Abstract
This review makes the case that gene discovery is a worthwhile approach to the study of ingestive behavior in general and to calcium appetite in particular. A description of the methods used to discover genes is provided for non-geneticists. Areas covered include the characterization of an appropriate phenotype, the choice of suitable mouse strains, the generation of a hybrid cross, interval mapping, congenic strain production, and candidate gene analysis. The approach is illustrated with an example involving mice of the C57BL/6J and PWK/PhJ strains, which differ in avidity for calcium solutions. The variation between the strains can be attributed to at least seven quantitative trait loci (QTLs). One of these QTLs is most likely accounted for by Tas1r3, which is a gene involved in the detection of sweet and umami tastes. The discovery of a novel function for a gene with no previously known role in calcium consumption illustrates the power of gene discovery methods to uncover novel mechanisms.
Keywords: Calcium appetite, QTL, Gene discovery, Taste preference, Tas1r3, Sweet, Review
1. Introduction
This review is based on a talk given at the annual meeting of the Society for the Study of Ingestive Behavior (SSIB), in Steamboat Springs, Colorado, in July 2007. Two aspects of the talk were unusual for members of the Society: First, was the topic; second, was the use of gene discovery methods. My goal here is to lay out for the non-geneticist the steps involved in gene discovery, together with some of the advantages and disadvantages of the approach. I will use my work on calcium intake as an example but the same methods can be applied to just about any ingestive behavior. For those looking for general overviews of the physiological and behavioral controls of calcium consumption, a comprehensive review [84] and a book [75] describes the animal literature, and there is a book chapter outlining some examples of calcium appetite in humans [85].
Before describing the methods used to discover genes, it is worth considering why calcium appetite has been largely ignored in the past and why the gene discovery method is a particularly appropriate way to approach it.
1.1. Why so little research on calcium appetite?
According to a quick count of abstracts of presentations at the 2007 SSIB meeting [53], there were 227 presentations on food intake or obesity, 12 on sodium or water intake, and only one on calcium intake. I doubt this is a bias of the Society or its membership; it is fairly typical of the field of ingestive behavior in general. According to PubMed, in 2007, there were published 9515 papers on “obesity”.1515 papers on “food intake”, 22 papers on “sodium appetite”, and none on “calcium appetite” [54].
Why is the large preponderance of research on ingestive behavior focused on food rather than salt or calcium? The first thing that comes to mind is that food intake and obesity may be bigger clinical problems than either sodium or calcium intake. There are, after all, an estimated 59 million obese Americans and another 65 million overweight. However, this argument by incidence is a misperception because 58 million Americans have hypertension [34], at least some of which is linked to high sodium intakes, and 44 million Americans have low bone mass or osteoporosis [55], at least some of which is linked to low calcium intakes. Moreover, low calcium intakes have been implicated in several other diseases, including hypertension, obesity, premenstrual syndrome, and various cancers. Thus, if research effort were to be dictated solely by disease prevalence some emphasis on studying energy intake would be warranted but not anywhere near the current level.
Instead, I believe the bias is part historical but largely irrational. It is easy to see that eating too much food will lead to obesity but not so easy to see the connection between mineral intake and hypertension or osteoporosis. Of course, simply because the connection is not obvious does not imply it is not important. More tellingly, obesity is historically considered a disease of energy balance (energy in-energy out) but hypertension has historically focused on the kidney, and osteoporosis on bone. I make it a habit to inspect the schematic diagrams of calcium homeostasis provided in textbooks. Many include sources of calcium loss (i.e., fecal and urinary loss, and sweat) but I have yet to find one that shows where this calcium enters the body. It seems strange that few would consider obesity as entirely a disease of energy loss whereas this is the norm for diseases of mineral homeostasis.
Another complication, at least in my opinion, is that research on the controls of sodium intake has lost contact with its clinical implications. Early work in this field emphasized the uniqueness of sodium appetite, particularly in contrast to the appetites for other minerals and vitamins. An unfortunate consequence of this was to dissuade investigation of calcium and other appetites. I have written about this elsewhere [84] so will not repeat it here. More recently, the main focus of sodium consumption research has been on controls exerted by the rennin-angiotensin-aldosterone system. This too is unfortunate because there is little evidence to suggest that circulating levels of angiotensin or aldosterone are relevant for human sodium consumption [15]. The evidence suggests that the rennin-angiotensin-aldosterone system is crucially involved in the motivation to ingest sodium during sodium deficiency but the human problem is one of avoiding, not finding, sodium [see [82] for discussion].
1.2. Why use a gene discovery approach to study calcium appetite?
There are three factors that have led my group to approach the study of calcium appetite using a genetic approach. The first is that we have tried other approaches and, although they have been useful, they reveal how things can get complicated very easily. For example, a seemingly simple question is whether 1,25-dihydroxyvitamin D, the active metabolite of vitamin D, effects calcium intake of rats. The “obvious” approach is to infuse 1,25-dihydroxyvitamin D and observe the behavior. However, 1,25-dihydroxyvitamin D levels influence parathyroid hormone and calcitonin production, and these hormones may influence behavior, either by direct action on the brain or indirectly, by altering circulating calcium concentrations. Thus, parathyroid hormone and calcitonin must be controlled and the only effective way to do this is to remove the parathyroid and thyroid glands and infuse replacement doses. An unintended consequence of destroying the thyroid glands is that this removes the source of thyroxin, which also influences calcium metabolism, so this hormone must be replaced as well. There is another problem because 1,25-dihydroxyvitamin D influences calcium absorption from the gut, which in turn will influence circulating calcium concentrations, so the infused rats need special diets formulated so that final circulating calcium concentrations are normal. The original question is simple but the experiment needed to test it requires thyroparathyroidectomized rats receiving four hormone infusions simultaneously and a special diet. It would be necessary but technically formidable to replicate the circadian dynamics of each hormone, and even if this could be done it would be difficult to ensure the result would tell us much about normal behavior.
The second reason to use a genetic approach is that there is very little already known about the physiological controls of calcium appetite. There are hypotheses concerning circulating calcium levels, calcium-regulatory hormones, aching bones, and changes in taste sensitivity, but none that prove completely compelling [reviewed in [84]]. With virtually a blank slate, it is difficult to know where to look first One of the strongest advantages of using a gene discovery approach is that it is, at least in the early stages, hypothesis-free. The results suggest the hypotheses, not the other way around.
The third factor that motivated us to take a gene discovery approach was the fortunate coincidence of time and place. The main field of ingestive behavior appears to have overlooked the gene discovery approach but there are three sub-fields that are exceptions: the study of taste receptors, alcohol consumption, and obesity genes. All three of these areas have been explored by my colleagues at Monell and their successes are clear [e.g., [3,4,6,64,65]]. Moreover, starting a gene discovery project even 10 years ago would have been difficult because of the limited number of polymorphic markers available and the high costs of genotyping. Now, there are large databases of markers and the costs of genotyping have dropped precipitously.
2. The gene discovery approach to study calcium appetite
The distinguishing feature of the gene discovery approach is the use of a genome screen to discover quantitative trait loci (QTLs) but to conduct a successful genome screen requires preparation, and to interpret the results requires follow up. Consequently, there are several steps involved, which are summarized below.
2.1. Choosing a phenotype
One of the most crucial decisions to be made at the start of a gene discovery program is to determine the trait or traits to measure. The gene discovery method relies on differences between individuals (or groups of identical individuals), and it is an unfortunate paradox that to study individuals requires large numbers of subjects. The project described here involved phenotyping over 1500 mice, and many projects in the “taste” or “alcohol” fields have involved more. With 1500 subjects, adding just 10 min of work to test each mouse translates into about 7 weeks of work for a full-fime technician. To avoid heroic efforts, it is crucial that simple and fast measurements are used. The trade off is that, in general, the shorter the test the less accurate and less reliable it is. There is a delicate balance between simplicity and reliability.
For this project, we chose to use either 48-h or 96-h two-bottle choice tests. For these, the mice received two drinking tubes, one containing water and the other a “taste solution”. Using 96-h tests seems contrary to the advice just given to use short tests but in fact the time required by the experimenter to conduct the test is ~3 min per mouse (i.e., place the tubes on the cage, read the fluid levels, 24 h later switch tube positions, 24 h later read fluid levels, etc.) and there is an economy of scale because several hundred mice can be tested in parallel in their home cages, without special equipment.
“Taste solution” here is used for convenience; the tests were 48-or 96-h long (depending on the experiment) so there was plenty of opportunity for olfactory, trigeminal and postingestive effects to influence intakes. There are “purer” phenotypes but the inclusion of non-gustatory factors is not necessarily a disadvantage if the goal is to look for genes underlying calcium appetite, which undoubtedly encompasses more than just gustatory effects. We considered and rejected using a gustometer or lickometer because these require mice to be water-deprived (in order to drink on demand), which adds a complex variable and, at least in our hands, the tests have low reliability. We also considered measuring calcium intake after mice were deprived of calcium but this required time-consuming procedures. It is difficult to calcium-deprive a mouse without special housing because there is calcium in the bedding, urine and cage walls of home cages.
A phenotype is the product of genes and environment. In order to discover genes it is crucial to maximize the variation due to genes and minimize the variation due to the environment. We therefore invested considerable effort in investigations of the environmental factors that influence preferences in two-bottle choice tests. For example, one of the problems with the two-bottle test is that most strains of mice prefer to drink from the bottle to their left [5] so that a preference may result from either a solution’s taste or its spout position. The standard way to control for this is to switch drinking spout positions half way through a test, which for practical purposes means that a test must be at least 48-h long because switching earlier splits a diurnal cycle and intakes differ dramatically at different times of the day. To investigate ways to avoid this, we conducted parametric comparisons using two strains of mice (C57BL/6J and 129X1/SvJ). We found that when these mice received two drinking tubes of water under our standard conditions, with spouts ~2-cm apart, the B6 strain showed no side preference but the 129 strain strongly preferred the left. This side preference did not depend on where food was placed in the cage but it could be eliminated by placing the drinking tubes 8- or 16-cm apart [90]. There were two other methods we found to eliminate or control for side preferences. The first was to arrange the drinking tubes vertically; mice drank equal volumes of water from spouts stacked one above the other at 2.5- and 4.5-cm above the cage floor [90]. The second was to provide three drinking tubes with the taste solution either in the center or in both outer tubes [91]. Despite finding these improved methods, we decided that the cost of modifying cages to accept non-standard placement of drinking tubes, and the resistance to change we experienced from reviewers, outweighed the advantages and so all our tests were conducted using drinking tubes placed ~2 cm apart with their positions switched every 24 h.
Based on studies with B6 and 129 mice, we found that the optimal duration for a taste preference test was either 48 h or 96 h, depending on the taste solution being used, and that counterintuitively, longer (144-h) tests were sometimes less sensitive than shorter tests [88]. Based on this series of studies, we chose to use for subsequent work 96-h tests in most cases but 48-h tests if we gave several concentrations of the same taste compound. In concentration-preference studies, the additional benefit of multiple tests was sufficient to outweigh the slight advantage of 96-h over 48-h tests. We also compared the taste preferences of mice fed with six different maintenance diets (four forms of cereal-based “chows” and two semisynthetic diets) and found that diet had little concerted effect on the variability of most two-bottle choice tests, although in some cases it had a large influence on strain means [[94]; see also [86]]. Based largely on the fact that the ingredients of chow diets are unknown and variable, and thus experiments using them cannot be replicated, we decided the best diet for our purposes was AIN-76A [1,32], a semisynthetic diet recommended by the American Institute of Nutrition (now called the American Society for Nutrition). There are “improved” versions of this diet [AIN-93G and AIN-93M; [66]] but our experience is that mice spill more of these than the AIN-76A diet, making them inconvenient to use when food intake measurements are required.
Since the project involved breeding mice, and this can be fickle, it was not always possible to have large batches of offspring of the same age available all at one time. To determine if differences in age were likely to influence taste preferences we tested B6 and 129 mice aged between 6 and 125 weeks. We found that, with few exceptions, age made no difference to taste preferences [86].
Having determined that for our project, the two-bottle test was the method of choice, and refined the method as much as we could, the next step was to determine what taste solutions to test. The use of multiple tests in the same animal forces a balance between efficient design and the possibility that early tests will confound later ones. The problems of within-subject designs are well known, and they are a consideration with taste preference tests. In particular, there is a “concentration” effect such that mice tested with a series of progressively increasing concentrations of a taste solution drink more of the solution than do mice given the same solutions in progressively decreasing order of concentration [e.g., [11]]. These and other carryover effects can cloud interpretation. To investigate this, we gave groups of B6 or 129 mice a series of four 48-h two-bottle choice tests (2 mM saccharin, 5 mM citric acid, 30 μM QHCl and 75 mM NaCl) and compared the results to those of separate groups of mice given each of the latter three solutions for the first time. Prior experience with these solutions in this order did not influence solution intakes or preferences. In similar work, we demonstrated that there were no carryover effects after drinking various concentrations of CaCl2 or calcium lactate (CaLa). Nevertheless, to be sure, in genetic experiments, we always interposed at least one day with just water to drink between tests with different taste compounds. We also gave all mice their taste solutions in the same order so that any carry-over effects affected each mouse to the same extent.
2.2. Choosing the mice
Most of our work on this project so far involved mice of the C57BL/6J and 129X1/SvJ strains, which we chose for several reasons. One of the most important was that we have used the C57BL/6ByJ and 129P3/J mice in several previous studies to investigate the genetic basis of sweet, salt, umami, and alcohol consumption [e.g., [4,7–10,12,13]]. The switch from C57BL/6ByJ to C57BL/6J was made because there are few differences between these substrains and the C57BL/6J substrain was the one used to sequence the mouse genome; it is now used almost universally in genetics experiments. On the other hand, there are much larger differences between various 129 substrains [27,76]. We switched from the 129P3/J to 129X1/SvJ substrain for the practical reason that when we started the method validation experiments, 129P3/J mice were unavailable.
Ideally, parental strains for gene discovery should have large phenotypic differences. The larger the strain difference, the more likely there are to be genes with large effects (or lots of genes with small effects). Unfortunately, the B6 and 129 strains did not differ much in calcium preferences [e.g., [86]]. This does not necessarily negate their use, but it does suggest that other strains might be more suitable. To assess this, we conducted two strain screens. The first involved male mice of 28 strains, which were chosen because (a) they were genetically diverse, (b) they had been used in behavioral studies previously, and (c) they were relatively easy to obtain. The primary goal of this experiment was to find mice differing in NaCl preference so they were tested with several concentrations of NaCl and KCl before CaCl2 (3, 10, 30 and 100 mM), and then with several concentrations of NH4Cl [2,5]. As expected, there were some large differences in preferences between the strains particularly for the 10 and 30 mM CaCl2 concentrations, but none of the strains showed a strong liking (i.e., preferences higher than 50%) for any concentration of calcium.
The second strain screen involved 10 males and 10 females of 40 strains [62,92,93]. The 40 strains form the core of the Mouse Phenome Project (MPD), which is an ambitious attempt to populate a database with phenotype information about inbred strains. It allows comparison between strains across phenotypes and, more recently, the database has added single nucleotide polymorphism (SNP) data, which are useful for choosing markers (see below), and as a tool for gene discovery using strain association methods. The 40 core strains were chosen by MPD staff and consultants primarily on the basis of genetic diversity.
Our experiment was designed specifically to investigate calcium preferences so it involved tests of three concentrations of CaCl2 and CaLa (7.5, 25 and 75 mM in each case) as well as three concentrations of NaCl and sodium lactate (25, 75 and 225 mM in each case). This 40-strain survey revealed much larger strain differences in calcium preferences than did our earlier 28-strain survey. One reason for this was that most of the additional strains tested were “wild-derived”; they came from more diverse genetic backgrounds than did most of the strains used in the 28-strain survey. Genetic diversity can be assessed by consulting mouse genealogies based on either historical records [16] or SNPs [60,79]. The more diverse the ancestry, the more opportunity for phenotypic differences to accrue.
A typical result of the 40-strain screen is shown in Fig. 1; more complete descriptions are available elsewhere [89,92]. The PWK strain had the highest preference scores for all three concentrations of CaCl2 and CaLa tested. In addition to the phenotype, there were at least three other reasons to consider the PWK strain as a good “calcium-liker” strain for genetic analysis. First, in contrast to most laboratory strains, which are Mus musculus domesticus, it belongs to the Mus musculus musculus subspecies. This difference suggests relatively ancient divergence from most other strains and thus plenty of opportunity for genetic polymorphisms to have accrued. Second, a strain closely related to the PWK strain, the PWD/Ph strain, has been used as the basis of a set of B6-chr nPWD consomic strains. Consomic strains (also called chromosome substitution strains) are mice that contain all genetic material from one strain (in this case the B6 strain) except for one chromosome (which in this case comes from the PWD strain). These animals have the potential to be a powerful tool for genetic and phenotypic analysis, not least because they can be used as a short-cut to producing congenic mice (see below). The third advantage of the PWK strain is arguably the most important from a practical standpoint: it breeds easily.
Fig. 1.
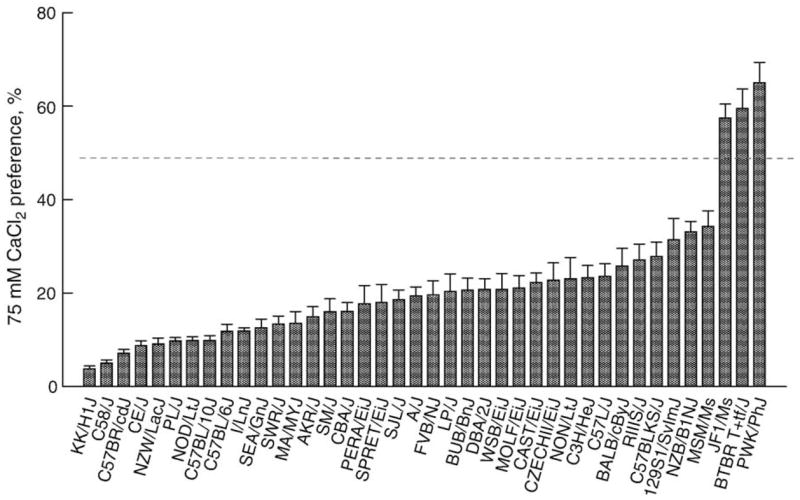
Mean ± SE preferences for 75 mM CaCl2 solution of 40 inbred strains of mice (n=0 males and 10 females per group). Note that three strains (JFl/Ms, BTBRT+ tf/J and PWK/PhJ) have preferences that were (a) above indifference (dotted line) and (b) notably higher than the other 37 strains.
The PWK strain is not without its problems. Male offspring from crosses between PWK mothers and domesticus fathers are sterile [33,77], which can complicate genetic analyses (see below). Inbred PWK mice are generally in short supply from the commercial breeder (JAX) and are relatively expensive (~$75/mouse). They are also less tame than many other strains. Animal care staff refer to them as “popcorn mice” which aptly describes their habit of leaping when the lid of their cage is removed. Another problem is their small size compared to most domesticus mice. A practical consideration is that young PWK mice require special cage lids because they can squeeze between the bars of a standard mouse cage. Their small size also limits the volume of blood or tissue that can be collected for physiological analyses.
Two other strains, the BTBR T+ tf/J and JF1/Ms, also had notably high calcium preferences (Fig. 1). The remaining 37 strains had a more-or-less continuous distribution of preferences. We could potentially have used any of these 37 as a “low-calcium-liker” strain for subsequent genetic analyses and chose the B6 strain, the advantages of which are given above. Out of the 40 strains tested, it ranked between 22nd and 34th in calcium preference, depending on the salt and concentration.
It is worth noting in passing that very few of the 40-strains surveyed had sex differences in calcium or sodium preference. This is surprising given several observations that female “rats” prefer higher concentrations of these minerals than do male “rats” [e.g., [23,73]]. I emphasize “rats” because most of this work has been with just one strain (Sprague Dawley) and the sex differences may well be strain-specific rather than species-specific. Irrespective of the underlying cause, the lack of sex differences is a strong advantage for genetic research because statistical power can be obtained from all offspring combined rather than smaller groups of males or females.
2.3. Comparison of parental strains
Another problem with using the PWK strain was that there was nothing known about its taste preferences [or, for that matter, virtually any behavior; see [33,79]]. Before using the PWK strain in a QTL study, it seemed worthwhile determining whether the avidity for calcium was specific. To do this, we gave series of 48-h two-bottle choice tests to several squads of mice, with each squad containing 10 B6 and 10 PWK male mice aged 7–9 week old. As a confirmation of the strain survey, we tested CaCl2 and CaLa. These salts were selected because they are soluble and both have been used to monitor calcium intake previously [e.g., [68,69,80,81]]. To determine whether strain differences in calcium preference extended to other minerals, we also tested MgCl2, NaCl, and sodium lactate. Human psychophysical research indicates that the predominant taste qualities of CaCl2 and CaLa are bitter and sour [83] so three representative bitter compounds (denatonium benzoate, quinine hydrochloride, and caffeine) and two representative sour compounds (HCl and citric acid) were tested. We also tested a representative sweet compound (saccharin) and umami compound (inosine monophosphate). Concentrations of each compound were chosen based on previous work in rats [e.g., [24,81]] and mice [e.g., [2,87]] with the aim of spanning the range from indifference to strong avoidance.
Relative to B6 mice, PWK mice had higher preferences for all concentrations tested of CaCl2, CaLa, and MgCl2 (Fig. 2). The PWK strain also had higher preferences for saccharin than did the B6 strain, but post hoc tests could not identify at which concentrations differences existed. The B6 and PWK strains responded similarly to all the other compounds tested. Based on these tests, it appears that the PWK strain has a specific avidity for calcium and magnesium, as well as an enhanced liking for sweetness. Of course, additional tests would provide a more detailed characterization but the important point is that the PWK mice show some selectivity in what they like; they do not drink more of everything.
Fig. 2.
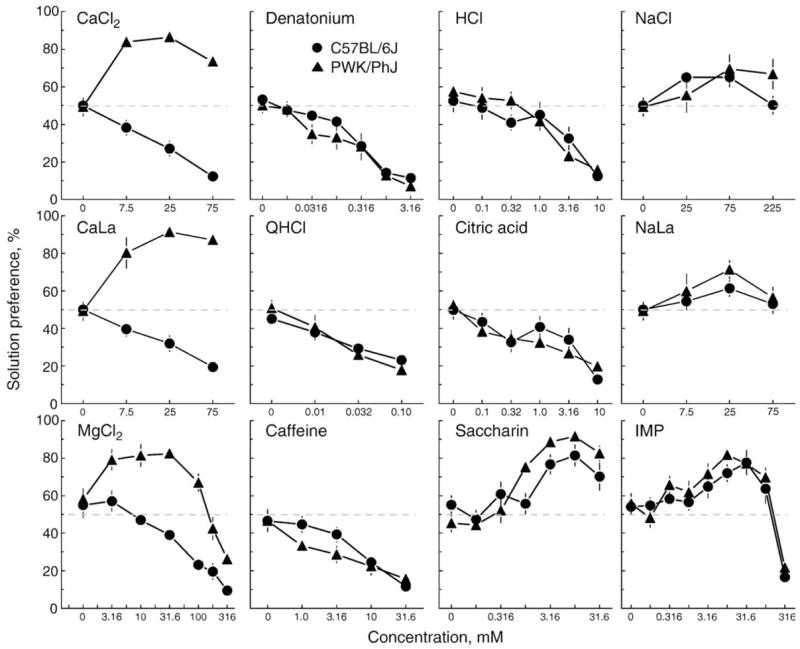
Preferences of male C57BL/6J and PWK/PhJ mice for various taste solutions. CaLa = calcium lactate, QHCl = quinine hydrochloride, NaLa = sodium lactate, IMP = inosine monophosphate.
2.4. Generation of segregating hybrids
The results of the comparisons of B6 and PWK mice were sufficiently interesting to warrant conducting a QTL study. We bred F2 hybrid mice using a standard mating scheme (Fig. 3) with allowance for the sterility of B6×PWK male F1, mice with PWK mothers [77]. To begin, female B6 mice were mated with male PWK mice, and female PWK mice were mated with male B6 mice to produce B6×PWK F1, hybrids. When adult, the male F1 hybrids with B6 mothers were mated with female F1 mice that had either strain as their mother to produce B6×PWK F2 hybrids. Although not necessary for QTL analysis, we also bred additional B6, PWK, and B6×PWK F1, mice so that mice of each genotype could be tested alongside the F2 mice. In total, we bred and phenotyped 31 B6, 29 PWK, 62 F1, and 484 F2 mice.
Fig. 3.
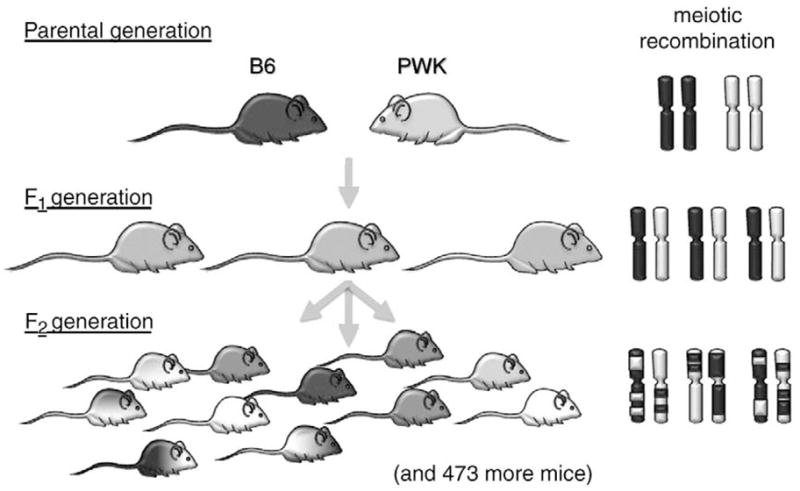
Representation of the mating scheme used to generate mice for a genome scan. C57BL/6J (B6) and PWK/PhJ (PWK) mice were mated to produce F1 mice and these were mated brother-to-sister to produce 484 F2 mice. Coat color is used to represent any phenotype (in fact, the B6 are black and PWK agouti). On the right is shown pairs of chromosomes from individual mice that are shaded based on parental origin. Note that all mice in the parental and F1 generations are isogenic but F2 mice are essentially random combinations of the B6 and PWK genomes. This is reflected in the phenotype which is expressed to varying degrees depending on whether the F2 mouse has inherited alleles that promote or detract from the phenotype. Interval mapping is used to associate the phenotype with particular locations in the genome.
Seven batches of 18–132 mice were tested over a 12-month period. Mice were weaned at 21–23 days old and housed in same-sex groups until aged 6–8 weeks when they were transferred to individual cages for taste phenotyping. After 7 days to adapt to their new housing, each mouse received a series of ten 96-h two-bottle choice tests with the following compounds in the order listed: 50 mM CaCl2, 50 mM CaLa, 50 mM MgCl2, 100 mM KCl, 100 mM NH4C1, 100 mM NaCl, 5 mM citric acid, 30 μM quinine hydrochloride (QHCl) and 2 mM saccharin.
The results of these tests are described in detail elsewhere [95]. Correlations between traits in the F2 mice can provide useful information about common genetic mechanisms. Differences in the parental strains between two phenotypes could be due to pleiotropy (that is, one gene having multiple effects; e.g., a body size gene that might also influence food intake) or to separate genes that happen to have been fixed during strain development (e.g., body size and coat color). In the F2 generation, meiotic recombination causes the association of phenotypes due to separate genes to be broken (Fig. 2). This is illustrated by the correlations between solution preferences. There were relatively strong correlations between CaCl2, CaLa, and MgCl2 preferences (r = 0.63–0.68), which is consistent with a common mechanism underlying these traits. Correlations between preferences for these compounds and preferences for the exemplars of saltiness (NaCl) sourness (citric acid), bitterness (QHCl), and sweetness (saccharin) were much lower (r = −0.14–0.36). Correlations between preferences for calcium-magnesium compounds and NH4Cl or KCl fell in between (r=0.31–0.39). Multidimensional scaling gives a view of the relationship between preferences for different taste solutions (Fig 4).
Fig. 4.
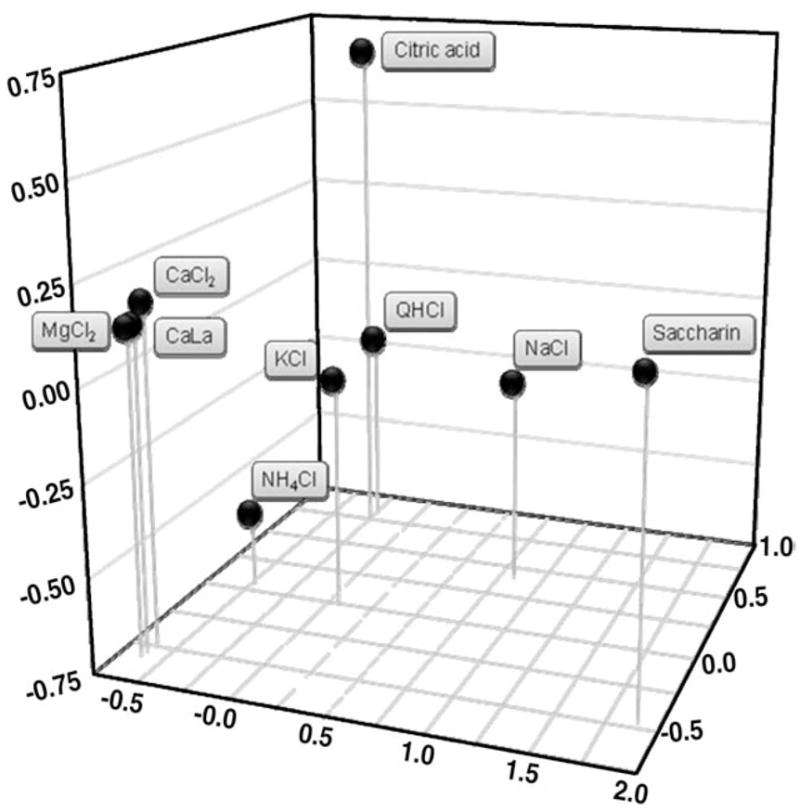
Three-dimensional solution of correlation coefficient matrix based on taste preferences of 484 B6 × PWK F2 mice. Values in each dimension are arbitrary units. The distance between taste solutions gives an idea of common mechanisms. CaCl2, CaLa, and MgCl2 fall close together which is consistent with a common underlying mechanism for calcium-magnesium taste. This cluster is distinct from the mechanisms underlying preferences for saltiness (NaCl), sourness (citric acid), bitterness (QHCl), and sweetness (saccharin). Preferences for NH4Cl and KCl fall between these extremes [redrawn from [95]]A
2.5. Genome screen
The next step in gene discovery is to determine each F2 mouse’s genotype. In an ideal world, or at least in just a few years, it may be possible to sequence the entire genome of each mouse, but for the time being this would be prohibitively expensive and is not necessary. For one thing, there is no point in looking at genomic regions that the two parental strains share in common because a region cannot be responsible for phenotypic variation if there is no genetic variation. In practice, this still leaves many different genetic loci, far more than can be sequenced economically. Instead, we resort to the use of polymorphic markers, which are regions of the genome that are known to differ between the parental strains. These markers have been discovered specifically for mapping or during sequencing efforts and they can be found in on-line databases [e.g., [79]]. In this project, we used as markers simple sequence length polymorphisms (SSLPs) but recently the technology has switched to single nucleotide polymorphisms (SNPs), which are more abundant and more conducive to detection by robotic methodologies so cheaper to genotype. In our genome scan, there were 4–10 markers on each of the 19 autosomes (non-sex chromosomes) of the mouse, giving a total of 117 markers with average spacing of 9.9 cM and no gaps greater than 26 cM. Genotyping all the 484 F2 mice at all 117 markers was a major undertaking (a total of 59,000 genotypes) that required the assistance of a dedicated laboratory [Center for Inherited Disease Research (CIDR)].
To link genotype with phenotype, we used statistical software designed for this purpose (MAPMAKER, J/QTL or R/qtl). This software produces interval maps of each chromosome showing the logarithm of odds (LOD) that a given locus is associated with the phenotype; the higher the LOD score, the more likely the underlying genetic region is responsible for a component of the phenotypic variation. Calculating the strength of this relationship for each marker is straightforward; the mice can be assigned to one of three groups by genotype (B6/B6, B6/PWK, or PWK/PWK) and the phenotypes of these groups compared, either by one-way ANOVA or by correlation (i.e., correlating the phenotype value with 0,1, or 2 PWK alleles). However, the interval mapping software is more powerful than this. It allows estimation of the strength of linkage not only at markers but between them by taking into account the rate of recombination between markers. Significant association or linkage between a genomic region and a phenotype is called a quantitative trait locus (QTL). Interval maps for several phenotypes are shown in Fig. 4 and a summary of QTLs is given in Table 1.
Table 1.
List of markers associated with QTL peaks for calcium and magnesium preference, with LOD scores and genotype means
| QTL ID | Position
|
Candidate gene | Solution | LOD score | %variance | Mean±SE preference, %
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Mb | Marker | B6/B6 | B6/PWK | PWK/PWK | |||||
| Drinkcacl24 | 1 | 104 | D1Mit1001 | Unknown | CaCl2 | 4.3** | 4.4% | 28±1a | 36±1b | 35±lb |
| Drinkmgcl24 | MgCl2 | 3.4* | 3.6% | 35±2a | 43±1b | 39±2a | ||||
| Drinkcala3 | 2 | 153 | D2Mit285 | Unknown | CaLa | 3.5* | 4.6% | 49±2b | 40±2a | 40±2a |
| Drinkmgcl23 | MgCl2 | - | 1.8% | 44±2b | 38±2a | 40±2a | ||||
| Drinkcacl21 | 4 | 155 | rs13478075 | Tas1r3 | CaCl2 | 5.7** | 7.1% | 27±2a | 34±1b | 40±2c |
| Drinkcala1 | CaLa | 6.8** | 10.1% | 34±2a | 42±2b | 51±2c | ||||
| Drinkmgcl21 | MgCl2 | 12.6** | 12.6% | 30±2a | 39±1b | 51±2c | ||||
| Drinksac1 | Saccharin | 8.9** | 10.3% | 83±1b | 83±1b | 74±1a | ||||
| Drinkmgcl25 | 10 | 24 | D10Mit86 | Unknown | MgCl2 | 5.7** | 5.4% | 33±2a | 40±1b | 45±2c |
| Drinkcacl23 | 10 | 92 | D10Mit95 | Unknown | CaCl2 | 4.3** | 4.2% | 29±1a | 34±1b | 38±1c |
| Drinkcacl25 | 11 | 90 | D11Mit285 | Unknown | CaCl2 | 4.3** | 4.1% | 37±2C | 35±1b | 28±1a |
| Drinkcacl22 | 16 | 33 | D16Mit60 | Casr | CaCl2 | 3.5* | 3.8% | 29±1a | 34±1b | 38±2C |
| Drinkcala2 | CaLa | 8.0** | 11.0% | 33±2a | 43±2b | 49±2C | ||||
| Drinkmgcl22 | MgCl2 | - | 2.2% | 36±2a | 39±la | 43±2b | ||||
Notes:
p<0.05,
p<0.01.
LOD values with a dash are non-significant for preference but significant for MgCl2 intake. %variance = percent of phenotypic variance accounted for, based on SSbetween/SStotal. Means±SE preference refers to the values for each genotype at the marker closest to the peak of the QTL. Different superscripts show significant differences, p<0.01. n’s=~230 for B6/PWK group and ~115 for B6/B6 and PWK/PWK groups. Both sexes are combined (there were no sex differences). (data extracted from Ref. 95)
2.6. Fine mapping and congenic strain production
The genome scan of CaCl2, CaLa, and MgCl2 preference revealed seven genetic loci of interest, and each region can contain several hundred genes. The exact number of genes under a QTL peak depends on gene density (which differs in different regions of the genome) and on the criterion used to limit the range. Typically, the QTL region is considered to encompass everything within a 1-or 1.5-LOD drop from the peak, but this is arbitrary. Depending on which criterion is used, the genome scan eliminated as causes of the variability in calcium preference around 24,000–25,000 of the estimated 25,613 genes in the mouse genome [17]. This is an important advance but it leaves open the question of which of the thousand or so genes that happen to fall close to a QTL peak are crucial for the phenotype.
With no other clues to work with, the next two steps most commonly used to progress toward discovering the culprit genes are fine mapping and generating congenic mice. Fine mapping involves peppering the QTL region with additional markers in order to define the QTL peak more precisely. This can be done relatively quickly and inxexpensively. Generating congenic mice is more involved. There are several methods but the general idea is to select hybrid mice with the appropriate genotype in the QTL region and then repeatedly backcross these and their offspring to the parental strain with the opposite genotype. According to chance, after 10 backcrosses the resulting mice should have a segment of the QTL region from one strain and over 99.9% of the remaining genome from the other. A series of strains can be made in this manner with congenic intervals that cover different segments of the QTL region. The expectation is that some of these will retain the phenotype and others will not. If a strain does not display the phenotype then it is assumed that its congenic interval does not contain the gene or genes responsible for the phenotype. If enough congenic strains can be produced and the congenic intervals are small enough then this approach can, at least theoretically, hone down the number of candidate genes to just a few dozen.
We did this successfully when cloning Tas1r3, the sweet preference gene [[4]; see below], and are currently producing congenics based on several other QTLs. However, making congenic strains is a perilous endeavor. Many nascent congenic strains stop breeding, presumably because alleles of genes from the donor strain that are lost during breeding are required for fertility. Other nascent congenic strains lose the phenotype, presumably because the gene underlying the QTL has epistatic interactions with alleles of genes of the donor strain (i.e., “background effects”). Moreover, even if the new strain breeds well and the phenotype survives, breeding 10 generations of mice requires many months, considerable patience and dedicated resources.
2.7. Candidate genes
Once a QTL is discovered, the question becomes which gene or genes are responsible for it. It is tempting to scan the list of genes in a QTL region and choose candidates based on prior hypotheses. There are two risks with this strategy. The first is that the culprit may be missed because its connection to the phenotype is not obvious to the investigator. The second is that considerable effort can be wasted while attempting to confirm the involvement of a candidate gene only to find out that it is not involved. In practice, the reduction from QTL to gene has been the most difficult step of gene discovery and there is not a consensus about what constitutes sufficient evidence to claim a particular gene is the source of a QTL. Candidates can be promoted or disqualified based on (a) sequence variation within the gene, particularly if sequence and phenotype correspond in many mouse strains, (b) appropriate expression patterns of the gene (e.g., a gene expressed only in testes is unlikely to be involved in ingestion) (c) co-variation of sequence variants and phenotypes in heterologous expression systems, and (d) data from knockout or transgenic mice. For behavioral phenotypes such as taste preferences, the latter evidence is arguably the strongest, particularly if the transgene can be turned on conditionally or is targeted at an appropriate organ or tissue.
2.7.1. Evidence that Tas1r3 underlies the QTL for calcium consumption on distal chromosome 4
In our calcium appetite project, we were fortunate to have a strong candidate gene that could potentially underlie the QTLs for CaCl2, CaLa, and MgCl2 consumption on the distal portion of chromosome 4 (Fig. 3; Table 1). The clue came from the observation that there was also a QTL for saccharin consumption in this region, and our previous work has shown that this QTL, Sac, was due to the sweet taste receptor gene, Tas1r3. Because we already had available in-house the genetic reagents and tools to study Tas1r3, the cost of investigating whether Tas1r3 could be involved in calcium intake was relatively small and so worth the risk.
Tas1r3 is a gene of considerable interest to our group. In 2000, we used a combination of a genome screen, fine mapping, and congenic mice to clone it [4]. Several other groups have used in silico methods to confirm the location and have provided in vitro evidence of the functional significance of Tas1r3 [43,48,49,51,56,72]. To summarize, the product of Tas1r3, T1R3, is a G-protein coupled receptor that dimerizes with T1R2 to produce a sweet taste receptor, and dimerizes with T1R1 to produce a glutamate or umami taste receptor. Not surprisingly, given the intense theoretical and commercial interest in sweet and umami taste, the gene has been the focus of considerable research activity to understand binding sites for sweet compounds, its functional specificity, and its evolutionary history and expression in different species.
To test whether Tas1r3 might underlie the Chr 4 QTL, we first determined the genotype of the B6 × PWK F2 mice at a SNP, rs13478075, that resides in Tas1r3 and can be used to distinguish between “sweet taster” and “sweet non-taster” haplotypes of Tas1r3 [63]. The B6 strain has the “sweet taster” allele whereas the PWK strain has the “sweet non-taster” allele. We found that mice with the PWK/PWK genotype at rs13478075 had significantly higher preferences for calcium and magnesium than did those with the B6/B6 genotype, whereas the opposite was true for saccharin intake and preference (Table 1). It is interesting that the PWK strain has higher saccharin preferences than the B6 strain (Fig. 1). This is probably because the PWK strain has other genes influencing sweet preference that override the effect of Tas1r3. The contribution of other genes to saccharin preference in the PWK strain may also explain why correlations between preferences for calcium and saccharin were low in the B6 × PWK F2 cross (Fig. 5).
Fig. 5.
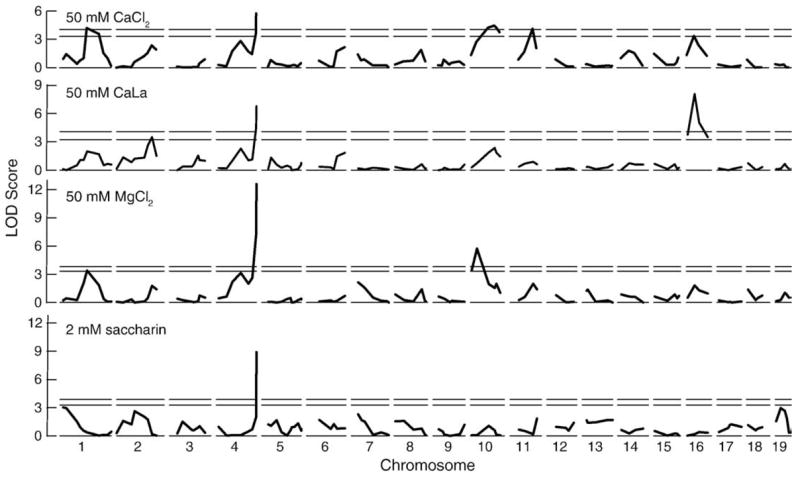
Interval maps based on preferences for CaCl2, CaLa, MgCl2 and saccharin. Horizontal dotted and filled lines shows significance at p<0.05 and p<0.01, respectively. Note that all four traits are linked to distal chromosome 4. (data extracted from Ref. 95)
The finding that differences in calcium preference are related to rs13478075 genotype supports the involvement of Tas1r3 in calcium consumption but it does not prove it. The genotype at a single marker gives information about the haplotype of a gene but this haplotype may extend over many megabases, including other nearby genes. In fact, more recent work based on comparison of the sequence of Toslr3 in 40 inbred strains of mice suggests that a more likely source for the cause of variation in calcium preference is a SNP responsible for a valine for alanine substitution at the 689th amino acid of T1R3 (unpublished results).
A more compelling case for the involvement of Tas1r3 in calcium consumption can be made using congenic mice. As part of our effort to clone Tas1r3, we generated a congenic strain based on the Sac QTL This involved using serial backcrossing to introgress a 194-kb chromosomal fragment containing the Tas1r3 allele from the “sweet taster” C57BL/6ByJ strain onto the genetic background of the “sweet non-taster” 129P3/J strain. In this congenic, the B6 haplotype is dominant and exerts a large effect on preference for a variety of sweeteners [38,44,47]. Although neither parental strain is the same as those involved in the rest of this project it seemed worthwhile to determine the calcium preferences of these animals.
The subjects were 11 heterozygous (B6/129) congenics and 19 homozygous (129/129) littermate controls. They received four series of 48-h two-bottle choice tests. Each series began with a choice between two bottles of water, and then between water and an ascending series of 11 concentrations of saccharin or 3 concentrations each of CaCl2, CaLa, and MgCl2. Relative to controls, the congenics consumed significantly more saccharin but less CaCl2, CaLa, and MgCl2 (Fig. 6). Given the very small (194-kb) introgressed fragment in the Sac congenic mice, the results are strong evidence that alleles of Tas1r3 influence calcium consumption.
Fig. 6.
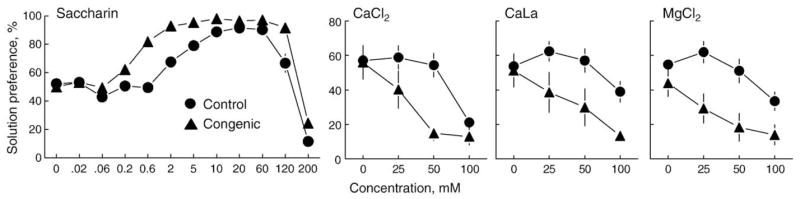
Preference for various concentrations of saccharin, CaCl2, calcium lactate (CaLa), and MgCl2 by 129.B6-Sac congenic mice and their littermate controls. The two types of mice are almost identical except for a 194-kb fragment containing Tas1r3. The congenics have a B6 allele at this locus whereas the controls are homozygous 129. Note that congenic mice more strongly avoid CaCl2, CaLa, and MgCl2 than do controls, which implies that the introgressed genetic fragment is involved in calcium consumption.
To confirm this finding and to determine the direction of the response imparted by the B6 allele, we next tested Tas1r3 knockout (−/−)mice. We mated Tas1r3 heterozygote (+/−) mice brother-to-sister to generate all three potential genotypes as littermate controls. The offspring received ascending series of 48-h preference tests with CaCl2, CaLa, MgCl2, and saccharin. The results show that the knockout mice consumed less saccharin and more CaCl2, CaLa and MgCl2 than did either Toslr3 +/+ or +/− controls (Fig. 7). We have confirmed this finding using different test series and compounds in two additional experiments (data not shown).
Fig. 7.
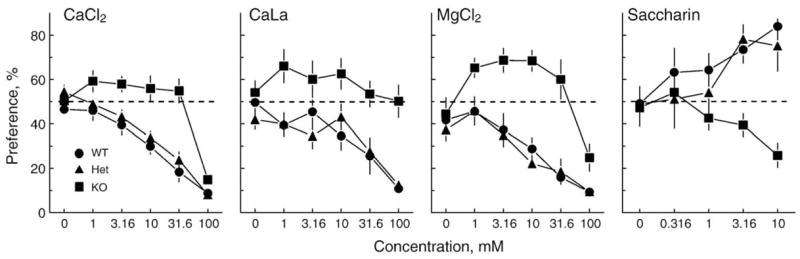
Mean ±SE preferences of Tas1r3 knockout mice (KO; n = 8) and their littermate controls (WT; n = 8, and Het; n = 15) for four solutions. Note that the KOs prefer some concentrations of CaCl2, CaLa, and MgCl2 more than water. Note also that the KOs dislike high concentrations of saccharin, presumably because its bitter taste component predominates.
2.8. From genes to hypotheses
The finding that Tas1r3 is responsible for some of the variation in calcium consumption between two strains of mice illustrates a tremendous advantage of using the genetic approach to understand behavior. The discovery was driven by the data not by hypotheses. Before we started the project, the possibility that a sweet taste receptor could contribute to the control of calcium consumption would not be given credence. Now that the gene has been implicated, we can turn our attention to understanding the mechanisms.
We do not yet know how alleles of Tas1r3 influence calcium consumption. On the genetic and molecular level, this involves understanding how sequence differences in Tas1r3 alter the conformation of T1R3. It will require using heterologous expression to isolate and test the response of T1R3 in a simple system, and mathematical modeling to predict how calcium interacts with different receptor conformations. On the anatomical level, work is needed to identify Tas1r3 expression patterns in tongue and other tissues, particularly in relation to the expression of other taste receptors and taste-related second messengers. This has been done to some extent for the B6 strain [51,56] but whether T1R3 has the same distribution in the PWK strain is unknown. There is also neuroanatomical work needed to determine how signals from T1R3 are integrated, and physiological work needed to investigate whether different forms of T1R3 influence the metabolic disposition of calcium. There is also behavioral work needed to understand more about the taste quality and hedonic value of calcium, particularly during calcium deficiency.
One of the most interesting questions is how can T1R3 act as a detector of calcium as well as sweetness and umami taste? One possibility is that there are separate binding pockets in T1R3 for calcium and sweet compounds, just as there are separate binding pockets for different sweeteners [21,31,39–41,45,52,78,96,98,99] If this is the case, there must be spatial organization or some form of intracellular mechanism that allows the brain to recognize the receptor is activated by calcium rather than sweetness. An alternative possibility depends on the dimerization of T1R3. T1R3 is an ineffective sweet or umami receptor on its own [except perhaps at very high sugar concentrations [101]]. Instead, it is sensitive to sweet compounds when dimerized with T1R2 and sensitive to L-amino acids (“umami” taste) when dimerized with T1R1. Both components of the heterodimer are required. If the combination of T1R3+T1R1 is responsible for the good taste of umami, and the combination of T1R3+T1R2 is responsible for the good taste of sweetness, it is a small step to hypothesize that the combination of T1R3 with a calcium-sensitive G-protein coupled receptor might be responsible for the good taste of calcium. Perhaps the PWK form but not B6 form of T1R3 allows dimerization in a manner that produces a functional calcium-sensitive receptor.
A good candidate to be a dimer with T1R3 is CaSR, the calcium-sensing receptor. CaSR is a G-protein coupled receptor that is moderately homologous to T1R3 and other taste receptors. Since its discovery in 1993 [18], it has been the focus of intense research and clinical interest, with over 900 research papers and 300 reviews [some good ones; [19,20,22,25,37,57,67]]. CaSR is abundantly expressed in parathyroid glands, kidney, gut, and bone, and has a key role in regulating the release of parathyroid hormone in response to circulating blood calcium concentrations. Several polymorphisms in the gene influence blood calcium concentrations and bone mineral density of humans [e.g., [26,28,29,35,36,42,46,71]]. In addition to its contribution to “classic” calcium homeostasis, it is also expressed in brain, particularly in the subfornical organ [70,100], a region that is intimately tied to mineral homeostasis [30,58,59,74]. Neural activity in the subfornical organ is modulated by extracellular calcium concentration within the physiological range, and by CaSR agonists [61,97]. Based on this and findings that lesions of the subfornical organ abolish calcium appetite of calcium-deprived rats [50], we hypothesized in 2001 that the CaSR might be involved in controlling calcium consumption [84]. Recent, unpublished, work from our lab has demonstrated CaSR is present in fungiform taste buds of the B6 mouse. Casr, the gene responsible for CaSR, is located on mouse chromosome 16 at 26.3 cM, which is close to the peak of one of the QTLs we found in the Be × PWK F2 cross (chromosome 16 at 19 cM; Table 1; Fig. 4). CaSR is believed to form a homodimer; that is, two molecules of CaSR cohere to form a functional receptor unit [14]. However, whether this is true for the PWK form of CaSR in combination with the PWK form of T1R3 is unknown.
3. What next?
Characterizing the contribution of Tas1r3 and Casr to the control of calcium consumption will take several years, but even if things turn out as expected, there will still be a lot left to learn. Tas1r3 accounts for only 7–13% of the phenotypic variance in calcium and magnesium preference in the B6 × PWK cross and there are at least 6 other QTLs involved. Crosses of other mouse strains will undoubtedly reveal even more QTLs, and there are likely to be epistatic and gene × environment interactions that have not yet been explored. Finally, it is worth remembering that understanding the mechanisms underlying calcium consumption in the mouse is important but the ultimate purpose of this project is to understand why many humans consume too little calcium to avoid osteoporosis and other diseases related to low calcium intake. The more we know about the mouse, the easier it will be to apply what we have learned to man.
Acknowledgments
This is a review based on a keynote address given at the Society for the Study of Ingestive Behavior annual meeting in Steamboat Springs, Colorado in July 2007. I thank Masterfoods (now Mars) for sponsoring the talk and the members of SSIB for their attention and questions. The following have supported the project: most funding was provided by NIH R01 grant DK-46791, and I particularly thank the program officer, Dr. Robert Karp, for taking an active interest in this project. Funding for research to improve methods of the two-bottle choice test was provided by NIH R01 grant AA-12715. Additional support was provided by unrestricted funds of the Monell Chemical Senses Center. The 40 strains of mice used for the initial strain screen were awarded by the Mouse Phenome Project and funded by AstraZeneca. Genotyping services were provided by the Center for Inherited Disease Research, which is funded through NIH contract NO1-HG-65403. Dr. A. Bachmanov conducted the experiment involving Sac congenic mice. Dr. R. Margolskee kindly donated breeding pairs of Tas1r3 knockout mice. Technical assistance was provided by Diane Pilchak, Fred Ollinger, Maureen Lawler, Stephanie Craw, Laura Alarcon, Matt Rosazza, and Hillary Ellis. Intellectual support was provided by several Monell faculty, in particular, Alexander Bachmanov, Gary Beauchamp, Stuart McCaughey, and Danielle Reed.
References
- 1.Ad hoc committee on standards for nutritional studies. Second report of the ad hoc committee on standards for nutritional studies. J Nutr. 1980;110:1726. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2 and NH4Cl solutions by 28 mouse strains. Behav Genet. 2002;32:445–57. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Li X, Li S, Neira M, Beauchamp GK, Azen EA. Highresolution genetic mapping of the sucrose octaacetate taste aversion (Soa) locus on mouse chromosome 6. Mamm Genome. 2001;12:695–9. doi: 10.1007/s00335-001-2061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–33. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, Tordoff MG. Voluntary ethanol consumption by mice: genome-wide analysis of quantitative trait loci and their interactions in a C57BL/6ByJ×_129P3/J F2 intercross. Genome Res. 2002;12:1257–68. doi: 10.1101/gr.129702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, et al. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mammal Genome. 1997;8:545–8. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet. 1996;26:563–73. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72:603–13. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20:201–6. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet. 1998;28:117–24. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr. 2000;130:935S–41S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–13. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem. 1998;1273:23605–10. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 15.Beauchamp GK, Bertino M, Engelman K. Human salt appetite. In: Friedman MI, Tordoff MG, Kare MR, editors. Appetite and nutrition. Chem Senses. New York: Marcel Dekker; 1991. pp. 85–107. [Google Scholar]
- 16.Beck JA, Lloyd S, Hafezparast M, Lennon-pierce M, Eppig JT, Festing MFW, et al. The genealogy chart of inbred strains. Nat Genet. 2000;24:23–5. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 17.Birney E, Andrews D, Caccamo M, Chen Y, Clarke L, Coates G, et al. Ensembl 2006. Nucleic Acids Res. 2006;34:D556–61. doi: 10.1093/nar/gkj133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown E, Gamba G, Riccardi D, Riccardi D, Lombardi M, Butters R, et al. Cloning and characterization of an extracellular Ca2+ -sensing receptor from bovine parathyroid. Nature. 1993;8:303–7. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 19.Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411. doi: 10.1152/physrev.1991.71.2.371. [DOI] [PubMed] [Google Scholar]
- 20.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–97. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 21.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 22.Chattopadhyay N, Mithal A, Brown E. The calcium-sensing receptor: a window into the physiology and pathophysiology of mineral ion metabolism. Endocr Rev. 1996;17:289–307. doi: 10.1210/edrv-17-4-289. [DOI] [PubMed] [Google Scholar]
- 23.Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav Neurosci. 1992;106:172–80. doi: 10.1037//0735-7044.106.1.172. [DOI] [PubMed] [Google Scholar]
- 24.Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am J Physiol. 1996;271:R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- 25.Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 2006;291:G753–61. doi: 10.1152/ajpgi.00189.2006. [DOI] [PubMed] [Google Scholar]
- 26.Conley YP, Finegold DN, Peters DG, Cook JS, Oppenheim DS, Ferrell RE. Three novel activating mutations in the calcium-sensing receptor responsible for autosomal dominant hypocalcemia. Mol Genet Metab. 2000;71:591–8. doi: 10.1006/mgme.2000.3096. [DOI] [PubMed] [Google Scholar]
- 27.Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–11. [PubMed] [Google Scholar]
- 28.D’Souza-Li L, Canaff L, Janicic N, Cole DE, Hendy GN. An acceptor splice site mutation in the calcium-sensing receptor (CASR) gene in familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Hum Mutat. 2001;18:411–21. doi: 10.1002/humu.1212. [DOI] [PubMed] [Google Scholar]
- 29.D’Souza-Li L, Yang B, Canaff L, Bai M, Hanley DA, Bastepe M, et al. Identification and functional characterization of novel calcium-sensing receptor mutations in familial hypocalciuric hypercalcemia and autosomal dominant hypocalcemia. J Clin Endocrinol Metab. 2002;87:1309–18. doi: 10.1210/jcem.87.3.8280. [DOI] [PubMed] [Google Scholar]
- 30.Denton D. The hunger for salt: an anthropological, physiological and medical analysis. Berlin: Springer-Verlag; 1984. [Google Scholar]
- 31.DuBois GE. Unraveling the biochemistry of sweet and umami tastes. Proc Natl Acad Sci U S A. 2004;101:13972–3. doi: 10.1073/pnas.0405991101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyets Inc. 100000 AIN-76A Purified rodent diet. 2003 http://www.dyets.com/100000.htm.
- 33.Gregorova S, Forejt J. PWD/Ph and PWK/Ph inbred mouse strains of Mus m. musculus subspecies-a valuable resource of phenotypic variations and genomic polymorphisms. Folia Biol (Praha) 2000;46:31–41. [PubMed] [Google Scholar]
- 34.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 35.Hendy GN, D’Souza-Li L, Yang B, Canaff L, Cole DE. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2000;16:281–96. doi: 10.1002/1098-1004(200010)16:4<281::AID-HUMU1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Hendy GN, Phommarinh M. CASRdb: calcium-sensing receptor. 2007 http://www.casrdb.mcgill.ca/
- 37.Herbert SC, Brown EM. The extracellular calcium receptor. Curr Opin Cell Boil. 1995;7:484–92. doi: 10.1016/0955-0674(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 38.Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129. B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang P, Cui M, Ji Q, Snyder L, Liu Z, Benard L, et al. Molecular mechanisms of sweet receptor function. Chem Senses. 2005;30:i17–i18. doi: 10.1093/chemse/bjh091. [DOI] [PubMed] [Google Scholar]
- 40.Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, et al. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem. 2005;280:34296–305. doi: 10.1074/jbc.M505255200. [DOI] [PubMed] [Google Scholar]
- 41.Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, et al. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279:45068–75. doi: 10.1074/jbc.M406779200. [DOI] [PubMed] [Google Scholar]
- 42.Kanazawa H, Tanaka H, Kodama S, Moriwake A, Kobayashi M, Seino Y. The effect of calcium-sensing receptor gene polymorphisms on serum calcium levels: a familial hypocalciuric hypercalcemia family without mutation in the calcium-sensing receptor gene. Endocr J. 2000;47:29–35. doi: 10.1507/endocrj.47.29. [DOI] [PubMed] [Google Scholar]
- 43.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–42. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, et al. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal chromosome 4. Mamm Genome. 2001;12:13–6. doi: 10.1007/s003350010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–6. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lorentzon M, Lorentzon R, Lerner UH, Nordstrom P. Calcium sensing receptor gene polymorphism, circulating calcium concentrations and bone mineral density in healthy adolescent girls. Eur J Endocrinol. 2001;144:257–61. doi: 10.1530/eje.0.1440257. [DOI] [PubMed] [Google Scholar]
- 47.Manita S, Bachmanov AA, Li X, Beauchamp GK, Inoue M. Is glycine”sweet”to mice? Mouse strain differences in perception of glycine taste. Chem Senses. 2006;31:785–93. doi: 10.1093/chemse/bjl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–4. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 49.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, et al. Taslr3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 50.McCaughey SA, Fitts DA, Tordoff MG. Lesions of the subfornical organ decrease the calcium appetite of calcium-deprived rats. Physiol Behav. 2003;79:605–12. doi: 10.1016/s0031-9384(03)00139-2. [DOI] [PubMed] [Google Scholar]
- 51.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–8. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 52.Morini G, Bassoli A, Temussi PA. From small sweeteners to sweet proteins: anatomy of the binding sites of the human T1R2_T1 R3 receptor. J Med Chem. 2005;48:5520–9. doi: 10.1021/jm0503345. [DOI] [PubMed] [Google Scholar]
- 53.Multiple authors. SSIB abstracts. Appetite. 2007;49:272–341. [Google Scholar]
- 54.National Library of Medicine and the National Institutes of Health. PubMed. 2007 www.pubmed.gov.
- 55.National Osteoporosis Foundation. Fast Facts. 2007 http://www.nof.org/osteoporosis/diseasefacts.htm.
- 56.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 57.Nemeth EF. Calcimimetic and calcilytic drugs: just for parathyroid cells? Cell Calcium. 2004;35:283–9. doi: 10.1016/j.ceca.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Noda M. The subfornical organ, a specialized sodium channel, and the sensing of sodium levels in the brain. Neuroscientist. 2006;12:80–91. doi: 10.1177/1073858405279683. [DOI] [PubMed] [Google Scholar]
- 59.Noda M. Hydromineral neuroendocrinology: mechanism of sensing sodium levels in the mammalian brain. Exp Physiol. 2007;92:513–22. doi: 10.1113/expphysiol.2006.035659. [DOI] [PubMed] [Google Scholar]
- 60.Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–11. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quinn SJ, Kifor O, Trivedi S, Diaz R, Vassilev P, Brown E. Sodium and ionic strength sensing by the calcium receptor. J Biol Chem. 1998;273:19579–86. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- 62.Reed DR, Bachmanov AA, Tordoff MG. Forty mouse strain survey of body composition. Physiol Behav. 2007;91:593–600. doi: 10.1016/j.physbeh.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, et al. Polymorphisms in the taste receptor gene (Taslr3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–46. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reed DR, Li X, Lu K, Li S, Tordoff MG, Price RA, et al. Loci on chromosomes 2,4,9 and 16 for body weight, body length and adiposity identified in a genome scan of an F2 intercross between 129P3/J and C57BL/6Byj mouse strains. Mamm Genome. 2003;14:302–13. doi: 10.1007/s00335-002-2170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed DR, McDaniel AH, Li X, Tordoff MG, Bachmanov AA. Quantitative trait loci for individual adipose depot weights in C57BL/6ByJxl29P3/J F2 mice. Mamm Genome. 2006;17:1065–77. doi: 10.1007/s00335-006-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 67.Riccardi D, Maldonado-Perez D. The calcium-sensing receptor as a nutrient sensor. Biochem Soc Trans. 2005;33:316–20. doi: 10.1042/BST0330316. [DOI] [PubMed] [Google Scholar]
- 68.Richter CP, Birmingham JR. Calcium appetite of parathyroidectomized rats used to bioassay substances which affect blood calcium. Endocrinol. 1941;29:655–66. [Google Scholar]
- 69.Richter CP, Eckert JF. Mineral appetite of parathyroidectomized rats. Am J Med Sci. 1932;9:9–16. [Google Scholar]
- 70.Rogers K, Dunn C, Hebert S, Brown E. Localization of calcium receptor mRNA in the adult rat central nervous system by in situ hybridization. Brain Res. 1997;744:47–56. doi: 10.1016/s0006-8993(96)01070-0. [DOI] [PubMed] [Google Scholar]
- 71.Rubin LA, Peltekova V, Janicic N, Liew CC, Hwang D, Evrovski J, et al. Calcium sensing receptor gene: analysis of polymorphism frequency. Scand J Clin Lab Invest Suppl. 1997;227:122–5. [PubMed] [Google Scholar]
- 72.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77:896–903. doi: 10.1046/j.1471-4159.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 73.Schulkin J. The ingestion of calcium in female and male rats. Psychobiology. 1991;19:262–4. [Google Scholar]
- 74.Schulkin J. Sodium hunger: the search for a salty taste. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- 75.Schulkin J. Calcium hunger. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 76.Simpson E, Linder C, Sargent E, Davisson M, Mobraaten L, Sharp J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 77.Storchova R, Gregorova S, Buckiova D, Kyselova V, Divina P, Forejt J. Genetic analysis of X-linked hybrid sterility in the house mouse. Mamm Genome. 2004;15:515–24. doi: 10.1007/s00335-004-2386-0. [DOI] [PubMed] [Google Scholar]
- 78.Temussi PA. Why are sweet proteins sweet? Interaction of brazzein, monellin and thaumatin with the T1R2–T1R3 receptor. FEBS Lett. 2002;526:1–4. doi: 10.1016/s0014-5793(02)03155-1. [DOI] [PubMed] [Google Scholar]
- 79.The Jackson Laboratory. Mouse phenome database web site. 2001 http://www.jax.org/phenome.
- 80.Tordoff MG. Influence of dietary calcium on sodium and calcium intake of spontaneously hypertensive rats. Am J Physiol. 1992;262:R370–81. doi: 10.1152/ajpregu.1992.262.3.R370. [DOI] [PubMed] [Google Scholar]
- 81.Tordoff MG. Voluntary intake of calcium and other minerals by rats. Am J Physiol. 1994;267:R470–5. doi: 10.1152/ajpregu.1994.267.2.R470. [DOI] [PubMed] [Google Scholar]
- 82.Tordoff MG. The importance of calcium in the control of salt intake. Neurosci Biobehav Rev. 1996;20:89–99. doi: 10.1016/0149-7634(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 83.Tordoff MG. Some basic psychophysics of calcium salt solutions. Chem Senses. 1996;21:417–24. doi: 10.1093/chemse/21.4.417. [DOI] [PubMed] [Google Scholar]
- 84.Tordoff MG. Calcium: taste, intake and appetite. Physiol Rev. 2001;81:1567–97. doi: 10.1152/physrev.2001.81.4.1567. [DOI] [PubMed] [Google Scholar]
- 85.Tordoff MG. The case for a calcium appetite in humans. In: Weaver CM, Heaney RP, editors. Calcium in human health. Totowa, NJ: Humana Press; 2005. pp. 247–66. [Google Scholar]
- 86.Tordoff MG. Taste solution preferences of C57BL/6J and 129Xl/SvJ mice: influence of age, sex, and diet. Chem Senses. 2007;32:655–71. doi: 10.1093/chemse/bjm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project. Monell Chemical Senses Center. 2001 www.monell.org/MMTPP.
- 88.Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses. 2002;27:759–68. doi: 10.1093/chemse/27.9.759. [DOI] [PubMed] [Google Scholar]
- 89.Tordoff MG, Bachmanov AA. Survey of calcium and sodium intake and metabolism with bone and body composition data (MPD:103) Mouse Phenome Project. 2002 http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&id=103.
- 90.Tordoff MG, Bachmanov AA. Mouse taste preference tests: Influence of drinking spout position. 2003 doi: 10.1093/chemse/28.4.315. http://www.monell.org/MMTPP/Verification%20-%20Spout%20position.htm. [DOI] [PMC free article] [PubMed]
- 91.Tordoff MG, Bachmanov AA. Mouse taste preference tests: why only two bottles? Chem Senses. 2003;28:315–24. doi: 10.1093/chemse/28.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol Behav. 2007;91:632–43. doi: 10.1016/j.physbeh.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol Behav. 2007;91:620–31. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–97. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tordoff MG, Reed DR, Shao DR. Calcium taste preferences: genetic analysis and genome screen of C57BL/6JxxPWK/PhJ hybrid mice. Genes Brain Behav. doi: 10.1111/j.1601-183X.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walters DE. Homology-based model of the extracellular domain of the taste receptor T1R3. Pure Appl Chem. 2002;74:1117–23. [Google Scholar]
- 97.Washburn DL, Smith PM, Ferguson AV. Control of neuronal excitability by an ion-sensing receptor (correction of anion-sensing) Eur J Neurosci. 1999;11:1947–54. doi: 10.1046/j.1460-9568.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- 98.Winnig M, Bufe B, Meyerhof W. Valine 738 and lysine 735 in the fifth transmembrane domain of rTaslr3 mediate insensitivity towards lactisole of the rat sweet taste receptor. BMC Neurosci. 2005;6:22. doi: 10.1186/1471-2202-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1 R subunits in the heteromeric taste receptors. Proc Natl Acad Sci U S A. 2004;101:14258–63. doi: 10.1073/pnas.0404384101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yano S, Brown EM, Chattopadhyay N. Calcium-sensing receptor in the brain. Cell Calcium. 2004;35:257–64. doi: 10.1016/j.ceca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 101.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]


