. Although increasing expression of any of the four lipoproteins fully protected OspC-deficient B. burgdorferi from elimination, distinct phenotypes were observed during infection of SCID mice. Increased expression of OspA, OspE or VlsE allowed the ospC mutant to cause disseminated infection, albeit the dissemination process was much slower than that of the mutant restored with OspC expression. However, increasing DbpA expression protected OspC-deficient spirochetes in murine skin but could not make them disseminate to distal tissues. Analysis of bacterial loads revealed that increasing expression of OspA, OspE or VlsE restored the ability of the ospC mutant to effectively colonize both joint and skin tissues but not heart of SCID mice. During the course of infection of SCID mice, ospC is highly expressed in the heart tissue (Liang et al., 2004), where host ligands may exist (Antonara et al., 2007); potential interactions of B. burgdorferi with host components mediated by OspC may facilitate tissue colonization. When OspA was selected for examining the ability to replace OspC for restoration of infectivity, increasing OspA expression provided the ospC mutant with an ID50 value comparable to that of the mutant restored with OspC expression in SCID mice. Given that most of the roles of OspC can be replaced with other surface lipoproteins, the primary function attributed to OspC should be to facilitate dissemination.
The overall surface architecture of B. burgdorferi is collectively determined by each of the individual lipoproteins and their expression levels on the surface, and directly interacts with the environment, thus largely influencing the behavior of the pathogen in a specific microenvironment. After the surface is decorated dominantly with OspA and OspB, B. burgdorferi probably fits best to the environment of the tick midgut (Neelakanta et al., 2007; Yang et al., 2004), where the two lipoproteins may mediate interactions with gut ligands (Pal et al., 2004a). A fresh blood meal induces the down-regulation of OspA and OspB and the up-regulation of OspC as well as other surface antigens (Schwan et al., 1995), reshaping the surface architecture, an event that prepares the pathogen for the mammalian environment. These modifications may also prompt B. burgdorferi to migrate to salivary glands and finally into the dermis of a mammal (Pal et al., 2004b). Repression of OspA/B expression during infection of mammals is critical for the maintenance of the enzootic cycle because their expression would ultimately induce a strong humoral response and, as a result, may effectively block acquisition of B. burgdorferi by the vector (de Silva et al., 1997; Tsao et al., 2001; Tsao et al., 2004), regardless of whether OspA/B can be targeted by borreliacidal antibodies in mammalian tissues (Strother et al., 2007). As clearly shown in the current study, abundant OspC expression is critical for efficient dissemination to and quick colonization of distal tissues, and an establishment of systemic infection. Because OspC is a strong immunogen and an effective target of protective immunity, active OspC expression soon becomes a disadvantage as specific humoral immune responses are being elicited (Xu et al., 2006; Xu et al., 2007a). To evade the specific humoral response and proceed to persistent infection, B. burgdorferi down-regulates OspC and greatly up-regulates other surface antigens, such as VlsE (Liang et al., 2004). As a variable surface antigen, VlsE vigorously undergoes antigenic variation and is required for persistent infection of immunocompetent mice (Bankhead and Chaconas, 2007; Zhang et al., 1997; Zhang and Norris, 1998). Therefore, B. burgdorferi certainly benefits from these surface modifications during infection of immunocompetent hosts.
DbpA, a well-defined surface adhesin, binds both decorin and glycosaminoglycans (Fischer et al., 2003; Guo et al., 1998), and contributes significantly to the overall virulence of B. burgdorferi (Shi et al., 2006). Increasing DbpA expression significantly reduces the ID50 value but severely impairs dissemination, probably because an abundant presence of DbpA on the spirochetal surface facilitates the interaction of the pathogen with host decorin (Xu et al., 2007b). Consistent with these previous studies, the current study showed that increasing DbpA expression effectively protected OspC-deficient B. burgdorferi in murine skin but completely inhibited dissemination. In contrast, after OspC expression was restored, B. burgdorferi quickly disseminated to distal tissues. The identification of this extreme phenotype further underscores our notion that the overall surface architecture greatly determines the infectious behavior of B. burgdorferi. One may argue that increasing DbpA expression protected the ospC mutant only in skin so spirochetes might be quickly eliminated once they left the tissue. If this was the case, OspC-deficient B. burgdorferi with increased DbpA expression should be grown from remote skin specimens of infected SCID mice. As a matter of fact, however, bacteria with increased DbpA expression were not grown from any remote skin samples during the 4-wk period.
As the first surface lipoprotein that is shown binding the complement regulator factor H (Hellwage et al., 2001), OspE is one of five complement regulator-acquiring surface proteins that have been identified in B. burgdorferi to date (Hartmann et al., 2006; Kraiczy et al., 2004; McDowell et al., 2003; Metts et al., 2003; Stevenson et al., 2002), and one of the four that are persistently expressed during murine infection (Bykowski et al., 2007). Although an in vitro study showed a critical role of the regulator-binding proteins in contributing to complement resistance in B. burgdorferi (Brooks et al., 2005), a recent study indicated that binding of factor H does not significantly affect the infectivity of B. burgdorferi in mice (Woodman et al., 2007). While it remains to be addressed whether OspE functions as a regulator-binding protein to provide protection against complement, the current study showed that OspE, just like other surface lipoproteins, is able to protect OspC-deficient B. burgdorferi against elimination by host innate defenses.
Naïve and immune statuses constitute two distinct environments to microbial pathogens. The ability of OspA to replace OspC was investigated in the two environments by using immunodeficient and immunocompetent mice. OspC-deficient B. burgdorferi modified with OspA expression under control of the ospC regulatory elements, including both the promoter and operator (Xu et al., 2007a), registered only a 1.6-fold ID50 increase in SCID mice but an over 60-fold increase in immunocompetent mice than the mutant restored with OspC expression. The mutant with increased OspA expression colonized all tissues of infected SCID mice and generated similar bacterial loads in both joint and skin tissues as the mutant restored with OspC expression, but was grown only from less than 20% of the heart and joint samples of chronically infected immunocompetent mice. This reduced ability of OspA to replace OspC in evasion of adaptive immunity may result from their unequal effectiveness targeted by protective humoral responses. In fact, OspC is an effective target of protective immunity (Xu et al., 2006); to evade the anti-OspC immune response, B. burgdorferi reduces OspC expression to a baseline level (Liang et al., 2002a; Liang et al., 2004), probably via the induction of a yet unidentified repressor to interact with the ospC operator (Xu et al., 2007a). As the current study showed, the ospC promoter indeed drove phase-dependent ospA expression in cultured spirochetes. However, it remains to be addressed whether the ospC operator can effectively respond to environmental changes, including the development of a specific humoral response, when it controls ospA expression.
One unique function that was identified for OspC in the current study is to facilitate dissemination, which can be substituted to varying extents by other Osps. OspC is highly expressed during initial murine infection but is downregulated after the specific humoral response has developed (Liang et al., 2002a; Liang et al., 2004), consistent with this newly defined role. Given this unique role, the early abundant expression promotes bacterial dissemination; after distal tissues are colonized and specific humoral responses develop, OspC is downregulated. The downregulation allows B. burgdorferi not only to effectively evade humoral immunity but also to preserve the integrity of the critical gene for the subsequent enzootic cycle, because active ospC expression would result in either clearance of infection or selection of ospC mutants (Xu et al., 2006). It would be interesting to examine the influence of OspC from different OspC genotypes of B. burgdorferi on spirochetal dissemination by cloning their ospC gene into the same ospC mutant, given that a study by Seinost et al. suggested an association of selective OspC genotypes with invasive infection (Seinost et al., 1999), although such a correlation has been challenged by a recent report from Alghaferi et al. (Alghaferi et al., 2005).
OspC is absolutely not required for tick colonization, but it remains controversial whether it is essential for transmission from the vector to a mammal (Fingerle et al., 2007; Grimm et al., 2004; Pal et al., 2004b; Stewart et al., 2006). Rosa and colleagues repeatedly showed that OspC is not required for the enzootic cycle of B. burgdorferi in the tick vector (Grimm et al., 2004; Stewart et al., 2006). In contrast, neither Pal et al. nor Fingerle et al. were able to make OspC mutants cross the tick salivary gland barrier to the murine host (Fingerle et al., 2007; Pal et al., 2004b). The fact that the first group used fully competent ospC mutants in their studies while the ospC mutants generated by the latter groups were not restored with infectivity could be a reason causing the disparity. Because none of their OspC-deficient clones was infectious, they had to use artificial tick feeding, which may also be a contributing factor for the disparity. The strategy to overcome the essential role of OspC in mammalian infection developed in the current study may help resolve the controversy.
B. burgdorferi very actively expresses OspA and OspB during in vitro cultivation before inoculation into mice. Although the pathogen down-regulates OspA and OspB immediately after the contact of murine tissues, these already expressed on the surface should remain protective until they are degraded or diluted through spirochete replication. However, an entire inoculum of 105 organisms deficient for OspC was eradicated, in most cases, within 24 h after inoculation into the skin of SCID mice, indicating that B. burgdorferi with compromised surface lipoprotein expression is extremely vulnerable to the innate immune system, albeit it remains to be determined which components of the system, phagocytes, complement and/or others, play a major role in this regard. Increasing expression of any of the four randomly chosen but well- studied surface lipoproteins successfully protected the ospC mutant in SCID mice. Given that the four lipoproteins are completely unrelated to OspC or to each other but were able to protect the pathogen just as OspC, the study demonstrated one common function of the surface lipoproteins, which is to protect B. burgdorferi against elimination by host innate defense mechanisms. It would be interesting to address how these antigens can provide the pathogen with such protection while they serve as potent innate immune stimulators via Toll-like receptor-dependent signaling.
Experimental procedures
Strains and constructs that were generated previously and used in the current study
The ospC mutant, and the clones ΔospC/FL/1 and ΔospC/FL/2 were generated previously (Xu et al., 2007a) (Table 1). The constructs pBBE22-ospC' and pBBE22-dbpA' were constructed previously (Xu et al., 2006; Xu et al., 2007b). The shuttle vector pBBE22 was a gift from S. Norris (Purser et al., 2003).
Construction of pBBE22-ospA', pBBE22-ospE', and pBBE22-vlsE'
As illustrated in Fig. 1A, three primer pairs, P2F and P3R, P3F and P4R, and P4F and P5R (Table S1, Supplemental Materials), were used, respectively, to amplify 845-bp promoterless ospA, 1119-bp vlsE, and 593-bp ospE sequences from borrelial DNA. Two fragments, 254-bp and 256-bp, were amplified from the promoter region of flaB using a common forward primer, P1F, and two reverse primers, P1R and P2R, respectively. The 254-bp flaBp amplicon was pooled with the 845-bp ospA and 1119-bp vlsE amplicons, respectively, digested with NdeI, purified, then ligated to form flaBp-ospA and flaBp-vlsE. The 256-bp flaBp amplicon was pooled with the 593-bp ospE product, digested with NcoI and BspHI, purified, and ligated to create flaBp-ospE. The fragments flaBp-ospA, flaBp-vlsE, and flaBp-ospE were amplified with the use of a common forward primer, P5F, and three reverse primers, P6R, P7R and P8R, respectively, digested with BamHI and XbaI, purified, and cloned into the shuttle vector pBBE22 (Purser et al., 2003) after the vector was digested with the same enzymes. The inserts within pBBE22 were sequenced to ensure that the inserts and their flanking sequences were arranged as designed.
Generation of B. burgdorferi with increased Osp expression
The constructs pBBE22-ospC', pBBE22-ospA', pBBE22-ospE', pBBE22-vlsE' and pBBE22-dbpA', and their parental vector, pBBE22, were electroporated into the ospC mutant, which was generated and characterized in our previous study (Xu et al., 2007a), as described previously (Xuet al., 2005). pBBE22-ospC' and pBBE22-dbpA' were generated in our previous studies (Xu et al., 2006; Xu et al., 2007b). Resulting transformants were first surveyed for the presence of lp28−1 because this plasmid is essential for persistent infection of immunocompetent hosts (Labandeira-Rey et al., 2003; Purser and Norris, 2000; Xu et al., 2005). Only clones containing lp28−1 were further analyzed for plasmid content as described previously (Xu et al., 2005).
In vitro characterization of transformants
Transformants were grown in Barbour-Stoenner-Kelly H (BSK-H) complete medium (Sigma Chemical Co., St. Louis, MO) to late-log phase at 33°C, and harvested by centrifugation. Spirochete lysates were subjected to immunoblot analysis probed with FlaB (Barbour et al., 1986), OspA (Sears et al., 1991) or OspC mAb (Mbow et al., 1999), or mouse anti-DbpA sera (Shi et al., 2006), or mouse antisera raised against recombinant OspE or VlsE prepared as described below. Immunoblotting was performed as described previously (Xu et al., 2006).
Preparation of recombinant proteins and generation of mouse antisera
The coding regions excluding the signal peptide-coding sequence of the genes ospA, ospE and vlsE were PCR amplified and cloned into the expression vector pET16b, and transformed into the Escherichia coli strain BL21(DE3) (Novagen, La Jolla, CA). Recombinant protein was purified using the Hi-Trap affinity column (Amersham-Pharmacia Biotech, Piscataway, NJ). The protein purity and concentration were determined using SDS-PAGE and the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA), respectively. Approximately 70 μg of recombinant protein was dissolved in 100 μl of PBS (pH = 7.3) and emulsified with 30 μl of Freund's complete (first injection) or incomplete adjuvant (remaining injections), and subcutaneously administered into each BALB/c mouse (ages, 5 − 8 wk) at 3-wk intervals. The specific humoral response was monitored by immunoblotting with use of borrelial lysates as antigen. Mice were euthanized 3 wk after last immunization for antiserum preparation.
Inoculation of SCID mice
BALB/c SCID mice (ages of 4--8 wk, provided by the Division of LSU Laboratory Animal Medicine) were given two intradermal/subcutaneous injections of 105 spirochetes. The two inoculation sites were at least 2 cm apart. Animals were sacrificed 24, 48 or 72 h later; inoculation site skin tissues were harvested for spirochete isolation as described previously (Xu et al., 2005).
In a second experiment, SCID mice each received a single intradermal/subcutaneous injection of 105 spirochetes and were euthanized at 1, 2, 3, and 4 wk post-inoculation. Inoculation site and remote site skin, ear, heart, and joint specimens were harvested for spirochete isolation as described previously (Xu et al., 2005). Spirochetes were injected into the dermis of the chest so the skin from the back was harvested as remote sites. Isolated bacteria were grown to late-log phase, and were subjected to immunoblot analysis probed with a mixture of FlaB and OspC MAbs as described previously (Xu et al., 2006).
In a third experiment, SCID mice each received a single intradermal/subcutaneous injection of 105 spirochetes. Ear biopsies were taken for bacterial culture at 2 and 3 wk post-inoculation. Animals were examined for the development of arthritis at 2-d intervals, starting at d 7, and sacrificed 1 mo post-inoculation. Joint, heart, and skin specimens were collected for DNA isolation. DNA was quantified by qPCR for copy numbers of flaB and murine actin genes (Xu et al., 2005). The tissue spirochete burden was expressed as flaB DNA copies per 106 host cells (2 × 106 actin DNA copies).
Determination of ID50 values
Spirochetes were grown at 33°C to late-log phase (108 cells per ml) and 10-fold serially diluted with BSK-H complete medium. BALB/c or BALB/c SCID mice (age, 4 -- 8 wk; provided by the LSU Division of Laboratory Animal Medicine) each received a single intradermal/subcutaneous injection of 100 μl of spirochetal suspension. Ear biopsies were performed up to 5 wk post-inoculation, starting at wk 2, as described previously (Xu et al., 2006). SCID and wild-type mice were euthanized 1 mo and 6 wk post-inoculation, respectively; heart, tibiotarsal joint, and skin (not from inoculation site) specimens were harvested for bacterial culture as described previously (Xu et al., 2005). The ID50 value was calculated as described by Reed and Muench (Reed and Muench, 1938).
Persistent infection study in immunocompetent mice
BALB/c mice at ages of 4 -- 8 wk were given a single intradermal/subcutaneous injection of 104 spirochetes. All mice were sacrificed 4 mo after the initial inoculation; heart, tibiotarsal joint, and skin specimens were collected for spirochete culture as described previously (Xu et al., 2005).
Measurement of anti-OspA humoral immune response
Specific OspA antibody end-point titers were determined by an ELISA. Ninety-six-well plates (Fisher Scientific, Pittsburgh, PA) were coated with 100 μl of 2.0 μg/ml recombinant OspA per well. Sera were 2-fold serially diluted, starting at 1/200. Five samples drawn from naive BALB/c mice were used as a control. The ELISA was performed as previously described (Xu et al., 2006).
Statistical analysis
qPCR data and calculated ID50 values were analyzed by using a one-way analysis of variance (ANOVA), followed by a two-tailed Student t test to compare two treatments and calculate P values. Calculated P values of ≤ 0.05 were considered to be significant. Fisher's exact test was used to analyze tissue colonization data.
Acknowledgments
We thank S. Norris for providing pBBE22.
This work was supported in part by a career development award and a grant from NIH/NIAMS, an Arthritis Foundation Investigators award, and P20RR020159 (PI, Kousoulas) from NIH/NCRR.
Supplementary Material
REFERENCES
- Alghaferi MY, Anderson JM, Park J, Auwaerter PG, Aucott JN, Norris DE, Dumler JS. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J Clin Microbiol. 2005;43:1879–1884. doi: 10.1128/JCM.43.4.1879-1884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonara S, Chafel RM, LaFrance M, Coburn J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol. 2007;66:262–276. doi: 10.1111/j.1365-2958.2007.05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol. 2007;65:1547–1558. doi: 10.1111/j.1365-2958.2007.05895.x. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF, Heiland RA, Schrumpf ME, Tessier SL. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, Akins DR. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, Kraiczy P, Stevenson B. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect Immun. 2007;75:4227–4236. doi: 10.1128/IAI.00604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crother TR, Champion CI, Whitelegge JP, Aguilera R, Wu XY, Blanco DR, Miller JN, Lovett MA. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect Immun. 2004;72:5063–5072. doi: 10.1128/IAI.72.9.5063-5072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PA, Haake DA, Adler B. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol Rev. 2004;28:291–318. doi: 10.1016/j.femsre.2003.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Telford SR, 3rd, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65:3146–3150. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Hook M, Sacchettini JC. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem. 2001;276:10010–10015. doi: 10.1074/jbc.M010062200. [DOI] [PubMed] [Google Scholar]
- Fingerle V, Goettner G, Gern L, Wilske B, Schulte-Spechtel U. Complementation of a Borrelia afzelii OspC mutant highlights the crucial role of OspC for dissemination of Borrelia afzelii in Ixodes ricinus. Int J Med Microbiol. 2007;297:97–107. doi: 10.1016/j.ijmm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fischer JR, Parveen N, Magoun L, Leong JM. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2003;100:7307–7312. doi: 10.1073/pnas.1231043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung BP, McHugh GL, Leong JM, Steere AC. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006;61:1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- He M, Boardman BK, Yan D, Yang XF. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol. 2007;189:8377–8380. doi: 10.1128/JB.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJ, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci U S A. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, Brade V, Zipfel PF, Wallich R. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem. 2004;279:2421–2429. doi: 10.1074/jbc.M308343200. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. Embo J. 2001;20:971–978. doi: 10.1093/emboj/20.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28−1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003;71:4608–4613. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TT, Nguyen TP, Montgomery RR, Kantor FS, Fikrig E, Flavell RA. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci U S A. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu X, Beck DS, Kantor FS, Fikrig E. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect Immun. 2006;74:3305–3313. doi: 10.1128/IAI.02035-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Neelakanta G, Liu X, Beck DS, Kantor FS, Fish D, Anderson JF, Fikrig E. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect Immun. 2007;75:4237–4244. doi: 10.1128/IAI.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med. 2002a;195:415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002b;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun. 2004;72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, Gilmore RD, Jr., Titus RG. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection by Borrelia burgdorferi B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, Marconi RT. Comprehensive analysis of the factor h binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect Immun. 2003;71:3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Wolfgang J, Senty L, Sundy CM, Noto MJ, Marconi RT. Demonstration of the involvement of outer surface protein E coiled coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J Immunol. 2004;173:7471–7480. doi: 10.4049/jimmunol.173.12.7471. [DOI] [PubMed] [Google Scholar]
- Metts MS, McDowell JV, Theisen M, Hansen PR, Marconi RT. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect Immun. 2003;71:3587–3596. doi: 10.1128/IAI.71.6.3587-3596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakanta G, Li X, Pal U, Liu X, Beck DS, DePonte K, Fish D, Kantor FS, Fikrig E. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 2007;3:e33. doi: 10.1371/journal.ppat.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci U S A. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004a;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, Norgard MV, Fikrig E. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004b;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Bourell KW, Akins DR, Brusca JS, Norgard MV. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol. 1994;176:21–31. doi: 10.1128/jb.176.1.21-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AM, Muench H. A simple method of estimating fifty percent endpoint. Am J Hygiene. 1938;27:93–497. [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears JE, Fikrig E, Nakagawa TY, Deponte K, Marcantonio N, Kantor FS, Flavell RA. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991;147:1995–2000. [PubMed] [Google Scholar]
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, Skare JT. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Xu Q, Seemanapalli SV, McShan K, Liang FT. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect Immun. 2006;74:6509–6512. doi: 10.1128/IAI.00740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun. 2008;76:1239–1246. doi: 10.1128/IAI.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70:491–497. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun. 2006;74:3547–3553. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother KO, Hodzic E, Barthold SW, de Silva AM. Infection of mice with Lyme disease spirochetes constitutively producing outer surface proteins A and B. Infect Immun. 2007;75:2786–2794. doi: 10.1128/IAI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Rothenberg RJ, Barbour AG. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Bestor A, Jewett MW, Rosa P. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun. 2007;75:1517–1519. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J, Barbour AG, Luke CJ, Fikrig E, Fish D. OspA immunization decreases transmission of Borrelia burgdorferi spirochetes from infected Peromyscus leucopus mice to larval Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:65–74. doi: 10.1089/153036601750137705. [DOI] [PubMed] [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A. 2004;101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, Botto M, Hellwage J, Wooten RM, Stevenson B. Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun. 2007;75:3131–3139. doi: 10.1128/IAI.01923-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Seemanapalli SV, Lomax L, McShan K, Li X, Fikrig E, Liang FT. Association of linear plasmid 28−1 with an arthritic phenotype of Borrelia burgdorferi. Infect Immun. 2005;73:7208–7215. doi: 10.1128/IAI.73.11.7208-7215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Seemanapalli SV, McShan K, Liang FT. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun. 2006;74:5177–5184. doi: 10.1128/IAI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol Microbiol. 2007a;64:220–231. doi: 10.1111/j.1365-2958.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, Seemanaplli SV, McShan K, Liang FT. Increasing the interaction of Borrelia burgdorferi with decorin significantly reduces the 50 percent infectious dose and severely impairs dissemination. Infect Immun. 2007b;75:4272–4281. doi: 10.1128/IAI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Norris SJ. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66:3689–3697. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


