Abstract
Aims
To determine whether the novel non-AT1, non-AT2 binding site for angiotensins recently discovered in rodent brains occurs in the human brain.
Main methods
Radioligand binding assays of 125I-sarcosine1, isoleucine8 angiotensin II binding were carried out in homogenates of the rostral pole of the temporal cortex of human brains containing 0.3 mM parachloromercuribenzoate (PCMB), 10 µM losartan to saturate AT1 receptors, 10 µM PD123319 to saturate AT2 receptors, with or without 10 µM angiotensin II to define specific binding. Competition binding assays employed a variety of angiotensin peptides, specific angiotensin receptor antagonists, several neuropeptides and an endopeptidase inhibitor to determine pharmacological specificity for this binding site.
Key findings
The novel non-AT1, non-AT2 binding site was present in similar amounts in female and male brains: Bmax 1.77±0.16 and 1.52±0.17 fmol/mg initial wet weight in female and male brains respectively. The KD values, 1.79±0.09 nM for females, and 1.53±0.06 nM for males were also similar. The binding site shows pharmacological specificity similar to that in rodent brains: sarcosine1, isoleucine8 angiotensin II>angiotensin III>angiotensin II>angiotensin I’angiotensin IV>angiotensin 1–7. Shorter angiotensin fragments and non-angiotensin peptides showed low affinity for this binding site.
Significance
The presence in human brain of this novel non-AT1, non-AT2 binding site supports the concept that this binding site is an important component of the brain angiotensin system. The functional significance of this binding site, either as a novel angiotensin receptor or a highly specific angiotensinase remains to be determined.
Keywords: Brain angiotensin system, Angiotensin receptors, Gender, Parachloromercuribenzoate, Thiols, Radioligand binding
Introduction
The functional significance of the brain angiotensin system for cardiovascular regulation is well established. Brain angiotensinergic activity is associated with stimulation of sympathetic nerve activity, vasopressin release and stimulation of fluid and electrolyte intake, all of which contribute to elevation of blood pressure, see reviews (Johnson and Thunhorst, 1997; Phillips and Sumners, 1998; Fitzsimons, 1998; Dampney et al., 2002; Veerasingham and Raizada, 2003; Saavedra, 2005; Osborn et al., 2007).
However, many questions remain as to how the brain angiotensin system functions. For example, astrocytes are the primary source of angiotensinogen in the brain (Stornetta et al., 1988). However, it is unknown how this glial angiotensinogen becomes the primary active neurohormone angiotensin II (Ang II) in the brain. It has been suggested that angiotensin III (Ang III, des Asp1 Ang II) rather than Ang II is the angiotensin peptide in the brain that elevates blood pressure (Reaux-Le Goazigo et al., 2005) however this idea has been challenged (Kokje et al., 2007).
With recent discoveries of receptors for other peptide fragments of Ang II, e.g., Ang IV (Harding et al., 1992) and Ang 1–7 (Santos et al., 2003) there is now good evidence that these angiotensin peptides also act in the brain (Wright et al., 1995; Chappell, 2007). The demonstration of the existence of ACE2 in the brain (Doobay et al., 2007) provides a mechanism for formation of Ang 1–7 from Ang II in the brain. In concert with the increased number of receptors for angiotensins has also come the recognition that the actions of the brain angiotensin system extend beyond cardiovascular regulation (Wright and Harding, 1995; Chai et al., 2004; Takeda et al., 2008).
To add to this complexity, a high affinity, non-AT1, non-AT2 binding site for Ang II (and Ang III) was recently described in rat brain in our laboratory (Karamyan and Speth, 2007). This binding site has low affinity for Ang IV (des Asp1, des Arg2 Ang II) and Ang 1–7, suggesting that it is also distinct from receptors for these angiotensin peptide fragments (Harding et al., 1992;Santos et al., 2003). This binding site is also present in the mouse brain (Karamyan et al., in press).
This binding site is unmasked in the presence of the organomercurial sulfhydryl agent parachloromercuribenzoate (PCMB) or its sulfonic acid derivative parachloromercurisulfonate (PCMPS). This suggests that oxidation of cysteines in a heretofore unknown angiotensin-binding protein causes a conformational alteration that unmasks its ability to bind Ang II and Ang III. The signaling pathway of the AT1 receptor for Ang II involves activation of NADPH to form reactive oxygen species (ROS) (Zimmerman and Davisson, 2004; Williams and Griendling, 2007). These ROS can oxidize cysteines in proteins, acting as a control switch that activates or inactivates proteins (Barford, 2004). Thus Ang II-mediated generation of ROS could contribute to oxidative stress in the brain that may enable this protein's ability to bind active angiotensins.
A critical question is whether this binding site is only present in rodent species or if it occurs in higher mammals. This report describes the presence of a non-AT1, non-AT2 angiotensin binding site in human brain that has pharmacological specificity similar to the non-AT1, non-AT2 angiotensin binding site described in rodent brain.
Materials and methods
Brain tissue was obtained at autopsy at the Cuyahoga County Coroner's Office, Cleveland, OH. The study was performed according to a protocol approved by the Institutional Review Board of University Hospitals of Cleveland. Characteristics of the eight subjects are recorded in Table 1. There were four white and four African American subjects and four male and four female subjects. The average age was 53±3 years (mean±SEM); the average postmortem interval (hours between death and freezing tissue) was 17.4±2.5; the average tissue pH was 6.7±0.1. No therapeutic or illicit drugs were detected by the coroner's office in samples of blood or urine. The rostral pole of the temporal lobe (Brodmann area 38) was collected, frozen in isopentane cooled by dry ice and stored at −80 °C. Approximately 500 mg of gray matter was cut with a scalpel blade from the frozen block for radioligand binding assay.
Table 1.
Characteristics of the human subjects
| Age (y) | Race | Gender | PMI (h) | Tissue pH | Toxicology | Cause of death |
|---|---|---|---|---|---|---|
| 41 | White | Female | 18 | 6.5 | N/D | Pulmonary thromboembolism |
| 45 | AA | Female | 9 | 6.9 | N/D | CVD |
| 48 | AA | Male | 9 | 7.0 | N/D | CVD |
| 49 | White | Male | 28 | 6.8 | N/D | Morbid obesity, CVD |
| 55 | AA | Female | 14 | 6.3 | N/D | CVD |
| 61 | White | Male | 21 | 6.4 | N/D | Ischemic bowel necrosis |
| 62 | White | Male | 15 | 6.9 | N/D | CVD |
| 63 | AA | Female | 25 | 6.8 | N/D | Gunshot wound to chest |
Abbreviations: AA, African-American; CVD, cardiovascular disease; N/D, no drugs detected; PMI, postmortem interval.
Angiotensin and non-angiotensin peptides were obtained from Phoenix Pharmaceuticals, Bachem, or American Peptides. Losartan was a gift of Dr. Ron Smith of Dupont Merck. JA-2 (N-[1-(R, S)-carboxy-3-phenylpropyl]-Ala-Aib-Tyr-p-aminobenzoate) was a gift of Dr. Ian Smith of Monash University. Telmisartan was provided by Drs. Mitchell Avery and Amar Chittiboyina, Department of Medicinal Chemistry, School of Pharmacy, University of Mississippi. ZD7155 (5,7-Diethyl-3,4-dihydro-1-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1,6-naphthyridin-2 (1H)-one hydrochloride), and PD123319 (1-[[4-(Dimethylamino)-3-methylphenyl]methyl]-5-(dipheny lacetyl)-4,5,6,7-tetrahydro-1H-imi-dazo[4,5-c]pyridine-6-carboxylic acid ditrifluoroacetate), were purchased from Tocris Bioscience, PCMB sodium salt were purchased from MP Biomedicals. 125I-SI-Ang II was prepared in house by the chloramine T procedure and purified by reverse-phase HPLC as described previously (Speth and Harding, 2001).
The measurement of 125I-sarcosine1, isoleucine8 Ang II (125I-SI-Ang II) binding in human brain membranes was carried out using established procedures (Karamyan and Speth, 2007). Briefiy, a frozen piece of tissue from a male and a female brain was thawed, weighed and immediately homogenized in ice-cold hypotonic, 20 mM NaPO4, 5 mM EDTA, pH7.2 solution by a mechanical homogenizer (Tekmar Tissuemizer). The homogenates were centrifuged (40,000 ×g for 20 min at 4 °C) and the supernatants decanted. The membrane pellets were resuspended by homogenization in assay buffer (150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, 50 mM NaPO4, pH7.1–7.2). The homogenates were recentrifuged as before and the pellets resuspended by homogenization in the assay buffer (40mg/ml initial wet tissue weight). Losartan and PD123319 (final concentration 10 µM from a 10 mM stock in de-ionized water) were present to saturate AT1 and AT2 receptors, respectively. PCMB when present in the homogenates (0.3 mM final concentration) was added into the membrane homogenates from a 100 mM stock solution in 50 mM NaOH about 5–10 min before incubation to unmask the non-AT1, non-AT2 binding site.
Saturation binding studies were carried out by incubation of 50 µl membrane preparations with six concentrations of 125I-SI-Ang II (0.25–3 nM) in 100 µl total assay volume for 1 h at room temperature. Non-specific binding was estimated in the presence of 10 µM Ang II. Values shown represent specific (total minus non-specific) binding.
Competition binding studies were conducted by incubation of 50 µl of membrane preparation with ~1 nM 125I-SI-Ang II in the presence of four concentrations of angiotensin and non-angiotensin peptides (1 nM to 10 µM) in 100 µl total assay volume for 1 h at room temperature. Non-specific binding was determined in the presence of 10 µM Ang II.
Free and bound radioligands were separated on glass fiber filters (Whatman, Schleicher and Schuell, #32 glass), prewetted with 1mg/ml bovine serum albumin, using a cell harvester (Model M24R, Brandel, Gaithersburg, MD). The bound radioligand retained on the filter disks was assayed with a Beckman Gamma 5500 gamma counter at a counting efficiency of 67%. Determination of Bmax (fmol of radioligand bound per mg initial wet weight), KD and IC50 values were carried out using one-site saturation and competition (with top and bottom constraints of 100 and 0%, respectively) binding models of Prism software (Graphpad Software, San Diego, CA). Ki values were determined using the Cheng–Prusoff equation: Ki=IC50/(1+H/Kd) where H equals radioligand concentration and KD is the KD for the radioligand. Values reported are mean±SEM. Statistical comparisons between male and female brains used a paired Student's t test. A one-sample t test was used to assess the enhancement of 125I-SI Ang II binding by Ang 1–4.
Results
In the presence of 0.3 mM PCMB, the specific binding of 125I-SI Ang II to homogenates of the rostral pole of the temporal lobe showed high affinity and saturability (Fig. 1). 125I-SI Ang II binding capacity did not differ significantly between female brains (Bmax = 1.77±0.16 fmol/g wet weight, n=4) and male brains (Bmax = 1.52±0.17 fmol/g wet weight, n=4, t=1.07, p=0.33). There was also no significant difference in the binding affinity of 125I-SI Ang II between female (KD = 1.79±0.09 nM, n=4) and male brains (KD = 1.53±0.06 nM, n = 4, t = 1.94, p = 0.15). In the absence of PCMB the binding of 125I-SI Ang II (Bmax) or the KD was not significantly different from zero (Fig. 1).
Fig. 1.
Representative saturation binding analyses of 125I-SI-Ang II binding in the human cerebral cortical (rostral pole of the temporal lobe) membranes (obtained from the 9 h post mortem brains listed in Table 1) in the presence of 10 µM PD123319 and losartan in the absence or presence (indicated with “+”) of 0.3 mM PCMB. In “F+” Bmax=1.80±0.16 fmol/mg wet wt., and Kd=1.61±0.36 nM; “M+” Bmax=1.99±0.18 fmol/mg wet wt., and Kd=1.69±0.39 nM; in the absence of PCMB specific 125I-SI-Ang II binding was not significantly different from zero.
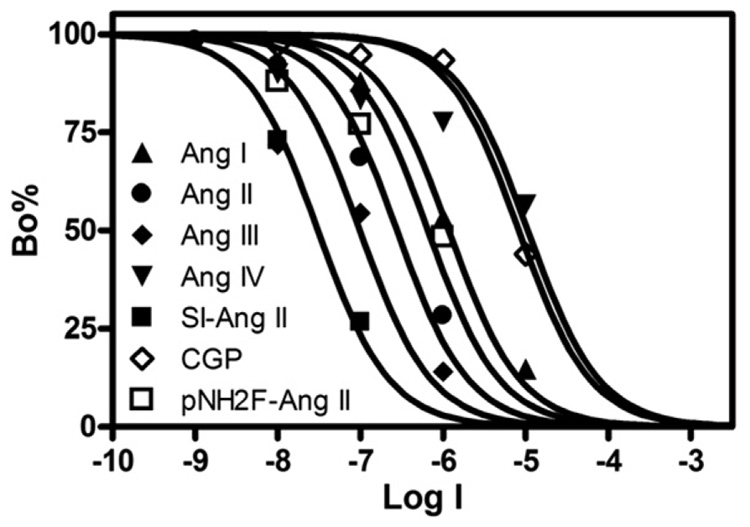
The pharmacological specificity of 125I-SI Ang II binding to homogenates of the rostral pole of the temporal lobe showed a pattern similar to that seen in rat and mouse brains (Fig. 2 and Table 2). SI Ang II was the most potent ligand for the non-AT1, non-AT2 binding site, followed by Ang III, and Ang II. Ang I and the AT2 receptor-selective Ang II analog p-aminophenylalanine Ang II had considerably lower affinity for the non-AT1, non-AT2 binding site. Ang IV, Ang 1–7, and CGP 42112 (another AT2 receptor-selective ligand) showed substantially lower affinity for the binding site. Other angiotensin fragments and selected non-angiotensin peptides also showed low affinity for the non-AT1, non-AT2 binding site in the rostral pole of the temporal lobe (Table 2).
Fig. 2.
Representative competition binding analyses of 125I-SI-Ang II with 4 natural and 3 synthetic angiotensin congeners in the human cerebral cortical (rostral pole of the temporal lobe) membranes in the presence of ~1 nM 125I-SI-Ang II, 10 µM PD123319 and losartan, and 0.3 mM PCMB. The complete list of ligands tested for competition with the binding of 125I-SI-Ang II to the non-AT1, non-AT2 angiotensin binding site are presented in Table 2.
Table 2.
Affinity of angiotensins, angiotensin analogs and non-peptidic AT1 receptor antagonists or the non-AT1, non-AT2 angiotensin binding site in the human cerebral cortical membranes
| Angiotensin peptides, analogs and angiotensin receptor ligands | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ang I | Ang II | Ang III | SI-Ang II | Ang IV | Ang (4–8) | Ang (5–8) | ||||
| 979±184 nM | 307±77.6 nM | 100±21.5 nM | 17.5±0.94 nM | >10 µM | >10 µM | >10 µM | ||||
| Ang (3–7) | Ang (1–7) | Ang (1–4)* | CGP42112A | pNH2F Ang II | Telmisartan | ZD7155 | ||||
| >10 µM | >10 µM | 33±4% | >5 µM | 988±242 nM | >10 µM | >10 µM | ||||
| Non-angiotensin peptides | ||||||||||
| Bradykinin | Neurotensin | VIP | LHRH | Substance P | JA-2** | |||||
| >10 µM | >10 µM | >10 µM | >10 µM | >10 µM | >10 µM | |||||
Summary of competition binding studies. Values presented are average Ki±S.E.M. (n=4) in the presence of ~1 nM 125I-SI-Ang II, 10 µM PD123319 and losartan, and 0.3 mM PCMB (1 h incubation at 24 °C).
Ang (1–4) increased binding of 125I-SI-Ang II by 33±4% at a concentration of 10 µM.
JA-2 is a nonselective inhibitor of endopeptidases EP 24.16 and EP 24.15 (also known as EC 3.4.24.15 and EC 3.4.24.16) (Shrimpton et al., 2000;Smith et al., 2000). VIP: vasoactive intestinal peptide; LHRH: luteinizing hormone-releasing hormone; pNH2F Ang II: p-aminophenylalanine-6-Ang II; JA-2: N-[1-(R,S)-carboxy-3-phenylpropyl]-Ala-Aib-Tyr-paminobenzoate.
Two other non-peptide AT1 receptor-selective antagonists, telmisartan and ZD7155, also showed low affinity for the non-AT1, non-AT2 binding site (Table 2). JA-2 a potent inhibitor of the endopeptidases EC 3.4.24.15 and EC 3.4.24.16 also showed low affinity for the non-AT1, non-AT2 binding site in the rostral pole of the temporal lobe (Table 2). As seen previously in rodent brains (Karamyan and Speth, 2007; Karamyan et al., in press), the amino terminal fragment of Ang II, Ang 1–4, significantly enhanced binding of 125I-SI Ang II to the non-AT1, non-AT2 binding site (33±4%, t=8.0, p=0.0039) when present at a concentration of 10 µM (Table 2).
Discussion
The brain angiotensin system may be one of the brain's most complex and poorly understood neurohormonal systems. The mechanisms whereby active angiotensins are synthesized in the brain, the actual active angiotensin peptides in the brain, and the pathways by which angiotensin peptides are inactivated or converted to other active angiotensins working on different receptors from the classical AT1 and AT2 subtypes remain in question.
Adding to this complexity is the recent discovery of an additional binding site for angiotensins in the rat (Karamyan and Speth, 2007) and mouse (Karamyan et al., in press) brains that differs from all four (AT1, AT2, AT4 and mas oncogene protein receptor for Ang 1–7) known receptors for angiotensin peptides. This report now demonstrates the presence of this fifth, selective, high affinity binding site for angiotensin peptides in membrane preparations of the human brain. The conservation of this binding site across species strongly suggests that this is a functional protein (Civelli, 1998).
The inability of AT1 selective antagonists (losartan, telmisartan and ZD7155) to compete for 125I-SI Ang II binding clearly indicates that this binding site is not the AT1 receptor. The presence of this binding site in AT1A and AT1B knockout mouse brains (Karamyan et al., in press) further indicates that this binding site is not the AT1 receptor.
Similarly, the inability of PD123319 to compete for 125I-SI Ang II binding, as well as the greatly reduced ability of other AT2 receptor-selective ligands to compete for this binding site (Table 2) indicates that this is not the AT2 receptor. The presence of this binding site in AT2 knockout mouse brains (Karamyan et al., in press) further indicates that this binding site is not the AT2 receptor.
The weak ability of Ang IV (des Asp1, des Arg2 Ang II) and Ang 1–7 to compete for 125I-SI Ang II binding to this site indicates that this binding site is not the Ang IV receptor (identified as being insulin-regulated aminopeptidase) (Albiston et al., 2001). The presence of this binding site in mas knockout mouse brains (Karamyan et al., in press) further indicates that this binding site is not the Ang 1–7 receptor. There did not appear to be any difference in binding as a function of the post-mortem interval. The average Bmax value for 125I-SI Ang II binding in the female brain was almost identical to that of the shortest post-mortem interval brain (9 h, Fig. 1). This is consistent with a report indicating that brain angiotensin receptors, determined in sheep, remain unchanged with post-mortem intervals up to 32 h (MacGregor et al., 1994).
The selectivity of this binding site for angiotensin peptides that bind with high affinity to the classical AT1 and AT2 binding sites suggests that this binding site is an important component of the classical brain angiotensin system. The high affinity of this binding site for SI Ang II, Ang II and Ang III, angiotensin peptides that have high affinity for both the AT1 and AT2 receptors relative to angiotensin and non-angiotensin peptides, indicates a strong selectivity for AT1/AT2 receptor-active angiotensins. The enhancement of 125I-SI Ang II binding by the amino-terminal tetrapeptide, Ang 1–4 cannot be explained at this time. It does not appear to be due to inhibition of metabolic inactivation of the radioligand (Karamyan and Speth, unpublished observations).
Despite the selectivity of this binding site for AT1/AT2 receptor-active angiotensins, it may mediate novel functions of brain angiotensins as a novel receptor. In the rat brain, the distribution of the non-AT1, non-AT2 binding site does not correlate with that of AT1 receptors, although there are some overlapping regions (Karamyan and Speth, in press). The brain region used for this study, the frontal pole of the temporal cortex, is not an area traditionally considered a site of action of Ang II. There is little (Barnes et al., 1993; Ge and Barnes, 1996) or no (MacGregor et al., 1995) evidence for the presence of AT1 or AT2 receptors in this brain region in humans.
The presence of high densities of this binding site in the substantia nigra, caudate nucleus, nucleus basalis of Meynert and nucleus accumbens of the rat brain suggests that this binding site could play role in Parkinson's Disease, Alzheimer's Disease and drug abuse (Karamyan and Speth, in press). Of note, in the human brain, moderate to high densities of AT1 receptors are found in the substantia nigra and caudate nucleus (Barnes et al., 1993; MacGregor et al., 1995). And, the density of AT1 receptors in these brain regions are profoundly reduced in Parkinson's Disease (Allen et al., 1992; Ge and Barnes, 1996). However, it will be necessary to determine whether the non-AT1, non-AT2 binding site is anatomically conserved from the rat to the human brain before making any conclusions regarding its functionality in this and other neurological disease states.
An important aspect of this binding site is the need for the presence of an organomercurial sulfhydryl-reactive agent to unmask its ability to bind Ang II and other angiotensins with AT1 and AT2 selectivity. This suggests that this binding site is a protein with a critical involvement of cysteine residues in its functionality. There is an increasing appreciation for the importance of cysteine oxidation in proteins to regulate the function of proteins. It has been suggested that cysteine may be a reversible “nano-switch” regulator of the functionality of a variety of proteins including transcription factors, molecular chaperones and enzymes, see review (Leichert and Jakob, 2006).
Organomercurial sulfhydryl-reactive agents such as PCMB oxidize the cysteine sulfhydryl of proteins (Karlin and Bartels, 1966; Ying et al., 2007). Since oxidative stress is a major cause of oxidation of protein cysteine side chains (see reviews (Ying et al., 2007)) the unmasking of this non-AT1, non-AT2 binding site by PCMB may represent the effect of oxidative stress in the brain. The ability of sulfhydryl reducing agents (SHRA) such as β-mercaptoethanol or dithiothreitol (DTT) to inhibit the binding of 125I-Ang II to the non-AT1, non-AT2 binding site in the rat brain, presumably by breaking disulfide bonds (Karamyan and Speth, 2007) further supports this concept. Of note, the AT-1 angiotensin receptor also requires intact disulfide bonds to bind Ang II (Chiu et al., 1989; Speth et al., 1991) and is inactivated in the presence of PCMB (Karamyan and Speth, 2007). By contrast, the AT2 receptor shows increased ability to bind Ang II in the presence of SHRA (Speth et al., 1991; Gehlert et al., 1991) and is not inhibited by PCMB (Karamyan, Speth unpublished observations). This suggests that the activity of both of the angiotensin receptors as well as the non-AT1, non-AT2 binding site are redox-sensitive.
Of note, genes encoding enzymes that reduce disulfide bonds and oxidized cysteines are induced by oxidative stress (Barford, 2004). An alternative mechanism for reducing oxidative stress would be for ROS to activate binding proteins capable of sequestering ROS generating signaling agents such as Ang II. Thus one possible function for the non-AT1, non-AT2 binding protein is to serve as a clearance receptor e.g., the C-type atrial natriuretic peptide receptor (Maack et al., 1987; Anand-Srivastava and Trachte, 1993) or transporter to translocate it from the extracellular milieu into cells during times of oxidative stress. If the non-AT1, non-AT2 binding protein is present on neurons this might explain a long-standing controversy regarding the presence of Ang II in neurons. Neurons, which for the most part lack the ability to synthesize angiotensinogen, the precursor of Ang II (Stornetta et al., 1988), possessing such a putative carrier protein could use it to internalize Ang II and subsequently sequester it intracellularly for lysosomal degradation, or for later use as a neurotransmitter.
The distribution of immunoreactive Ang II in the rat brain (Brownfield et al., 1982; Lind et al., 1985) has moderate overlap with the distribution of the non-AT1, non-AT2, binding site (Karamyan and Speth, in press). However, the distribution of Ang II positive cells is primarily in the basal forebrain. Labeling in the caudate nucleus, hippocampus and cerebral cortex is sparse and for the most part limited to nerve terminals. Whether the binding protein acts as a molecular chaperone in neurons delivering Ang II to lysosomes for degradation or to storage granules for subsequent use will require further investigation.
The possibility that this binding site could be an enzyme cannot be eliminated. A previous demonstration of a soluble angiotensin binding protein from liver that was unmasked by PCMB (Kiron and Soffer, 1989; Hagiwara et al., 1989) was later identified as endopeptidase EC 3.4.24.15 (thimet oligopeptidase) and/or EC 3.4.24.16 (neurolysin) (McKie et al., 1993; Kato et al., 1994). However, the inability of the selective EC 3.4.24.15 and EC 3.4.24.16 inhibitor JA-2 (Shrimpton et al., 2000; Smith et al., 2000) to compete for 125I-SI Ang II binding to the non-AT1, non-AT2 binding site in this study argues against this binding site being either of those endopeptidases. In addition, non-angiotensin peptide substrates for EC 3.4.24.15 and EC 3.4.24.16 show low affinity for this binding site (Table 2). Furthermore, this binding site is not present in membranes from liver, adrenal, and kidney of the rat (Karamyan and Speth, 2007) which also argues against it being EC 3.4.24.16. Of note, the non-AT1, non-AT2 binding site is present in the brains of mice in which the gene for EC 3.4.24.11 (neprilysin, neutral endopeptidase) is knocked out (Karamyan et al., in press) suggesting that the non-AT1, non-AT2 binding site is not this peptidase either. However, there are many other enzymes in brain for which Ang II is substrate (Karamyan and Speth, 2007;Speth and Karamyan, 2008) which might be this binding site.
Conclusion
The conserved presence of this novel, non-AT1, non-AT2 binding protein in the human brain suggests that it is a functional component of the brain angiotensin system. Future studies directed at identifying this protein should provide insights into the way in which it affects brain angiotensinergic activity.
Acknowledgments
The authors acknowledge the support of Elizabeth K. Balraj, M.D., and the staff of the Cuyahoga County Coroner's Office, Cleveland, Ohio. The authors also acknowledge the assistance of Gouri Mahajan in identifying and preparing the tissue samples. The authors thank Drs Ron Smith, Ian Smith, Mitchell Avery and Amar Chittiboyina for generously providing losartan, JA-2, and telmisartan used for these studies. Supported in part by NIH grants RR17701 and MH67996, and by the Peptide Radioiodination Service Center of the University of Mississippi.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.lfs.2008.07.003.
References
- Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM, Chai SY. Evidence that the angiotensin IV (AT4) receptor is the enzyme insulin regulated aminopeptidase. Journal of Biological Chemistry. 2001;276:48623–48626. doi: 10.1074/jbc.C100512200. [DOI] [PubMed] [Google Scholar]
- Allen AM, MacGregor DP, Chai SY, Donnan GA, Kaczmarczyk S, Richardson K, Kalnins R, Ireton J, Mendelsohn FA. Angiotensin II receptor binding associated with nigrostriatal dopaminergic neurons in human basal ganglia. Annals of Neurology. 1992;32:339–344. doi: 10.1002/ana.410320306. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacological Reviews. 1993;45:455–497. [PubMed] [Google Scholar]
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Current Opinion in Structural Biology. 2004;14:679–686. doi: 10.1016/j.sbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Steward LJ, Barber PC, Barnes NM. Identification and characterisation of angiotensin II receptor subtypes in human brain. European Journal of Pharmacology. 1993;230:251–258. doi: 10.1016/0014-2999(93)90558-y. [DOI] [PubMed] [Google Scholar]
- Brownfield MS, Reid IA, Ganten D, Ganong WF. Differential distribution of immunoreactive angiotensin and angiotensin converting enzyme in rat brain. Neuroscience. 1982;7:1759–1769. doi: 10.1016/0306-4522(82)90033-1. [DOI] [PubMed] [Google Scholar]
- Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, Albiston AL. The angiotensin IV/AT4 receptor. Cellular and Molecular Life Sciences. 2004;61:2728–2737. doi: 10.1007/s00018-004-4246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- Chiu AT, McCall DE, Nguyen TT, Carini DJ, Duncia JV, Herblin WF, Uyeda RT, Wong PC, Wexler RR, Johnson AL, Timmermans PBMWM. Discrimination of angiotensin II receptor subtypes by dithiothreitol. European Journal of Pharmacology. 1989;170:117–118. doi: 10.1016/0014-2999(89)90145-3. [DOI] [PubMed] [Google Scholar]
- Civelli O. Functional genomics: the search for novel neurotransmitters and neuropeptides. FEBS Letters. 1998;430:55–58. doi: 10.1016/s0014-5793(98)00524-9. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clinical and Experimental Pharmacology and Physiology. 2002;29:261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin–angiotensin system. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiological Reviews. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Ge JA, Barnes NM. Alterations in angiotensin AT(1) and AT(2) receptor subtype levels in brain regions from patients with neurodegenerative disorders. European Journal of Pharmacology. 1996;297:299–306. doi: 10.1016/0014-2999(95)00762-8. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Schober DA. Angiotensin II receptor subtypes in rat brain: dithiothreitol inhibits ligand binding to AII-1 and enhances binding to AII-2. Brain Research. 1991;546:161–165. doi: 10.1016/0006-8993(91)91173-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Sugiura N, Wakita K, Hirose S. Purification and characterization of angiotensin-binding protein from porcine liver cytosolic fraction. European Journal of Biochemistry. 1989;185:405–410. doi: 10.1111/j.1432-1033.1989.tb15129.x. [DOI] [PubMed] [Google Scholar]
- Harding JW, Cook VI, Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, Stobb JW, Swanson GN, Coleman JK, Wright JW. Identification of an AII (3–8) [AIV] binding site in guinea pig hippocampus. Brain Research. 1992;583:340–343. doi: 10.1016/s0006-8993(10)80047-2. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Thunhorst RL. The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Frontiers in Neuroendocrinology. 1997;18(3):292–353. doi: 10.1006/frne.1997.0153. [DOI] [PubMed] [Google Scholar]
- Karamyan VT, Gembardt F, Rabey FM, Walther T, Speth RC. Characterization of the brain-specific non-AT(1), non-AT(2) angiotensin binding site in the mouse. European Journal of Pharmacology. doi: 10.1016/j.ejphar.2008.05.035. (In press) [DOI] [PubMed] [Google Scholar]
- Karamyan VT, Speth RC. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Research. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- Karamyan VT, Speth RC. Distribution of the Non-AT1, Non-AT2 Angiotensin-Binding Site in the Rat Brain: Preliminary Characterization. Neuroendocrinology. doi: 10.1159/000140635. (In press) [DOI] [PubMed] [Google Scholar]
- Karlin A, Bartels E. Effects of blocking sulfhydryl groups and of reducing disulfide bonds on the acetylcholine-activated permeability system of the electroplax. Biochimica et Biophysica Acta. 1966;126:525–535. doi: 10.1016/0926-6585(66)90010-0. [DOI] [PubMed] [Google Scholar]
- Kato A, Sugiura N, Hagiwara H, Hirose S. Cloning, amino acid sequence and tissue distribution of porcine thimet oligopeptidase. A comparison with soluble angiotensin-binding protein. European Journal of Biochemistry. 1994;221:159–165. doi: 10.1111/j.1432-1033.1994.tb18725.x. [DOI] [PubMed] [Google Scholar]
- Kiron MA, Soffer RL. Purification and properties of a soluble angiotensin II-binding protein from rabbit liver. Journal of Biological Chemistry. 1989;264:4138–4142. [PubMed] [Google Scholar]
- Kokje R, Wilson W, Brown T, Karamyan V, Wright J, Speth R. Pressor Actions of Aminopeptidase-Resistant Analogs of Angiotensin II in the Rat Brain: Challenging the Angiotensin III Hypothesis. Hypertension. 2007;49:1328–1335. doi: 10.1161/HYPERTENSIONAHA.107.087130. [DOI] [PubMed] [Google Scholar]
- Leichert LI, Jakob U. Global methods to monitor the thiol-disulfide state of proteins in vivo. Antioxidant and Redox Signaling. 2006;8:763–772. doi: 10.1089/ars.2006.8.763. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten G. Organization of angiotensin II immunor-eactive cells and fibers in the rat central nervous system. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- MacGregor DP, Murone C, Mendelsohn FA. The stability of angiotensin receptors and angiotensin converting enzyme in post mortem brain. Neurochemistry International. 1994;25:413–417. doi: 10.1016/0197-0186(94)90016-7. [DOI] [PubMed] [Google Scholar]
- MacGregor DP, Murone C, Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin II receptor subtypes in the human central nervous system. Brain Research. 1995;675:231–240. doi: 10.1016/0006-8993(95)00076-3. [DOI] [PubMed] [Google Scholar]
- McKie N, Dando PM, Rawlings ND, Barrett AJ. Thimet oligopeptidase: similarity to 'soluble angiotensin II-binding protein' and some corrections to the published amino acid sequence of the rat testis enzyme. Biochemical Journal. 1993;295:57–60. doi: 10.1042/bj2950057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Current Hypertension Reports. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Sumners C. Angiotensin II in central nervous system physiology. Regulatory Peptides. 1998;78:1–11. doi: 10.1016/s0167-0115(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Iturrioz X, Fassot C, claperon C, Roques BP, Llorens-Cortes C. Role of angiotensin III in hypertension. Current Hypertension Reports. 2005;7:128–134. doi: 10.1007/s11906-005-0087-z. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cellular and Molecular Neurobiology. 2005;25:485–512. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RA, Simoes e Silva A, Maric C, Silva DM, Machado RP, de IB, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angioten-sin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton CN, Abbenante G, Lew RA, Smith I. Development and characterization of novel potent and stable inhibitors of endopeptidase EC 3.4.24.15. Biochemical Journal. 2000;345(Pt 2):351–356. [PMC free article] [PubMed] [Google Scholar]
- Smith AI, Lew RA, Shrimpton CN, Evans RG, Abbenante G. A novel stable inhibitor of endopeptidases EC 3.4.24.15 and 3.4.24.16 potentiates bradykinin-induced hypotension. Hypertension. 2000;35:626–630. doi: 10.1161/01.hyp.35.2.626. [DOI] [PubMed] [Google Scholar]
- Speth RC, Harding JW. Radiolabeling of Angiotensin Peptides. In: Wang DH, editor. Angiotensin Protocols. Totowa, NJ: Humana Press; 2001. pp. 275–295. [DOI] [PubMed] [Google Scholar]
- Speth RC, Karamyan VT. The significance of brain aminopeptidases in the regulation of the actions of angiotensin peptides in the brain. Heart Failure Reviews. 2008;13:299–309. doi: 10.1007/s10741-007-9078-2. [DOI] [PubMed] [Google Scholar]
- Speth RC, Rowe BP, Grove KL, Carter MR, Saylor DL. Sulfhydryl reducing agents distinguish two subtypes of angiotensin II receptors in the rat brain. Brain Research. 1991;548:1–8. doi: 10.1016/0006-8993(91)91098-l. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Hawelu-Johnson CL, Guyenet PG, Lynch KR. Astrocytes synthesize angiotensinogen in brain. Science. 1988;242:1444–1446. doi: 10.1126/science.3201232. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Ogihara T, Morishita R. The renin–angiotensin system, hypertension and cognitive dysfunction in Alzheimer's disease: new therapeutic potential. Frontiers in Bioscience. 2008;13:2253–2265. doi: 10.2741/2839. [DOI] [PubMed] [Google Scholar]
- Veerasingham SJ, Raizada MK. Brain renin–angiotensin system dysfunction in hypertension: recent advances and perspectives. British Journal of Pharmacology. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? Journal of Cardiovascular Pharmacology. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Brain angiotensin receptor subtypes AT(1), AT(2), and AT(4) and their functions. Regulatory Peptides. 1995;59:269–295. doi: 10.1016/0167-0115(95)00084-o. [DOI] [PubMed] [Google Scholar]
- Wright JW, Krebs LT, Stobb JW, Harding JW. The angiotensin IV system: functional implications. Frontiers in Neuroendocrinology. 1995;16:23–52. doi: 10.1006/frne.1995.1002. [DOI] [PubMed] [Google Scholar]
- Ying J, Clavreul N, Sethuraman M, Adachi T, Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radical Biology in Medicine. 2007;43:1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Progress in Biophysics and Molecular Biology. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]