Summary
Many nervous system pathologies are associated with increased levels of Apolipoprotein D (ApoD), a lipocalin also expressed during normal development and aging. An ApoD homologous gene in Drosophila, Glial Lazarillo, regulates resistance to stress, and neurodegeneration in the aging brain. Here we study for the first time the protecting potential of ApoD in a vertebrate model organism. Loss of mouse ApoD function increases the sensitivity to oxidative stress and the levels of brain lipid peroxidation, and impairs locomotor and learning abilities. Human ApoD over-expression in the mouse brain produces opposite effects, increasing survival and preventing the raise of brain lipid peroxides after oxidant treatment. These observations, together with its transcriptional up-regulation in the brain upon oxidative insult, identify ApoD as an acute response protein with a protective and therefore beneficial function mediated by the control of peroxidated lipids.
Keywords: lipocalin, oxidative stress, learning, locomotor behavior, paraquat, lipid peroxidation
Introduction
Apolipoprotein D (ApoD) is a Lipocalin, an ancient family of small proteins engaged in a diverse array of physiological processes, whose general molecular function is binding and transporting hydrophobic ligands. ApoD is known to bind arachidonic acid, progesterone, retinol, cholesterol and other lipophilic ligands. Elevations in its expression level have been associated with a number of pathological and tissue damage conditions including neurodegenerative diseases. Its levels increase as well during physiological aging (see Rassart et al. 2000; Van Dijk et al. 2006 for review). Although the correlation between those states and increased gene expression is consistent, the causal link with ApoD function is still unclear. Whether ApoD is one of the factors contributing to the degenerative or aging processes, or its function is part of a defense mechanism providing protection to cells must be solved.
Our previous work on an ApoD homologue in insects, the Drosophila Glial Lazarillo (GLaz), shows that it regulates longevity, resistance to stress, locomotor behavior, and degeneration in brain and other tissues upon aging (Sanchez et al. 2006; see also Walker et al. 2006). We therefore undertook a genetic analysis of the ApoD function in a vertebrate model organism by generating knockout and transgenic over-expressing mice.
Results and Discussion
An ApoD knockout mouse (ApoD-KO) was generated by standard homologous recombination techniques. We replaced the wild-type ApoD gene with a copy interrupted by the insertion of the neomycin resistance gene (Neo) in the opposite direction to ApoD transcription (Fig. 1S A), which rendered a transcriptional null mutant (Fig. 1S B–D) without deleting any genomic fragment potentially containing regulatory regions. We also generated transgenic mice over-expressing the human orthologue of ApoD (HApoD) under the control of the neuronal human Thy-1 promoter (HApoD-Tg line; Fig. 2S A), thus expressing HApoD in neurons in all regions of the nervous system (Fig. 2S B–E). General features of ApoD-KO and HApoD-Tg mice are described in Supporting Information available online.
Both ApoD-KO and HApoD-Tg mice are viable and breed normally. Three independent lines were obtained for the Thy-1 transgenic, but a single line was used in this work due to their similar phenotypes based on general health, and biochemical and molecular characterization (Do Carmo & Rassart, unpublished observations). No apparent genotypic differences were observed during development when comparing sibling ApoD-KO and WT mice (see Supporting Online Information). We therefore turn to study ApoD function during adulthood, both in normal conditions, and when exposing the organism to oxidative stress situations mimicking the many pathological situations in which ApoD is up-regulated.
ApoD alters locomotor activity and learning abilities in young adult animals
Open field analysis revealed a significant decrease in horizontal and vertical locomotor activity in ApoD-KO mice (25% and 36% mean reduction in horizontal and vertical activity respectively; Fig. 1A, left and right panels), while no differences were observed in anxiety related parameters (relative time spent in the center vs. periphery of the open field arena; Fig. 1A central panel). On the other hand, over-expression of human ApoD causes an increase in vertical activity (number of rearings, Fig. 1B, left panel), while differences in horizontal activity were not significant (Fig. 1B, right panel).
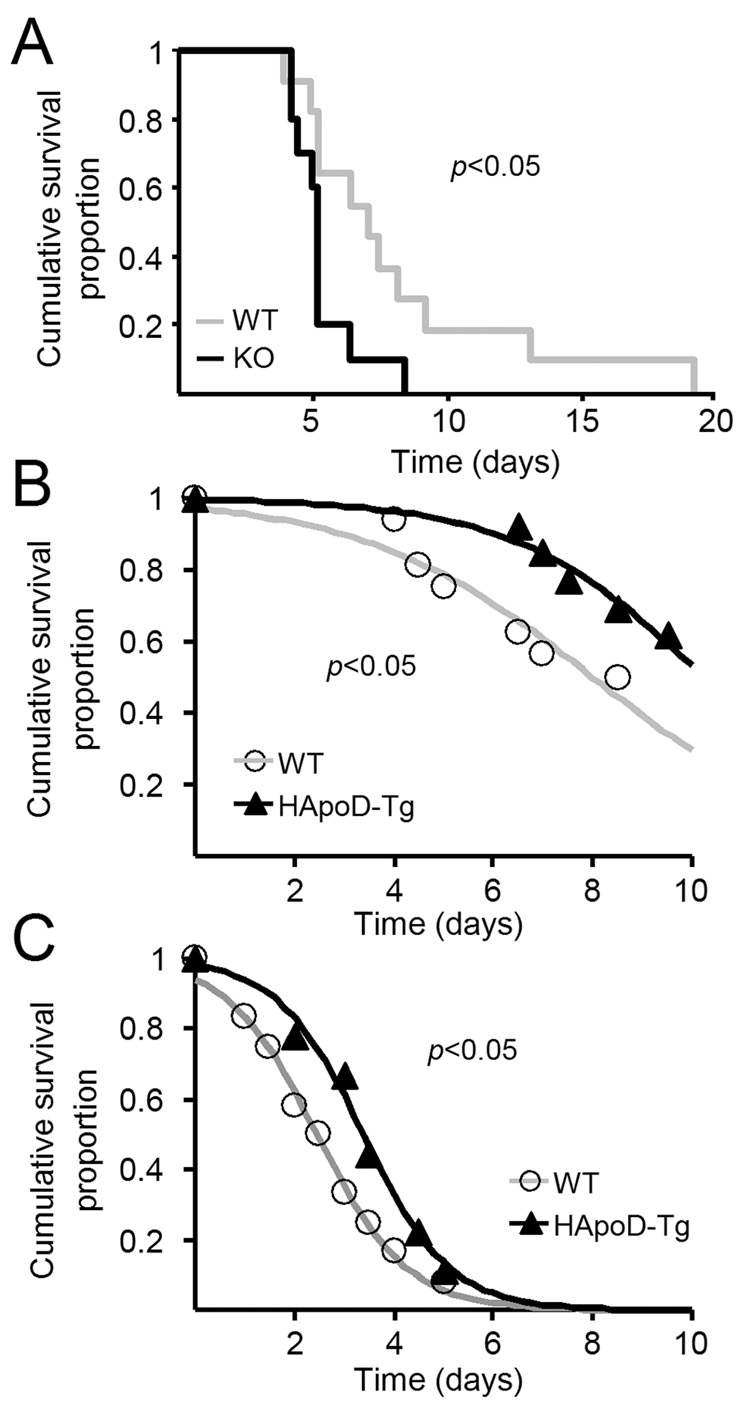
Figure 1. Alterations in behavioral output in ApoD-KO and HApoD-Tg mice.
(A) Open field test on locomotor exploratory behavior in ApoD-KO mice. Both horizontal (number of squares visited) and vertical (number of rearings) activities are decreased in the ApoD-KO mice, while an anxiety-related behavior (exploration of the center of the 1 m2 arena) is not altered. N= 12 mice/genotype (6th backcross generation onto C57BL/6 background). Unpaired two-sided Student’s t-test, *p < 0.05, **p < 0.01.
(B) Open field test in HApoD-Tg mice. Vertical activity increase in HApoD-Tg mice (left panel), while horizontal activity differences are not significant (right panel). A smaller arena was used in this case, and the anxiety parameter was not measured. WT: N = 16; HApoD-Tg: N = 13 (11th backcross generation onto C57BL/6 background). Unpaired two-sided Student’s t-test, *p < 0.05.
(C) Motor learning abilities were tested as the increment of the Rotarod test score over a 2 hours interval, and is represented normalized to the score of the first test. ApoD-KO mice show a lower learning ability under normal conditions. N= 14 mice/genotype (11th backcross generation onto C57BL/6 background). Sign non-parametric test, *p < 0.05.
(D) Barnes maze test on spatial learning. ApoD-KO mice fail to increase their rate of success in finding a safe escape hole, while wild type mice learn and remember using distal spatial clues. Mice were tested daily for 70 days. Results from each five consecutive daily sessions were combined in blocks. N= 6 mice/genotype (5th backcross generation onto C57BL/6 background). ANOVA test, p < 0.0001.
(A–D) Data are represented as mean ± SD.
Visual ability, assessed by darkness preference and object recognition, was not significantly affected. Other behavioral tests exploring pain and touch sensitivity, gait, and muscular strength gave no significant differences between ApoD-KO mice and their wild-type littermates. Similarly, the walking pole test exploring sensory-motor coordination gave no differences in ApoD-KO nor HApoD-Tg mice (see Supporting Online Material).
ApoD-KO mice and their wild-type control perform equally well in the Rotarod test (also exploring sensory-motor coordination) when first tested on the accelerated rod after a set of training sessions. However, a parameter that estimates the ability to learn a motor task (improvement of performance over consecutive trials) was much smaller in the ApoD-KO mice (Fig. 1C).
Hippocampal-dependent memory was assessed with the spatial version of the Barnes maze. Wild-type mice showed an average learning time of 64 days, while none of the ApoD-KO mice were able to meet the learning criteria (7 out of 8 sessions with 3 or less errors) for the test period of 70 days (Fig. 1D).
Therefore, mice lacking ApoD expression reduce their locomotor and exploratory activity, while over-expression of HApoD in the brain renders the animals slightly more active. In addition, ApoD-KO mice show deficits in learning either a motor task or an orientation-based task (dependent on visual cues). Decreased locomotor performance was also shown for Drosophila GLaz null mutants (Sanchez et al. 2006), illustrating interesting functional conservation of these homologous genes throughout evolution.
Resistance to experimentally induced oxidative stress is undermined in the absence of ApoD and increased by the over-expression of human ApoD
The regulation of ApoD in physiological and pathological states where reactive oxygen species (ROS) are generated (reviewed in Rassart et al. 2000), and the higher sensitivity to oxidative stress experienced by the Drosophila GLaz mutants (Sanchez et al. 2006) motivated us to test the overall survival of ApoD-KO and HApoD-Tg mice exposed to an oxidative insult. Sensitivity to the ROS generator 1,1’-dimethyl-4,4’-bypiridinium (paraquat; PQ) was assayed using different paradigms of intraperitoneal injections (see Methods in Supporting Online Material). The ApoD null mutant mice show a higher sensitivity to PQ-induced chronic oxidative stress than wild-type controls (Fig. 2A). The survival curves show a reduction in both the median and the maximum lifespan (26% and 56% respectively, Fig. 2A) reflecting an early onset of functional decline in the ApoD-KO mice. This effect is observed independently of the genetic background (C57BL6/J or Balb/C), indicating that the reduction observed is originated by the lack of ApoD.
Figure 2. Oxidative stress-compromised survival is decreased in the absence of ApoD and increased when human ApoD is over-expressed in the mouse brain.
(A) Survival analysis of mice treated daily with the ROS generator paraquat (15 mg/kg of body weight; Protocol A). KO and WT genotypes in C57BL/6 background (8th backcross generation) are compared. N= 11 mice/genotype. The absence of ApoD shortens the mice survival (Gehan-test, p = 0.026; Cox’s F-test, p = 0.028). A decrease of 26% in mean lifespan and 56% in maximum lifespan is observed. The survival phenotype is stable after changing the genetic background (Balb/C, 8th outcross generation) with 11% decrease in mean lifespan and 9% in maximum lifespan. N= 12 mice/genotype. Gehan-test, p = 0.031; Cox’s F-test, p = 0.043 (curve not shown).
(B and C) Survival analysis of WT and transgenic (HApoD-Tg) mice treated with single PQ doses of 30 (B) or 50 (C) mg/kg of body weight (Protocol B). Death occurrence was recorded up to 10 days. Surviving animals were sacrificed at day 10. Data was fitted to a Gompertz function. (B) Over-expression of ApoD increases the mice survival. WT: N = 16; HApoD-Tg: N = 13. Gehan-test, p = 0.029; Cox’s F-test, p = 0.043. (C) Over-expression of ApoD also protects mice at higher doses of paraquat. WT: N = 12; HApoD-Tg: N = 9. Gehan-test, p = 0.045; Cox’s F-test, p = 0.033. All transgenic mice are backcrossed on the C57Bl/6 background (11th generation).
These results will predict that mice over-expressing ApoD would be more protected against the stress produced by PQ. Indeed, HApoD-Tg mice showed improved survival upon single systemic exposures to PQ at two different concentrations, with an increase in median survival time of 41.6 and 27.5% respectively (Fig. 2B,C). Thus the human ApoD protein is able to exert a protective role when expressed in the mouse brain. This general effect on survival might be due to either the presence of the neuron-expressed ApoD in the systemic circulation, or to low levels of non-neuronal ApoD expression in other tissues.
The protective effect of ApoD is also consistent with the results obtained in Drosophila over-expressing the endogenous ApoD homologue GLaz (Walker et al. 2006) or the human ApoD gene (Muffat et al. personal communication). All these results strongly suggest that the protecting role in oxidative stress-compromised survival is a common factor in all ApoD homologues, reflecting that it is probably part of the ancestral function of this lipocalin. Therefore, the analyses in model organisms can be very useful to understand the human ApoD function.
ApoD expression is induced in the brain upon experimental oxidative stress
The up-regulation of ApoD in ROS-related pathological conditions predicts that an experimentally produced oxidative stress would also regulate ApoD mRNA expression in the mouse. Since ApoD is up-regulated in neurological pathologies, and our behavioral studies in the mouse indicate that nervous system functionality is compromised in the absence of ApoD, we assayed the transcription of ApoD in mouse brain after a single exposure to PQ. An acute up-regulation of ApoD (1.86-fold induction) is detected 3 hours after exposure, and the expression returns to baseline by 24 hours (Fig. 3A). Similar transient effects of PQ on gene transcription have been shown in lung and kidney for several genes with antioxidant roles (Tomita et al. 2006a; Tomita et al. 2006b). We therefore assayed the transcription of ApoD in several tissues 3 hours after PQ exposure, when the expression peaks in the brain. No up-regulation is produced in the lung or the liver, while the brain tissue shows a strong ApoD induction upon PQ treatment (Fig. 3B). These results suggest a specific function of ApoD in the response of the nervous system to oxidative injury.
Figure 3. The endogenous mouse ApoD is transiently up-regulated by exposure to PQ while the human transgene is expressed constitutively in the brain.
(A) Northern blot analysis of mouse ApoD mRNA shows a transient increase in response to a single injection of paraquat (30 mg/kg; Protocol B). Quantification of mRNA expression by band densitometry. Values were normalized with the GAPDH gene. Error bars represent SD (N=3).
(B) Mouse ApoD is specifically up-regulated in the brain upon acute PQ treatment (3 hours after a single PQ dose; 30mg/kg; Protocol B). No induction is observed in liver or lung. The GAPDH gene was used as a control.
(C) Northern blot analysis shows a comparable expression of the mouse ApoD endogenous mRNA in the brain of WT and HApoD-Tg mice 3 hours after a single PQ dose (30mg/kg; Protocol B), and the absence of response to PQ of the human transgene. The GAPDH gene was used as a control.
The higher sensitivity to oxidative stress of mice lacking ApoD parallels our results obtained in the Drosophila GLaz null mutant (Sanchez et al. 2006) and in null mutants of the ApoD homologue in plants (Frenette-Charron et al. personal communication) which are more sensitive to oxidative and other forms of stress. We can therefore predict that the regulation of these lipocalin genes must also be conserved (a transient up-regulation of GLaz also occurs in Drosophila, our unpublished observations also confirmed by J. Muffat et al. personal communication), and that the ApoD up-regulation in the mouse brain upon PQ-induced ROS generation is part of the normal protective response to stress.
The increased survival of the transgenic animals over-expressing human ApoD in the nervous system should be due to a higher and longer-lasting presence of ApoD in the tissues. Since the human transgene is placed in a wild-type genetic background (C57BL6/J mice), we measured the expression level of the mouse endogenous ApoD gene in the brain of transgenic animals. Mouse ApoD transcript levels are not affected by the presence of the human transgene either under normal conditions or upon PQ treatment. As expected, the human transgene does not show a response by PQ (Fig. 3C).
ApoD controls the levels of brain lipid peroxidation under normal conditions
PQ treatment is known to generate protein and lipid peroxidation (Beal 2002). By evaluating the oxidation state of these macromolecules in the brain, both in the control situation and in the presence of PQ-induced oxidative stress, we should gain insight into the biochemical actions of ApoD in the brain tissue when it is exerting the protecting effects described above at the organism level.
We assayed lipid peroxidation levels upon the different paradigms used for PQ injection. In agreement with McCormack et al. (McCormack et al. 2005), lipid peroxide adducts accumulate in the wild type brain over time at a relatively slow pace (Fig. 3S A). As it is the case for tissues like lung, kidney and liver (Sato et al. 1992), no increase in lipid peroxidation is observed in the brain 3 hours after a single high dose of PQ (acute PQ treatment, protocol B), while a low-dose chronic treatment (protocol C) does produce a significant increase after two weeks. In contrast, protein carbonylation, a marker of oxidative modification of proteins, is much faster, showing a patent increase by 3 hours upon acute treatment (Fig. 3S B).
In control conditions (sham injection, Fig. 4A) we observe that the lack of ApoD results in increased levels of lipid peroxides in the brain, but not in the lung, in accordance with the normal tissue distribution of ApoD. Moreover, as expected for a lipid managing lipocalin, the effect is specific for lipids, since no alterations are seen in protein carbonylation. On the other hand, the amount of lipid peroxidation in the brain of HApoD-Tg mice is similar to the wild-type levels (Fig. 4C), indicating that an excess of human ApoD cannot reduce the basal levels of oxidation products related to normal cellular metabolic activity. As expected, lung lipid peroxidation levels are also unchanged by the human transgene (not shown).
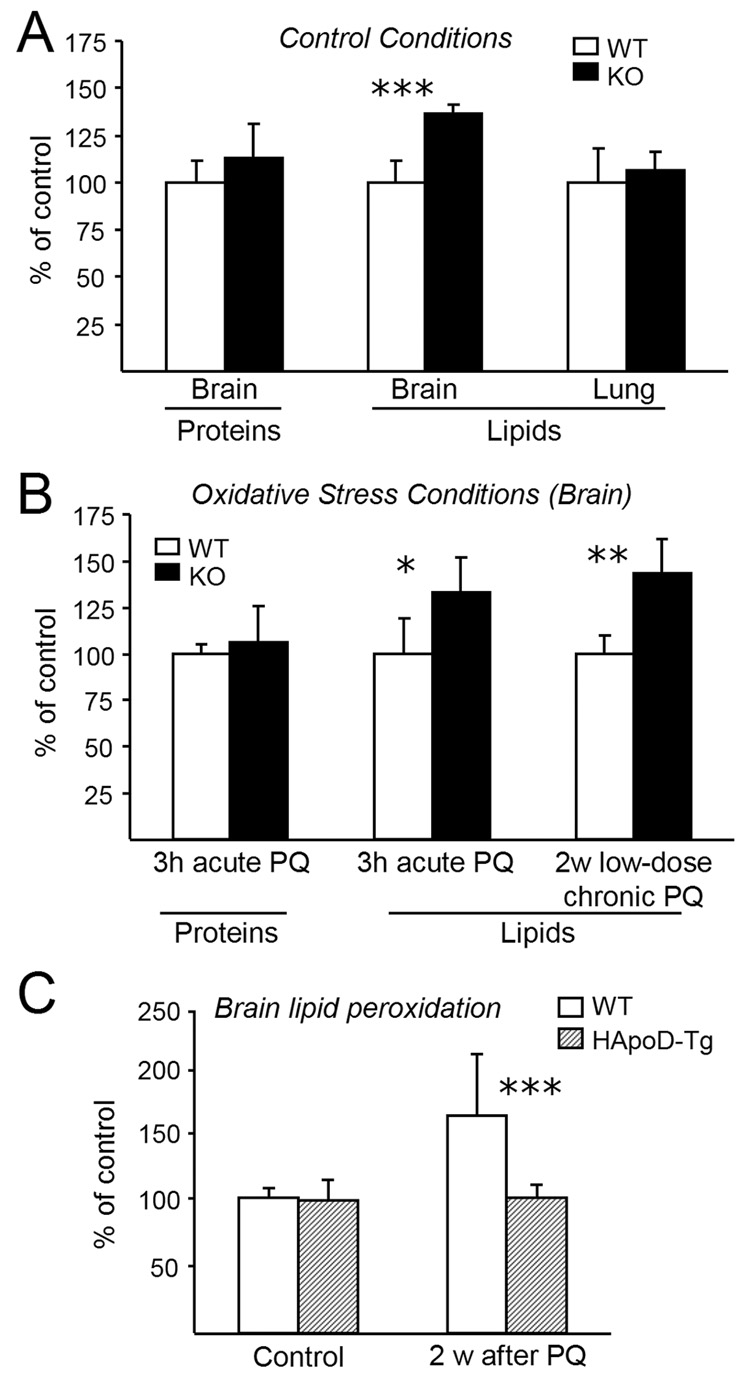
Figure 4. Loss of ApoD function specifically alters lipid peroxidation in the brain and an over-dose of human ApoD prevents their accumulation upon oxidative insult.
(A) Oxidation of proteins (Carbonyls-ELISA assay) and lipids (TBARS assay) in control conditions (sham injection of PBS). Lipid peroxidation, and not protein carbonylation, is increased in the ApoD-KO brains, while lipid peroxidation in the lung remains unchanged.
(B) Brain oxidation status assayed upon acute (3 hours, Protocol B) or chronic (2 weeks low-dose, Protocol C) exposure to paraquat. ApoD-KO mouse brain show a higher level of lipid peroxidation while protein carbonylation levels do not change with genotype.
(A and B) N= 7 mice/genotype/sex (11th backcross generation onto C57BL/6 background). Data are represented as mean ± SD normalized to the wild-type value. Unpaired two-sided Student’s t-test, *p < 0.05, **p < 0.01 ***p < 0.001.
(C) Analysis of lipid peroxidation two weeks after PQ treatment (30 mg/kg of body weight). Human ApoD expression prevents the rise of oxidized lipids accumulation in the brain. WT: N = 16; HApoD-Tg: N = 13 (11th backcross generation onto C57BL/6 background). Unpaired two-sided Student’s t-test, ***p < 0.001. Data are represented as mean ± SD normalized with respect to the control values (sham injection of PBS).
An increased level of lipid peroxidation under basal conditions was also observed in the GLaz null mutant fruit flies (Sanchez et al. 2006). These results suggest that ApoD and its homologues can condition the peroxidation state of lipids, even in normal non-stress situations. The maintenance of cellular membrane integrity can be expected to be compromised in the ApoD-KO and, since ApoD absence is particularly noticed in the brain, it can result in neural functional alterations that we are able to detect by behavioral tests.
The data by Thomas and Yao (Thomas & Yao 2006) also support the above proposal. They performed a brain lipid profile analysis in our ApoD-KO mice to understand the role of ApoD in the mechanisms of antipsychotic drug action. Their results in the control animals (without drug addition) show that ApoD-KO brains have increased levels of some polyunsaturated fatty acids (dienes and hexanenes). Since polyunsaturated fatty acids are more prone to peroxidation, the susceptibility of membranes to peroxidation (Hulbert 2005) must be increased in the ApoD-KO brains, and therefore membranes could be more susceptible to the effects of normal oxidative metabolism.
ApoD is able to prevent the lipid peroxidation increase upon oxidative insult
Upon oxidative insult (either acute or low-dose chronic PQ treatment) lipid peroxidation increases (33% and 43% respectively) over the wild-type levels in the ApoD-KO brain (Fig. 4B). This change can be correlated with the decrease in survival time observed in the ApoD-KO mice upon PQ treatment.
Again, no genotype-dependent differences in protein carbonylation were observed under PQ treatment (Fig. 4B), suggesting that the function of ApoD is confined to lipid management.
On the other hand, human ApoD over-expression abolishes the long-term accumulation of lipid peroxides after 2 weeks of PQ treatment, maintaining the levels at control values (Fig. 4C).
These results show that an excess of human ApoD in the brain can counteract the exogenously-induced oxidative damage to lipids. Therefore, we can expect that a supplement of ApoD might be beneficial for human conditions where lipid oxidation is a key factor in the functional decline associated to physiological aging or neural pathologies.
Lipocalins as part of the oxidative stress response system
Our data support the notion that ApoD is part of the mechanisms regulating protection from diverse forms of stress, including oxidation.
We show in this report that ApoD specifically controls the levels of lipid peroxidation in an organ as vulnerable as the brain. The structural property of ApoD shared with all other lipocalins is the presence of a binding pocket that can bind diverse molecules, mostly hydrophobic (Flower et al. 2000; Akerstrom et al.. Eds.) (2006), therefore it is probable that its mechanism of action involves this lipid-binding property. The control of lipid peroxidation can be exerted directly, either a priori by preventing the oxidation of bound lipids, or a posteriori by removing peroxidated lipids from membranes, and therefore avoiding the positive feedback loops leading to more oxidative damage. Alternatively, the control of lipid oxidation can be a consequence of the regulation of lipid composition in membranes, which will determine the susceptibility of membranes to peroxidation.
Scavenging roles have been proposed for other lipocalins. Lcn-1 (tear lipocalin), expressed in secretory glands, has been shown to bind lipid peroxidation products in vitro and is also up-regulated upon oxidative stress in an epithelial cell line (Lechner et al. 2001). α-1 microglobulin is able to reduce and then trap ABTS radicals by covalent binding (Akerstrom et al. 2007).
However, can our data also be compatible with a direct antioxidant activity of ApoD?
The antioxidant defense system is currently known to comprise low molecular mass agents (e.g. glutathione or melatonin), iron or copper sequestration proteins (such as transferrin, ferritin, or albumin) and antioxidant enzymes (like superoxide dismutase or catalase). With the data available so far, ApoD belongs to none of these categories. While other lipocalins are well known for their iron sequestration properties (Goetz et al. 2002; Flo et al. 2004), binding to toxic heme (Allhorn et al. 2002) or antioxidant catalytic activities (Allhorn et al. 2005), neither metal sequestration properties nor enzymatic activity have been shown for ApoD, or its orthologues in fruitflies or plants.
The lipocalin α-1 microglobulin (Allhorn et al. 2002; Allhorn et al. 2005; Akerstrom et al. 2007) exerts reductase and dehydrogenase activities through a free cysteine group located in a flexible loop. ApoD has four cysteine residues that form two disulfide bonds at the core of the protein fold in all species studied so far. However, human ApoD has an extra cysteine (Cys116) known to bind covalently to Apolipoproteins A-II and B-100 (Blanco-Vaca et al. 1992). Whether the thiol group of Cys116 confers additional antioxidant properties to human ApoD remains to be investigated. If this were the case, then this property would be human-specific. In order to explain the results obtained in mouse (this study), fly (Sanchez et al. 2006) and plants (Frenette-Charron et al. personal communication), additional antioxidant properties of ApoD that are conserved among these divergent species must be invoked. Moreover, the fact that only lipid peroxidation, but not protein carbonylation, is altered in the absence of ApoD argues against a direct antioxidant activity of mouse ApoD.
The oxidative modification of membrane lipids also triggers intracellular signaling cascades that direct the cell fate after an oxidative insult. Particularly, arachidonic acid (AA) is known to be mobilized from membranes upon oxidative stress (Balboa & Balsinde 2006). ApoD is able to bind a variety of lipidic ligands (Rassart et al. 2000; Breustedt et al. 2006; Eichinger et al. 2007) of which the highest affinity is shown for AA. Data supporting the participation of ApoD in regulating the mobilization of AA has been shown in a cell culture system (Thomas et al. 2003b), and both ApoD and AA are regulated in patients with schizophrenia in response to antipsychotic drug treatment (Thomas et al. 2001; Thomas et al. 2003a). The lipid analysis of ApoD-KO brains upon treatment with clozapine (Thomas & Yao 2006) has shown that the absence of ApoD alters the levels of AA upon drug treatment (even though levels are not modified by the mutation in basal conditions). Thus by modulating important signaling lipids like AA, ApoD can influence the patient’s response to different treatments.
All data available so far point to a common biochemical function for ApoD, directly linked to membrane physiology, which is essential for a proper nervous system function. By controlling the levels of lipid peroxidation ApoD could influence parameters as important as neurotransmitter release, in turn affecting learning abilities or locomotor activity (Vajragupta et al. 2000) throughout life. Likewise, by performing the same biochemical function, ApoD can control the rate of brain functional decline upon normal aging or in pathological conditions, although it is unlikely to be the only such factor.
Functional significance of ApoD: its influence on normal and pathological nervous system decline upon aging
We have shown that ApoD expression in the mouse brain is boosted by experimental oxidative stress, mimicking what happens in many human neurodegenerative and psychiatric diseases, nervous system injuries, as well as during normal aging. The main known effect of lacking ApoD is to become more vulnerable to stress, while an ApoD overdose makes animals less sensitive to the same stress. The fact that the human ApoD gene can produce effects that oppose those observed in the ApoD-KO mouse strongly suggests a conserved function, and therefore the utility of the mouse model to test potential therapeutic uses of ApoD. This study is the first showing that an excess of ApoD results in seemingly beneficial effects upon experimental oxidative stress in the mouse. Testing the effects of over-expressing ApoD in mouse models of disease should therefore become a priority.
Experimental procedures
Animals
The experimental procedures were approved by the Animal Care and Use Committees of the University of Utah, University of Valladolid and Université du Québec à Montréal, and were in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2001). Mice were maintained in positive pressure-ventilated racks at 25±1°C with a 12 h light/dark cycle, fed a standard rodent pellet diet (Global Diet 2014; Harlan), and allowed free access to filtered and UV-irradiated water. Experiments were carried out with 3–5 months old animals of mixed sexes.
Targeted disruption of the mouse ApoD gene
In order to silence the mouse ApoD gene we constructed a targeting vector with the neomycin phosphotransferase (Neo) gene interrupting exon 6 of the ApoD gene (Fig. 1S A). G418r-GANCs 129/SvJ ES cells were transfected with the construct and tested for the presence of the disrupted ApoD allele by BamHI digestion of their genomic DNA followed by Southern hybridization with an external probe comprising exon 4 (Fig. 1S A). Genomic DNA sequence of the region surrounding the targeting vector confirmed the proper homologous recombination of the positive lines injected into female mouse blastocysts at the U. of Utah Transgenics Facility. Subsequent crosses with the chimeric mice showing germline transformation of the targeted allele generated heterozygous ApoD+/− mice that were further confirmed by Southern blot with a 3’ probe (Fig. 1S A). Standard genotyping was performed from tail DNA and PCR with Neo and ApoD-specific primers: ApoD-for: 5’ CCA CCG GCA CCC TAC TGG ATC 3’; ApoD-rev: 5’ CGG GCA GTT CGC TTG ATC TGT 3’; and Neo-for: 5’ CGA TTG TCT GTT GTG CCC AGT 3’.
Phenotype stability was assessed by backcrossing and outcrossing the ApoD mutated allele into the C57BL6/J and Balb/C genetic backgrounds respectively. The mutant allele was selected for ten generations of crosses with the corresponding wild type females. An additional generation was performed by crossing mutant females with wild type males to introduce the Y chromosome of the wild type strain.
Generation of human ApoD transgenic mouse
The human ApoD transgenic mouse (HApoD-Tg) carries a construct (~ 4.5 kb) containing the promoter, the first exon, the first intron and the 5’ non-coding region of the second exon of the human Thy-1 gene (generous gift from J. Silver, New York University Medical Center). This fragment was fused to the human ApoD coding sequence followed by the bovine growth hormone (BGH) polyadenylation signal (Fig. 2S A). The presence of the transgene was verified by PCR using an ApoD-specific primer (5’ CCC AAT CCT CCG GTG CAG GAG AA 3’) and a BGH-specific primer (5’ GAA GGC ACA GTC GAG GCT GAT CAG 3’), producing a 0.6 kb fragment (Fig. 2S B). The HApoD-Tg mice used in this study were backcrossed onto C57/BL6 wild-type mice as explained above.
RNA extraction and northern blot analysis
Animals were euthanized by inhalation of CO2/O2 (2:1). Tissues were collected, frozen in dry ice, and kept at −80°C until extraction of total RNA using the Trizol reagent™ (Invitrogen). Total RNA (10µg) was separated on 1.5% (wt/vol) agarose-formaldehyde gels and blotted to a nylon membrane. The membranes were hybridized with [α-32P]dCTP-labeled mouse or human ApoD, or GAPDH cDNAs, exposed to Biorad Imaging screen K and revealed with a PhosphorImager (Biorad Molecular Imager FX) and Quantity One software (Biorad). For each value, the optical density measured for each gene tested was divided by that of the GAPDH mRNA.
Protein extraction and western blot analysis
Frozen tissues were thawed on ice and homogenized in lysis buffer (50 mM Tris-HCl pH 7.3, 150 mM NaCl, 5 mM EDTA, 0.2% Triton X-100, and 10% Complete protease inhibitors (Roche)). After 30 min of incubation at 4°C, lysates were sonicated and cleared by centrifugation. The protein concentration was determined using a protein assay reagent (Bio-Rad Laboratories). All extracts were stored at −80°C.
For western blot analysis, protein extracts (10µg) were separated on a 12% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. A prestained size marker (Biorad prestained SDS-PAGE standard, low range) was included in each run. Membranes were blocked in PBS containing 0.2% Tween-20 and 5% skim milk powder before incubation with the primary antibodies diluted as follows: polyclonal anti-human ApoD (Caro2), 1:4000; anti-GAPDH, 1:4000. Subsequently, the blots were incubated under gentle agitation at room temperature with a HRP-conjugated secondary antibody diluted 1:5000 in blocking buffer. The blots were developed using the enhanced chemoluminescence method (Amersham-Pharmacia) with X-ray film.
Behavioral analyses
Open field tests in the ApoD-KO and their wild type controls were performed by video recording in a 1m2 arena for a single session of 10 min. A total of 6 mice of each genotype and sex were analyzed. The subsequent analysis explored horizontal (Number of squares visited) and vertical (Number of rearings) activities, as well as anxiety, measured as relative time spent in the central area of the arena. Horizontal and vertical activity was measured in the HApoD-Tg mice and control littermates in an open field arena measuring 30 × 44.5 cm.
A novel-object recognition test was used to assay for visual ability and short-term memory recognition. Mice habituated to the test arena are presented with two plastic objects of different shapes. The object recognition was evaluated by the ratio of time spent exploring the novel vs. the familiar object 10 sec and 1 min after first exposure.
Hippocampal-dependent memory was explored in six WT and six ApoD-KO mice subjected to the spatial version of Barnes maze test (Barnes 1979), where the mouse needs to learn and remember distal cues in the environment, and associate them with the location of an escape tunnel. The mice were tested daily for 70 days, and the number of holes searched, perseverations and time was recorded. The average for each genotype was recorded and grouped into session blocks of 5 testing days.
Rotarod analysis was performed in a Ugo Basile (Varese, Italy) apparatus with acceleration from 4 to 40 rpm over 5 minutes. Each mouse was subjected to two training sessions separated by a 15 min rest. Each session consisted of a 2 min period of exposure to the rod rotating at minimum speed (4 rpm). After another 15 min resting period a first test was performed, consisting of three runs with the rod accelerating from the starting 4 rpm speed. Time of permanence in the rod was recorded. A second test of three runs was performed 2 hours later, after the mice had been injected intraperitoneally either with PBS or paraquat (see Protocol B below).
Other tests such as pain and touch sensitivity, gait, and muscular strength were performed as detailed in (Crawley 2000).
Oxidative stress toxicity by 1,1’-dimethyl-4,4’-bypiridinium (paraquat)
The following protocols of doses, administration patterns and analysis have been used:
Protocol A
Chronic paraquat (PQ) treatment. Mice were daily injected intraperitoneally with a solution of PQ in phosphate buffered saline (PBS) at 15 mg/kg of body weight. Death occurrence was scored at least every 12 hours for the survival analysis. Morphological analyses were performed on brains from mice euthanized on day 5 of treatment (when the ApoD-KO mice survival is at the 50% level).
Protocol B
Acute PQ treatment. A single dose of either 30 or 50 mg/kg of PQ was injected as described above. Behavioral tests were performed just before injection (t =0), 1.5 hours after injection, and 24 hours after injection. Mice were then euthanized either 3 hours or 24 hours post-injection, and their brains extracted for biochemical and immunohistochemical analyses.
Protocol C
Low-dose, neurotoxic chronic PQ treatment. Following Thyruchelvam et al. (Thiruchelvam et al. 2000), mice were injected twice a week, for a total of 2 weeks, with a 15 mg/kg of PQ as described before. Animals were euthanized 3 hours after the last injection, and their brains extracted for biochemical and immunohistochemical analyses. We confirmed the absence of lung toxicity by histopathological analysis looking for alterations in alveoli, respiratory ducts, and bronchioles (results not shown).
Lipid and protein oxidation assays
TBARS assay
The concentration of thiobarbituric acid (TBA) reactive species was assayed to monitor the level of lipid peroxidation. Brain tissue was homogenized in PBS in the presence of an antioxidant (butylated hydroxytoluene, BHT) to prevent new lipid peroxidation to occur during homogenization. A 12µl aliquot of each extract was incubated with 390µl of 0.2 M glycine-HCL, pH 3.6 and 250µl of fresh TBA reagent (0.5% TBA, 0.5% SDS). After 15 min incubation at 90°C, samples were cooled on ice and transferred to a 96 well microplate for triplicate readings. Absorbance was monitored at 532 nm in a Versamax microplate reader (Molecular Devices). Values were normalized to the protein concentration of each sample, measured with the Micro BCA Protein Assay (Pierce).
Protein carbonylation-ELISA assay
Protein carbonylation was performed as previously reported (Buss et al. 1997). Briefly, triplicate protein samples were allowed to react with dinitrophenylhydrazine (DNP) and then adsorbed to wells of an ELISA plate. A biotinylated anti-DNP antibody (Molecular Probes Inc.) was used for detection of the protein-bound DNP followed by an amplification step with streptavidin-biotinylated horseradish peroxidase (Amersham). Color reaction was performed by supplying o-phenylenediamine, hydrogen peroxide and citric acid in phosphate buffer. Absorbance at 490 nm was measured in a Versamax microplate reader (Molecular Devices). Values were normalized to the protein concentration of each sample.
Supplementary Material
Acknowledgments
We thank J.R. Acebes, V. Pomies and S. Thompson for technical assistance. Polyclonal antibody anti-rat ApoD was kindly provided by Dr. S. Patel. This work was supported by start-up grants to D.S. and M.D.G. from the “Ramon y Cajal Program”(MEC-Spain and FEDER-FSE); the SGFRH Foundation; NIH grant 2/R01/NS25387-10A1 to M.J.B.; CIHR grant MOP-15677 to E.R.; FRSQ and CRSNG studentships to S.d.C.; STINT fellowship KU2003-4308 to M.A.; DGICyT grant BFU2004-06394 and JCyL grant VA011C05 to C.G.; and MEC grant BFU2005-00522 and JCyL grant VA049A05 to M.D.G. and D.S.
Contributor Information
Maria D. Ganfornina, Email: opabinia@ibgm.uva.es.
Sonia Do Carmo, Email: do_carmo.sonia@courrier.uqam.ca.
Jose M. Lora, Email: jmlora@comcast.net.
Sonia Torres-Schumann, Email: soniat@comcast.net.
Marci Vogel, Email: marci@relativegenetics.com.
Maria Allhorn, Email: maria.allhorn@medkem.lu.se.
Constancio González, Email: constanc@ibgm.uva.es.
Michael J. Bastiani, Email: bastiani@bioscience.utah.edu.
References
- Akerstrom B, Borregaard N, Flower DR, Salier J-P, editors. Lipocalins. Georgetown, Texas: Landes Biosciences; 2006. [Google Scholar]
- Akerstrom B, Maghzal G, Winterbourn CC, Kettle AJ. The lipocalin alpha 1-microglobulin has radical scavenger activity. J Biol Chem. 2007;282:31493–31503. doi: 10.1074/jbc.M702624200. [DOI] [PubMed] [Google Scholar]
- Allhorn M, Berggard T, Nordberg J, Olsson ML, Akerstrom B. Processing of the lipocalin alpha(1)-microglobulin by hemoglobin induces heme-binding and heme-degradation properties. Blood. 2002;99:1894–1901. doi: 10.1182/blood.v99.6.1894. [DOI] [PubMed] [Google Scholar]
- Allhorn M, Klapyta A, Akerstrom B. Redox properties of the lipocalin alpha1-microglobulin: reduction of cytochrome c, hemoglobin, and free iron. Free Radic Biol Med. 2005;38:557–567. doi: 10.1016/j.freeradbiomed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Balsinde J. Oxidative stress and arachidonic acid mobilization. Biochim Biophys Acta. 2006;1761:385–391. doi: 10.1016/j.bbalip.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Blanco-Vaca F, Via DP, Yang CY, Massey JB, Pownall HJ. Characterization of disulfide-linked heterodimers containing apolipoprotein D in human plasma lipoproteins. J Lipid Res. 1992;33:1785–1796. [PubMed] [Google Scholar]
- Breustedt DA, Schonfeld DL, Skerra A. Comparative ligand-binding analysis of ten human lipocalins. Biochim Biophys Acta. 2006;1764:161–173. doi: 10.1016/j.bbapap.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Crawley J. What's wrong with my mouse?: behavioral phenotyping of transgenic and knockout mice. New York: Wiley-Liss; 2000. [Google Scholar]
- Eichinger A, Nasreen A, Kim HJ, Skerra A. Structural insight into the dual ligand specificity and mode of high density lipoprotein association of apolipoprotein D. J Biol Chem. 2007;282:31068–31075. doi: 10.1074/jbc.M703552200. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Flower DR, North ACT, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J Theor Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. Epub 2005 Jan 2024. [DOI] [PubMed] [Google Scholar]
- Lechner M, Wojnar P, Redl B. Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem J. 2001;356:129–135. doi: 10.1042/0264-6021:3560129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, Milne R. Apolipoprotein D. Biochim Biophys Acta. 2000;1482:185–198. doi: 10.1016/s0167-4838(00)00162-x. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Lopez-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD, Walker DW, Muffat J, Rundel C, Benzer S. Loss of glial lazarillo, a homolog of apolipoprotein D, reduces lifespan and stress resistance in Drosophila. Curr Biol. 2006;16:680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sato N, Fujii K, Yuge O, Morio M. Changes in lipid peroxidation levels and lipid composition in the lungs, livers, kidneys and brains of mice treated with paraquat. J Appl Toxicol. 1992;12:365–368. doi: 10.1002/jat.2550120513. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Copolov DL, Sutcliffe JG. From pharmacotherapy to pathophysiology: emerging mechanisms of apolipoprotein D in psychiatric disorders. Curr Mol Med. 2003a;3:408–418. doi: 10.2174/1566524033479681. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA. 2001;98:4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, George RC, Sutcliffe JG. Apolipoprotein D modulates arachidonic acid signaling in cultured cells: implications for psychiatric disorders. Prostaglandins Leukot Essent Fatty Acids. 2003b;69:421–427. doi: 10.1016/j.plefa.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Yao JK. Clozapine specifically alters the arachidonic acid pathway in mice lacking apolipoprotein D. Schizophr Res. 2006;89:147–153. doi: 10.1016/j.schres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Tomita M, Okuyama T, Katsuyama H, Hidaka K, Otsuki T, Ishikawa T. Gene expression in rat lungs during early response to paraquat-induced oxidative stress. Int J Mol Med. 2006a;17:37–44. [PubMed] [Google Scholar]
- Tomita M, Okuyama T, Katsuyama H, Ishikawa T. Paraquat-induced gene expression in rat kidney. Arch Toxicol. 2006b;80:687–693. doi: 10.1007/s00204-006-0092-2. [DOI] [PubMed] [Google Scholar]
- Vajragupta O, Monthakantirat O, Wongkrajang Y, Watanabe H, Peungvicha P. Chroman amide 12P inhibition of lipid peroxidation and protection against learning and memory impairment. Life Sci. 2000;67:1725–1734. doi: 10.1016/s0024-3205(00)00762-1. [DOI] [PubMed] [Google Scholar]
- Van Dijk W, Do Carmo S, Rassart E, Dalhlback B, Sodetz J. The plasma Lipocalins α1-acid glycoprotein, apolipoprotein D, apolipoprotein M and complement C8γ. In: Akerstrom B, Borregaard N, Flower D, Salier J, editors. Lipocalins. Georgetown, Texas: Landes Bioscience; 2006. pp. 140–166. [Google Scholar]
- Walker DW, Muffat J, Rundel C, Benzer S. Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Curr Biol. 2006;16:674–679. doi: 10.1016/j.cub.2006.01.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.