Abstract
Fusion of post-Golgi secretory vesicles with the plasma membrane in yeast requires the function of a Rab protein, Sec4p, and a set of v- and t-SNAREs, the Snc, Sso, and Sec9 proteins. We have tested the hypothesis that a selective interaction between Sec4p and the exocytic SNAREs is responsible for ensuring that secretory vesicles fuse with the plasma membrane but not with intracellular organelles. Assembly of Sncp and Ssop into a SNARE complex is defective in a sec4-8 mutant strain. However, Snc2p binds in vivo to many other syntaxin-like t-SNAREs, and binding of Sncp to the endosomal/Golgi t-SNARE Tlg2p is also reduced in sec4-8 cells. In addition, binding of Sncp to Ssop is reduced by mutations in two other Rab genes and four non-Rab genes that block the secretory pathway before the formation of secretory vesicles. In an alternate approach to look for selective Rab–SNARE interactions, we report that the nucleotide-free form of Sec4p coimmunoprecipitates with Ssop. However, Rab–SNARE binding is nonselective, because the nucleotide-free forms of six Rab proteins bind with similar low efficiency to three SNARE proteins, Ssop, Pep12p, and Sncp. We conclude that Rabs and SNAREs do not cooperate to specify the target membrane.
INTRODUCTION
Eukaryotic cells contain a dynamic network of membrane-bound organelles that are constantly remodeled by the budding and fusion of transport vesicles and tubules as well as the homotypic fusion of like organelles. The specificity of membrane fusion events must be carefully regulated to allow proper communication between the organelles of the secretory and endocytic pathways while avoiding inappropriate fusion events that might degrade the organization of membranes within a cell. The identification by genetic and biochemical means of proteins involved in membrane trafficking led to the realization that many proteins required only for a specific trafficking step are members of protein families and have homologues localized to diverse sites within the cell and to diverse cell types in multicellular organisms (Bennett and Scheller, 1993; Ferro-Novick and Jahn, 1994). It has been proposed that although the general mechanism for intracellular membrane fusion is conserved, specific interactions between particular members of protein families ensure that membranes fuse only with an appropriate target. The Rabs and the SNAREs are the largest of the protein families involved in membrane trafficking, and both have been proposed to ensure the fidelity of fusion (Botstein et al., 1988; Rothman and Warren, 1994).
Rabs are guanine nucleotide-binding proteins whose conformation is regulated by GTPase-activating proteins, which stimulate GTP hydrolysis, and by nucleotide exchange proteins, which promote the disassociation of GDP and subsequent binding of GTP (Bourne et al., 1990; Novick and Zerial, 1997; Schimmoller et al., 1998). Rab effectors bind exclusively to the GTP-bound conformation of Rab proteins (Novick and Zerial, 1997). Sec4p, the first Rab protein to be implicated in secretion, is found on post-Golgi secretory vesicles in yeast and is required for their fusion with the plasma membrane (Goud et al., 1988). After fusion, Sec4p-GDP is extracted from the plasma membrane by the cytosolic protein Gdi1p and recycled for subsequent rounds of transport (Garrett et al., 1994). Complete sequencing of the Saccharomyces cerevisiae genome has revealed 11 Rab proteins, including 9 that have been associated with a specific membrane trafficking step (Table 1) (Jedd et al., 1995; Lazar et al., 1997). Although some Rabs such as Ypt51p, Ypt52p, and Ypt53p have overlapping distributions and functions, in general each membrane transport step requires the participation of a specific Rab protein (Lazar et al., 1997).
Table 1.
Rab proteins in S. cerevisiae
| Rab protein | Transport step |
|---|---|
| Ypt1p | ER to Golgi, intra-Golgi |
| Ypt31p, Ypt32p | Exit from trans-Golgi |
| Sec4p | Golgi to plasma membrane |
| Ypt51p, Ypt51p, Ypt53p | Endocytosis |
| Ypt6p | trans-Golgi to endosomes |
| Ypt7 | Fusion with vacuoles |
SNAREs were originally identified as membrane proteins that bind to the in vitro fusion factors N-ethylmaleimide-sensitive factor (NSF) and α soluble NSF attachment protein (αSNAP) and NSF (Sollner et al., 1993b). In situations in which fusion occurs between a transport vesicle and a larger organelle, the SNAREs can be classified as v-SNAREs on vesicles or t-SNAREs on fusion targets. SNAREs are now known to assemble into a thermodynamically stable, parallel four-helix bundle known as a SNARE complex, which bridges the gap between opposing membranes before fusion (Nichols et al., 1997; Sutton et al., 1998). SNARE complex assembly either directly catalyzes membrane fusion or recruits other factors required for fusion (Ungermann et al., 1998b; Weber et al., 1998). SNARE complexes can be disassembled by the ATPase activity of NSF (Sollner et al., 1993a). NSF also has a priming activity that is necessary before the docking stage in an in vitro fusion assay (Mayer et al., 1996). All SNARE complexes identified to date include a SNARE protein homologous to the synaptic t-SNARE syntaxin 1. In yeast, the eight syntaxin homologues are each involved in fusion with a distinct subset of membranes (Table 2) (Holthuis et al., 1998a,b). The exocytic SNARE complex in yeast is composed of a secretory vesicle v-SNARE, Snc1p or Snc2p, and the plasma membrane t-SNAREs, Sec9p and Sso1p or Sso2p (Brennwald et al., 1994). Because the Snc1p and Snc2p v-SNAREs are 83% identical and functionally redundant (Protopopov et al., 1993), they will be referred to collectively as Snc proteins. Similarly, because the syntaxin-like t-SNAREs Sso1p and Sso2p are 72% identical and have a redundant function during exocytosis (Aalto et al., 1993), we will refer to them as Sso proteins.
Table 2.
Syntaxin homologues in S. cerevisiae
| SNARE protein | Localization |
|---|---|
| Ufe1p | Endoplasmic reticulum |
| Sso1p, Sso2p | Plasma membrane |
| Vam3p | Vacuoles |
| Pep12p | Endosomes |
| Sed5p | cis-Golgi |
| Tlg1p | trans-Golgi, endosomes |
| Tlg2p | trans-Golgi, endosomes, chitosomes |
Neither Rabs nor SNAREs are, by themselves, sufficient to ensure the fidelity of membrane fusion. For the Rab proteins, a single Sec4p/Ypt1p chimeric Rab protein can fulfill the essential functions of both Ypt1p and Sec4p without allowing fusion of vesicles derived from the endoplasmic reticulum (ER) with the plasma membrane (Brennwald and Novick, 1993). In addition, a single Rab, Ypt1p, is required for at least two distinct transport steps: transport between the ER and Golgi and intra-Golgi transport (Jedd et al., 1995). For SNAREs, it has been shown that multiple v-SNAREs are often present in a single class of transport vesicle (Chilcote et al., 1995; Grote et al., 1995), and the same v-SNARE often participates in both anterograde and retrograde vesicle traffic between two organelles (Gotte and von Mollard, 1998). Conversely, when vesicles originating from different sources fuse with a common target organelle, a single t-SNARE must bind to diverse v-SNAREs (Gotte and von Mollard, 1998). Finally, a recent study has documented that there are no preferential high-affinity interactions between particular combinations of v- and t-SNARE proteins (Yang et al., 1999).
The insufficiency of either Rabs or SNAREs acting alone to ensure the fidelity of membrane fusion stimulated us to consider the hypothesis that the specificity of membrane fusion is mediated via combinatorial interactions between Rab and SNARE proteins. Genetic evidence suggests that SNAREs may be Rab effectors, because Rab mutations can often be suppressed by overexpression of SNAREs (Dascher et al., 1991; Brennwald et al., 1994), and Rab activity is required for the assembly of a SNARE complex (Lian et al., 1994; Sogaard et al., 1994). Recently, a direct interaction has been reported between a Rab, Ypt1p, and the syntaxin-like t-SNARE Sed5p (Lupashin and Waters, 1997). The authors suggest that Ypt1p may activate Sed5p to allow its subsequent binding to Sec22p. An extension of this model is that specific and direct interactions between Rabs and t-SNAREs regulate the assembly of SNARE complexes. We have tested this model by examining the effect of Rab mutations on different v-SNARE/t-SNARE pairs and by testing the specificity of the direct Rab–t-SNARE interaction. We report that several mutations that blocks membrane transport upstream of SNARE complex assembly can prevent coimmunoprecipitation of v- and t-SNAREs. Furthermore, we find that the binding of Rab proteins to t-SNAREs involves the presumably inactive, nucleotide-free state of the Rab and is inefficient and nonspecific.
MATERIALS AND METHODS
Plasmid and Strain Constructions
Strains used are listed in Table 3. Construction of the GAL1p-SNC2-HA3 yeast integrating plasmid pNRB841 and the GAL1p-SNC2-HA ΔSNC1 sec18-1 strain NY1643 has been previously described (Abeliovich et al., 1998). The myc-SSO2 SSO1::LEU2 sec18-1 strain NY1727 was created by a three-step process. First, the SSO2 gene of NY605 was modified with an N-terminal myc3 tag by the method of Schneider et al. (1995) to create EGY244. Second, the SSO1 disruption from H826 [MATα SSO2::leu2::(GAL1p-SSO1 HIS3) SSO1::LEU2 ade2-1 can1-100 his3-11,15 leu2-3, 112 trp1 ura3-1; a gift from S. Keranen, VTT] was crossed into EGY244 to create EGY248. Third, the sec18-1 gene from NY1228 (MATα sec18-1 leu2-3, 112) was crossed into EGY248 to yield NY1727.
Table 3.
Strain list
| NY605 | MATa leu2-3,112 ura3-52 |
| NY1643 | MATα sec18-1 SNC2-HA3∷LEU2-GAL1p-SNC2-HA3 SNC1∷URA3 leu2-3,112 ura3-52 trp1 |
| NY1727 | MATα sec18-1 myc3-SSO2 SSO1∷LEU2 leu2-3,112 ura3-52 his3-Δ200 |
| NY1722 | MATa LEU2∷GAL1p-SNC2-HA ura3-52 |
| NY1726 | PEP12∷URA3 LEU2∷GAL1p-SNC2-HA ura3 his4 ade6 |
| DBY1034 | MATa ura3-5 lys2 his4 |
| NSY222 | MATα ypt1-A136D ura3-52 his4 |
| NSY348 | MATa ΔYPT31∷HIS3 ypt32-A141D ura3-52 lys2 his4 |
| NY405 | MATa sec4-8 ura3-52 |
| NY13 | MATa ura3-52 |
| NY415 | MATa sec16-2 ura3-52 |
| NY424 | MATα sec21-1 ura3-52 |
| NY420 | MATa sec19-1 ura3-52 |
| NY430 | MATa sec14-3 ura3-52 |
| NY1262 | MATa ypt1-3 ura3-52 |
| NY1084 | MATα LEU2∷GAL1p-SEC4 ura3-52 |
| NY1085 | MATα LEU2∷GAL1p-SEC4-S34N ura3-52 |
| NY1088 | MATα LEU2∷GAL1p-SEC4-N133I ura3-52 |
| NY1089 | MATα LEU2∷GAL1p-SEC4-Q79L ura3-52 |
| NY1090 | MATα LEU2∷Empty vector ura3-52 |
| NY1724 | MATa leu2-3,112 ura3-52 (2μ URA3 3xmyc-DSS4) (pNRB632) |
| NY1723 | MATa leu2-3,112 ura3-52 (CEN URA3 GAL1p-SEC2-GFP) (pBEB19) |
| NY1725 | MATa ΔDSS4∷URA3 LEU2∷GAL1p-sec4-N133I ura3-52 |
| NY1705 | MATa ura3-52 LEU2∷pNB529 |
| NY1706 | MATa ura3-52 LEU2∷GAL1p-HA-YPT1 |
| NY1707 | MATa ura3-52 LEU2∷GAL1p-HA-ypt1-N121I |
| NY1708 | MATa ura3-52 LEU2∷GAL1p-HA-YPT32 |
| NY1709 | MATa ura3-52 LEU2∷GAL1p-HA-ypt32-N126I |
| NY1710 | MATa ura3-52 LEU2∷GAL1p-HA-SEC4 |
| NY1711 | MATa ura3-52 LEU2∷GAL1p-HA-sec4-N133I |
| NY1712 | MATa ura3-52 LEU2∷GAL1p-HA-YPT51 |
| NY1713 | MATa ura3-52 LEU2∷GAL1p-HA-ypt51-N120I |
| NY1714 | MATa ura3-52 LEU2∷GAL1p-HA-YPT6 |
| NY1715 | MATa ura3-52 LEU2∷GAL1p-HA-ypt6-N124I |
| NY1716 | MATa ura3-52 LEU2∷GAL1p-HA-YPT7 |
| NY1717 | MATa ura3-52 LEU2∷GAL1p-HA-ypt7-N126I |
| NY1719 | MATa/α myc-SSO2/SSO2 LEU2∷GAL1p-HA-SEC4-N133I/leu2-3,112 ura3-52/URA3 his4/HIS4 gal2/GAL2 |
| NY1720 | MATa/α myc-SSO2/SSO2 leu2-3,112/leu2-3,112 ura3-52/URA3 his4/HIS4 gal2/GAL2 |
| NY1721 | MATa/α LEU2∷GAL1p-HA-SEC4-N133I/leu2-3,112 ura3-52/URA3 his4/HIS4 gal2/GAL2 |
| NY1718 | MATa sec18-1 LEU2∷GAL1p-HA-sec4-N133I ura3-52 |
NY1726, the ΔPEP12 GAL1p-SNC2-HA strain, was created by digesting pNRB841 with EcoRI to direct integration of the GAL1p-SNC2-HA3 gene at the LEU2 locus of RPY106 (Robert Piper, University of Iowa). The NSY222 and NSY348 strains were a generous gift from Nava Segev (University of Chicago) (Jedd et al., 1995, 1997). EGY375 was created by transformation of NY605 with pBEB19 (GAL1p-GFP-SEC2 in a 2μ URA3 vector; a gift from N. Barry Elkind, Yale Unversity). NY1724 was created by transformation of NY605 with pNRB632 (myc3-DSS4 in the 2μ URA3 vector pRS426); 2μ plasmids containing the DSS4, HSC82, SSO2, and HSC82 + SSO2 genes transformed into NY1088 were a gift from J. Shannon (Yale University). NY1725 (GAL1p-sec4-N133I ΔDSS4) was created by dissecting a cross of NY1088 with NY929 (MATa leu2-3112 ura3-52 DSS4::URA3).
The hemagglutinin (HA)-tagged Rab protein expression vectors pNRB829–pNRB840 were created by PCR-based subcloning and mutagenesis using 6HIS-tagged bacterial expression vectors (Du et al., 1998) as templates. A BamHI site and the HA epitope YPYDVPDYA were fused to the N terminus of each coding sequence using a primer beginning with the sequence GGATCCACCATGTACCCATACGATGTCCCAGACTACGCTATG, where the final ATG corresponds to the start of the open reading frame. The PCR products were inserted between the BamHI and HindIII (YPT 1, YPT32, YPT51, and YPT7) or PstI (SEC4 and YPT6) sites of pNB529 to create plasmids pNRB829–pNRB840. Nucleotide sequencing confirmed the presence of asparagine to isoleucine mutations where appropriate and revealed several differences between the pNRB529 subclones and sequences available from the Saccharomyces Genomic Database. A total of six missense mutations were found, and each mutation was present in both the wild-type and N→I mutant plasmids. Because three of the mutations did not affect the protein sequence, it seems likely that the source of the mutations is natural variation between the strains used by Novagen (Madison, WI) (Du et al., 1998) and the yeast genomic sequencing consortium. The remaining three mutations resulted in the following changes to the protein sequence: K111E in YPT51, D81G in YPT6, and D51E in YPT7. PNRB529 and pNRB829–pNRB840 were digested with ClaI to direct integration into the LEU2 gene of NY605 to create strains NY1705–NY1717. NY1720 (a/α myc3-SSO2) and NY1719 (a/α myc3-SSO2 HA3-sec4-N133I) were created by crossing EGY244 to NY871 (MATα leu2 his4)- and to pNRB834 (HA-sec4-N133I)-transformed NY871. Strain NY1718 (sec18-1 HA-sec4-N133I) was created by transforming NY1217 (MATa sec18-1 leu2-3112 ura3-52) with pNRB834 (HA-sec4-N133I).
Antibodies
Antiserum against purified Sso1p (a gift from Axel Brunger, Yale University) was generated by Cocalico. The anti-Ssop serum was affinity purified using glutathione S-transferase-Ssop (Rice et al., 1997) bound to glutathione-agrose beads (Amersham Pharmacia Biotech, Uppsala, Sweden). Biotinylated anti-Sso for immunoblotting was prepared using NHS-LC-Biotin (Pierce, Rockford, IL) according to the manufacturer’s protocol. Affinity-purified anti-Pep12p and anti-Vam3p antibodies were from Robert Piper (University of Iowa, Iowa City, IA). Anti-Sed5p and anti-Tlg2p sera were from Susan Fero-Novick (Yale University). Anti-HA and biotinylated anti-HA monoclonal antibodies (12CA5) were purchased from Boehringer Mannheim (Indianapolis, IN). The anti-Sncp antibody has been previously described (Rossi et al., 1997). HRP-conjugated Goat anti-mouse and Goat anti-rabbit antibodies were purchased from Jackson ImmunoResearch (West Grove, PA) and Streptavidin-HRP was purchased from Amersham Pharmacia Biotech.
Lysis, Immunoprecipitation, and Western Blotting
Under standard conditions, early log phase yeast cultures grown at 25°C in YPD were collected by centrifugation and washed with 20 ml of ice-cold TAF buffer (20 mM Tris, pH 7.5, 20 mM NaN3, 20 mM NaF). The cells were then transferred to 2-ml screw capped tubes in 1 ml of TAF buffer and pelleted. Ice-cold immunoprecipitation (IP) buffer (20 mM HEPES, pH 7.4, 150 mM KCl, 1 mM DTT, 0.5% N-P40, 1 mM EDTA), proteinase inhibitors (1 mM PMSF, 1 μM pepstatin A) and 2 g of zirconia-silica beads were added, and the tubes were completely filled with liquid and sealed. The cells were lysed by homogenization in a mini-Bead Beater (Biospec Products, Bartlesville, OK) at full power for 4 min and then returned to an ice-water bath.
When temperature shifting was required, cultures at elevated temperatures were diluted 1:10 in ice cold TAF buffer, and cells were collected by centrifugation at 4°C. When appropriate, SNARE complex disassembly was promoted by collecting cells in ice-cold 20 mM Tris buffer, pH 7.5, without NaN3 or NaF and homogenizing in IP buffer supplemented with an ATP-regenerating system (1 mM ATP, 5 mM creatine phosphate, 10 μg/ml creatine phosphokinase, 3 mM MgCl2).
The lysates were diluted with IP buffer into 1.4-ml, 2-mg/ml aliquots and spun for 5 s in a microfuge to remove unbroken cells and cellular debris and then for 30 min at 16,000 × g. The cleared lysate was transferred to a fresh tube, an aliquot was reserved, and then primary antibody was added. After incubating on a rocking platform at 4°C for 2–16 h, protein G-Sepharose beads (Amersham Pharmacia Biotech) were added, and the incubation was continued for an additional 45 min. The immunoprecipitates were collected by centrifugation, and an aliquot of the supernatant was reserved. The immunoprecipitates were washed five to eight times with IP buffer, boiled for 5 min in gel-loading buffer with 1% SDS, run on either 12 or 15% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. The membranes were stained with Ponceau S to observe the quality of the transfer. Antigens on the membrane were detected by incubating the filter with blocking buffer (5% nonfat dry milk in PBS, 0.05% Tween 20), adding primary antibodies in blocking buffer, washing five times, adding HRP-conjugated detection reagent in blocking buffer, washing five times, incubating in chemiluminescent substrate (ECL from Amersham Pharmacia Biotech or BLAZE from Pierce), and then exposing the filter to BioMax MR film (Eastman Kodak, Rochester, NY). Lysates and depleted supernatant fractions from each immunoprecipitation were also assessed by immunoblotting. SNARE expression and immunoprecipitation efficiencies were identical for wild-type and mutant strains.
GTP Overlays
Lysates or anti-HA immunoprecipitates were run on 15% SDS-polyacrylamide gels and transferred to nitrocellulose. The blots were preincubated for 30 min in GTP buffer (50 mM NaH2PO4, pH 7.5, 2 mM DTT, 10 μM MgCl2, 0.2% Tween 20, 4 μM ATP) to allow renaturation of low-molecular-weight GTP-binding proteins, probed with 1 μCi/ml [α-32P]GTP for 2 h in GTP buffer, washed five times over 1 h with GTP buffer, air dried, and exposed to film overnight at −80°C with an intensifying screen.
RESULTS
Sncp Binds to Many t-SNAREs
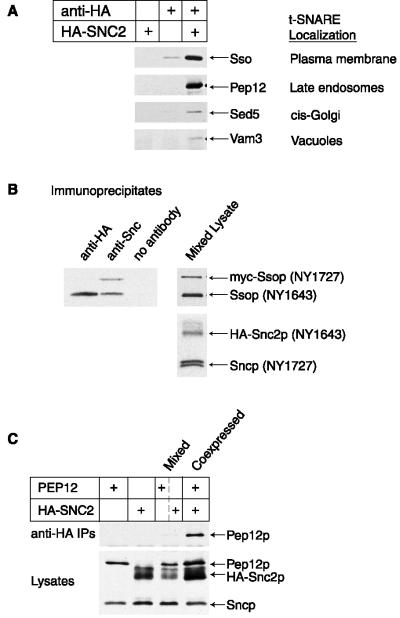
The Snc proteins Snc1p and Snc2p are best known as the v-SNAREs on secretory vesicles that interact with the plasma membrane t-SNAREs Sso1p, Sso2p, and Sec9p (Protopopov et al., 1993; Brennwald et al., 1994). More recently, Sncp has been shown to bind to two other t-SNAREs, Tlg1p and Tlg2p (Abeliovich et al., 1998; Holthuis et al., 1998a), which are localized to endosomal and/or Golgi membranes. We tested whether Snc2p binds to additional t-SNAREs by looking for coimmunoprecipitation of t-SNAREs with HA-tagged Snc2p. The three Ssop homologues tested, Pep12p, Vam3p, and Sed5p, each coimmunoprecipitated with HA-Snc2p but were not present in control immunoprecipitations either without the anti-HA antibody or from an untagged strain (Figure 1A). Although these interactions are specific, only ∼1% of the total amount of each t-SNARE in the lysate coimmunoprecipitated with HA-Snc2p. A similar small percentage of the total amount of Sncp coimmunoprecipitated with Ssop, Tlg2p, or Pep12p. The observation that only a small percentage of each t-SNARE protein coimmunoprecipitates with Sncp is consistent with the proposal that assembled SNARE complexes are transient intermediates in the process of membrane fusion.
Figure 1.
Snc2p binds to diverse t-SNAREs. (A) Ssop, Pep12p, Sed5p, and Vam3p coimmunoprecipitate with HA-Snc2p. Lysates were prepared from either wild-type yeast (NY605) or sec18-1 yeast expressing HA-Snc2p (NY1643). The cells were shifted to 37°C for 10 min before lysis to allow SNARE complexes to accumulate in the sec18-1 mutant strain. An immunoblot from anti-HA and no antibody control immunoprecipitates was probed for coprecipitating t-SNAREs. The minimal amount of nonspecific Ssop precipitation from the untagged strain is independent of Sec18p function. (B) myc-Sso2 does not bind to HA-Snc2p in vitro. HA-Snc2p and myc-Ssop were expressed in different populations of sec18-1 cells (NY1643 and NY1727), which were mixed, shifted to 37°C for 10 min, and then lysed. HA-Snc2 was immunoprecipitated with anti-HA antibodies, native and HA-tagged Sncp were immunoprecipitated with anti-Sncp antibodies, and a control precipitation was performed without antibody. The immunoprecipitates were probed for coprecipitation of myc-Sso2p and native Sso proteins with biotinylated anti-Sso antibodies. The anti-Sncp antibody reacts with an epitope conserved between Snc1p and Snc2p. Likewise, the anti-Ssop antibody reacts with an epitope conserved between Sso1p and Sso2p. (C) Pep12p does not bind to HA-Snc2p in vitro. Pep12p and HA-Snc2p were either coexpressed in the same SEC+ cells (NY1722) or expressed in different cells that were mixed before lysis (NY605and NY1726). Anti-HA immunoprecipitates were probed for coprecipitation of Pep12p. An immunoblot of the lysates was probed with antibodies against Sncp and Pep12p.
The original SNARE hypothesis proposed that the formation of specific v-SNARE/t-SNARE pairs ensures the fidelity of vesicle targeting (Rothman and Warren, 1994). Our observation that Sncp binds to multiple t-SNAREs, like similar observations for the v-SNAREs Sec22p and Vti1p (Lewis et al., 1997; von Mollard et al., 1997), was not predicted by this early model. One interpretation of these results is that nonspecific SNARE pairs assemble during homogenization or in the lysate. To test this possibility, we have used a “mixing” assay that examines the binding of tagged proteins expressed in different cell populations. We first examined the interaction between Snc and Sso proteins (Figure 1B). A mixed lysate was prepared from cells expressing myc-Sso2p and native Snc proteins (NY1643) and cells expressing native Sso proteins and HA-Snc2p (NY1643). Both native and myc-tagged Sso proteins precipitated in an anti-Sncp immunoprecipitation, demonstrating that myc-Sso2p can bind to Sncp. However, myc-Sso2p was absent from an anti-HA immunoprecipitate. Therefore, Sncp and Ssop do not assemble into a SNARE complex during or after homogenization. Because Sncp does not bind to Ssop in lysates, the Sncp/Ssop complex we have observed must have assembled in vivo. The two strains in the experiment shown have a mutation in Sec18p, the yeast NSF homologue, which enhances the recovery of SNARE complexes. Comparable results have been obtained in experiments with strain expressing wild-type Sec18p (Carr et al., 1999).
A similar experiment was performed to examine the interaction between HA-Snc2p and Pep12p (Figure 1C). Pep12p coimmunoprecipitated with HA-Snc2p if the two proteins were expressed in the same cells. However, if HA-Snc2p expressed in a Δpep12 strain was mixed with Pep12p from an untagged strain during homogenization of the yeast, the two proteins did not coimmunoprecipitate. We conclude that the Pep12p–HA-Snc2p interaction forms in vivo before homogenization and is stable in the lysate. A third mixing experiment indicated that HA-Sncp binds Tlg2p in vivo but not in lysates (Abeliovich et al., 1998). In summary, log phase yeast cells have SNARE complexes involving Snc2p binding to Ssop, Pep12p, Tlg2p, and possibly also Tlg1p, Vam3p, and Sed5p. Therefore, although Sncp is normally required for fusion of secretory vesicles with the plasma membrane, it is unlikely to play a role in secretory vesicle targeting, because Sncp can bind to t-SNAREs present on a variety of potential target organelles.
Three Rab Mutations Inhibit Membrane Traffic Upstream of Sncp–Ssop SNARE Complex Assembly
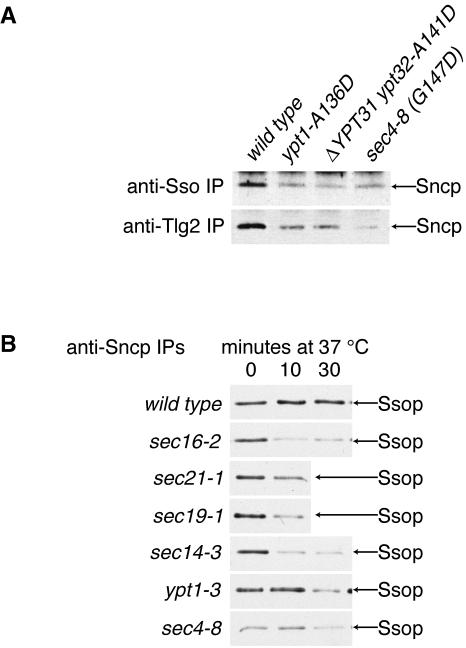
Because a selective interaction between Sncp and Ssop cannot be responsible for targeting secretory vesicles to the plasma membrane, we considered the hypothesis that the mechanism of secretory vesicle targeting involves a selective interaction between the secretory vesicle protein Sec4p and the plasma membrane protein Ssop that promotes the assembly of a SNARE complex between Sncp and Ssop. Sogaard et al. (1994) reported that mutations in the ER to Golgi Rab protein Ypt1p inhibited coimmunoprecipitation of a SNARE complex between the appropriate v- and t-SNARE proteins, Sec22p and Sed5p. This result was interpreted as evidence that Ypt1p activates Sec22p/Sed5p complex formation. We examined the effect of the sec4-8 mutation on Sncp/Ssop SNARE complex assembly and found that coimmunoprecipitation of Ssop with Sncp was reduced in a sec4-8 mutant strain. To test whether this result reflects a specific interaction between Sec4p and Ssop, we examined the effect of mutations in three different Rab genes, SEC4, YPT1, and YPT32, on the association between Sncp and two different t-SNAREs, Ssop and Tlg2p. The sec4 and ypt1 mutant strains accumulate Golgi to plasma membrane vesicles and ER to Golgi vesicles, respectively, when shifted to temperatures >30°C (Novick et al., 1981; Segev et al., 1988). YPT32 has a functionally redundant homologue, YPT31. If both Ypt31p and Ypt32p are mutated, secretory vesicles fail to bud from the Golgi (Jedd et al., 1997). The ypt1, ypt31 and sec4 mutant alleles used for Figure 2A are similar to each other, because each results in an aspartate for alanine (or glycine) substitution at a conserved position in the nucleotide binding domain that causes a recessive loss of function.
Figure 2.
SNARE complex assembly depends on membrane transport. (A) Mutations in three different Rab proteins inhibit Sncp association with both Ssop and Tlg2. Wild-type (DBY1034), ypt1-A136D, ΔYPT31 ypt32-A141D, and sec4-8 cells were shifted to the restrictive temperature of 33°C for 10 min before homogenization. Anti-Ssop and anti-Tlg2p immunoprecipitates were probed for coprecipitation of Sncp. (B) Early secretion blocks inhibit Ssop association with Sncp. Wild-type (NY13) and sec mutant strains were grown to log phase at 25°C and then shifted to 37°C for 0, 10, or 30 min before homogenization. Anti-Sncp immunoprecipitates were probed for coprecipitation of Ssop.
If the Sncp/Ssop assembly defect in sec4-8 cells reflects a specific interaction between Sec4p and Ssop, one would predict that the sec4-8 mutation would not affect the binding of Sncp to Tlg2p. Perhaps Sncp/Tlg2p binding would be inhibited by loss of Ypt31p and Ypt32p activity. In contrast to this prediction, the results show that instead of each Rab mutation affecting assembly of a specific SNARE complex, there was reduced coprecipitation of Sncp with both Ssop and Tlg2p in all three Rab mutant strains (Figure 2A). Based on these results, we cannot conclude that inactivation of Rab proteins affects the interaction of Sncp with specific t-SNAREs.
One explanation for the observation that all three rab mutations affect both Sncp-containing SNARE complexes is that these mutations are acting upstream to block flux through several pathways involving Sncp-dependent fusion. A prediction from this model is that any mutation that blocks the secretory pathway upstream of the fusion of secretory vesicles with the plasma membrane will inhibit assembly of the Sncp/Ssop complex. To test this prediction, we examined several temperature-sensitive strains with early blocks in the secretory pathway and compared them with a sec4-8 strain. The mutant strains used include sec16-2, which is defective in budding from the ER (Kaiser and Schekman, 1990); sec21-1 (COPI), defective in assembly of a coat required for the budding of retrograde transport vesicles from the Golgi that fuse to the ER and also in selective aspects of anterograde ER to Golgi transport (Hosobuchi et al., 1992; Letourneur et al., 1994; Orci et al., 1997); sec19-1 (gdi1), which is inhibited at multiple steps in the secretory pathway including ER to Golgi transport because of a defect in Rab protein recycling (Garrett et al., 1994); and sec14-3 (phophatidyl inositol/phosphatidyl choline transfer protein), which is unable to bud secretory vesicles from the Golgi (Bankaitis et al., 1989). As predicted, there was a reduction in the amount of Ssop coprecipitating with Sncp in each of the mutant strains if the cells were shifted to 37°C before lysis (Figure 2B). Notably, for ypt1-3 cells no reduction in the amount of Ssop associated with Sncp was observed until 30 min after the shift to 37°C. The difference between the ypt1-A131D and ypt1-3 alleles after 10 min at 37°C can be explained by supposing that the Ypt1-3 mutant protein is slowly inactivated. Also notable is the reduced association of Ssop with Sncp at 25°C in the sec4-8 mutant strain. This observation is consistent with the reduced abundance of the Sec4-8 protein and the reduced growth rate of sec4-8 cells under these nominally permissive conditions (our unpublished observation). The association of Ssop with Sncp is also reduced in wild-type yeast strains if they are grown in synthetic media or in rich media with a nonfermentable carbon source. Assuming that the rate of secretion correlates with the reduced doubling in suboptimal growth media, these observations are all consistent with the model that SNARE complex assembly depends on flux through the secretory pathway.
Nucleotide-free Sec4p Binds to Ssop
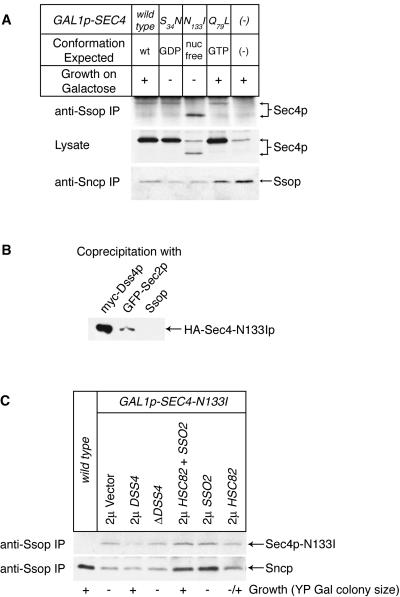
Because experiments performed with the Rab mutants do not provide evidence for a specific functional interaction between Sec4p and Ssop, we chose to look for a physical interaction between the two proteins by coimmunoprecipitation as an alternative test of our hypothesis that combinatorial interactions between Rabs and SNAREs ensure correct vesicle targeting. In preliminary experiments with wild-type yeast,we failed to detect Sec4p coprecipitating with Ssop either by probing an immunoblot with anti-Sec4p antibodies or with an [α-32P]GTP overlay assay. We then looked for binding to Ssop of wild-type and mutant forms of Sec4p expressed at high levels under control of a galactose-regulated promotor (Walworth et al., 1989). As a GTPase, Sec4p is thought to act as a molecular switch with three conformations regulated by GTP binding, hydrolysis, and release. Mutations were engineered in Sec4p based on well-known mutations in the Ras oncogene that affect its nucleotide binding and hydrolysis cycle. These mutations include sec4-S34N, which is predicted to be locked in its GDP-bound conformation, sec4-N133I, which fails to bind nucleotide, and sec4-Q79L, which is defective in GTP hydrolysis (Walworth et al., 1989, 1992; Collins et al., 1997). Growth is inhibited by overexpression of the sec4-S34N and sec4-N133I alleles (Walworth et al., 1989; Collins et al., 1997). These dominant negative effects are thought to occur because the mutant Sec4 proteins bind and sequester factors that are essential for secretion. Cells were shifted to media containing 3% galactose for 6 h to induce expression of the Sec4 proteins without killing the cells.
Among the Sec4 proteins, the nucleotide-free Sec4-N133I mutant protein coimmunoprecipitated most efficiently with Ssop (Figure 3A). The significance of this result is strengthened by the observation that Sec4-N133Ip was expressed at lower levels than wild-type Sec4p and the other mutant Sec4 proteins. We also examined the effect of Sec4 proteins on the Ssop/Sncp SNARE complex. Expression of the two dominant-negative Sec4 proteins, Sec4-S34Np and Sec4-N133Ip, reduced the amount of Ssop coprecipitating with Sncp. This result was anticipated because these mutants reduce flux through the secretory pathway. Ssop/Sncp coimmunoprecipitation was also slightly reduced by overexpressing wild-type Sec4p. High levels of Sec4p expression (behind the GAL1 promotor on a high-copy-number plasmid) are known to have a dominant negative growth phenotype (Kabcenell et al., 1990). Similarly, overproduction of Ypt1p has been reported to reduce the coimmunoprecipitation of Sec22p with Sed5p (Lupashin and Waters, 1997). In contrast to the results with the other Sec4 proteins, expression of Sec4-Q79Lp did not reduce Sncp/Ssop coimmunoprecipitation or have a dominant-negative growth phenotype.
Figure 3.
Binding of Sec4-N133Ip to Ssop. (A) Binding of overexpressed Sec4 mutant proteins to Ssop. Cells overexpressing wild-type or mutant Sec4 proteins were grown to log phase in YP raffinose media and then shifted to YP galactose media for 6 h before homogenization. An immunoblot from the lysates was probed for Sec4 proteins; anti-Sso immunoprecipitates were probed for coprecipitating Sec4 proteins; and anti-Sncp immunoprecipitates were probed for coprecipitating Ssop. (B) Binding of HA-Sec4-N133Ip to myc-Dss4p, GFP-Sec2p, and Ssop. Cells expressing myc-Dss4p (NY1724) or GFP-Sec2p (NY1723) were mixed with HA-Sec4-N133Ip-expressing cells (NY1710) before homogenization. Myc-Dss4p was immunoprecipitated from the myc-Dss4p + HA-Sec4-N133Ip mixed lysate with anti-myc antibodies. The GFP-Sec2p from the GFP-Sec2p + HA-Sec4-N133Ip mixed lysate was immunoprecipitated with anti-GFP antibodies. For comparison, Ssop was also immunoprecipitated from the GFP-Sec2p + HA-Sec4-N133Ip mixed lysate. Coprecipitating HA-Sec4-N133Ip in the three immunoprecipitates was detected with anti-HA antibodies. HA-Sec4-N133Ip was detectable in the anti-Ssop immunoprecipitate on a longer exposure using the BLAZE detection system. (C) Differential effects in Sec4-N133Ip-overexpressing strains of 2μ DSS4 and SSO plasmids on growth and coprecipitation of Sec4-N133Ip and Sncp with Ssop. Strains were grown for 6 h in YP galactose media before lysis and immunoprecipitation with anti-Ssop antibodies. The immunoprecipitates were probed for coprecipitation of Sec4-N133Ip and Sncp. Suppression of the dominant-negative growth phenotype of Sec4-N133Ip overexpression was measured by observing colony sizes 3 d after streaking on YP galactose plates.
In addition to Ssop, two other proteins, Dss4p and Sec2p, are known to bind Sec4-N133Ip. These other proteins regulate the nucleotide binding status of Sec4p. Dss4 promotes GDP release, and Sec2p promotes both GDP release and GTP binding (Moya et al., 1993; WalchSolimena et al., 1997). We were intrigued by the possibility that Ssop might also function as a nucleotide exchange factor. As a preliminary test of this idea, we compared the binding of Dss4p, Sec2p, and Ssop to Sec4-N133Ip by mixing cells expressing HA-Sec4-N113Ip (see below) with cells expressing either myc-Dss4p or GFP-Sec2p and then immunoprecipitating with antibodies against myc, GFP, or Ssop. We found that >10% of the HA-Sec4-N133Ip coprecipitated with myc-Dss4p, 2% coprecipitated with GFP-Sec2p, and 0.05% coprecipitated with Ssop (Figure 3B). The extremely inefficient binding of Ssop to Sec4-N133Ip suggests that Ssop is unlikely to regulate Sec4p’s nucleotide binding state. A second indication that Ssop is not a Sec4p exchange factor is that, unlike Dss4p and Sec2p (Collins et al., 1997; Walch-Solimena et al., 1997), Ssop does not bind to the Sec4-S34N mutant protein, which mimics the GDP-bound state of Sec4p.
We were able to examine the relationship between Sec4-N133Ip binding to Ssop, Sncp/Ssop SNARE complex assembly, and growth inhibition in Sec4-N133Ip expressing strains by comparing Sec4-N133Ip and Sncp binding to Ssop in a variety of genetic backgrounds (Figure 3C). Dss4p binds to the Sec4-N133I mutant protein and, when overproduced, will suppress the dominant-negative growth phenotype of Sec4-N133Ip expression (Collins et al., 1997). Overproduction of Dss4p reduced the binding of Sec4-N133Ip to Ssop but did not restore normal levels of Sncp/Ssop binding (Figure 3C). Because overproduction of Dss4p in Sec4-N133Ip-expressing cells results in a normal growth rate with reduced levels of Sncp/Ssop SNARE complexes, the absolute number of Sncp/Ssop SNARE complexes cannot be rate limiting for growth. Deletion of DSS4, a nonessential gene (Moya et al., 1993), from sec4-N133I yeast had no effect on growth or binding of either Sncp or Sec4-N133Ip to Ssop.
A second suppressor of the dominant-negative growth phenotype of Sec4-N133Ip was isolated in a multicopy genomic DNA library screen (Shannon and Novick, unpublished results). This plasmid contained two genes, HSC82 and SSO2, that are adjacent to each other on chromosome XIII. The HSC82 gene codes for a constitutively expressed homologue of the heat shock-induced chaperonin, Hsp70p. Sec4-N133Ip-overexpressing cells containing this plasmid have a wild-type growth rate and have partially restored binding of Sncp to Ssop. However, cooverexpression of Hsc82p and Sso2p does not prevent binding of Sec4-N133Ip to Ssop (Figure 3C). Thus, binding of Sec4-N133Ip to a small fraction of the total Ssop does not prevent Ssop/Sncp SNARE complex assembly when excess t-SNARE is available.
Individually, SSO2 and HSC82 are poor suppressors of the dominant negative sec4-N133I growth phenotype (Shannon, unpublished results). Hsc82p overexpression has no effect on the binding of Sncp or Sec4-N133Ip to Ssop. In contrast, although Sso2p overproduction does not restore growth, the amount of both Sncp and Sec4-N133Ip bound to Ssop increases, presumably by mass action (Figure 3C). Therefore, restoration of Sncp/Ssop binding is not sufficient to suppress the growth defect of Sec4-N133Ip-overexpressing cells. In summary, Sec4-N133Ip overexpression reduced both the amount of Sncp bound to Ssop and the growth rate, but these two phenotypes are not intimately related, because Ssop overexpression restores SNARE binding but not growth, whereas Dss4p overexpression restores growth but not SNARE binding.
All Nucleotide-free Rab Proteins Bind to v- and t-SNAREs
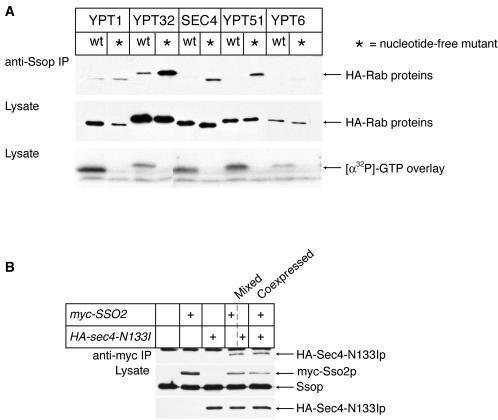
If Rabs interact with t-SNAREs to ensure the fidelity of transport, one would expect that each Rab would interact with specific t-SNAREs. To address the specificity of the coimmunoprecipitation of Sec4-N133Ip with Ssop, we constructed a series of strains with genes coding for a representitive selection of N-terminally HA-tagged Rab proteins integrated at the LEU2 locus of a wild-type yeast strain behind a galactose-regulated GAL1 promotor. These Rab proteins were either wild-type or carried an Asn to Ile mutation at a position in their sequence analogous to the N133I mutation of sec4-N133I. The plasmids containing the HA-tagged Rab genes were sequenced to confirm the presence of the mutations and that no errors were introduced during the PCR-based subcloning and mutagenesis (see MATERIALS AND METHODS). Expression was confirmed with an anti-HA immunoblot of lysates prepared from cultures grown overnight in YP galactose media (Figure 4A). An [α-32P]GTP overlay assay of proteins in the lysate (Figure 4A) and in anti-HA immunoprecipitates confirmed that the wild-type, but not the mutant, Rab proteins bound GTP. Because overproduction of several of the HA-tagged Rab proteins inhibited growth, the HA-Rab-transformed strains were maintained in YP raffinose media and grown for 6 h in YP galactose media to induce HA-Rab expression before lysis.
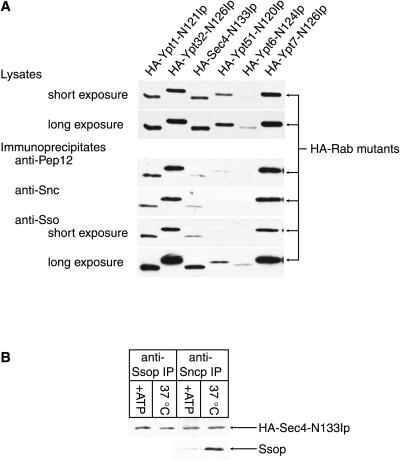
Figure 4.
Six nucleotide-free HA-Rab proteins bind to Ssop in lysates. (A) Binding of HA-Rab proteins to Ssop. Strains were grown for 6 h in YP galactose media before homogenization. Anti-Ssop immunoprecipitates and aliquots of the lysates were probed for the HA-Rab proteins. A blot from a second aliquot of the lysate was probed with [α-32P]GTP to detect GTP binding proteins. (B) HA-Sec4-N133Ip binds myc-Ssop in vitro. HA-Sec4-N133Ip and myc-Ssop were either coexpressed in the same cells (NY1719) or expressed in different populations of cells (NY1720 and NY1721) that were mixed before homogenization. An aliquot of the lysates was probed with antibodies against Ssop to detect both myc-Sso2 and native Sso1 and Sso2 proteins and with anti-HA antibodies to detect HA-Sec4-N133Ip. Anti-myc immunoprecipitates were probed to detect coprecipitating HA-Sec4-N133Ip with anti-HA antibodies.
To assay for specificity in the binding interactions of Rabs and t-SNAREs, we looked for coimmunoprecipitation of each of the wild-type and nucleotide-free mutant HA-Rab proteins with Ssop (Figure 4A). All of the nucleotide-free mutant Rabs coimmunoprecipitated with Ssop, but their wild-type counterparts did not. As a negative control, we confirmed that the nucleotide-free HA-Rab proteins do not bind to protein G-agarose beads in the absence of immunoprecipitating antibody. The quantity of each mutant HA-Rab protein in the anti-Ssop immunoprecipitates was approximately proportional to its expression level. We conclude that there are not significant differences in the efficiency with which of each of the Rab proteins, in their nucleotide-free conformations, binds Ssop.
We also compared the binding of each of the HA-Rab mutant proteins to Pep12p, the t-SNARE on the prevacuolar compartment (Figure 5A). Pep12p might be predicted to have a specific interaction with the Rab protein Ypt51p, because Pep12p and Ypt51p are both involved in fusion to the prevacuolar compartment. Ypt51-N120Ip did coimmunoprecipitate with Pep12p, but there was not significantly more Ypt51-N120Ip in the Pep12 immunoprecipitate than the amount that bound to Ssop. Furthermore, in addition to Ypt51-N120Ip, the other mutant HA-tagged Rab proteins coimmunoprecipitated with Pep12p in amounts approximately proportional to their expression levels. Thus, Rab proteins do not preferentially bind to those t-SNAREs with which they functionally interact.
Figure 5.
HA-tagged nucleotide-free mutant Rab proteins bind to Pep12p, Sncp, and Ssop but not to SNARE complexes. (A) Nonpreferential binding of nucleotide-free HA-Rab mutant proteins to SNAREs. Expression of the HA-tagged mutant Rab proteins was induced by growth for 6 h in YP galactose media. Lysates were divided into aliquots for immunoprecipitation with antibodies against Pep12p, Sncp, and Ssop. The immunoprecipitates were probed for coprecipitation of the HA-tagged mutant Rab proteins with anti-HA antibody. Similar amounts of untagged Sec4-N133I coimmunoprecipitated with Ssop and Sncp. Thus, the possibility that the lack of specificity we have observed in HA-Rab coprecipitation with SNAREs is an artifact of the N-terminal HA-tag is excluded. (B) HA-Sec4-N133Ip/SNARE binding is insensitive to SNARE complex disassembly. sec18-1 yeast expressing HA-Sec4-N133Ip (NY1718) were either lysed in buffer supplemented with ATP and an ATP-regenerating system or shifted to 37°C for 10 min, collected in ice-cold buffer with NaN3 and NaF, and lysed in buffer containing EDTA. Anti-Snc and anti-Ssop immunoprecipitates were probed with anti HA antibodies for coprecipitating HA-Sec4-N133Ip. Disassembly of the Sncp/Ssop SNARE complex in lysates with ATP was confirmed by probing the anti-Sncp immunoprecipitates with antibodies against Ssop.
Because it has been established that Rab proteins are located on different membranes within a cell, the nonpreferential coimmunoprecipitation that we have observed suggests that Rabs and t-SNAREs may bind in the lysate after homogenization. A mixing experiment was carried out to determine whether HA-Sec4-N133Ip is able to bind to myc-Ssop after lysis (Figure 4B). HA-Sec4-N133Ip and myc-Ssop were either coexpressed in the same cells or expressed in separate populations of cells that were then mixed before homogenization. Expression of HA-Sec4-N133Ip in a separate population of cells than myc-Ssop did not reduce the amount of HA-Sec4-N133Ip coprecipitated in an anti-myc immunoprecipitate when compared with a strain in which the two proteins are coexpressed. This result suggests not only that HA-Sec4-N133Ip and Ssop can bind after lysis, but also that most of the complexes we observed formed after lysis. If any complexes present before lysis still remained, more HA-Sec4-N133Ip would have bound to myc-Ssop when the two proteins were expressed in the same cells than bound when the proteins were expressed in different cells. Thus, we find no evidence for the specific interaction between Sec4p and Ssop that would be expected if Rabs and t-SNAREs interact to ensure the fidelity of membrane fusion.
To determine whether Rab proteins bind to free t-SNAREs or to SNARE complexes, we first looked for coimmunoprecipitation of the overexpressed mutant Rab proteins with the Snc v-SNARE proteins (Figure 5A). Sncp bound in similar amounts to each of the mutant HA-Rabs. The binding of the mutant Rabs to both Sncp and Ssop suggested that Rabs bind to SNARE complexes. However, the result is also consistent with binding of Rabs to free v- and t-SNARE proteins. To distinguish between these possibilities, we compared the binding of HA-Sec4-N133Ip to Sncp and Ssop under lysis conditions that promote or inhibit SNARE complex disassembly (Figure 5B). The Sncp/Ssop SNARE complex is disassembled by the NSF homologue Sec18p, an ATPase that can be activated in lysates by addition of ATP and an ATP-regenerating system (Carr et al., 1999). To inhibit disassembly, cells are collected in ice-cold buffer containing azide and fluoride to lower cellular ATP levels and then lysed in buffer containing EDTA to chelate Mg2+, which is an essential cofactor for Sec18p. Addition of ATP to the lysate eliminated detectable binding of Ssop to Sncp but did not affect the coimmunoprecipitation of HA-Sec4N133Ip with either Sncp or Ssop. Although we cannot exclude the possibility that HA-Sec4-N133Ip binds SNARE complexes, we conclude that HA-Sec4-N133Ip is able to bind to free v- and t-SNAREs and that SNARE complex assembly is not a prerequisite for binding.
DISCUSSION
SNAREs and Vesicle Targeting
In the original formulation of the SNARE hypothesis, specific interactions between SNARE proteins were proposed to mediate vesicle targeting. Each class of transport vesicle was defined by a unique v-SNARE, which could bind only to its cognate t-SNARE on the appropriate fusion target (Rothman and Warren, 1994). One difficulty with this model is that, over its lifetime, each v-SNARE protein is found on several different classes of transport vesicles destined to fuse with different targets. Newly synthesized v-SNAREs are translocated into the ER (Kutay et al., 1995) and transported to their donor compartments via the secretory pathway. Under steady-state conditions, v-SNAREs are recycled after fusion from the acceptor organelle back to the donor to be incorporated into a new transport vesicle. At the end of their lifetimes, v-SNAREs are likely to be transported to proteolytic organelles such as the yeast vacuole for degradation. For v-SNAREs to function as targeting molecules, they must be active only in those transport vesicles destined to fuse with the acceptor organelle containing their cognate t-SNARE (Pfeffer, 1996). We find no evidence for such regulation, because the v-SNARE Snc2p binds to a variety of t-SNAREs including Sso1p, Sso2p, Tlg1p, Tlg2p, Pep12p, Sed5p, and Vam3p. Because these t-SNAREs are each localized to a distinct set of acceptor organelles, we propose that Snc2p is active at all times and participates in diverse fusion events. The accumulation of post-Golgi secretory vesicles, but not ER to Golgi vesicles, in a snc mutant strain (Protopopov et al., 1993) can be explained by postulating that other v-SNAREs that function in the early secretory pathway are excluded from post-Golgi secretory vesicles. We conclude that Snc2p does not have a fundamental role in vesicle targeting.
This conclusion is only valid if the v-SNARE–t-SNARE interactions we have observed by coimmunoprecipitation actually occur within the cell. This is a serious concern, because monomeric Sncp is predicted to have an amphipathic helix with an exposed hydrophobic surface that might interact with t-SNAREs after the constraints of subcellular localization have been removed by lysing the cells with detergent. We tested for SNARE complex assembly during or after lysis by mixing populations of cells expressing either HA-tagged Snc2p or various t-SNAREs before preparing lysates for immunoprecipitation. The results established that HA-Snc2p does not bind to myc-Sso2p, Pep12p, or Tlg2p after lysis. The failure of SNARE complexes to assemble in vitro is an unusual property. We have observed that myc-Dss4p and GFP-Sec2p bind to HA-Sec4-N133Ip in lysates. In addition, the binding of myc-Sec1p to Sncp/Ssop/s9p SNARE complexes can also occur in lysates (Carr et al., 1999). Therefore, in contrast to other protein–protein interactions, the SNARE complexes we have observed between Sncp and Ssop and Pep12 and Tlg2p only assemble within living cells.
Regulation of SNARE Complex Assembly
The purified core α-helical domains of Snc1p, Sso1p, and Sec9p have been shown to spontaneously assemble into a stable, high-affinity complex (Rice et al., 1997). Although dilution of cellular proteins with lysis buffer might slow the rate of SNARE complex assembly, the failure of Snc2p to bind to Ssop in lysates during an overnight incubation suggests that SNARE complex assembly is subject to negative regulation. Synaptophysin and n-Sec1 (munc18 or rb-sec1) have been proposed to act as negative regulators of neuronal exocytic SNARE complex assembly because they bind to monomeric SNARE proteins but not to the exocytic SNARE complex (Pevsner et al., 1994; Edelmann et al., 1995). This type of negative regulation is unlikely to inhibit Sncp assembly into SNARE complexes because there are no known synaptophysin homologues in yeast, and Sec1p cannot act as a negative regulator of SNARE complex assembly because it preferentially binds to assembled SNARE complexes (Carr et al., 1999). SNARE complex assembly is also negatively regulated by an intramolecular interaction between the N- and C-terminal helical domains of syntaxin-like t-SNAREs (Calakos et al., 1994; Hanson et al., 1995; Nicholson et al., 1998). Promoting a conformational change in the t-SNARE necessary for the assembly of SNARE complexes may be one component of the priming activity provided by NSF/Sec18p in staged cell-free fusion assays (Mayer et al., 1996; Ungermann et al., 1998a). However, we find that the SNARE complex disassembly activity of Sec18p predominates over any potential assembly-promoting activity in lysates, so it was not possible for us to examine the role of Sec18p in SNARE complex assembly.
Rab–SNARE Interactions
Rab proteins have been proposed to activate SNARE complex assembly based on strong genetic interactions between Rabs and SNARE genes (Dascher et al., 1991; Brennwald et al., 1994) and the observation that the ER to Golgi SNARE complex fails to assemble in ypt1 mutants (Sogaard et al., 1994). However, we have now demonstrated that inhibition of SNARE complex assembly is not a unique phenotype of Rab mutations, because the Sncp/Ssop SNARE complex fails to assemble in a variety of mutants that inhibit secretion upstream of the docking of secretory vesicles to the plasma membrane. Thus, no conclusions about the mechanism of SNARE complex assembly can be drawn from the observation that SNARE complexes fail to assemble in sec4-8 mutant cells, because sec4-8 fits within the large category of mutations that block secretion upstream of secretory vesicle docking.
A more recent observation suggested a direct physical interaction between Rab and SNARE proteins that might activate SNARE complex assembly. Lupashin and Waters (1997) reported that the Rab protein Ypt1p binds to Sed5p, the syntaxin-like t-SNARE, on the cis-Golgi. These authors proposed that transient binding of Ypt1p to Sed5p might activate Sed5p by promoting the release of the negative regulator Sly1p (a Sec1p homologue), thereby allowing Sed5p to bind to the v-SNARE Sec22p (Lupashin and Waters, 1997). Because Rabs and syntaxin-like t-SNAREs each function in discrete membrane trafficking steps, this model suggested to us that interactions between Rabs and syntaxin-like t-SNAREs might regulate vesicle targeting. We therefore examined in detail the binding of Rabs to SNAREs.
We observed that six different Rabs, in their nucleotide-free conformation, bind with similar efficiency to three different SNAREs including two t-SNAREs, Ssop and Pep12p, and the v-SNARE Sncp. These results do not support the model that specific interactions between Rabs and t-SNAREs regulate vesicle targeting. In fact, because the interactions we have observed are promiscuous, inefficient and involve the unstable, nucleotide-free Rab conformation, it is possible that they simply reflect the ability of amphipathic helicies to bind to partially unfolded Rab proteins. However, because specific Rab and SNARE proteins are likely to be brought in close proximity to each other during membrane fusion, it remains plausible that the Rab/SNARE binding we and others have observed is physiologically significant. Further experiments will be required to determine whether physical interactions between Rabs and SNAREs at the high concentrations available during membrane fusion regulate either Rab function or SNARE complex assembly.
In vitro, Rab proteins promote tethering of vesicles to larger organelles (Cao et al., 1998; Ungermann et al., 1998b). Close apposition of vesicles and their target membranes is likely to accelerate SNARE assembly by increasing the local concentration of v- and t-SNARE proteins. In addition to their vesicle-tethering function, Rab proteins have also been implicated in regulation of vesicle budding (Woodman, 1998), transport of vesicles on the cytoskeleton (Walch-Solimena et al., 1997), and other cytoskelletal rearrangements (Echard et al., 1998). As multifunctional proteins, Rabs are likely to interact with numerous regulatory and effector proteins.
Functional and Nonfunctional SNARE Complexes
Assembly of a SNARE complex in trans between a v-SNARE on a transport vesicle and a t-SNARE on its fusion target is essential for fusion (Nichols et al., 1997). Nevertheless, SNARE complexes can also form in cis between v- and t-SNARE proteins located in the same membrane (Otto et al., 1997). One indication that the Sncp/Ssop SNARE complexes we have identified by coimmunoprecipitation are functional (trans) complexes is that the amount of Ssop bound to Sncp is reduced by ∼75% within 10 min after a block in transport is imposed early in the secretory pathway. The remaining complexes may be remnants of earlier fusion events that have not yet been disassembled by Sec18p or cis complexes assembled by a mechanism independent of membrane transport.
The amount of Ssop bound to Sncp is also reduced when growth is inhibited by overexpression of Sec4-N133Ip. This reduction in SNARE complex assembly may be an indirect consequence of reduced flux through the secretory pathway analogous to the reduction in SNARE complex assembly seen in early sec mutants rather than a direct consequence of overproducing Sec4-N133Ip. Overexpression of Sso2p leads to an increase in the amount of Ssop coprecipitating with Sncp without restoring growth. These SNARE complexes are likely to be nonproductive cis complexes. In contrast, overproducing Dss4p restores normal growth without increasing the amount of Ssop bound to Sncp. This result suggests that under normal conditions, more trans-SNARE complexes are assembled than are necessary for growth. However, it is also possible that partial restoration of secretory function by Dss4p overproduction is sufficient to allow a wild-type growth rate.
Conclusion
We have tested the hypothesis that specific interactions between Rabs and syntaxin-like t-SNAREs are responsible for ensuring that transport vesicles fuse only with appropriate target organelles. The attraction of this hypothesis is primarily based on its adherence to the theoretical expectation that fusion specificity is mediated by evolutionarily related proteins. In a test for specificity of the interaction between the exocytic Rab and t-SNARE proteins Sec4p and Ssop, we have found that Sec4p is but one of many proteins in the secretory pathway whose function is required upstream of the assembly of Sncp and Ssop into SNARE complexes. We have also found that the direct binding of Sec4p to Ssop involves the nucleotide-free form of Sec4p and is inefficient and nonspecific. Based on these results, we propose that the fidelity of vesicle fusion originates from factors unique to each type of transport event rather than from conserved components evolved from a common primordial fusion machine. One such stage-specific factor is the exocyst (TerBush et al., 1996). The exocyst is a heteroligomeric protein complex essential for growth that has been proposed to ensure that post-Golgi secretory vesicles fuse only with the plasma membrane. The subunits of the exocyst include Sec15p, which binds to the GTP-bound form of Sec4p on secretory vesicles (Guo et al., 1999), and Sec3p, which is localized to sites on the plasma membrane where secretion normally occurs independently of flux through the secretory pathway (Finger et al., 1998). Candidate targeting complexes have also been identified for ER to Golgi transport and for homotypic endosome fusion (Cao et al., 1998; Sacher et al., 1998; Christoforidis et al., 1999). In addition to factors that regulate fusion itself, interactions between vesicles and the cytoskeleton are also likely to have a fundamental role in vesicle targeting.
ACKNOWLEDGMENTS
Thanks to Nava Segev, Robert Piper, Axel Brunger, Sirkka Keranen, Susan Ferro-Novick, Jeffrey Shannon, Li Lin Du, N. Barry Elkind, and Ruth Collins for strains and reagents. Thanks to Ruth Collins, Chavela Carr, Hagai Abeliovich, Jeffrey Shannon, N. Barry Elkind, and Li Lin Du for fruitful discussions and/or comments on the manuscript. This work was supported by a National Research Service Award to E.G. and by a grant from the National Institutes of Health to P.N.
REFERENCES
- Aalto MK, Ronne H, Keranen S. Yeast syntaxins Sso1p and Sso2p belong to a family of related membrane proteins that function in vesicular transport. EMBO J. 1993;12:4095–4104. doi: 10.1002/j.1460-2075.1993.tb06093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeliovich H, Grote E, Novick P, Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J Biol Chem. 1998;273:11719–11727. doi: 10.1074/jbc.273.19.11719. [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Scheller RH. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci USA. 1993;90:2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D, Segev N, Stearns T, Hoyt MA, Holden J, Kahn RA. Diverse biological functions of small GTP-binding proteins in yeast. Cold Spring Harb Symp Quant Biol. 1988;53:629–636. doi: 10.1101/sqb.1988.053.01.072. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Brennwald P, Novick P. Interactions of three domains distinguishing the Ras-related GTP-binding proteins Ypt1 and Sec4. Nature. 1993;362:560–563. doi: 10.1038/362560a0. [DOI] [PubMed] [Google Scholar]
- Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998;17:2156–2165. doi: 10.1093/emboj/17.8.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcote TJ, Galli T, Mundigl O, Edelmann L, McPherson PS, Takei K, De Camilli P. Cellubrevin and synaptobrevins: similar subcellular localization and biochemical properties in PC12 cells. J Cell Biol. 1995;129:219–231. doi: 10.1083/jcb.129.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Collins RN, Brennwald P, Garrett M, Lauring A, Novick P. Interactions of nucleotide release factor Dss4p with Sec4p in the post-Golgi secretory pathway of yeast. J Biol Chem. 1997;272:18281–18289. doi: 10.1074/jbc.272.29.18281. [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Collins RN, Novick PJ. Identification of a Sec4p GTPase-activating protein (GAP) as a novel member of a Rab GAP family. J Biol Chem. 1998;273:3253–3256. doi: 10.1074/jbc.273.6.3253. [DOI] [PubMed] [Google Scholar]
- Echard A, Jollivet F, Martinez O, Lacapere JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. doi: 10.1126/science.279.5350.580. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Hanson PI, Chapman ER, Jahn R. Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J. 1995;14:224–231. doi: 10.1002/j.1460-2075.1995.tb06995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M, von Mollard GF. A new beat for the SNARE drum [see comments] Trends Cell Biol. 1998;8:215–218. doi: 10.1016/s0962-8924(98)01272-0. [DOI] [PubMed] [Google Scholar]
- Goud B, Salminen A, Walworth NC, Novick PJ. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988;53:753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Otto H, Barton N, Jahn R. The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998a;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Nichols BJ, Pelham HRB. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol Biol Cell. 1998b;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. JCell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell AK, Goud B, Northup JK, Novick PJ. Binding and hydrolysis of guanine nucleotides by Sec4p, a yeast protein involved in the regulation of vesicular traffic. J Biol Chem. 1990;265:9366–9372. [PubMed] [Google Scholar]
- Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Kutay U, Ahnert-Hilger G, Hartmann E, Wiedenmann B, Rapoport TA. Transport route for synaptobrevin via a novel pathway of insertion into the endoplasmic reticulum membrane. EMBO J. 1995;14:217–223. doi: 10.1002/j.1460-2075.1995.tb06994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar T, Gotte M, Gallwitz D. Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem Sci. 1997;22:468–472. doi: 10.1016/s0968-0004(97)01150-x. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JP, Stone S, Jiang Y, Lyons P, Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994;372:698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Moya M, Roberts D, Novick P. DSS4–1 is a dominant suppressor of sec4-8 that encodes a nucleotide exchange protein that aids Sec4p function. Nature. 1993;361:460–463. doi: 10.1038/361460a0. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Munson M, Miller RV, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Rice LM, Brennwald P, Brunger AT. Formation of a yeast SNARE complex is accompanied by significant structural changes. FEBS Lett. 1997;415:49–55. doi: 10.1016/s0014-5793(97)01091-0. [DOI] [PubMed] [Google Scholar]
- Rossi G, Salminen A, Rice LM, Brunger AT, Brennwald P. Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J Biol Chem. 1997;272:16610–16617. doi: 10.1074/jbc.272.26.16610. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Sacher M, Jiang Y, Barrowman J, Scarpa A, Burston J, Zhang L, Schieltz D, Yates JR, III, Abeliovich H, Ferro-Novick S. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller F, Simon I, Pfeffer SR. Rab GTPases, directors of vesicle docking. J Biol Chem. 1998;273:22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- Schneider BL, Seufert W, Steiner B, Yang QH, Futcher AB. Use of PCR epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast. 1995;11:1265–1274. doi: 10.1002/yea.320111306. [DOI] [PubMed] [Google Scholar]
- Segev N, Mulholland J, Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988;52:915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HR, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998a;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs [see comments] Nature. 1998b;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- von Mollard GF, Nothwehr SF, Stevens TH. The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of postGolgi vesicles. J Cell Biol. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Brennwald P, Kabcenell AK, Garrett M, Novick P. Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. Mol Cell Biol. 1992;2:2017–2028. doi: 10.1128/mcb.12.5.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, Goud B, Kabcenell AK, Novick PJ. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989;8:1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Woodman P. Vesicle transport: more work for the Rabs? Curr Biol. 1998;8:R199–R201. doi: 10.1016/s0960-9822(98)70124-1. [DOI] [PubMed] [Google Scholar]
- Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani RJ, Scheller RH. SNARE interactions are not selective. Implications for membrane fusion specificity. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]