Abstract
The inferior colliculus is a major relay nucleus in the ascending auditory pathways that receives multiple glutamatergic inputs. Vesicular glutamate transporters 1 and 2 (VGLUT1, VGLUT2) most often have complementary non-overlapping distributions and can be used to differentiate glutamatergic inputs. The present study therefore examined co-immunolabeling of VGLUT1 and VGLUT2 in three divisions of the inferior colliculus. Additional co-immunolabeling of microtubule associated protein 2 and neuronal class III β-tubulin provided visualization of neuronal soma and processes and allowed identification of axo-somatic versus axo-dendritic contacts. Results showed numerous VGLUT1 and 2 immunolabeled terminals in the central nucleus, lateral cortex and dorsal cortex. In all three divisions there was little to no co-containment of the two vesicular glutamate transporters indicating a complementary distribution. VGLUT1 made predominantly axo-dendritic connections in the neuropil, while VGLUT2 had many axo-somatic contacts in addition to axo-dendritic contacts. VGLUT2 immunolabeled terminals were numerous on the soma and proximal dendrites of many medium-to-large and large neurons in the central nucleus and medium to large neurons in the dorsal cortex. There were more VGLUT2 terminals than VGLUT 1 in all divisions and more VGLUT2 terminals in dorsal and lateral cortices than in the central nucleus. This study shows that VGLUT1 and VGLUT2 differentiate complementary patterns of glutamatergic inputs into the central nucleus, lateral and dorsal cortex of the inferior colliculus with VGLUT1 endings VGLUT1 endings predominantly on the dendrites and VGLUT2 on both dendrites and somas.
Keywords: auditory, auditory brain stem, co-localization
Glutamate is the major excitatory transmitter in the ascending auditory pathway, used by multiple neurons and multiple projections at all the different levels. The presence of vesicular glutamate transporters (VGLUTs) which mediate the packaging of the excitatory neurotransmitter glutamate into synaptic vesicles has proven to be a useful immunocytochemical marker for glutamatergic terminals. There are at least three subtypes of VGLUTs: VGLUT1, VGLUT2 and VGLUT3. VGLUT1 and VGLUT2 are both associated with glutamatergic terminals and their localizations are generally complementary without overlap (e.g. Kaneko and Fujiyama. 2002; Kaneko et al, 2002; Hioki et al, 2003; Fremeau et al, 2004a,b) allowing their use to differentiate among glutamatergic systems. Zhou et al (2007) recently found that VGLUT1 immunolabeled auditory nerve terminals in the guinea pig cochlear nucleus (CN) while VGLUT2 immunolabeled non-auditory nerve glutamatergic inputs to the CN including spinal trigeminal nerve terminals. Blaesse et al (2005) examined VGLUT1 and 2 immunolocalization in the rat superior olivary complex and found VGLUT1 and 2 had unique cellular labeling patterns on neurons in the medial, ventral and lateral nuclei of the trapezoid body, the medial and lateral superior olivary complexes and the superior paraolivary nucleus, while Billups et al (2005) noted co-containment in some terminals and not others. Although VGLUT1 and 2 correlate well with glutamatergic terminals, VGLUT3 is often found in terminals associated with other neurotransmitters, where glutamate may not be playing the major role (Herzog et al, 2004).
The inferior colliculus (IC) receives multiple inputs from ascending and descending auditory pathways as well as from non-auditory inputs (Adams 1979, 1980, 1983, Aitkin et al, 1981; Bajo and Moore, 2005; Cant & Benson, 2006; Faye Lund, 1985; Jain & Shore, 2006; Loftus et al, 2004; Malmierca et al, 2005; Marsh et al, 2002; Merchan et al, 1994; Moore et al, 1998; Okayama et al, 2006; Oliver, 2000; Oliver et al, 1997; Osen, 1972; Saldana et al, 1996; Shofield, 2002; Shofield and Cant, 1996; Shore and Zhou, 2006, Tokunaga et al, 1984; Zhou and Shore, 2006) many of which are glutamatergic (Malmierca, 2003 for review; Alibardi, 1998; Feliciano and Potashner, 1995; Ross et al, 1995; Saint Marie, 1996, Suneja et al, 1995). The present study used co-immunolabeling to determine if VGLUT1 and VGLUT2 would differentiate unique and characteristic patterns of glutamatergic inputs onto neurons in the rat IC and to determine if there would be differences in the patterns in three sub-regions of the IC; the central nucleus (CIC), the lateral (external) cortex (LCIC) and the dorsal cortex (DCIC). The CIC is a major relay station in the ascending auditory pathway while the LCIC and DCIC are para-lemniscal/shell regions involved in integration with descending auditory and non-auditory pathways (Malmierca, 2003, Morest & Oliver, 1984, Oliver 2005 for reviews).
Experimental Procedures
Distribution of VGLUT1 and VGLUT2 in CIC, LCIC, DCIC
Studies were conducted in accordance with the rules of animal care and use of the University of Michigan Committee on the Use and Care of Animals. Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) weighing between 200–280 g were used in the studies. Auditory brainstem response (ABR) thresholds were measured at frequencies of 2, 10 and 20 kHz from 0 to 100dB to confirm that all animals had normal hearing before their inclusion in the study. Rats were deeply anesthetized with a single intraperitoneal injection of 350 mg/kg Fatal Plus (Vortech Pharmaceuticals Dearborn, MI) and then perfused transcardially with 0.12 M phosphate buffer (pH 7.3) followed by 4% paraformaldehyde in phosphate buffer fixative. The brains were then removed and postfixed for 2 h in the same fixative at 4°C. Coronal sections (150μm) containing the inferior colliculus (IC) were cut on a vibrating microtome (Vibratome). Slices were selected that contained three regions of the IC - central nucleus (CIC), lateral cortex LCIC) and dorsal cortex (DCIC) - in caudal, mid caudal-rostral and rostral levels.
The sections were pre-treated with Universal Blocking Reagent (BioGenex, San Ramon, CA) for 10 minutes at room temperature and then incubated overnight in a mixture of four primary antibodies from three species. A mixture of monoclonal mouse anti-TUJ-1 (1:200; Covance, Berkeley, CA); monoclonal mouse anti-MAP-2 (1:200, Chemicon, Temecula, CA); polyclonal rabbit anti-VGLUT-1 (1:500; Synaptic Systems, Germany) and polyclonal guinea pig anti-VGLUT-2 (1:500; Chemicon, Temecula, CA) in phosphate buffered saline (PBS) with 0.2% triton X-100 was applied to sections for 16 – 20 hours at 4°C on a rotator. Sections were rinsed three time in PBS and then received a second incubation for indirect immunoflourescence staining, with a fluorescence-labeled secondary antibody mixture of Alexa 355-, Alexa 488-, or Alexa 568- goat anti-rabbit, anti-guinea pig and anti-mouse Immunoglobulin G (IgG) (Molecular Probes, Carlsbad, CA), each diluted 1:100 in PBS and incubated together for 1 hour at room temperature. The slices were then rinsed and mounted on VWR superfrost plus slides. The sections were covered with FluoroMount G (EM Sciences, Inc, Hatfield, PA) and then by a coverslip. Primary antibodies were omitted in negative control experiments.
Digital pseudocolor images were captured on a Zeiss LSM 510 Meta confocal laser scanning microscope (software version 3.2 SP2, Carl Zeiss, Oberkochen, Germany) with optical slice thicknesses approximately 1.0 um, using a 63x objective water immersion lens (Zeiss C-Apochromat, 1.20 NA, 0.24mm WD). The images were collected in multi-track scanning mode to reduce the cross-talk between emission channels. AlexaFluor 350, 488 and 568 were excited with 364nm, 488nm and 543 nm laser beams and observed through band pass 385–470nm, 505–550nm and long pass 560nm emission filters, respectively. When the primary antibodies were omitted in negative control experiments, no immunofluorescence was detected.
Assessment
Digitized images from comparable regions in four animals were imported into a Metamorph Image Analysis workstation (Universal Imaging) for a quantitative assessment. Terminals were considered “positive” if fluorescent intensity of immunolabeling was at least 3 times over background. Two assessments were carried out. The number of positively immunolabeled terminal was determined for VGLUT1 and VGLUT2 in randomly assigned regions of interest from the high frequency region of the central nucleus of the inferior colliculus and from layer 2 of the lateral cortex. This was expressed as density of immunolabeled terminals per 1000 μm2. Statview ® software was used to compare the density of VGLUT1 versus VGLUT terminals with an ANOVA and a Scheffe test for post-hoc comparisons. A second “semi-quantitative” assessment determined the number of immunopositive VGLUT1 and VGLUT axosomatic on profiles of neuronal somata containing nuclei, in the high frequency region of the central nucleus of the inferior colliculus.
Results
General
Neurons in the IC were differentiated based on size (Faye-Lund and Osen, 1985; Malmierca et al, 1993, 1995; Morest & Oliver, 1984; Oliver refs. Peruzzi et al, 2000). Neurons less than 10μm were considered small sized, neuron that were 10–15μm were termed medium sized, neurons that were 15–20μm were considered medium-to-large sized, and neurons that were greater than 20μm were termed large. Antibody to TUIJ1 immunolabeled neuronal class III β-tubulin, present in neuronal somata and processes. Antibody to MAP2 immunolabeled microtubule associated protein 2 which cross links microtubules with intermediate filaments and other microtubules, and which is enriched in dendrites and spines. Together the combination of the two antibodies (both rabbit polyclonal antibodies and visualized together with an anti-rabbit secondary antibody using one chromophore) provided a robust labeling of somata and dendrites of many IC neurons in all divisions. This allowed differentiation of axo-somatic versus axo-dendritic terminals when sections were also labeled with antibodies to VGLUT1 and VGLUT2 using two additional chromophores. Labeling VGLUT1 and VGLUT2 with different chromophores also allowed assessment of co-labeling within terminals. There was, however, little overlap (less than 5%) of immunostaining for VGLUT1 and VGLUT2 in terminals on neurons or dendrites in any of the three regions of the IC assessed. This indicates that VGLUT1 and VGLUT2 are enriched in different terminals suggesting they are associated with different projections and pathways onto IC neurons. The VGLUT1 terminals generally appeared larger than VGLUT2 labeled terminals in all divisions.
Central Nucleus of the Inferior Colliculus (CIC)
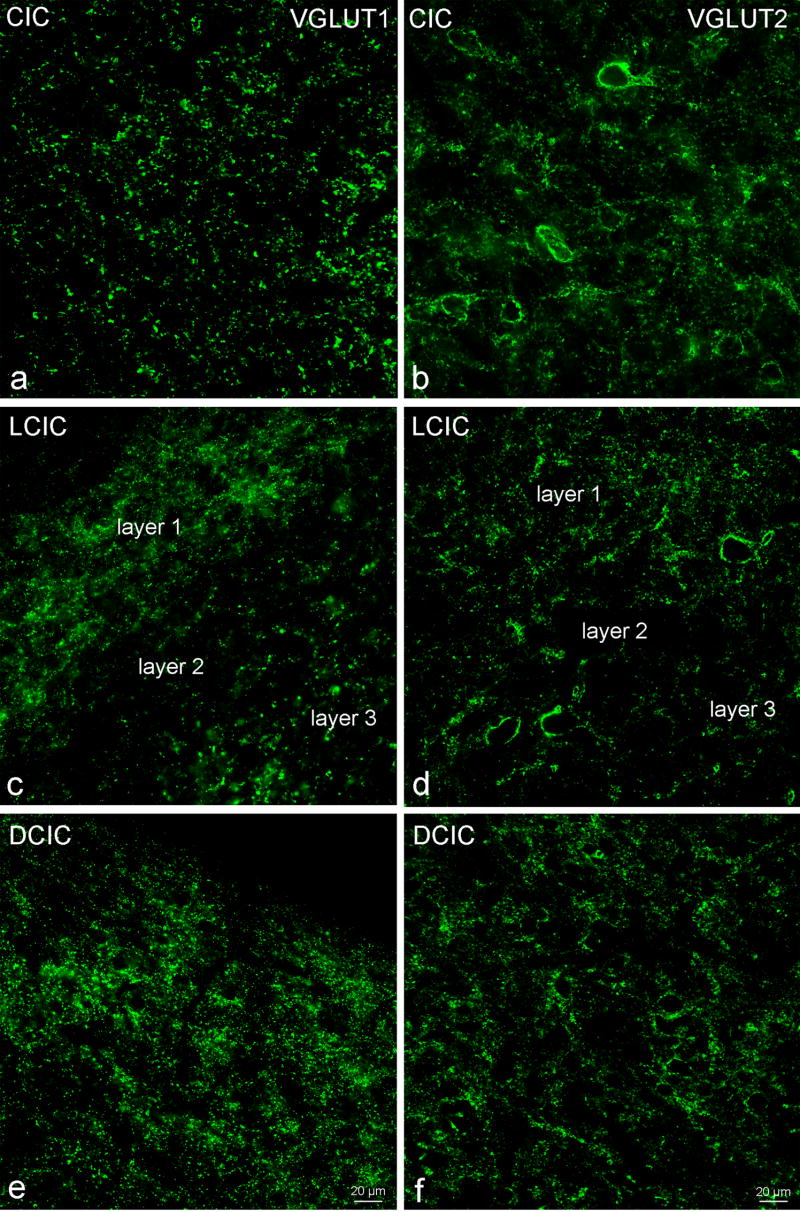
In the CIC there were many VGLUT1 and VGLUT2 immunolabeled terminals (Figure 1). Triple immunofluorescence (Figures 2,3) showed little (less than 5%) co-labeling for VGLUT1 and VGLUT2 suggesting their enrichment in different and discrete inputs. The VGLUT1 terminals were predominantly axo-dendritic, with very few VGLUT1 terminals (0–3) on somatic profiles of CIC neurons of all sizes. In contrast there were many (17–30 on a somatic profile) axo-somatic VGLUT2 terminals on medium-to-large and large (18 – 28 um long diameter) neurons in all regions of the central nucleus. Large numbers of VGLUT2 terminals were also seen onto the proximal dendrites of these neurons. The smaller neurons in the CIC had fewer (0–3) VGLUT2 terminals on somatic profiles, comparable to the pattern seen for VGLUT1. There were also many VGLUT1 and VGLUT2 immunolabeled axo-dendritic terminals throughout the neuropil that could not be attributed to specific neurons. When the density of VGLUT1 and VGLUT2 immunolabeled puncta/terminals was compared there was a significantly greater (p ≤ .05) density of VGLUT2 terminals (40.8 +/− 9.4 per 1000 μm2) than VGLUT1 (26.5 +/−5.7 per 1000 μm2), perhaps reflecting the many VGLUT2 axo-somatic contacts. On the other hand, the VGLUT1 terminals were larger.
Figure 1.
Figure 1a–f: Low magnification view of immunolabeling for VGLUT1 (a,c,e) and VGLUT2 (b,d,f) immunolabeling in the central nucleus of the inferior colliculus (CIC) (a,b), layers 1,2 and 3 of the lateral cortex of the inferior colliculus (LCIC) (c,d,) and the dorsal cortex of the inferior colliculus (DCIC) (e,f). Bar = 20 microns.
Figure 2.

Figure 2a–d: Co-immunolabeling for VGLUT1 (blue), VGLUT2 (green) and TUJ1/MAP2 (red) in the central nucleus of the inferior colliculus. Many VGLUT2 immunolabeled ending are seen surrounding the soma and proximal dendrites of medium to large and large neurons. Both VGLUT1 and VGLUT2 labeled puncta corresponding to axo-dendritic endings are seen throughout the neuropil. Bar = 20 microns.
Figure 3.
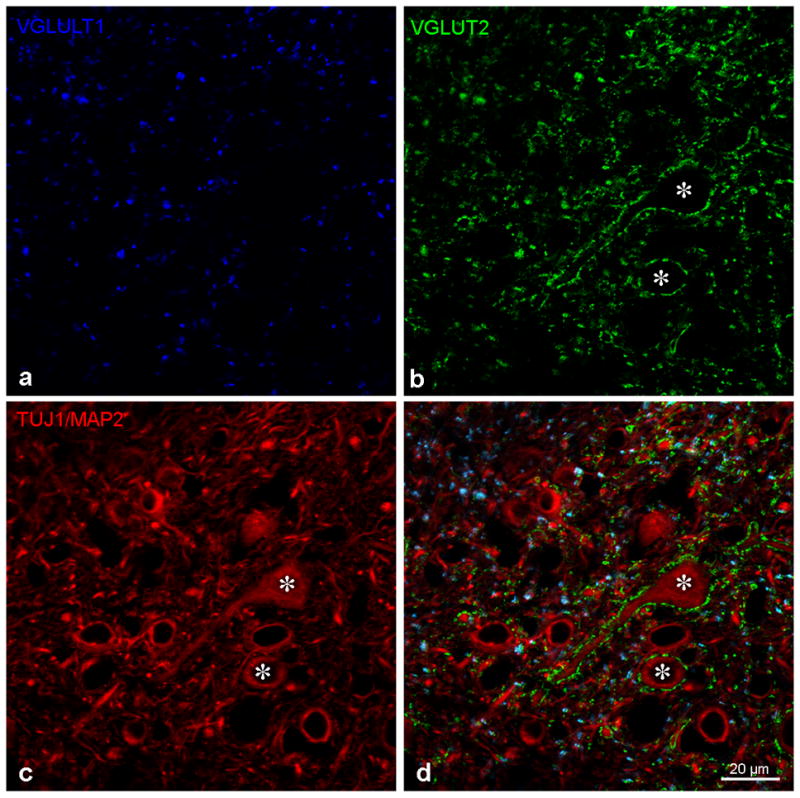
Figure 3a–d: The photomicrograph from Figure 2a with immunolabeling for VGLUT1 (3a - blue), VGLUT2 (3b- green) and TUJ1/MAP2 (3c red) in the central nucleus of the inferior colliculus presented separately and then together as in Figure 2a. Asterisks mark two neurons receiving multiple VGLUT2 immunolabeled puncta on their soma. Bar = 20 microns.
Lateral (External) Cortex of the Inferior Colliculus (LCIC)
There were also many VGLUT1 and VGLUT2 immunolabeled terminals (Figures 1,4) in all layers of the LCIC. VGLUT1 puncta were more abundant than VGLUT2 in layers 1 and 3 while VGLUT2 puncta were more abundant than VGLUT1 in layer 2 (Figure 1). Only layer 2 was quantitatively assessed for density and VGLUT2 (58.5 +/− 8.6 per 1000 μm2) was significantly higher (p ≤ .05) than VGLUT1 (36.4 +/− 7.3 per 1000 μm2). Densities for both VGLUT1 and VGLUT2 were both higher in layer 2 of the LCIC than in the high frequency CIC, but only VGLUT2 was significantly higher (p ≤ .05).
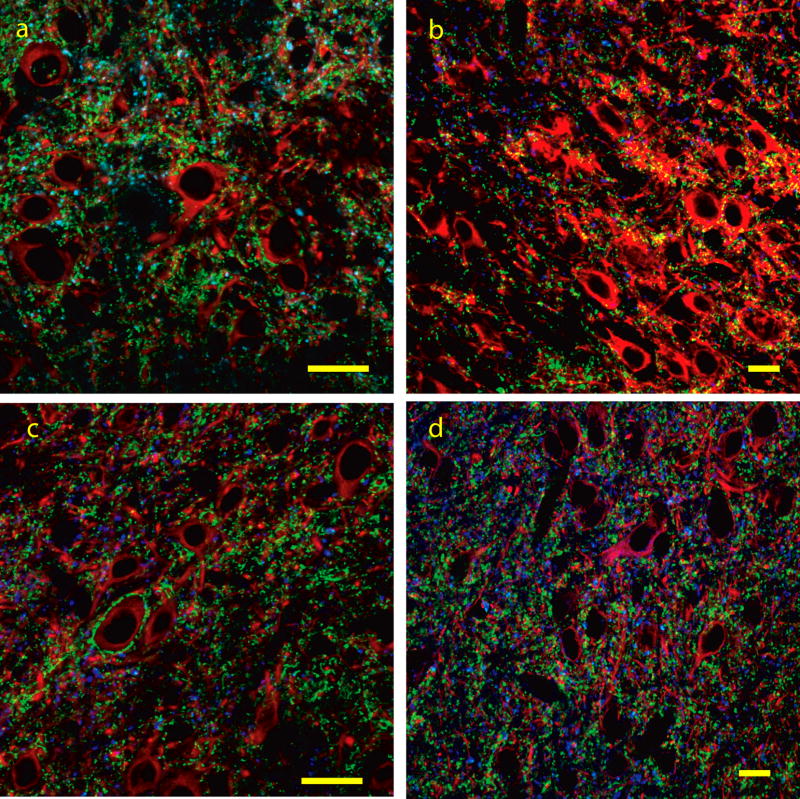
Figure 4.
Figure 4a–d: Co-immunolabeling for VGLUT1 (blue), VGLUT2 (green) and TUJ1/MAP2 (red) in the lateral cortex (a,b) and dorsal cortex (c,d) of the inferior colliculus. In the lateral cortex both VGLUT1 and VGLUT2 labeled puncta corresponding to axo-dendritic endings are seen throughout the neuropil. In the dorsal cortex many VGLUT2 immunolabeled ending are seen surrounding the somata and proximal dendrites of medium to large neurons. Both VGLUT1 and VGLUT2 immunolabeled puncta corresponding to axo-dendritic endings are seen throughout the neuropil. Bars = 20 microns
A large number of VGLUT2 terminals on somatic profiles was less common than in the CIC, rarely covering the entire somatic profile and only seen in layer 2. Axo-somatic VGLUT2 terminals (2–12 per profile) were seen on neurons in layers 2 and 3 but not in layer 1. Axo-somatic VGLUT1 immunolabeled terminals were not commonly found in any layers of the lateral cortex, with these terminals predominantly observed making axo-dendritic endings in the neuropil. Triple immunofluorescence revealed little (less than 5%) co-labeling for VGLUT1 and VGLUT2 in the lateral cortex suggesting they label discrete inputs in this region.
Dorsal Cortex of the Inferior Colliculus (DCIC)
The DCIC contained many VGLUT1 and VGLUT2 immunolabeled fibers with a distribution closer to that seen in the CIC than in the LCIC. VGLUT1 immunolabeled endings were observed throughout the neuropil but were only rarely observed on somatic profiles. VGLUT2 immunolabeled endings were both axo-somatic and axo-dendritic and multiple endings were often seen on somatic profiles and proximal dendrites of neurons, though less common than in the CIC. The neurons receiving many axo-somatic VGLUT2 endings were slightly smaller than the comparable neurons in the central nucleus, falling in the medium-to-large category. As in CIC and LCIC there was little co-enrichment of VGLUT1 and VGLUT2 in the same endings, indicating they label discrete inputs.
Discussion
The results of this study, showing complementary, non-overlapping immunolabeling for VGLUT1 and VGLUT2 in terminals with different patterns of termination in the inferior colliculus, is consistent with what is seen in other systems as well as in the lower auditory brain stem regions. Other studies have also reported that VGLUT1 terminals are larger than VGLUT2 (e.g. Fremeau et al, 2004, Zhou et al, 2007), as seen in the current study. Previous studies by Zhou et al (2007) in the cochlear nucleus and Blaesse et al (2005) in the superior olivary complex, show VGLUT1 is closely associated with the classical or lemniscal ascending auditory pathway and Zhou et al (2007) found VGLUT2 is more associated with non-classical paralemniscal pathways such as somatosensory inputs. This would predict that inputs to the central nucleus from the cochlear nucleus and superior olivary complex will be more associated with VGLUT1. On the other hand, many VGLUT2 terminals were observed around soma and proximal dendrites of neurons in central nucleus and dorsal cortex which both receive ascending classical/leminiscal input and this pattern of termination was not found on neurons in lateral cortex which receive more non-classical/paralemniscal input as well as input from central nucleus. Malmierca et al (2005) reported different patterns of terminals in the IC originating in the CN, with large CN neurons giving rise to large fibers and boutons, comparable to larger VGLUT1 immunolabeled terminals seen in the current study and a wider lamina exclusively of small boutons more comparable to the VGLUT2 immunostaining seen in the current study. The IC receives glutamatergic input from multiple ascending sources including several cell types in the cochlear nucleus, the lateral and medial superior olives and smaller input from the ventral nucleus of the lateral lemniscus. There is also glutamatergic input from the contralateral IC, intrinsic glutamatergic connections, non-auditory multi-modal glutamatergic input as well as descending glutamatergic input from the auditory cortex. It will be interesting and important to combine tract tracing to the IC with VGLUT immunolabeling in the different divisions of the IC in future studies.
The functional significance of having different VGLUTs enriched in different terminals, with VGLUT2 having its greatest expression in brain stem and thalamus (Moechars et al, 2006), is not completely understood. Increasing VGLUT1 increases quantal size and decreasing VGLUT1 or VGLUT2 decreases the amount of glutamate loaded into vesicles (Moechars et al, 2006; Fremeau et al, 2004; Wojcik et al, 2004). Fremeau et al (2004) suggested that there are different properties of release for VGLUT1 and VGLUT2 with more rapid recycling and recovery for VGLUT1. The C terminals of VGLUT1 and VGLUT2 have significant sequence differences leading Herzog et al (2001) to suggest VGLUT1 and VGLUT2 might have differences in protein targeting. Zhou et al (2007) suggested that VGLUT1 was more associated with fibers conveying precise temporal information in the cochlear nucleus with enrichment in auditory nerve terminals and Blaesse et al (2005) also reported VGLUT1 enrichment in end-bulb terminals in the superior olivary complex. While the present study found that VGLUT1 was also enriched in the larger terminals in the IC, any association with specific inputs and functions awaits future studies.
Acknowledgments
These studies were supported by NIDCD Grant DC00383 and core center grant P30 DC05188 for RA and NIDCD Grant DC00189 for DO. We would like to acknowledge the valuable input of Susan Shore and the technical assistance of Mike Ross and Cathy Lomax Martin.
List of abbreviations
- IC
inferior colliculus
- CIC
central nucleus of the inferior colliculus
- LCIC
lateral cortex of the inferior colliculus
- DCIC
dorsal cortex of the inferior colliculus
- CN
cochlear nucleus
- DCN
dorsal cochlear nucleus
- TUJ1
neuronal class III β-tubulin
- MAP2
microtubule associated protein 2
- VGLUT
vesicular glutamate transporter
- ANOVA
analysis of variance
References
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol. 1979;1;183(3):519–38. doi: 10.1002/cne.901830305. [DOI] [PubMed] [Google Scholar]
- Adams JC. Crossed and descending projections to the inferior colliculus. Neurosci Lett. 1980;19(1):1–5. doi: 10.1016/0304-3940(80)90246-3. [DOI] [PubMed] [Google Scholar]
- Adams JC. Multipolar cells in the ventral cochlear nucleus project to the dorsal cochlear nucleus and the inferior colliculus. Neurosci Lett. 1983;37(3):205–8. doi: 10.1016/0304-3940(83)90431-7. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol. 1981;196(1):25–40. doi: 10.1002/cne.901960104. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Ultrastructural and immunocytochemical characterization of neurons in the rat ventral cochlear nucleus projecting to the inferior colliculus. Ann Anat. 1998;180(5):415–26. doi: 10.1016/S0940-9602(98)80102-7. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486(2):101–16. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Billups B. Colocalization of vesicular glutamate transporters in the rat superior olivary complex. Neurosci Lett. 2005;382(1–2):66–70. doi: 10.1016/j.neulet.2005.02.071. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Ehrhardt S, Friauf E, Nothwang HG. Developmental pattern of three vesicular glutamate transporters in the rat superior olivary complex. Cell Tissue Res. 2005;320(1):33–50. doi: 10.1007/s00441-004-1054-8. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495(5):511–28. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol (Berl) 1985;171(1):1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Faye Lund H. The neocortical projection to the inferior colliculus in the albino rat. Anat Embryol (Berl) 1985;171:1–20. doi: 10.1007/BF00707304. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Potashner SJ. Evidence for a glutamatergic pathway from the guinea pig auditory cortex to the inferior colliculus. J Neurochem. 1995;65(3):1348–57. doi: 10.1046/j.1471-4159.1995.65031348.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004a;304(5678):1815–9. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004b;27(2):98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Herzog E, Gilchrist J, Gras C, Muzerelle A, Ravassard P, Giros B, Gaspar P, El Mestikawy S. Localization of VGLUT3, the vesicular glutamate transporter type 3, in the rat brain. Neuroscience. 2004;123(4):983–1002. doi: 10.1016/j.neuroscience.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The existence of a second vesicular glutamate transporter specifies subpopulations of glutamatergic neurons. J Neurosci. 2001;21(22):181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Fujiyama F, Taki K, Tomioka R, Furuta T, Tamamaki N, Kaneko T. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience. 2003;117(1):1–6. doi: 10.1016/s0306-4522(02)00943-0. [DOI] [PubMed] [Google Scholar]
- Jain R, Shore S. External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett. 2006;\395(1):71–5. doi: 10.1016/j.neulet.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42(4):243–50. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;25;444(1):39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol. 2004;472:330–44. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Saint Marie RL, Merchan MA, Oliver DL. Laminar inputs from dorsal cochlear nucleus and ventral cochlear nucleus to the central nucleus of the inferior colliculus: two patterns of convergence. Neuroscience. 2005;136:883–94. doi: 10.1016/j.neuroscience.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK, Karagulle T, Molowny RL. The central nucleus of the inferior colliculus in rat: a Golgi and computer reconstruction study of neuronal and laminar structure. J Comp Neurol. 2003;333(1):1–27. doi: 10.1002/cne.903330102. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Seip KL, Osen KK. Morphological classification and identification of neurons in the inferior colliculus: a multivariate analysis. Anat Embryol (Berl) 1995;191(4):343–50. doi: 10.1007/BF00534687. [DOI] [PubMed] [Google Scholar]
- Malmierca MS. The structure and physiology of the rat auditory system: an overview. Int Rev Neurobiol. 2003;56:147–211. doi: 10.1016/s0074-7742(03)56005-6. [DOI] [PubMed] [Google Scholar]
- Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci. 2002;22(23):10449–60. doi: 10.1523/JNEUROSCI.22-23-10449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan MA, Saldana E, Plaza I. Dorsal nucleus of the lateral lemniscus in the rat: concentric organization and tonotopic projection to the inferior colliculus. J Comp Neurol. 1994;342(2):259–78. doi: 10.1002/cne.903420209. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kotak VC, Sanes DH. Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J Neurophysiol. 1998;80(5):2229–36. doi: 10.1152/jn.1998.80.5.2229. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol. 1984;222(2):209–36. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Okoyama S, Ohbayashi M, Ito M, Harada S. Neuronal organization of the rat inferior colliculus participating in four major auditory pathways. Hear Res. 2006;218(1–2):72–80. doi: 10.1016/j.heares.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Ascending efferent projections of the superior olivary complex. Microsc Res Tech. 2000;51(4):355–63. doi: 10.1002/1097-0029(20001115)51:4<355::AID-JEMT5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382(2):215–29. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neural Organization in the Inferior Colliculus. Springer-Verlag; NY: [Google Scholar]
- Osen KK. Projection of the cochlear nuclei on the inferior colliculus in the cat. J Comp Neurol. 1972;144(3):355–72. doi: 10.1002/cne.901440307. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Sivaramakrishnan S, Oliver DL. Identification of cell types in brain slices of the inferior colliculus. Neuroscience. 2000;2000;101(2):403–16. doi: 10.1016/s0306-4522(00)00382-1. [DOI] [PubMed] [Google Scholar]
- Ross CD, Godfrey DA, Parli JA. Amino acid concentrations and selected enzyme activities in rat auditory, olfactory, and visual systems. Neurochem Res. 1995;20(12):1483–90. doi: 10.1007/BF00970598. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL. Glutamatergic connections of the auditory midbrain: selective uptake and axonal transport of D-[3H]aspartate. J Comp Neurol. 1996;16;373(2):255–70. doi: 10.1002/(SICI)1096-9861(19960916)373:2<255::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Saldana E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371(1):15–4. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Ascending and descending projections from the superior olivary complex in guinea pigs: different cells project to the cochlear nucleus and the inferior colliculus. J Comp Neurol. 2002;453(3):217–25. doi: 10.1002/cne.10402. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Projections from the ventral cochlear nucleus to the inferior colliculus and the contralateral cochlear nucleus in guinea pigs. Hear Res. 1996;102(1–2):1–14. doi: 10.1016/s0378-5955(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hear Res. 2006;216–217:90–9. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Gross J, Potashner SJ. Uptake and release of D-aspartate, GABA, and glycine in guinea pig brainstem auditory nuclei. J Neurochem. 1995;64(1):147–60. doi: 10.1046/j.1471-4159.1995.64010147.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Sugita S, Otani K. Auditory and non-auditory subcortical afferents to the inferior colliculus in the rat. J Hirnforsch. 1984;25(4):461–72. [PubMed] [Google Scholar]
- Wojcik SM, Rhee JSm Herzog E, Siglaer A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci USA. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500(4):777–87. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495(1):100–12. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]