Abstract
Human succinic semialdehyde dehydrogenase (SSADH) deficiency is an autosomal recessive disorder of GABA metabolism associated with motor impairment and epileptic seizures. Similarly, mice with targeted deletion of the Aldh5a1 gene (Aldh5a1−/−) exhibit SSADH deficiency and seizures early in life. These seizures begin as absence seizures the second week of life, but evolve into generalized convulsive seizures that increase in severity and become lethal during the fourth postnatal week. The seizures are alleviated and survival is prolonged when the mutant animals are weaned onto a ketogenic diet (KD). The persistence of spontaneous, recurrent, generalized tonic–clonic seizures in KD-treated adult Aldh5a1−/− mice allowed us to quantify their daily (circadian) distribution using a novel behavioral method based on the detection of changes in movement velocity. Adult KD-treated Aldh5a1−/− mice exhibited a seizure phenotype characterized by fits of wild running clonus accompanied by jumping and bouncing. These hypermotor seizures were largely spontaneous and occurred daily in a nonrandom pattern. The seizure rhythm showed a peak shortly after dark phase onset (2008 hours) with near-24-hour periodicity. Age-matched wild-type littermates showed no evidence of abnormal motor behavior. These new data suggest that generalized tonic–clonic seizures in Aldh5a1−/− mice are more frequent during a specific time of day and will provide useful information to clinicians for the treatment of seizures associated with human SSADH deficiency.
Keywords: Aldh5a1 gene, Succinic semialdehyde dehydrogenase, Hypermotor seizures, Brainstem, Daily rhythms
1. Introduction
The Aldh5a1 gene encodes for mitochondrial NAD+-dependent succinate semialdehyde dehydrogenase (SSADH) (OMIM 271980, EC 1.2.1.24, Gene ID: 7915), an enzyme that catalyzes the last step of the GABA shunt pathway and irreversibly oxidizes succinate semialdehyde (SSA) to succinate for entry into the Krebs cycle [1]. Two specific exon-skipping mutations have been identified in the human Aldh5a1 gene that result in SSADH deficiency [2], a rare autosomal recessive metabolic disorder associated with severe neurological morbidity. Affected individuals experience a progression of cognitive and language impairment, ataxia, hypotonia, behavioral hyperactivity and aggressiveness, sleep disturbances [3], and epileptic seizures in the form of absences and tonic–clonic convulsions [4]. This phenotype is due largely to the accumulation of SSA, an increase in brain γ-aminobutyric acid (GABA), and its subsequent conversion to γ-hydroxybutyrate (GHB), the key biomarker of SSADH deficiency [4]. GHB is an endogenous constituent of the mammalian brain but at a level <1% of its precursor (GABA). The increased levels of GHB associated with SSADH deficiency over-stimulate GABA and GHB-specific receptors to induce significant behavioral and physiological effects[5].
Targeted deletion of Aldh5a1 in mice (Aldh5a1−/−) leads to SSADH deficiency and a behavioral phenotype consistent with the human form of the disease, including the development of epileptic seizures [6]. Absence-like seizure activity with 7-Hz spike-and-wave discharges first appear in Aldh5a1−/− mice by the second postnatal week and then rapidly evolve into generalized convulsive seizures and lethal status epilepticus by 3 weeks of age [6–8]. However, with the introduction at weaning of the ketogenic diet (KD), a high-fat, adequate-protein, low-carbohydrate diet commonly used in the treatment of drug-resistant forms of epilepsy [9], it is possible for Aldh5a1−/− mice to survive well into adulthood [10], albeit with significant motor and metabolic dysfunction, allowing for further study relevant to their corresponding clinical population. An understanding of how biological timing mechanisms influence seizure expression (i.e., what time of day seizures are more likely to occur) is of great clinical import. To that end we employed a novel seizure detection method and conducted a longterm behavioral study of adult Aldh5a1−/− mice to characterize daily (i.e., circadian) patterns of spontaneous motor seizures.
2. Experimental methods
2.1. Animal husbandry
The Aldh5a1 null Aldh5a1−/− mice with a C57/129Sv background were obtained from the Oregon Health and Science University, Portland, OR, USA [7], and the mutant mouse line was maintained by inbreeding of heterozygotes. Genotypic analysis of the mutants was performed as described [7]. Before and after weaning, mice were group-housed in a pathogen-free facility under a 12-hour:12-hour light:dark photocycle (lights on at 0700 hours). For wild-type (WT) mice, food and water were freely available throughout the study. Mutant mice were obtained from litters maintained exclusively on the KD [9,10] from weaning day onward. The rationale for using KD-treated animals is that absent this dietary treatment, no mutant animals survived to adulthood. All experimental procedures were performed in compliance with the Hospital for Sick Children Lab Animal Services and Canadian Council on Animal Care.
2.2. Actimetry
The behavioral activity of male (Aldh5a1−/−) (N = 6) and wild-type (WT, N = 8) mice was recorded using a TruScan movement sensor (Coulbourn Instruments, Allentown, PA, USA) as described [11]. Briefly, at the start of each experiment, the animal was placed in a 12 × 12 × 16-in. transparent plastic arena and then left undisturbed for 3–4 hours to habituate to the test environment. To minimize the potential confound of isolation stress on behavioral activity [12], each subject's littermates were placed in a separate plastic cage adjacent to the movement sensor to provide visual and olfactory stimuli. We also took measures to minimize the amount of noise and traffic within the procedure room. The arena used for behavioral recording was positioned inside a square frame containing a sensor array that counted interruptions in infrared beams as the animal moved about in the horizontal plane. Twenty-four-hour rhythms of behavioral activity were recorded for each animal over a 4-day period, during which time three specific variables were calculated and averaged at 1-hour intervals by a computer system (TruScan Version 2.0): total movements (TM), movement velocity (MV, expressed in arbitrary units), and jumps above the floor plane (JFP).
After data collection was completed for each subject, we employed a novel method for the detection of seizures based on the assumption that during a hypermotor seizure, there will be a substantial increase in MV well above the background (mean) of the respective sampling period. In effect, our aim was to identify statistical outliers among the individual 1-hour data bins and then to classify the outliers as behavioral seizure events. By definition, statistical outliers represent those cases that are separated from the majority by virtue of their unusually high (or low) scores. To that end, for each subject we treated the hourly MV values as separate cases and performed z-score transformations (zMV) to determine how far and in what direction the hourly MV values deviated from their 24-hour mean. Those zMV scores that were ≥2SD, which would represent <5% of the data under the normal distribution curve, were classified as seizure events. As a measure of validity, at least one spontaneous tonic–clonic seizure was visually confirmed for each mouse. The seizure episode was then cross-referenced with MV data for that particular time of day to confirm that seizures involving pronounced locomotor activity registered with the movement sensor as an increase in MV. Additional technical considerations regarding the temporal resolution of this method are discussed below.
2.3. Cosinor analysis
The seizure events for all Aldh5a1−/− mice were summed, and the 24-hour distribution was calculated using Cosinor analysis in which a nonlinear least-squares regression technique is used to fit single or multiple cosine functions to time-series data. The models used for single and multiple simultaneous oscillators were defined by the equations
| 1 |
| 2 |
Here, Y = value at time t; M = mean (24-hour mean, i.e., the midline estimating statistic of the rhythm, or mesor); A = amplitude (half the distance between the peak and nadir of the rhythm); (2π/τ)t = degrees per unit of time; and Φ = acrophase (peak time of rhythm) in Eq. (1). The mesor, amplitude, acrophase, and frequency of the rhythms were calculated using Cosifit Biological Rhythm Analysis Software (Circesoft Inc., Waltham, MA, USA) [13]. For multiple oscillators (Eq. (2)), N subharmonics (i), are incorporated into the model to obtain estimates of M, A, and Φ for each cosine term entering the equation [13, 14]. The degree to which the temporal distribution of seizures followed an established curve (i.e., goodness-of-fit to the cosine function) was estimated by the Cosinor-derived coefficient of determination, R2.
3. Results
3.1. Seizure phenotype of Aldh5a1−/− mice
Spontaneous generalized tonic–clonic seizures in adult Aldh5a1−/− mice typically evolved into fits of wild running and jumping, hereafter referred to as hypermotor seizures. Occasionally, we found this type of seizure to be triggered by stimuli such as loud noises and handling. Spontaneous hypermotor seizures typically began with a slight loss of righting reflex and brief (<5-second) episodes of forelimb clonus with tonic hindlimb extension, which was difficult to distinguish amidst a background of severe ataxia. This was followed by a gradual increase in ambulation culminating in a hypermotor seizure. This sequence of behaviors was seldom observed in standard open-rack cages due to physical interference by littermates and a lack of space, but was easily identified within the open area of the movement sensor. Wild-type littermates did not exhibit any seizures or motor impairment.
3.2. Circadian distribution of hypermotor seizures
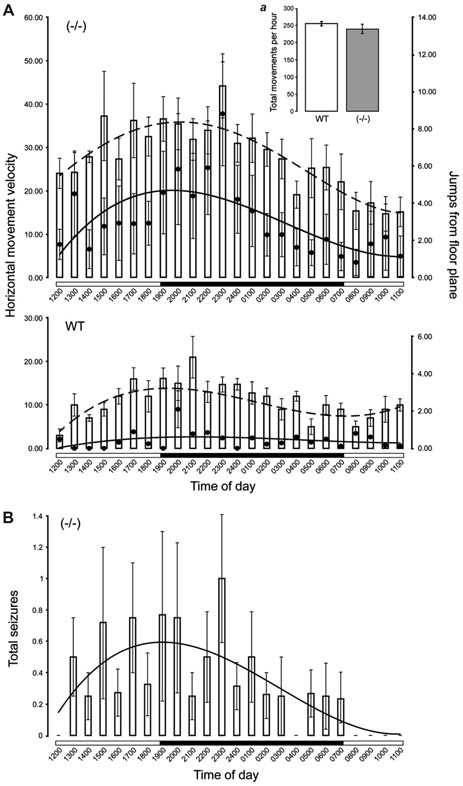
We first considered the behavioral variables from which the number and distribution of hypermotor seizures could be estimated. There were significant between-group differences in light phase and dark phase MV and JFP (Table 1). During both the light and dark phases, the Aldh5a1−/− mice exhibited higher levels of MV and JFP as compared with WT mice. Within-group comparisons indicated that only mutant mice exhibited higher MV during the dark phase (t5 = 3.71, P < 0.05), whereas WT mice exhibited higher JFP during the dark phase (t7 = 4.26, P < 0.05]. Repeated-measures analysis indicated there were also significant differences in MV and JFP between and within groups over time (MV,F[23,336] = 112.39, P< 0.01; JFP, F[23,336] = 84.12, P < 0.01). A post hoc analysis revealed significantly higher MV and JFP for the Aldh5a1−/− mice across all time points except 2000 and 0800 hours (JFP only)(Fig. 1A and B).
TABLE 1.
Summary of the Cosinor analysis of the daily rhythms of seizure behavior in adult Aldh5a1−/−and WT mice
| Variable | Group | N | Light phase | Dark phase | Mesor | Amplitude | Phase | Period (h) | R2 |
|---|---|---|---|---|---|---|---|---|---|
| MV | WT | 8 | 9.08 ± 2.14 | 11.17 ± 3.19 | 10.13 ± 2.65 | 5.67 ± 2.01 | −5.08 (1855) | 24.55 ± 0.78 | 0.43 |
| −/− | 6 | 24.42 ± 2.55b | 30.86 ± 2.06*,b | 27.23 ± 1.17b | 10.42 ± 1.65b | −4.16 (1950) | 25.26 ± 0.28 | 0.76b | |
| JFP | WT | 0.25 ± 0.02 | 0.57 ± 0.12* | 0.44 ± 0.071 | 0.17 ± 0.07 | −2.16 (2150) | 24.88 ± 0.33 | 0.16 | |
| −/− | 2.07 ± 1.06a | 3.89 ± 1.47b | 2.99 ± 0.22b | 2.04 ± 0.31b | −4.28 (1943) | 24.74 ± 0.19 | 0.72b | ||
| Seizures | −/− | 0.25 ± 0.05 | 0.42 ± 0.03 | 0.32 ± 0.06 | 0.24 ± 0.08 | −3.86 (2008) | 25.53 ± 1.02 | 0.73 |
Note. Data are expressed as means ± SEM. Phase data are expressed as hours before (−) subjective midnight with the clock time in parentheses. MV, movement velocity; JFP, jumps above the floor plane; WT, wild type. R2 = coefficient of determination.
P < 0.05.
P < 0.01 for between-group comparisons of rhythm parameters (ANOVA).
P < 0.05 for within-group comparisons of dark/light phase MV and JFP (two-tailed, paired sample t test).
Fig. 1.
Twenty-four-hour distributions of motor behavior and generalized tonic–clonic seizures in Aldh5a1−/− mice. (A) Daily patterns of movement velocity (MV, vertical bars) and jumps above the floor plane (JFP, filled circles) in Aldh5a1−/− (upper panel) and WT (lower panel) mice. Mutant mice exhibited significantly higher MV and JFP compared with the WT group across nearly all time points, but both groups of mice displayed similar levels of overall behavioral activity (i.e., total movements, inset a). (B) The distribution of spontaneous seizures in Aldh5a1−/− mice based on standardized MV (zMV). For each mouse, seizures for which the zMV scores were ≥2SD above their respective means were categorized as spontaneous hypermotor seizures.
The Cosinor analysis of MV and JFP is summarized in Table 1. For both MV and JFP, the mutant mice exhibited a significantly higher mesor (MV, F1,13 = 14.58, P < 0.01; JFP, F[1,13] = 11.95, P < 0.01) and amplitude (MV, F[1,13] = 17.32, P < 0.01; JFP, F[1,13] = 6.89, P < 0.01), whereas both groups of mice showed comparable peaks and periodicity in their MV and JFP profiles. However, in WT mice, the single-oscillator model provided poor fits of the MV and JFP rhythms to the cosine function compared with mutant mice. This suggested that the MV and JFP of WT mice varied little over the 24-hour photocycle (Table 1, Fig. 1B). There was no significant increase in the R2 of MV or JFP for WT mice using a two-oscillator model (not shown). Interestingly, the MV and JFP rhythms of Aldh5a1−/− mice appear phase-locked to one another, with peaks separated by only 7 minutes (i.e., 1950 hours for MV and 1943 hours for JFP). This suggested a strong temporal association between rapid locomotor activity and jumping.
It is important to note that despite significantly higher MV and JFP in mutant mice, both groups showed comparable levels of general behavioral activity (i.e., TM) (Fig. 1A, inset a),). Intuitively, higher MV would suggest that mutant mice moved around the arena at a faster rate, covering more distance and thus registering more TM. However, hypermotor seizures are invariably followed by a postictal depression of behavior, which can last anywhere from a few minutes to well over an hour (even after this period, the mutant mice still remain relatively immobile). So although each hypermotor seizure may be accompanied by a large increase in TM, these behavioral fits are infrequent so that overall both groups exhibit statistically equivalent levels of TM.
We next z-transformed MV data (zMV) for each Aldh5a1−/− mouse and classified data points that were ≥2SD above their respective mean as “probable seizures.” Using this method, we identified 42 seizures among 576 1-hour MV data bins. Nine of 42 (21%) hypermotor seizures were visually identified as such and corresponded to a >2SD increase in zMV. Though we did not visually confirm the remaining 79% of seizure events, fits of hyperactivity distinct from hypermotor seizures that might register in the movement sensor as a “probably seizure” simply do not occur in the mutant mice, which remain relatively inactive during interictal periods. Based on this method, seizures were found to occur with a higher frequency during the dark phase (Fig. 1B), with a peak at 2008 hours and a variable period of 25.53 ± 1.02 hours (Table 1). The single-oscillator model provided a good fit of the seizure rhythm to the cosine function (R2 = 0.73).
3.3. Technical considerations
It is possible to improve on the temporal resolution of this seizure detection method by decreasing the width of the data bins during which MV is calculated. Averaging MV at 60-minute intervals permits only a binary response to whether or not a seizure occurred during that 1-hour time frame and not their frequency. Therefore, the maximum number of seizures any one mouse could exhibit during the 24-hour day is 24. As a hypermotor seizure in Aldh5a1−/− mice typically lasts <20 seconds, the optimal bin width for seizure detection in this particular model might be between 20 and 30 seconds. However, from random visual observations we have found that if left undisturbed, the mutant mice seldom exhibit more than one behavioral event per hour that would satisfy our statistical criteria for a hypermotor seizure, but we do not exclude the possibility that seizures associated with a subtle motor component also occur. Our method estimates the daily occurrence of severe behavioral seizures that might pose a serious health risk in a clinical setting.
Also in this study, only the Aldh5a1−/− mice were maintained on the KD. This raises an important question regarding the possible effects of a high-fat diet in wild-type littermates and whether this dietary modification might have influenced the behavior of the mutants. Burnham and co-workers conducted a series of studies on the effects of different KD formulas on locomotor activity in different strains of rats [15,16]. Diets with fat:carbohydrate plus protein ratios of 6.3:1, 4:1 (present study also), and 3:1 resulted in a reversible decrease in exploratory activity unrelated to anxiety, sedation, or ataxia. Therefore, the relative inactivity of the mutants may be attributable to the diet itself, but lack of sedation accounts for why hypermotor seizures are still observed in their daily pattern.
4. Discussion
The Aldh5a1−/− mouse exhibits SSADH deficiency, brain GABA and GHB accumulation, motor dysfunction, and severe epileptic seizures [6–8]. In the present study adult Aldh5a1−/− mice maintained on the KD showed daily patterns of increased movement velocity and jumping behavior from which we derived a circadian rhythm of spontaneous hypermotor seizures. Cosinor analysis revealed that this seizure rhythm occurred with near-24-hour periodicity and an acrophase during the early dark phase (2008 hours).
The average age of Aldh5a1−/− mice used in our behavioral analysis was 100.51 ± 15.29 days. The study of adult mutant mice was possible only after maintaining them exclusively on the KD from weaning day (P20) onward [9,10]. Aldh5a1−/− pups that continue to suckle beyond P20 survive longer than their weaned mutant littermates (unpublished), suggesting that the high-fat dam's milk exerts a beneficial effect in this disorder. As Aldh5a1−/− mice exhibit impaired mitochondrial function [17], we have hypothesized that the KD enhances survival by providing an alternative energy source. Recently, the KD has been shown to increase the number and function of mitochondria [18]. Thus, the KD may aid in the restoration of impaired mitochondrial function in the mutant mice. This hypothesis remains to be tested.
Our group has recently identified changes in GABAA-R function that coincide with the development of epileptic activity in Aldh5a1−/− mice [6]. There was a progressive decrease in the binding of the GABAA-R antagonist t-butyl-bicyclo-[35S]phosphorthionate in cortex, hippocampus, and thalamus from P7 and P19, at which age the ongoing absence seizure activity evolves into tonic–clonic convulsions and lethal status epilepticus. There was also a significant reduction in expression of the β2 subunit of GABAAR and decreased hippocampal GABAA-R-mediated inhibitory postsynaptic potentials. Because Aldh5a1−/− mice exhibit increased brain GABA levels [19], a decrease in β2 subunit protein may relate to usedependent down-regulation of the GABAA-R due to prolonged occupancy of the ligand binding site. We propose that these region-specific decreases in GABAA-R function are at least a partial determinant of the seizure phenotype of Aldh5a1−/− mice, as seizure activity in forebrain structures would not account for running and jumping fits. Several strains of epileptic rodents exhibit wild running seizures [20]. Faingold and co-workers [21] have reported that during the initial running phase of audiogenic seizures in the genetically epilepsy-prone rat (GEPR-9), epileptiform discharges appear abruptly and are restricted to neurons in deep layers of the superior colliculus (DLSC), which then project to the spinal cord by way of the tectospinal tract to generate the wild running behavior. The DLSC is the recipient of GABAergic fibers originating in the substantia nigra pars reticulata (SNpr) [22]. Therefore, an inhibitory nigrotectal projection system may serve to exert control over the expression of wild running seizures. Deransart and coworkers [23] reported that bilateral injection of the GABAA-R agonist muscimol into the SNpr, which would effectively disinhibit the nigrotectal projection and as a result increase inhibition of DLSC neurons, decreased the duration of wild running clonus in audiogenic Wistar rats. Similarly, disinhibition of DLSC neurons by local application of the GABAA-R antagonist bicuculline protects against maximal electroshock-induced convulsions [24], limbic motor seizures [25], and absence seizures in GAERS rats [26]. These data suggest an important modulatory role for GABAA-Rs in the SNpr and DLSC against diverse seizure types. It stands to reason that impaired GABAA-R function within the nigrotectal projections system of Aldh5a1−/− mice would enhance DLSC excitability and render these animals susceptible to the caudal transfer of seizure activity into circuits involved with wild running behavior.
Daily variation in the frequency and severity of epileptic seizures in animal models has been described for kainic acid [27]- and pilocarpine [28]-induced motor seizures, and recently in AY-induced atypical absence seizures [29]. The purported involvement of the hippocampal formation in each of these seizure models suggests that its connectivity with the suprachiasmatic nucleus (SCN)[30], a region of the hypothalamus that regulates the expression of physiological and behavioral events in response to environmental time cues [31], may form the substrate for circadian rhythmicity in seizure activity. However, given that seizures in Aldh5a1−/− mice appeared to cluster during a time of day that would be associated with increasing levels of arousal (i.e., onset of the activity phase), daily variation in seizures might result from activity within a related neural circuit involving the SCN → dorsomedial hypothalamus → locus coeruleus (LC) pathway. This pathway regulates circadian rhythms in LC neuronal activity such that LC neurons fire significantly faster during the dark phase of the photocycle. This pattern of activity is thought to play an important role in the regulation of sleep–wake states [32]. Therefore, stimulation of cortical neurons by norepinephrine [33] during periods of increased LC activity may be sufficient to initiate seizure activity in the presence of dysfunctional GABAA-Rs, which then recruits additional forebrain structures and eventually propagates into the midbrain to trigger running behavior.
Taken together, these data suggest that the 24-hour distribution of spontaneous tonic–clonic seizures in adult Aldh5a1−/− mice is not random. Seizures occurred with greater frequency around the early dark phase, a time of day that corresponded to the onset of the animal's activity cycle and would likely be associated with increases in neuronal activation and arousal. This study will provide useful information for clinicians to better understand the temporal distribution of seizures associated with human SSADH deficiency and will aid in the development of more effective treatment strategies.
Acknowledgments
The authors thank Dr. Zhengping Jia and Dr. Lily Shen for assistance. This work was supported by the National Institutes of Health (NS 40270, K.M.G. and O.C.S.) and the Canadian Institutes of Health Research (O.C.S.). L.S.S. was supported by the Natural Sciences and Engineering Research Council of Canada (PDF-313950-2005). K.J.N. was the recipient of a Hospital for Sick Children Restracomp scholarship.
References
- 1.Cash CD, Maitre M, Mandel P. Purification from human brain and some properties of two NADPH-linked aldehyde reductases which reduce succinic semialdehyde to 4-hydroxybutyrate. J Neurochem. 1979;33:1169–75. doi: 10.1111/j.1471-4159.1979.tb05261.x. [DOI] [PubMed] [Google Scholar]
- 2.Chambliss KL, Hinson DD, Trettel F, et al. Two exon-skipping mutations as the molecular basis of succinic semialdehyde dehydrogenase deficiency (4- hydroxybutyric aciduria) Am J Hum Genet. 1998;63:399–408. doi: 10.1086/301964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson KM, Christensen E, Jakobs C, et al. The clinical phenotype of succinic semialdehyde dehydrogenase deficiency (4-hydroxybutyric aciduria): case reports of 23 new patients. Pediatrics. 1997;99:567–74. doi: 10.1542/peds.99.4.567. [DOI] [PubMed] [Google Scholar]
- 4.Gibson KM. Gamma-hydroxybutyric aciduria: a biochemist's education from a heritable disorder of GABA metabolism. J Inherit Metab Dis. 2005;28:247–65. doi: 10.1007/s10545-005-7053-4. [DOI] [PubMed] [Google Scholar]
- 5.Snead OC., 3rd Evidence for a G protein-coupled gamma-hydroxybutyric acid receptor. J Neurochem. 2000;75:1986–96. doi: 10.1046/j.1471-4159.2000.0751986.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Buzzi A, Frantseva M, et al. Status epilepticus in mice deficient for succinate semialdehyde dehydrogenase: GABAA receptor mediated mechanisms. Ann Neurol. 2006;59:42–52. doi: 10.1002/ana.20686. [DOI] [PubMed] [Google Scholar]
- 7.Hogema BM, Gupta M, Senephansiri H, et al. Pharmacological rescue of lethal seizures in mice deficient in succinate semialdehyde dehydrogenase. Nat Genet. 2001;29:212–6. doi: 10.1038/ng727. [DOI] [PubMed] [Google Scholar]
- 8.Cortez MA, Wu Y, Gibson KM, Snead OC., 3rd Absence seizures in succinic semialdehyde dehydrogenase deficient mice: a model of juvenile absence epilepsy. Pharmacol Biochem Behav. 2004;79:547–53. doi: 10.1016/j.pbb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–43. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- 10.Nylen K, Velazquez JLP, Likhodii SS, et al. A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp Neurol. 2008;210:449–57. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart LS, Shukla R, Serbanescu I, et al. Daily rhythms of seizure activity and behavior in a model of atypical absence epilepsy. Epilepsy Behav. 2006;9:564–72. doi: 10.1016/j.yebeh.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Wu CF, Liu W, Yang JY, Chen D. Sex difference in psychological behavior changes induced by long-term social isolation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:115–21. doi: 10.1016/j.pnpbp.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Teicher MH, Barber NI. COSIFIT: an interactive program for simultaneous multioscillator analysis of time-series data. Comput Biomed Res. 1990;23:283–95. doi: 10.1016/0010-4809(90)90022-5. [DOI] [PubMed] [Google Scholar]
- 14.Kastania AN, Bekakos MP. Multioscillator cosinor models for optimal curve-fit of time series data. Nonlinear Anal. 2001;47:2293–300. [Google Scholar]
- 15.Murphy P, Likhodii SS, Hatamian M, Burnham WM. Effect of the ketogenic diet on the activity level of wistar rats. Pediatr Res. 2005;57:353–7. doi: 10.1203/01.PDR.0000150804.18038.79. [DOI] [PubMed] [Google Scholar]
- 16.Murphy P, Burnham WM. The ketogenic diet causes a reversible decrease in the activity level of Long-Evans rats. Exp Neurol. 2006;201:84–9. doi: 10.1016/j.expneurol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Sauer SW, Kolker S, Hoffman GF, et al. Enzymatic and metabolic evidence for a region specific mitochondrial dysfunction in the brains of murine succinic semialdehyde dehydrogenase deficiency (Aldh5a1−/− mice) Neurochem Int. 2007;50:653–9. doi: 10.1016/j.neuint.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 19.Gupta M, Polinsky M, Senephansiri H, et al. Seizure evolution and amino acid imbalances in murine succinate semialdehyde dehydrogenase (SSADH) deficiency. Neurobiol Dis. 2004;16:556–62. doi: 10.1016/j.nbd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Ross KC, Coleman JR. Developmental and genetic audiogenic seizure models: behavior and biological substrates. Neurosci Biobehav Rev. 2000;24:639–53. doi: 10.1016/s0149-7634(00)00029-4. [DOI] [PubMed] [Google Scholar]
- 21.Faingold CL, Randall ME. Neurons in deep layers of superior colliculus play a critical role in the neuronal network for audiogenic seizures: mechanisms for production of wild running behavior. Brain Res. 1999;815:250–8. doi: 10.1016/s0006-8993(98)01136-6. [DOI] [PubMed] [Google Scholar]
- 22.Depaulis A, Vergnes M, Marescaux C. Endogenous control of epilepsy: the nigral inhibitory system. Prog Neurobiol. 1994;42:33–52. doi: 10.1016/0301-0082(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 23.Deransart C, Le-Pham BT, Hirsch E, Marescauz C, Depaulis A. Inhibition of the substantia nigra suppresses absences and clonic seizures in audiogenic rats, but not tonic seizures: evidence for seizure specificity of the nigral control. Neuroscience. 2001;105:203–11. doi: 10.1016/s0306-4522(01)00165-8. [DOI] [PubMed] [Google Scholar]
- 24.Dean P, Gale K. Anticonvulsant action of GABA receptor blockade in the nigrotectal target region. Brain Res. 1989;477:391–5. doi: 10.1016/0006-8993(89)91434-0. [DOI] [PubMed] [Google Scholar]
- 25.Gale K, Pazos A, Maggio R, Japikse K, Pritchard P. Blockade of GABA receptors in superior colliculus protects against focally evoked limbic seizures. Brain Res. 1993;603:279–83. doi: 10.1016/0006-8993(93)91248-q. [DOI] [PubMed] [Google Scholar]
- 26.Depaulis A, Liu Z, Vergnes M, Marescaux C, Micheletti G, Warter JM. Suppression of spontaneous non-convulsive seizures in the rat by microinjection of GABA antagonist into the superior colliculus. Epilepsy Res. 1990;5:192–8. doi: 10.1016/0920-1211(90)90038-w. [DOI] [PubMed] [Google Scholar]
- 27.Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainite-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- 28.Stewart LS, Leung LS, Persinger MA. Diurnal variation in pilocarpine-induced generalized tonic–clonic seizure activity. Epilepsy Res. 2001;44:207–12. doi: 10.1016/s0920-1211(01)00192-9. [DOI] [PubMed] [Google Scholar]
- 29.Stewart LS, Bercovici E, Shukla R, et al. Daily rhythms of seizure activity and behavior in a model of atypical absence epilepsy. Epilepsy Behav. 2006;9:564–72. doi: 10.1016/j.yebeh.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus in the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- 31.Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–19. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- 32.Aston-Jones G, Chen S, Zhu Y, Oshinsky MI. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 33.Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci BioBehav Rev. 1998;22:243–57. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]