Abstract
We examined rotavirus-specific IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cell responses in gnotobiotic pigs infected with a virulent human rotavirus (VirHRV) or vaccinated with an attenuated (Att) HRV vaccine (AttHRV3x or AttHRV2x) or an AttHRV oral priming and 2/6-virus-like particle (VLP) intranasal boosting (AttHRV-2/6VLP) regimen. In VirHRV infected pigs, HRV-specific IFN-γ producing T cells reside primarily in ileum. AttHRV-2/6VLP induced similar frequencies of intestinal IFN-γ producing T cells as the VirHRV, whereas AttHRV3x or 2x vaccines were less effective. Protection rates against rotavirus diarrhea upon VirHRV challenge significantly correlated (r = 0.97–1.0, p < 0.005) with frequencies of intestinal IFN-γ producing T cells, suggesting their role in protective immunity.
Keywords: Human rotavirus vaccine, T cell responses, Gnotobiotic pigs
1. Introduction
Group A rotaviruses are the single most important etiologic agent of dehydrating diarrhea in infants and young children worldwide, causing approximately 611,000 deaths yearly [1]. To improve the efficacy of rotavirus vaccines, an improved understanding of both B and T cell arms of rotavirus protective immunity is essential. The distribution and magnitude of antibody-secreting cell (ASC) and memory B cell responses to virulent (Vir) human rotavirus (HRV) Wa strain (P1A[8]G1), the attenuated (Att) Wa HRV or the combined vaccine regimen of AttHRV oral priming followed by 2/6-virus-like particle (2/6VLP) intranasal (IN) boosting (AttHRV-2/6VLP) have been delineated in our previous studies of gnotobiotic (Gn) pigs [2-6]. The protective role of rotavirus-specific IgA effector and memory B cells in intestinal lymphoid tissues and IgA antibodies in the serum, intestinal contents or feces against rotavirus diarrhea have been indicated in the Gn pig model of HRV diarrhea [3,5-8] and in studies of rotavirus natural infection of humans [9-11]. In humans, serum IgA and sometimes IgG antibody titers were correlated with protection after rotavirus natural infection [12]; but not after vaccination with Rotashield™ [9,13], implying a potential role for other effectors including T cell mediated immunity in the protection induced by this tetravalent reassortant rotavirus vaccine.
In studies of adult mice, CD8+ T cells were shown to provide the most important but not exclusive mechanism mediating clearance of a primary rotavirus infection [14]. Also in studies of adult mice, CD4+ T cells were shown to be the only lymphocytes needed to protect mice against rotavirus infection after the mice were vaccinated with VP6 peptide vaccines [15,16]. Despite a large number of studies on the role of T cells in mediating protection against rotavirus infection in adult mice with various gene knockouts (protection against disease cannot be assessed in the adult mice model) [14-18], limited data is available on T cell immune responses to rotavirus in humans [19,20] or other out-bred native hosts, i.e. calves and pigs [21-23]. However, because of the potentially important role for CD4+ and CD8+ T cells in rotavirus immunity, especially in heterotypic protection as suggested by studies of influenza virus infections [24], it is important to quantify and characterize antigen-specific CD4+ and CD8+ T cells in an animal model of rotavirus disease as well as in human clinical trials for the development of new rotavirus vaccines. The objective of this study was to identify the potential correlation between HRV-specific IFN-γ producing or proliferating T cell responses with protective immunity induced by rotavirus infection and vaccination using our well-established Gn pig model of HRV infection and disease [25,26].
Production of IFN-γ within hours of antigenic restimulation is a functional characteristic of virus-specific effector-memory T cells [27]. We chose to use functional characteristics to define T cell subpopulations in vitro to better reflect their in vivo functions. IFN-γ was recently identified to be the only cytokine produced by restimulated CD4+ T cells from immunized mice that directly inhibited rotavirus replication in vitro [28]. We postulated that protective efficacy against rotavirus is associated with IFN-γ producing T cell responses induced by rotavirus infection or vaccines. We used flow cytometry to detect intracellular accumulation of IFN-γ by CD4+, CD8+ and CD4+CD8+ T cells activated by intact homologous HRV antigen. This assay allows quantitation of HRV-specific IFN-γ producing T cells, at the single cell level [29]. Studies of the interaction of rotavirus with human myeloid dendritic cells (MDCs) demonstrated that intact peripheral blood mononuclear cell (PMNC) populations containing antigen presenting cells are equally efficient compared to rotavirus-infected MDC, in stimulating IFN-γ-producing rotavirus-specific effector-memory T cells [30]. Furthermore, the frequencies and patterns of cytokines produced by effector-memory CD4+ T cells after stimulation of peripheral blood MNC with the purified rhesus rotavirus (RRV) or MDC infected with RRV were similar. Thus intracellular IFN-γ staining assays using purified intact rotavirus as stimulating antigen with mononuclear cell (MNC) populations can provide comparative information regarding the tissue distribution and magnitude of rotavirus-specific anti-viral T cell responses elicited by rotavirus infection and vaccines. The CD4+CD8+ double positive T cells are mainly found in swine and also in humans, nonhuman primates and mice [31,32]. Studies have suggested that CD4+CD8+ T cells can respond to recall antigens and are effector-memory or memory T cells in swine [31,33] and humans [34]. The role of virus-specific double positive T cells in rotavirus immunity in pigs or humans has not been studied before.
Compared to effector-memory T cells, memory T cell subpopulations are more difficult to define due to the dynamics of the T cell progressive differentiation from effector to effector-memory (resting effector) to memory T cells [35]. Because of the lack of definitive memory T cell surface markers for swine and the lack of other functional markers in general, proliferation has been the chosen parameter to measure memory T cell responses to rotavirus infection and vaccination in humans and pigs [20,21]; however the role of proliferating T cells in rotavirus protective immunity has not been defined. Immunofluorescent staining of incorporated bromodeoxyuridine (BrdU) and flow cytometry is a high-resolution technique to measure T cell proliferation. Cytokine (i.e. IFN-γ)producing proliferating T cells can be assessed at the same time in a multicolor flow cytometry [36].
In this study, we determined the magnitude and distribution of rotavirus-specific IFN-γ producing or proliferating T cell responses in intestinal (ileum) and systemic (spleen) lymphoid tissues and blood in Gn pigs orally infected with a VirHRV (mimic natural infection) or vaccinated with two or three oral doses of live AttHRV (mimic currently licensed HRV vaccines) or a combined AttHRV-2/6VLP prime/boost vaccine. We found significant correlations between the magnitude of intestinal IFN-γ producing CD4+, CD8+ or CD4+CD8+ T cell responses induced by the VirHRV infection or the various rotavirus vaccines and protection rates against rotavirus diarrhea upon challenging the Gn pigs with the VirHRV.
2. Materials and methods
2.1. Viruses
The VirHRV Wa strain (G1P1A[8]) was passaged in Gn pigs and the pooled intestinal contents of pigs from the 23rd passage were used for inoculation at a dose of ∼106 fluorescence forming units (FFU). The virus titers were determined using a cell culture immunofluorescence (CCIF) assay [3]. The 50% infectious dose (ID50) and diarrhea dose (DD50) of Wa VirHRV was determined previously in Gn pigs as ≤1 FFU [37]. The 37th cell culture passage of Wa AttHRV was propagated in the African green monkey kidney cell line MA104 and used for vaccination at a dose of 5×107 FFU and as stimulating antigen (HRV antigen) in intracellular cytokine staining of IFN-γ producing or proliferating T cells. The HRV antigen was semipurified from AttHRV-infected MA104 cell-culture supernatants (titer = approximately 107 FFU/ml) by centrifugation (112,700×g) through a 40% (w/w) sucrose cushion as described previously [5] and the protein concentration of the preparations was determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA).
2.2. Virus-like particles with ISCOM adjuvant
Rotavirus 2/6-virus-like particles (2/6VLP) with VP2 derived from RF and VP6 from Wa strain rotaviruses were produced in Spodoptera frugiperda nine insect cells. The assembled VLPs were purified using CsCl2 gradients and characterized as previously described [38,39]. The protein concentration of the purified 2/6VLP was determined using the Bio-Rad protein assay (Bio-Rad). The endotoxin level in each 2/6VLP preparation was quantitated (<0.05 units/dose) with the Limulus amebocyte assay (Associates of Cape Cod, Inc., Woods Hole, Mass.). The 2/6VLP (250μg/dose) were incorporated into immunostimulating complexes (ISCOM) (1.25 mg/dose) as previously described [2]. Electron microscopy or immune electron microscopy was performed on each of the 2/6VLP-ISCOM preparations just prior to inoculation to confirm the integrity of the vaccine.
2.3. Gnotobiotic pigs and experimental design
Gnotobiotic pigs were derived by hysterectomy of near term sows, maintained aseptically in isolation units and fed a sterile commercial infant formula (Similac®, Ross laboratories, Columbus, OH) as described previously [40]. Pigs were assigned randomly to 1 of 5 groups. All pigs were orally inoculated at 5–6 days of age with a single dose (∼105 FFU) of VirHRV (group 1, VirHRV, n = 5) or a priming dose (5×107 FFU) of the AttHRV. The AttHRV primed pigs were boosted with two intranasal (IN) doses of 2/6VLP-ISCOM (group 2, AttHRV-2/6VLP, n = 4), or two or one oral booster doses of AttHRV (group 3, AttHRV3x, n = 8, or group 4, AttHRV2x, n = 4, respectively) at 10 and 21 days after the first inoculation. The 2/6VLP dose used in the AttHRV-2/6VLP vaccine regimen was 250μg 2/6VLP incorporated into 1.25 mg ISCOM matrix. Pigs mock-inoculated with diluent or ISCOM matrix was included as controls (group 5, control, n = 8). Protective efficacies against diarrhea conferred by the VirHRV infection, AttHRV-2/6VLP, AttHRV3x, or 2x vaccines after challenge with the Wa VirHRV (1×106 FFU) at postinoculation day (PID) 28 were assessed as previously reported [3,5,6]. The MNC from ileum, spleen and peripheral blood were isolated from pigs euthanized at PID28 (PID27-29) for characterization of T cell responses. The animal experimental protocol was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University.
2.4. Collection of lymphoid tissues and isolation of MNC
After euthanasia, the distal portion of the small intestine (ileum), spleen and blood were collected [3]. The MNC were extracted from the ileum using EDTA and collagenase and enriched by discontinuous Percoll gradient [3]. The MNC of the spleen were extracted by mechanical separation and enriched by discontinuous Percoll gradient. Peripheral blood lymphocytes (PBLs) were purified using Ficoll-PaqueTM plus (Amersham Biosciences, Uppsala, Sweden) [3,21]. The purified MNC from all tissues were resuspended in complete medium consisting of RPMI-1640 (Gibco, BRL) supplemented with 8% fetal bovine serum, 20 mM HEPES (N-2-hydroxyethyl-piperazine-Nk-2-ethanesulphonic acid), 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 100μg/ml of gentamicin, 10μg/ml of ampicillin and 50 mM 2-mercaptoethanol (E-RPMI).
2.5. Intracellular cytokine staining and flow cytometry analysis of frequencies of IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cells
Flow cytometry was used to determine frequencies of HRV-specific IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cells in the intestinal (ileum) and systemic (spleen) tissues and blood in Gn pigs in each group. The MNC extracted from the pigs were restimulated in vitro in 12-well cell culture plates (Costar, Corning Incorporated, Corning, New York) with semi-purified HRV antigen (12μg/ml), positive control PHA (10μg/ml) or mock stimulated (medium-only) in E-RPMI media for 17 h at 37°C [29]. A protein transport inhibitor, Brefeldin A (10μg/ml; Sigma) was added for the last 5 h to block secretion of cytokines produced by the T cells. An anti-human CD49d (0.5μg/ml) monoclonal antibody (MAb) (BD Pharmingen, San Diego, CA) was added to each sample as a costimulator [19], because an anti-porcine CD49d MAb was not available. The anti-human CD49d MAb cross-reacts with the molecules on porcine leukocytes [41]. The MNC were washed with staining buffer (3%FBS and 0.09% sodium azide in 1× DPBS (0.2 mg/ml KCl, 0.2 mg/ml KH2PO4, 8 mg/ml NaCl and 2.16 mg/ml Na2HPO4·7H2O, pH 7.2–7.4)), centrifuged at 500×g for 5min at 4°C before and after each staining step prior to permeabilizing the cells. The MNC (2×106 cells/tube) were stained at room temperature (RT) for 15 min with the following MAbs in sequential steps: (1) fluorescein isothiocyanate (FITC) conjugated mouse anti-porcine CD4 (IgG2b, Clone 74-12-4, BD Pharmingen) and purified mouse anti-porcine CD8α (IgG1, clone PT36B, VMRD, Inc, Pullman, WA), followed by (2) allophycocyanin (APC) conjugated rat anti-mouse IgG1 (BD Pharmingen), and then (3) biotinylated mouse anti-porcine CD3 (IgG1, clone PPT3, SouthernBiotech, Birmingham, AL) and (4) peridinine chlorophyll protein (PerCP) conjugated streptavidin (BD Pharmingen). After staining of cell surface markers, the MNC were permeabilized with BD cytofix/cytoperm™ buffer (BD pharmingen) for 15 min at RT. Then the MNC were washed with BD perm/wash buffer (BD pharmingen) and stained with R-phycoerythrin (PE) conjugated mouse anti-porcine IFN-γ (IgG1, clone P2G10, BD Pharmingen) for 15 min at RT. The MNC were washed with perm/wash buffer and resuspended in staining buffer and kept in the dark at 4°C before flow cytometry analysis the next day. The appropriate isotype-matched irrelevant control antibodies (BD pharmingen, VMRD or SouthernBiotech) were included for MNC of each antigen-stimulation from each tissue in each staining as negative controls to set the quadrant markers for the bivariate dot plots. All MAbs were titrated and used at optimal concentrations. At least 20,000 cells were acquired on a FACSCalibur flow cytometer (BD Biosciences). Flow cytometry data were analyzed using CellQuest software (BD) or FlowJo 7.2.2 software (Tree Star, Ashland, Oregon). Only samples in which the IFN-γ staining was at least twice that of the medium-stimulated controls (background) were considered positives. The frequencies of IFN-γ+CD4+, IFN-γ+CD8+ and IFN-γ+CD4+CD8+ T cells were expressed as percentages of total CD3+ T cells. All mean frequencies in Fig. 4 are reported after subtraction of the background frequencies.
2.6. BrdU staining and flow cytometry analysis of proliferating CD4+ and CD8+ T cells
Immunofluorescent staining of incorporated BrdU is a high-resolution technique to measure T cell proliferation after virus infection or vaccination. Bromodeoxyuridine, a pyrimidine analogue, is incorporated into the proliferating S-phase cells. After in vitro stimulation of 2×106 MNC from each tissue with semipurified HRV antigen (12μg/ml), PHA (10μg/ml) or mock (medium-only) for 5 days, 10μg/ml BrdU were added to each cell culture for the last 18 h. The MNC were harvested, washed with staining buffer and stained with the following MAbs: PE conjugated mouse anti-porcine CD4 (clone 74-12-4, BD Pharmingen), FITC conjugated mouse anti-porcine CD8α (clone 76-2-11, BD Pharmingen) and biotinylated mouse anti-porcine CD3 (clone PPT3, SouthernBiotech, Birmingham, AL) followed by PerCP conjugated streptavidin (BD Pharmingen). After staining of the T cell surface markers, the MNC were permeabilized with BD cytofix/cytoperm™ buffer for 15 min at RT. Then the MNC were washed with BD perm/wash buffer and stained with APC conjugated anti-BrdU antibody (BD Pharmingen) for 20 min at RT. Isotype matched control antibodies were included for MNC of each antigen-stimulation from each tissue in each staining. Four-color flow cytometry analysis was performed to determine the frequencies of BrdU+CD3+CD4+ and BrdU+CD3+CD8+ cells using a FACSCalibur Flow Cytometer and CellQuest™ Pro software following the manufacturer's instructions (Becton Dickinson). At least 20,000 cells were acquired. Frequencies of proliferating T cells from medium-only stimulated MNC (background) were subtracted from the frequencies in HRV stimulated MNC. The HRV-specific proliferating T cell responses were presented as the frequencies of BrdU+CD4+ or BrdU+CD8+ T cells among total CD3+ MNC. After 5 days in vitro stimulation of MNC with HRV antigen or PHA, the frequencies of CD4+CD8+ double positive T cells become very low to undetectable among proliferating T cells. Thus CD4+CD8+ T cells were not analyzed as a separate subpopulation for T cell proliferation.
2.7. BrdU and intracellular staining and flow cytometry analysis of IFN-γ or IL-13 producing CD4+ and CD8+ T cells among proliferating and non-proliferating T cells
A flow cytometry assay was developed to detect IFN-γ or IL-13 producing CD4+ and CD8+ T cells among proliferating (BrdU+) or non-proliferating (BrdU−) CD3+ MNC. The MNC were stimulated with HRV antigen, PHA or medium-only for 5 days as described above. Mouse anti-porcine CD3 (IgG1κ, clone PPT3) MAb conjugated with SpectralRed™ (SPRD, SouthernBiotech), mouse anti-porcine CD4 (IgG2bκ, clone 74-12-4) or CD8 (IgG1, clone PT36B) conjugated with biotin (SouthernBiotech) and then streptavidin conjugated with APC-Cy7 (eBioscience) were added in the initial two steps to stain T cell surface markers. The cells were then permeabilized with cytofix/cytoperm™ buffer and stained for intracellular BrdU, IFN-γ and IL-13 with anti-BrdU conjugated with APC, anti-porcine IFN-γ PE (BD) and anti-human IL-13 FITC (IgG1, clone PVM13-1, eBioscience) MAbs, respectively. Isotype-matched control MAbs were included for MNC from each tissue in each staining. An upgraded five-color FACSCalibur Flow Cytometer (the 3rd violet laser added by Cytek Flow Cytometry Products, CA), CellQuest™ Pro (BD) and Rainbow™ (Cytek) software were used to analyze the stained cells. At least 20,000 cells were acquired. The stained cells were first gated for CD3 and BrdU and then analyzed on CD4 vs. IFN-γ or IL-13 and CD8 vs. IFN-γ or IL-13 bivariate dot plots. The frequencies of IFN-γ producing CD4+ or CD8+ T cells were expressed as percentages of total CD3+BrdU+ or CD3+BrdU− MNC.
2.8. Statistical analysis
Comparisons of frequencies of T cells among treatment groups or tissues were performed for all data presented in Figs. 4-6 using the Kruskal–Wallis test (SAS Institute Inc., Cary, NC). When differences among the groups or tissues were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Spearman's correlation was used for assessment of association between mean frequencies of IFN-γ+ producing or proliferating T cells and protection rates against rotavirus diarrhea. Protection rates among treatment groups were compared using Fisher's Exact Test. Statistical significance was assessed at p < 0.05 for all comparisons.
3. Results
3.1. Detection of HRV-specific IFN-γ+CD4+, IFN-γ+CD8+ and IFN-γ+CD4+CD8+ T cells
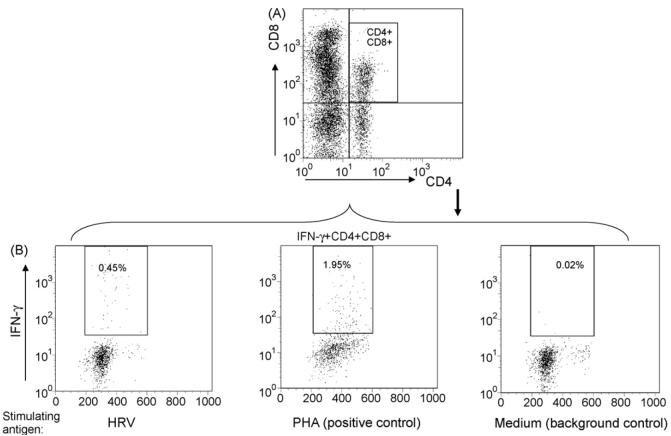
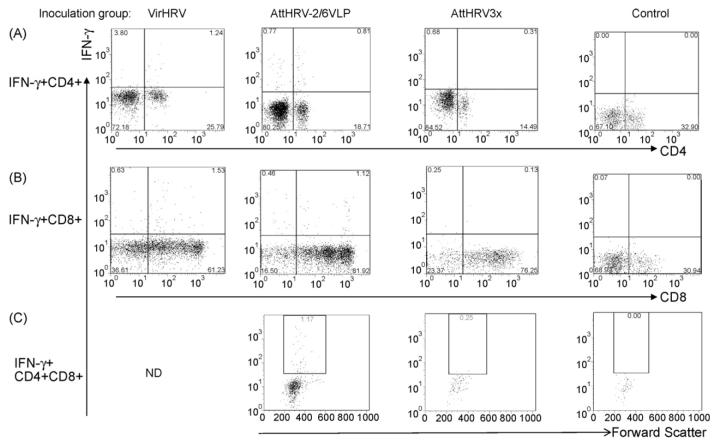
The intracellular staining and flow cytometry analysis for detection of HRV-specific IFN-γ-producing CD4+ and CD8+ T cells is illustrated in Fig. 1. The detection of HRV-specific IFN-γ-producing CD4+CD8+ double positive T cells is illustrated in Fig. 2. The three T cell subpopulations CD4+, CD8+ and CD4+CD8+ were identified by gating through MNC and then CD3+ lymphocytes (Figs. 1A and 2A). PHA and medium-only stimulated MNC were included (for all samples in the study) as positive and background controls. Low frequencies of spontaneous IFN-γ producing T cells were observed among the medium-only stimulated MNC (right panels in Figs. 1B and 2B), which may reflect the presence of T cells that constitutively produce IFN-γ (background, non-HRV specific). Their frequencies were generally substantially lower than those among the HRV antigen-stimulated MNC (left panels in Figs. 1B and 2B). Examples of IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cell responses among different inoculation groups are given in Fig. 3. Representative dot plots were shown for ileum of one pig in the VirHRV, AttHRV-2/6VLP, AttHRV3x or control groups, respectively.
Fig. 1.
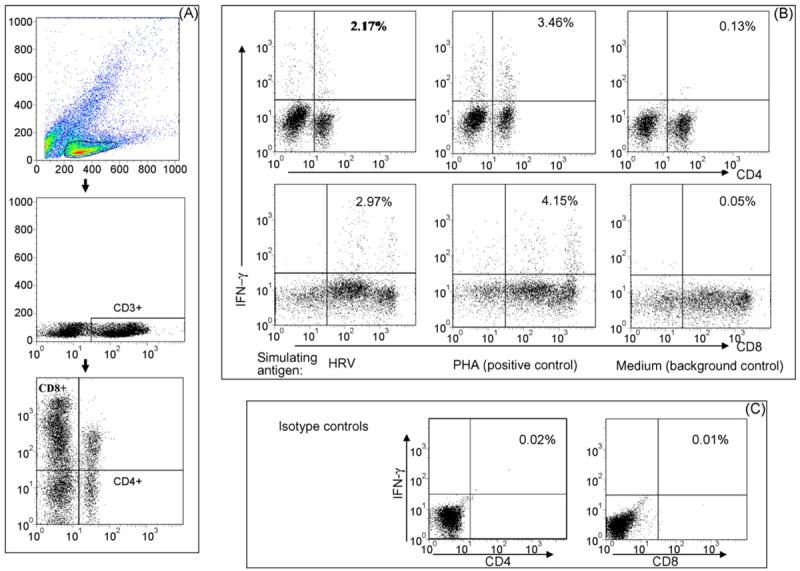
Detection of HRV-specific IFN-γ producing CD4+ and CD8+ T cells by intracellular staining and flow cytometry. Stained cells were gated first for mononuclear cell (MNC) populations based on forward scatter and side scatter profile followed by gating on CD3+ T cells. Then a bivariate dot plot was drawn to define CD4+, CD8+ and CD4+CD8+ subpopulations within CD3+ T cells (A). Dot plots in (B) depict IFN-γ producing CD4+ and CD8+ T cells among CD3+ MNC from spleen of a HRV-infected gnotobiotic pig. The MNC were stimulated in vitro with purified HRV antigen, PHA (positive control), or medium (background control). The frequencies of IFN-γ+CD4+ or IFN-γ+CD8+ cells are labeled on the upper right corner of each dot plot. Quadrants are defined by using MNC stained with isotype-matched irrelevant antibodies (C).
Fig. 2.
Detection of HRV-specific IFN-γ producing CD4+CD8+ double positive T cells by intracellular staining and flow cytometry. After gating on the CD3+CD4+CD8+ subpopulation similarly to Fig. 1, IFN-γ PE and forward scatter dot plots were drawn within the CD4+CD8+ double positive T cell subpopulation. The frequencies of IFN-γ+CD4+CD8+ (labeled on the dot plot) are among CD3+ MNC that were stimulated in vitro with HRV antigen, PHA (positive control), or medium (background control), respectively.
Fig. 3.
Examples of HRV-specific IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cell responses in ileum of gnotobiotic pigs at PID 28. Ileal MNC was from pigs infected with VirHRV, or vaccinated with AttHRV-2/6VLP, AttHRV3x or mock-inoculated controls. MNC were stimulated in vitro with HRV antigen for 17 h. A representative dot plot was shown for each inoculation group. The responses in AttHRV2x group (not shown) were similar to AttHRV3x group. The numbers at the upper right corners of dot plots of panels (A) and (B) are the frequencies of IFN-γ+CD4+ or IFN-γ+CD8+ T cells. The numbers in the rectangles in dot plots of panel (C) are the frequencies of IFN-γ+CD4+CD8+ double positive T cells. ND, undetermined.
3.2. IFN-γ producing T cells induced by virulent HRV infection reside primarily in the intestine
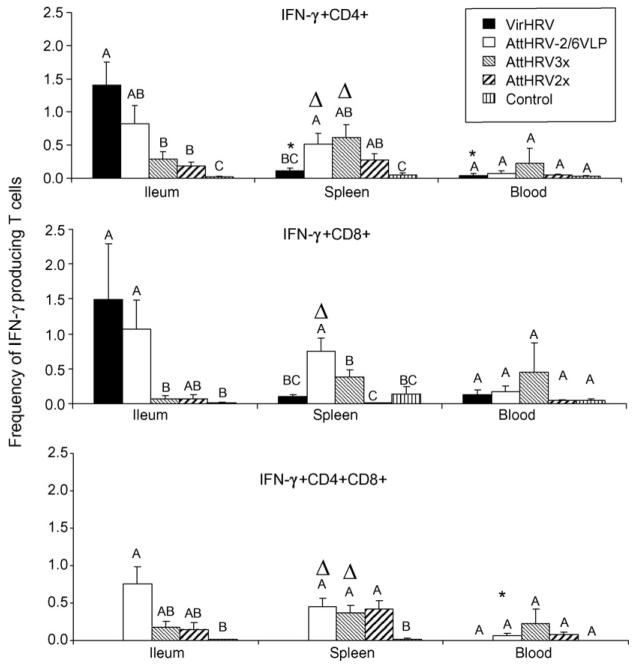
The mean frequencies of HRV-specific IFN-γ+CD4+, IFN-γ+CD8+ and IFN-γ+CD4+CD8+ T cells among the inoculation groups are depicted in Fig. 4. Mean frequencies of IFN-γ producing T cells in each tissue of each group were calculated using adjusted frequencies in which the frequencies from the medium-only stimulated MNC were subtracted from the HRV stimulated MNC. The IFN-γ+CD4+CD8+ T cell responses in the VirHRV group were not determined because the CD4 and CD8 were stained in separate tubes for this group. In the VirHRV infected pigs, higher frequencies of HRV-specific IFN-γ+CD4+ (statistically significant) and IFN-γ+CD8+ T cells were detected in ileum compared to spleen (13-fold and 15-fold higher, respectively) and blood (35-fold and 11-fold higher, respectively), indicating that the IFN-γ producing T cells after VirHRV infection reside primarily in the intestinal lymphoid tissues at PID28, as we showed previously for memory B cells [5]. The pigs receiving AttHRV-2/6VLP or AttHRV3x vaccine had significantly higher frequencies of IFN-γ+CD4+, IFN-γ+CD8+ (for AttHRV-2/6VLP only) and IFN-γ+CD4+CD8+ T cells in spleen compared to blood. Blood had the lowest frequencies of IFN-γ producing T cells compared to ileum and spleen in all inoculation groups (except for AttHRV3x) and the frequencies in VirHRV infected or vaccinated pigs did not differ from that of the mock-inoculated control pigs, suggesting that few HRV-specific effector-memory T cells traffic through the circulation at PID 28 after HRV infection or vaccination.
Fig. 4.
HRV-specific IFN-γ producing T cell responses in Gn pigs. Data are presented as mean frequency±standard error of the mean (n = 4–8). The frequencies are calculated by subtracting the frequencies detected in medium-only stimulated MNC from HRV antigen-stimulated MNC. Black bars, VirHRV1× group; white bars, AttHRV-2/6VLP group; light hatched bars, AttHRV3x; heavy hatched bars, AttHRV2x group; and vertical lined bars, mock-inoculated controls. Different letters on top of bars indicate significant differences in frequencies among groups for the same cell type and tissue. Asterisks denote significant difference when compared to ileum; and Δ indicates significant difference when compared to blood for the same cell type in the same group (Kruskal–Wallis test, p < 0.05).
3.3. AttHRV-2/6VLP vaccine induced higher intestinal HRV-specific IFN-γ producing T cell responses than the AttHRV3x and 2x vaccines
In pigs receiving the AttHRV-2/6VLP2x vaccine, frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells in ileum were slightly lower, but statistically similar to those of the VirHRV-infected pigs and they (including IFN-γ+CD4+CD8+ T cells) were higher or significantly higher than those of pigs vaccinated with the AttHRV3x or 2x vaccines and controls (Fig. 4). The AttHRV-2/6VLP vaccine is more effective in inducing intestinal IFN-γ producing T cell responses and protection (Table 1) than the AttHRV vaccines, possibly due to the multiple mucosal induction sites involved in the AttHRV-2/6VLP prime/boost vaccine regimen (i.e. oral: gut associated lymphoid tissues [GALT] followed by IN: nasal associated lymphoid tissues [NALT]). In spleen, the AttHRV-2/6VLP vaccine induced significantly higher IFN-γ+CD4+ and IFN-γ+CD8+ T cell responses than the VirHRV, which may reflect the effect of the 2/6VLP booster doses. Antigen-specific T or B cells primed in NALT reside predominately in the lungs and in systemic over intestinal sites [42]. The AttHRV-2/6VLP vaccinated pigs were also the only inoculation group that developed significantly higher splenic IFN-γ+CD8+ T cell responses than controls. The frequencies of IFN-γ+CD4+ T cells in spleen of AttHRV3x or 2x vaccinated pigs did not differ significantly from those of the VirHRV pigs, but they (including the IFN-γ+CD4+CD8+ T cells) were significantly higher than controls. The trend for the higher frequencies (not significantly vs. VirHRV group) of IFN-γ+ T cell responses in spleen and blood of AttHRV3x pigs may be explained also by the booster doses. Higher rate of nasal virus shedding than fecal shedding was observed after the AttHRV oral inoculation in our previous studies [43]. This fact may also help to explain the higher IFN-γ+CD4+ and IFN-γ+CD4+CD8+ T cell responses observed in the systemic site (spleen) than in the intestine (ileum) after inoculation with the AttHRV3x or 2x vaccine.
Table 1.
Correlation between magnitude of intestinal and systemic IFN-γ producing T cell responses and protection rates against HRV diarrhea
| Group | Protection rate against HRV diarrheaa | Mean frequencies of IFN-γ producing T cells in ileum |
Mean frequencies of IFN-γ producing T cells in spleen |
||||
|---|---|---|---|---|---|---|---|
| IFN-γ+CD4+ T cells | IFN-γ+CD8+ T cells | IFN-γ+CD4+CD8+ T cells | IFN-γ+CD4+ T cells | IFN-γ+CD8+ T cells | IFN-γ+CD4+CD8+ T cells | ||
| VirHRV | 87%Ab | 1.40Ac | 1.49A | ndd | 0.11BC | 0.10BC | nd |
| AttHRV-2/6VLP | 71%A | 0.82AB | 1.07A | 0.76A | 0.51A | 0.75A | 0.45A |
| AttHRV3x | 62%A | 0.28B | 0.07B | 0.18AB | 0.61AB | 0.38B | 0.38A |
| AttHRV2x | 34%AB | 0.18B | 0.07AB | 0.15AB | 0.28AB | 0.01C | 0.42A |
| Control | 0%B | 0.02C | 0.01B | 0.01B | 0.05C | 0.14BC | 0.02B |
| Correlation coefficiente | r=1.0 p < 0.0001 | r = 0.97468 p = 0.0048 | r=1.0 p < 0.0001 | r = 0.3000 p = 0.6238 | r = 0.2000 p = 0.7471 | r = 0.8000 p = 0.2000 | |
At PID 28, subsets of pigs (7–25 pigs/group) were challenged orally with ∼106 ID50 of VirHRV. Rectal swabs were collected and fecal scores were observed from post-challenge day (PCD) 1–6 for assessment of diarrhea (fecal scores of 0, normal; 1, pasty; 2, semi-liquid; and 3, liquid). Scores of greater or equal to 2 were considered as diarrhea. The pigs were considered as completely protected against diarrhea upon challenge with VirHRV only when they did not have diarrhea during the entire observation period. Control pigs were inoculated with diluent or ISCOM matrix only and challenged with VirHRV. Protection rate = [1−(percentage of VirHRV-inoculated or vaccinated pigs in each group with diarrhea/percentage of control pigs with diarrhea)]×100. Protection data from present study and previously published studies [3–6] were combined.
Numbers with different superscript capital letters differ significantly (Fisher's exact test, p < 0.05).
Mean frequencies in the same column with different superscript capital letters differ significantly (Kruskal–Wallis test, p < 0.05).
nd, not determined.
Correlation coefficient between protection rates and mean frequencies of IFN-γ producing T cells (Spearman's correlation).
3.4. Frequencies of intestinal HRV-specific IFN-γ producing T cell responses correlated with protection rates against HRV diarrhea
We analyzed the correlation between mean frequencies of IFN-γ producing T cells in the lymphoid tissues and blood and protection rates against diarrhea in all inoculation groups (Table 1). The protection rates against HRV diarrhea upon VirHRV challenge were significantly correlated with the mean frequencies of ileal IFN-γ+CD4, IFN-γ+CD8+ and IFN-γ+CD4+CD8+ double positive T cells in Gn pigs, demonstrating the important role of virus-specific intestinal IFN-γ producing T cells in protective immunity against rotavirus disease. A slightly stronger correlation was found between protection rates and mean frequencies of IFN-γ+CD4+ or IFN-γ+CD4+CD8+ (r = 1.0, p < 0.0001) than IFN-γ+CD8+ T cell (r = 0.975, p = 0.0048). There were no significant correlations between systemic IFN-γ producing T cell responses and protection rates against diarrhea when all groups were included for calculation of the correlation coefficients (Table 1). However, when only the AttHRV groups and the control group were included for the calculation, the mean frequencies of IFN-γ+CD4+ T cells in both ileum and spleen of AttHRV3x and 2x inoculated pigs were significantly correlated (r = 1.0, p < 0.0001) with protection rates against diarrhea.
3.5. HRV-specific intestinal proliferating T cell responses were highest in the vaccinated pigs but they were not correlated with protection
To examine the potential role of HRV-specific proliferating T cell responses in rotavirus protective immunity, mean frequencies of HRV-specific BrdU+CD4+ and BrdU+CD8+ T cells in ileum, spleen and blood of the five inoculation groups were compared (data not shown). Overall higher frequencies of BrdU+CD4+ than BrdU+CD8+ T cells were detected in all tissues of all groups, confirming that CD4+ T cells are the major proliferating T cell population. Unlike the IFN-γ producing T cell responses, the distribution of BrdU+CD4+ and BrdU+CD8+ T cells in VirHRV-infected pigs did not differ significantly among tissues at PID28. There were also no significant differences in frequencies among tissues of the vaccinated or control groups. The AttHRV-2/6VLP vaccine induced the highest intestinal proliferating T cell responses whereas AttHRV3x or 2x vaccine induced the highest systemic proliferating T cell responses. However, there were no significant differences in frequencies of ileal BrdU+CD4+ or BrdU+CD8+ T cells among vaccine groups and there were no significant correlations between protection rates against diarrhea and frequencies of BrdU+CD4+ or BrdU+CD8+ T cells in any tissues (data not shown).
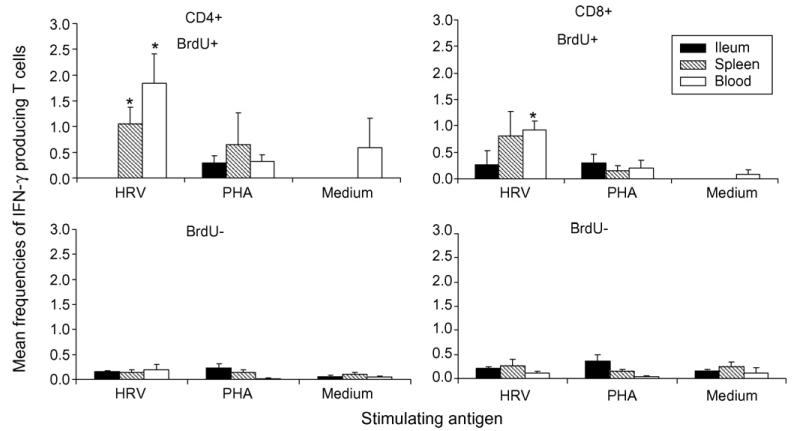
3.6. Low frequency of HRV-specific IFN-γ producing T cells were detected among proliferating CD4+ and CD8+ T cells in spleen and blood
To help us understand why the magnitude of HRV-specific proliferating T cell responses was not directly associated with protection, the frequencies of IFN-γ producing T cells among proliferating (BrdU+) and non-proliferating (BrdU−) CD4+ and CD8+ T cells were analyzed in Gn pigs receiving the AttHRV3x vaccine using a five-color flow cytometry developed later in the study. This assay was only applied to pigs vaccinated with AttHRV3x vaccine which was the last treatment group for the study. The mean frequencies of HRV-specific BrdU+ or BrdU−, IFN-γ+CD4+ and IFN-γ+CD8+ T cells in ileum, spleen and blood are depicted in Fig. 5, along with PHA stimulated and medium-only stimulated controls. Among HRV-specific proliferating T cells, no or very low frequencies of IFN-γ+CD4+ and IFN-γ+CD8+ T cells were detected in ileum, but higher frequencies were detected in spleen and blood. Significantly higher frequencies of HRV-specific IFN-γ+CD4+ and IFN-γ+CD8+ T cells were detected among proliferating T cells compared to non-proliferating T cells in spleen (CD4+ only) and blood. Thus, after stimulation with recall antigen for 5 days, HRV-specific IFN-γ producing T cells are mainly found among proliferating T cells and resided primarily in spleen and blood.
Fig. 5.
Mean frequencies of HRV-specific IFN-γ producing CD4+ and CD8+ T cells among proliferating and non-proliferating T cells in pigs vaccinated with AttHRV3x. Data are presented as mean frequency±standard error of the mean (n = 4). Black bars, ileum, hatched bars, spleen; and white bars, blood. Asterisks denote significant difference between BrdU+ and BrdU− IFN-γ producing T cells for the same tissue (Kruskal–Wallis test, p < 0.05).
3.7. Frequencies of total CD4+ and CD8+ T cells in blood were reduced in VirHRV infected pigs compared to the vaccinated or control pigs at PID28
Frequencies of total CD3+CD4+ and CD3+CD8+ T cells among MNC were analyzed to assess whether VirHRV infection and vaccination have an effect on total T cell frequencies in Gn pigs at PID28. The mean frequencies of total CD4+ and CD8+ T cells in ileum, spleen and blood in the VirHRV, AttHRV3x and control pigs are depicted in Fig. 6. The frequencies of total T cells in the AttHRV-2/6VLP and AttHRV2x groups were similar to those of AttHRV3x group for all the tissues, therefore data are not shown. The CD4/CD8 ratio in ileum of all pig groups were <1.0, and in spleen and blood were >1.5, but CD4/CD8 ratios did not differ significantly among groups (data not shown). The frequencies of total CD4+ T cells in all tissues and CD8+ T cells in blood increased significantly in the AttHRV3x group, but not in the VirHRV group, compared to the controls. These increases may reflect the effect of booster doses (repeated antigen stimulation) received by the vaccine group on the development/maturation of the T cell compartment in neonatal Gn pigs. However, increased total T cell numbers did not correlate with increased protection rates against HRV diarrhea. For total CD8+ T cells, a noteworthy observation was the significantly reduced frequency in the blood of VirHRV pigs compared to the controls. Total blood CD4+ T cells also showed the same trend, although it was not statistically significant. Frequencies of total CD4+CD8+ double positive T cells were also compared among the vaccine groups and the controls. In ileum, the frequencies of double positive T cells were increased or significantly increased in the vaccine groups compared to the controls. In spleen and blood, the frequencies among groups did not differ significantly (data not shown).
Fig. 6.
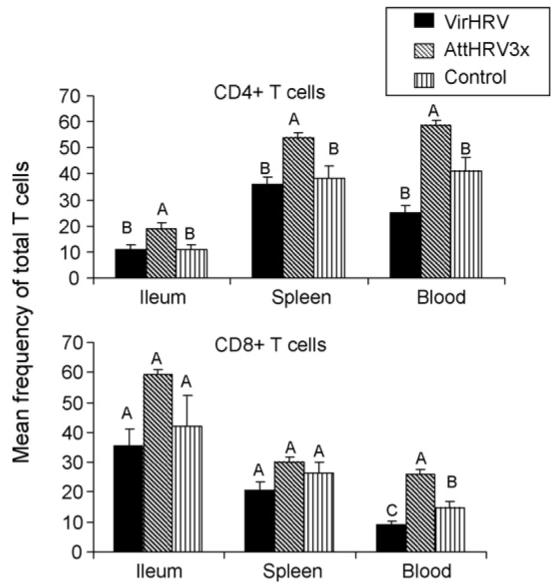
Mean frequency of total CD4+ and CD8+ T cells in VirHRV infected or AttHRV3x vaccinated pigs and the controls. Frequencies of CD3+CD4+ and CD3+CD8+ T cells were analyzed among the MNC that were cultured for 17 h with medium-only. Data are presented as mean frequency ± standard error of the mean (n = 5–8). Black bars, VirHRV group; hatched bars, AttHRV3x; and vertical lined bars, mock-inoculated controls. Different letters on top of bars indicate significant differences among groups for the same cell type and tissue (Kruskal–Wallis test, p < 0.05).
4. Discussion
Using intracellular staining and flow cytometry, we examined frequencies of HRV-specific IFN-γ producing T cells, HRV-specific proliferating T cells and total T cells in ileum, spleen and blood of Gn pigs after VirHRV infection or inoculation with various vaccines. We also examined frequencies of IFN-γ producing CD4+ and CD8+ T cells among proliferating and non-proliferating T cells from the AttHRV3x vaccinated pigs. After VirHRV infection or vaccinations, the frequencies of intestinal HRV-specific IFN-γ producing CD4+ T cells in Gn pigs at the time of challenge (PID28) correlated significantly with protection rates against VirHRV-induced diarrhea. IFN-γ producing T cells induced by VirHRV infection in Gn pigs reside primarily in the intestine as we showed previously for memory B cells [5]. This finding agrees with the existence of a population of extralymphoid effector-memory T cells poised for immediate response to infection [44,45]. In mice, antigen-specific CD4+ effector-memory T cells were found to reside primarily in the gut, lungs and salivary glands at 11–60 days post-antigen exposure and these cells readily produced IFN-γ upon antigen restimulation [44]. Our study demonstrated a direct correlation between the existence of extralymphoid IFN-γ producing T cells and protection at the site of virus entry (intestine). In studies of mice, intestinal IFN-γ producing CD4+ T cells were suggested to be the only lymphocytes required for protection against rotavirus infection after the mice were immunized with a chimeric VP6 vaccine using attenuated E. coli labile toxin as an adjuvant [15,28,46,47]. Their data and ours collectively emphasize the role of intestinal IFN-γ producing CD4+ T cells as correlates of rotavirus protective immunity, possibly more important than CD8+ T cells. The finding that IFN-γ producing T cell responses measured after in vitro antigen restimulation correlated with protection against rotavirus diarrhea confirms that the memory responses detected in vitro are reflective of protective immunity in vivo. Intestinal IFN-γ producing CD4+CD8+ T cells induced by various HRV vaccines also significantly correlated with protection. To our knowledge this is the first study showing that intestinal double positive T cells may play an important role in rotavirus protective immunity. Intestinal double positive T cells in rhesus macaques have been shown to be highly activated memory T cells with increased capacity to produce cytokines [48].
AttHRV elicited stronger systemic than local IFN-γ producing T cell response which concurred with the stronger systemic than local memory B cell response [5]. There were significant correlations between protection rates against diarrhea and IFN-γ+CD4+ T cell responses in ileum as well as in spleen of pigs inoculated with the AttHRV vaccines. These results suggest that systemic immune responses may also play a role in protective immunity after AttHRV vaccination. Such findings may have implications in identifying the immune determinant of protection conferred by the currently licensed live oral vaccines in humans.
In pigs, blood had the lowest frequencies of IFN-γ producing T cells compared to ileum and spleen in all pigs (except for CD8+ T cells in the AttHRV3x group) and the frequencies did not differ among treatment groups; therefore, they did not reflect the magnitude of HRV-specific T cell responses in the ileum and spleen at PID 28. Correlations, therefore, were analyzed between mean frequencies of IFN-γ producing T cells and protection rates among all inoculation groups, instead of using the more direct approach (i.e. between IFN-γ producing T cell frequencies in blood at challenge and clinical signs post-challenge in the same pigs). Recent studies of T cell responses in human PBL showed that in rotavirus infected children, virus-specific CD4+ and CD8+ T cells that secreted IFN-γ were very low or undetectable [19,49]. Similar results were found in studies of rotavirus-infected juvenile rhesus macaques monkeys [50]. Studies of intestinal and systemic rotavirus-specific ASC and lymphocyte proliferation responses in Gn pigs at various time points indicated that blood mirrored the IgA ASC and lymphocyte proliferation responses in the gut, but at a lower level, and could serve as a temporary “window” for monitoring intestinal immune responses to rotavirus. A correlation between ratios of HRV-specific IgA ASC and total IgA ASC in the blood and the small intestinal lamina propria was found in children; the study was done during annual rotavirus epidemic season [51]. Thus this window may be limited to a short time post-infection, when presumably the lymphocytes activated by rotavirus antigen in intestinal induction sites traffic (homing) to the effector sites via the circulation [3,21]. At PID 28, the only time examined in this study, the frequencies of IFN-γ producing T cells in blood did not reflect the magnitude of intestinal T cell responses induced by infection or the various vaccines. Thus, studies of T and B cell responses to rotavirus infection or vaccination using blood samples need to carefully select the optimal time point; blood samples collected outside the temporary “window” will not accurately reflect the magnitude/patterns of the intestinal immune responses to rotavirus infection or vaccines.
Studies by Reinhardt et al. [44] suggested that antigen recognition in the context of infection generates memory T cells that are specialized to proliferate in secondary lymphoid tissues or to traffic and fight infection in the peripheral non-lymphoid tissues at the sites of microbial entry. In our study, HRV-specific proliferating CD4+ and CD8+ T cells in Gn pigs after HRV infection or vaccination were distributed similarly among intestinal and systemic tissues and in the blood. The frequencies of HRV-specific proliferating T cells did not correlate with protection. The low frequencies of IFN-γ+ T cells (∼1%) among proliferating T cells indicate that only a very small percent of proliferating T cells have effector functions after restimulation with HRV antigen, which may explain the lack of a correlation between protection and magnitude of proliferating T cell responses. However, further studies including all other four-treatment groups are needed to determine if frequencies of HRV-specific proliferating IFN-γ+CD4+ or IFN-γ+CD8+ T cells are correlated with protection. We also attempted to measure HRV-specific IL-13 producing T cells between proliferating and non-proliferating T cells using an anti-human IL-13 antibody (porcine IL-13 antibody is not available). IL-13 was indicated to replace IL-4 as the major T helper (Th) 2 cytokine in swine [52]. The frequencies of CD4+IL-13+ or CD8+IL-13+ T cells were very low, only detectable in non-proliferating T cells and did not differ significantly between medium-only stimulated and HRV stimulated MNC (data not shown). Similar low frequencies of CD4+IL-13+ T cells were reported in a study of human adults and young children with natural HRV infection [19].
The proliferating T cells are a heterogeneous population. They include IFN-γ+ cells as we detected, that are likely the effector T cells derived from memory T cells after antigen re-stimulation and may play a direct role in rotavirus immunity. The majority of proliferating T cells, however, may be Th cells that have different functional capabilities (i.e. facilitate/regulate T or B cell response). The magnitude of Th cell responses is not likely to directly correlate with protection because these cells are not cytotoxic T cells; although they are an intrinsic part of the immune responses induced by rotavirus infection or vaccination. Further studies are needed to dissect the T cell types of HRV-specific proliferating T cells induced by infection and vaccines to identify if a subpopulation of proliferating T cells correlates with the short-term (PID28) or longer-term protection.
The frequencies of blood CD4+ and CD8+ T cells were reduced or significantly reduced in the VirHRV infected pigs compared to mock-inoculated control pigs at PID28. A recent study of children with acute rotavirus diarrhea also reported moderate to severe reduction in the frequencies of CD4+ and CD8+ T cells from peripheral blood [53]. The reduction in frequency of T cells in children was only detected in the acute-phase blood samples. In convalescent-phase blood samples (3 weeks after the acute sample) the frequencies of T lymphocytes recovered to almost normal levels in most patients. This difference between rotavirus infected Gn pigs and children may be due in part to the Gn status of pigs that lack of commensal microflora or environmental antigen stimulation may have delayed the recovery from the VirHRV-induced T cell lymphopenia. The mechanism for the rotavirus-induced T cell lymphopenia in pigs and children are unclear and requires further studies [53].
In conclusion, this study demonstrated a direct correlation between protection rates against rotavirus diarrhea and the magnitude of HRV-specific IFN-γ producing CD4+, CD8+ and CD4+CD8+ T cell responses in the intestine of Gn pigs induced by VirHRV infection and various HRV vaccines. Our findings suggest that HRV-specific intestinal IFN-γ producing T cells are an important determinant of rotavirus immunity. This knowledge should improve our understanding of rotavirus protective immunity and will facilitate development and evaluation of effective rotavirus vaccines.
Acknowledgements
We thank Peggy Lewis for technical assistance and Rich McCormick and Dr. Juliette Hanson for animal care. This work was supported by grants (R01A133561 to LS and R21AT002524 to LY) from the National Institutes of Health. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
References
- 1.Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iosef C, Van Nguyen T, Jeong K, Bengtsson K, Morein B, Kim Y, et al. Systemic and intestinal antibody secreting cell responses and protection in gnotobiotic pigs immunized orally with attenuated Wa human rotavirus and Wa 2/6-rotavirus-like-particles associated with immunostimulating complexes. Vaccine. 2002;20(13–14):1741–53. doi: 10.1016/s0264-410x(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 3.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70(5):3075–83. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan L, Iosef C, Azevedo MS, Kim Y, Qian Y, Geyer A, et al. Protective immunity and antibody-secreting cell responses elicited by combined oral attenuated Wa human rotavirus and intranasal Wa 2/6-VLPs with mutant Escherichia coli heat-labile toxin in gnotobiotic pigs. J Virol. 2001;75(19):9229–38. doi: 10.1128/JVI.75.19.9229-9238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan L, Geyer A, Saif LJ. Short-term immunoglobulin A B-cell memory resides in intestinal lymphoid tissues but not in bone marrow of gnotobiotic pigs inoculated with Wa human rotavirus. Immunology. 2001;103(2):188–98. doi: 10.1046/j.1365-2567.2001.01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez AM, Nguyen TV, Azevedo MS, Jeong K, Agarib F, Iosef C, et al. Antibody responses to human rotavirus (HRV) in gnotobiotic pigs following a new prime/boost vaccine strategy using oral attenuated HRV priming and intranasal VP2/6 rotavirus-like particle (VLP) boosting with ISCOM. Clin Exp Immunol. 2004;135(3):361–72. doi: 10.1111/j.1365-2249.2004.02395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To TL, Ward LA, Yuan L, Saif LJ. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79(Pt 11):2661–72. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo MS, Yuan L, Iosef C, Chang KO, Kim Y, Nguyen TV, et al. Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin Diagn Lab Immunol. 2004;11(1):12–20. doi: 10.1128/CDLI.11.1.12-20.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez R, Franco M, Sarmiento L, Romero M, Schael IP. Serum IgA levels induced by rotavirus natural infection, but not following immunization with the RRV-TV vaccine (Rotashield), correlate with protection. J Med Virol. 2005;76(4):608–12. doi: 10.1002/jmv.20404. [DOI] [PubMed] [Google Scholar]
- 10.Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproanti-body in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30(7):1678–84. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182(6):1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 12.Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis. 2002;34(10):1351–61. doi: 10.1086/340103. [DOI] [PubMed] [Google Scholar]
- 13.Ward RL, Bernstein DI. Lack of correlation between serum rotavirus antibody titers and protection following vaccination with reassortant RRV vaccines. US Rotavirus Vaccine Efficacy Group. Vaccine. 1995;13(13):1226–32. doi: 10.1016/0264-410x(95)00060-e. [DOI] [PubMed] [Google Scholar]
- 14.Franco MA, Greenberg HB. Immunity to rotavirus infection in mice. J Infect Dis. 1999;179(Suppl 3):S466–9. doi: 10.1086/314805. [DOI] [PubMed] [Google Scholar]
- 15.McNeal MM, VanCott JL, Choi AH, Basu M, Flint JA, Stone SC, et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G) J Virol. 2002;76(2):560–8. doi: 10.1128/JVI.76.2.560-568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeal MM, Stone SC, Basu M, Bean JA, Clements JD, Hendrickson BA, et al. Protection against rotavirus shedding after intranasal immunization of mice with a chimeric VP6 protein does not require intestinal IgA. Virology. 2006;346(2):338–47. doi: 10.1016/j.virol.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997;238(2):169–79. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 18.McNeal MM, Rae MN, Ward RL. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71(11):8735–42. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaimes MC, Rojas OL, Gonzalez AM, Cajiao I, Charpilienne A, Pothier P, et al. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol. 2002;76(10):4741–9. doi: 10.1128/JVI.76.10.4741-4749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Offit PA, Hoffenberg EJ, Pia ES, Panackal PA, Hill NL. Rotavirus-specific helper T cell responses in newborns, infants, children, and adults. J Infect Dis. 1992;165(6):1107–11. doi: 10.1093/infdis/165.6.1107. [DOI] [PubMed] [Google Scholar]
- 21.Ward LA, Yuan L, Rosen BI, To TL, Saif LJ. Development of mucosal and systemic lymphoproliferative responses and protective immunity to human group A rotaviruses in a gnotobiotic pig model. Clin Diagn Lab Immunol. 1996;3(3):342–50. doi: 10.1128/cdli.3.3.342-350.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons KR, Hall GA, Bridger JC, Cook RS. Number and distribution of T lymphocytes in the small intestinal mucosa of calves inoculated with rotavirus. Vet Immunol Immunopathol. 1993;39(4):355–64. doi: 10.1016/0165-2427(93)90067-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan RS, Singh NP. Cell-mediated immune response in rotavirus-infected calves: leucocyte migration inhibition assay. J Comp Pathol. 1992;107(1):115–8. doi: 10.1016/0021-9975(92)90101-y. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen HH, Moldoveanu Z, Novak MJ, van Ginkel FW, Ban E, Kiyono H, et al. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999;254:50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- 25.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87(3–4):147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saif L, Yuan L, Ward L, To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv Exp Med Biol. 1997;412:397–403. doi: 10.1007/978-1-4899-1828-4_62. [DOI] [PubMed] [Google Scholar]
- 27.Mayer KD, Mohrs K, Crowe SR, Johnson LL, Rhyne P, Woodland DL, et al. The functional heterogeneity of type 1 effector T cells in response to infection is related to the potential for IFN-gamma production. J Immunol. 2005;174(12):7732–9. doi: 10.4049/jimmunol.174.12.7732. [DOI] [PubMed] [Google Scholar]
- 28.McNeal MM, Stone SC, Basu M, Clements JD, Choi AH, Ward RL. IFN-gamma is the only anti-rotavirus cytokine found after in vitro stimulation of memory CD4+ T cells from mice immunized with a chimeric VP6 protein. Viral Immunol. 2007;20(4):571–84. doi: 10.1089/vim.2007.0055. [DOI] [PubMed] [Google Scholar]
- 29.He XS, Mahmood K, Maecker HT, Holmes TH, Kemble GW, Arvin AM, et al. Analysis of the frequencies and of the memory T cell phenotypes of human CD8+ T cells specific for influenza A viruses. J Infect Dis. 2003;187:1075–84. doi: 10.1086/368218. [DOI] [PubMed] [Google Scholar]
- 30.Narvaez CF, Angel J, Franco MA. Interaction of rotavirus with human myeloid dendritic cells. J Virol. 2005;79(23):14526–35. doi: 10.1128/JVI.79.23.14526-14535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuckermann FA. Extrathymic CD4/CD8 double positive T cells. Vet Immunol Immunopathol. 1999;72(1–2):55–66. doi: 10.1016/s0165-2427(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 32.Varas A, Jimenez E, Sacedon R, Rodriguez-Mahou M, Maroto E, Zapata AG, et al. Analysis of the human neonatal thymus: evidence for a transient thymic involution. J Immunol. 2000;164(12):6260–7. doi: 10.4049/jimmunol.164.12.6260. [DOI] [PubMed] [Google Scholar]
- 33.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87(3):500–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104(2):478–86. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- 35.Swain SL, Agrewala JN, Brown DM, Jelley-Gibbs DM, Golech S, Huston G, et al. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol Rev. 2006;211:8–22. doi: 10.1111/j.0105-2896.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta BA, Maino VC. Simultaneous detection of DNA synthesis and cytokine production in staphylococcal enterotoxin B activated CD4+ T lymphocytes by flow cytometry. J Immunol Methods. 1997;208(1):49–59. doi: 10.1016/s0022-1759(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 37.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–41. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 38.Crawford SE, Labbe M, Cohen J, Burroughs MH, Zhou YJ, Estes MK. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68(9):5945–52. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan L, Geyer A, Hodgins DC, Fan Z, Qian Y, Chang KO, et al. Intranasal administration of 2/6-rotavirus-like particles with mutant Escherichia coli heat-labile toxin (LT-R192G) induces antibody-secreting cell responses but not protective immunity in gnotobiotic pigs. J Virol. 2000;74(19):8843–53. doi: 10.1128/jvi.74.19.8843-8853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free swine for microbiological investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Summerfield A, McCullough KC. Porcine bone marrow myeloid cells: phenotype and adhesion molecule expression. J Leukoc Biol. 1997;62(2):176–85. doi: 10.1002/jlb.62.2.176. [DOI] [PubMed] [Google Scholar]
- 42.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 43.Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, et al. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79(9):5428–36. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410(6824):101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 45.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 46.Smiley KL, McNeal MM, Basu M, Choi AH, Clements JD, Ward RL. Association of IFN-{gamma} and IL-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) J Virol. 2007 doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNeal MM, Basu M, Bean JA, Clements JD, Choi AH, Ward RL. Identification of an immunodominant CD4(+) T cell epitope in the VP6 protein of rotavirus following intranasal immunization of BALB/c mice. Virology. 2007 doi: 10.1016/j.virol.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 48.Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36(3):583–92. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- 49.Rojas OL, Gonzalez AM, Gonzalez R, Perez-Schael I, Greenberg HB, Franco MA, et al. Human rotavirus specific T cells: quantification by ELISPOT and expression of homing receptors on CD4+ T cells. Virology. 2003;314(2):671–9. doi: 10.1016/s0042-6822(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 50.Sestak K, McNeal MM, Choi A, Cole MJ, Ramesh G, Alvarez X, et al. Defining T-cell-mediated immune responses in rotavirus-infected juvenile rhesus macaques. J Virol. 2004;78(19):10258–64. doi: 10.1128/JVI.78.19.10258-10264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown KA, Kriss JA, Moser CA, Wenner WJ, Offit PA. Circulating rotavirus-specific antibody-secreting cells (ASCs) predict the presence of rotavirus-specific ASCs in the human small intestinal lamina propria. J Infect Dis. 2000;182(4):1039–43. doi: 10.1086/315808. [DOI] [PubMed] [Google Scholar]
- 52.Bautista EM, Nfon C, Ferman GS, Golde WT. IL-13 replaces IL-4 in development of monocyte derived dendritic cells (MoDC) of swine. Vet Immunol Immunopathol. 2007;115(1–2):56–67. doi: 10.1016/j.vetimm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Dennehy PH, Keyserling HL, Tang K, Gentsch JR, Glass RI, et al. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J Virol. 2007;81(8):3904–12. doi: 10.1128/JVI.01887-06. [DOI] [PMC free article] [PubMed] [Google Scholar]