Abstract
Increases in the incidence and severity of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections have spawned efforts to define unique virulence properties among prevalent strains. Panton-Valentine leukocidin (PVL), a pore-forming cytotoxin, has garnered attention due to its epidemiologic association with CA-MRSA. Using the clinical isolate LAC, representative of the epidemic USA300 strain, and its isogenic PVL-negative strain in murine models of staphylococcal skin infection and pneumonia, we have extended recent studies by assessing the contribution of PVL in the BALB/c genetic background. The data herein support the observation that PVL does not contribute to the pathogenesis of staphylococcal infection of mice.
Keywords: Community-associated methicillin resistant Staphylococcus aureus (CA-MRSA), Panton-Valentine leukocidin (PVL), USA300, skin infection, pneumonia, animal models
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections in the United States are primarily caused by isolates classified as pulsed-field gel electrophoresis type USA300 [1, 2]. CA-MRSA infections are typically skin and soft tissue infections, but may manifest as severe invasive disease, including pneumonia [3–5]. USA300 contains unique genetic loci postulated to encode virulence factors that contribute to the success of this pathogen [6]. One such locus encodes Panton-Valentine leukocidin (PVL), an exotoxin comprised of LukF-PV and LukS-PV protein subunits that assemble into cytolytic pore-forming octamers [7] on the surface of susceptible cells. Epidemiologic studies indicate a tight association between PVL-encoding genes and CA-MRSA infections [1, 8–10], prompting investigation of the role of PVL in pathogenesis through animal infection models.
Several recent reports have provided conflicting views on the role of PVL in the pathogenesis of CA-MRSA disease. An investigation of LAC/USA300 and MW2/USA400 clinical isolates and their isogenic lukS/F-PV (encoding PVL) deletion mutants (Δpvl) by Voyich et al. revealed that PVL is not required for the pathogenesis of S. aureus sepsis/bacteremia or skin abscess formation in CD1 Swiss or hairless Crl:SKH1-hrBR murine models of disease [11]. Labandeira-Rey et al. examined the role of PVL in a BALB/c model of S. aureus pneumonia [12] using the laboratory strain RN6390 and RN6390 variants expressing either native phage- or plasmid-encoded PVL. The study suggests PVL promotes staphylococcal lung infection. Importantly, mortality in this system was evident only when PVL was expressed from a plasmid, presumably yielding high levels of toxin expression. Most recently, we evaluated LAC and MW2 clinical isolates and Δpvl mutants in a C57BL/6J model of S. aureus pneumonia [13]. In contrast to the observations of Labandeira-Rey et al., we demonstrated that PVL is not essential for disease pathogenesis, as wild-type and Δpvl strains were equally virulent when mortality, bacterial load in infected tissue or pathologic evidence of disease were evaluated as experimental endpoints. These conflicting reports raise two alternative hypotheses about the role of PVL in disease. First, the effects of PVL may be most evident in particular host genetic backgrounds, perhaps owing to altered sensitivity/response to the toxin as dictated by the genetic makeup of the mouse strain. Alternatively, the apparent differences in the role of PVL in pathogenesis may relate solely to the experimental systems employed, either as a consequence of the S. aureus strains utilized or the experimental readouts. Discerning between these possibilities is essential, as PVL is frequently cited to be a critical virulence factor of CA-MRSA infections, and thus is a potential target for staphylococcal vaccine development.
Methods
USA300 wild-type and isogenic lukS/F-PV mutant S. aureus strains (LAC and LACΔpvl, respectively) were described previously [11].
Female BALB/cJ (The Jackson Laboratory) and BALB/cAnNHsd (Harlan) mice (7–9 weeks) were housed in microisolator cages and received food and water ad libitum. All studies conformed to guidelines set forth by the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases (NIAID).
For each experiment, overnight cultures of S. aureus grown in trypticase soy broth (TSB) were diluted 1:200 and grown at 37°C with shaking (250 rpm) to mid-exponential phase of growth (OD600 = 0.75). Staphylococci were washed in Dulbecco’s PBS (DPBS) and resuspended in DPBS at 2×108 colony forming units (CFUs)/mL. Two independent cultures were used to generate inocula for subsequent infection (e.g., 2 duplicate cultures×10 mice each = 20 mice total per S. aureus strain). Mice were anesthetized with isoflurane and inoculated by subcutaneous injection in the shaved right flank with 50 kL of DPBS containing 1×107 S. aureus, an inoculum determined in preliminary studies to produce consistent dermonecrotic abscesses within 2 days. Mice were weighed before inoculation and at 24-hour intervals for 14 days. Since abscesses were relatively flat, the area of each was calculated daily using the formula A = π(l × w)/2 as described [11].
To determine CFUs at the site of infection, a second set of mice (14–15 each of BALB/cJ and BALB/cAnNHsd) were inoculated as described above. Four mice were injected with DPBS. On day 4 post-infection, mice were euthanized and the abscessed skin and surrounding tissue were removed. Tissue samples were weighed, homogenized in DPBS, diluted in saline and plated on trypticase soy agar. CFUs were enumerated the following day. No bacteria were recovered from mice injected with DPBS.
For murine lung infections examining weight loss, lung CFU recovery and histopathology, LAC and LACΔpvl were prepared as described by Labandeira-Rey, et al. [12]. Briefly, overnight cultures grown in TSB were refreshed 1:100 in media and grown with shaking to an OD600 of 1.0. Bacteria were sedimented by centrifugation, washed in PBS, and suspended at a concentration of 4–6×107 CFUs per 20-μl volume of PBS for intranasal inoculation. For acute lethal disease studies, S. aureus strains were grown at 37°C in TSB to an OD660 of 0.5. Culture aliquots (50 ml) were centrifuged, washed in PBS, and suspended in 750 μl PBS (3–4×108 CFUs per 30 μl volume).
Animal experiments were reviewed, approved and supervised by the IACUC at the University of Chicago. For lung infection, 7 week old mice (BALB/cJ or BALB/cAnNHsd) were anesthetized prior to inoculation of the S. aureus suspension into the left nare as previously described [14]. Microbiologic and pathologic correlates of disease were assessed 48 hours post-infection, also as previously described [14].
Results and discussion
Prior studies of PVL in several distinct animal model systems raised the possibility that the role of this toxin may be most readily discerned in the BALB/c genetic background [11–13]. While the number of studies examining the role of PVL in disease pathogenesis have been limited, it is only within the BALB/c background that a role for this exotoxin has been appreciated. This suggests that the BALB/c lineage may be particularly susceptible to PVL, perhaps due to differences cellular susceptibility to the toxin or in the host immune response to toxin-expressing strains. Importantly, investigations to date have not examined CA-MRSA strains and their isogenic lukS/F-PV-negative mutants in BALB/c mice. Inasmuch as USA300 is the most prevalent CA-MRSA genotype in the United States, we investigated the virulence of LAC wild-type and LACΔpvl mutant strains in BALB/c models of staphylococcal skin infection and pneumonia. Infection via subcutaneous inoculation of BALB/cJ or BALB/cAnNHsd mice with LAC or LACΔpvl produced consistent dermonecrotic abscesses and a 2.0–2.3-gram weight loss over the first 2 days following inoculation (Fig. 1A and 1C). Weight loss was gradually recovered over the subsequent 12 days and comparable between wild-type and Δpvl strains. In general, abscess size in BALB/cJ and BALB/cAnNHsd mice was not significantly different following infection with either LAC or LACΔpvl (Fig. 1B and 1D). However, the average abscess area was slightly greater in mice infected with LACΔpvl, albeit this difference was significant on only 3 days (Fig. 1B and 1D, respectively). The average skin abscess area per day (over 14 days) was not different between groups of mice infected with wild-type or mutant strains, confirming that LAC and LACΔpvl are equally capable of causing skin and soft tissue infection (Fig. 1E and 1F).
Figure 1.
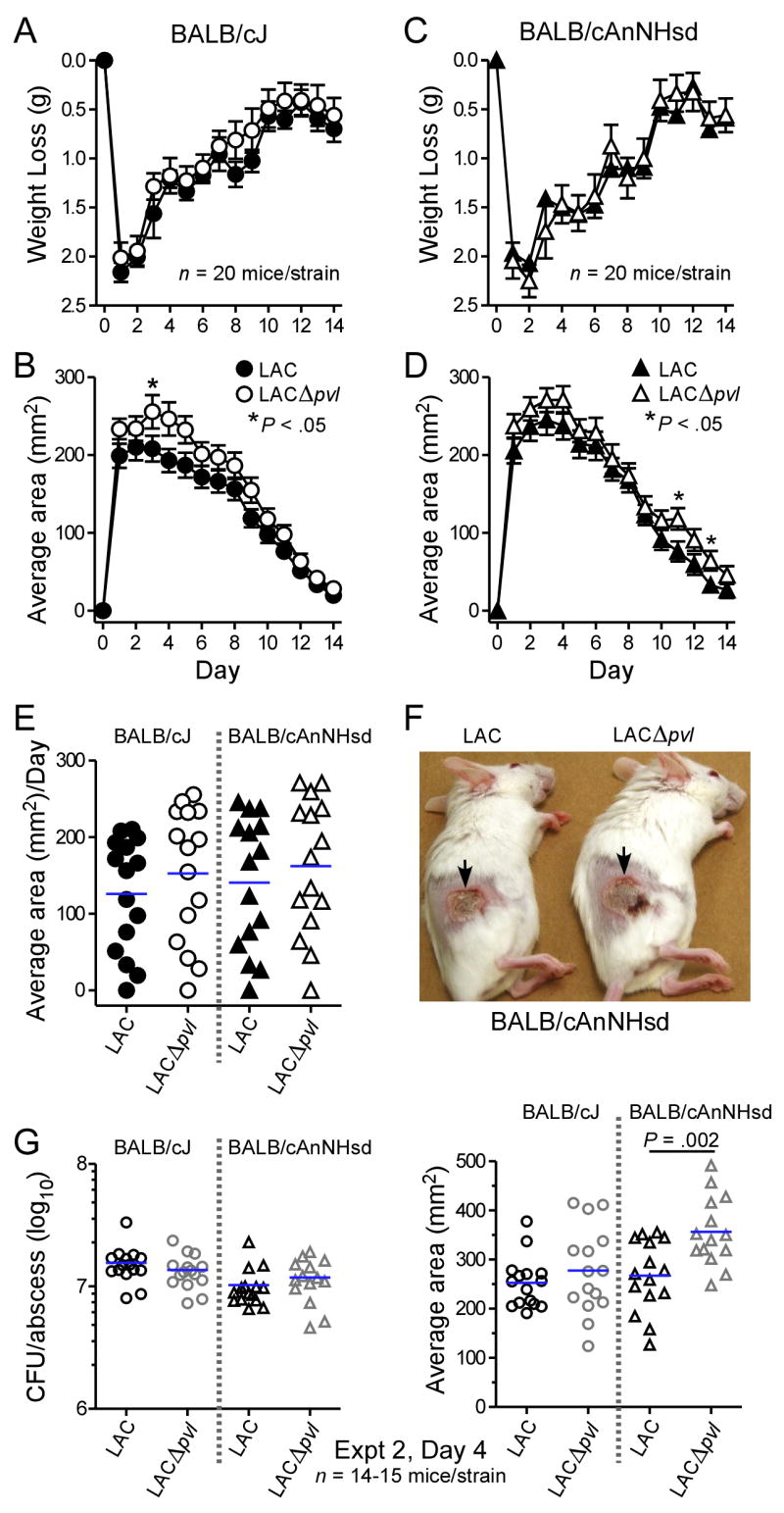
Virulence of LAC and LACΔpvl is comparable in BALB/cJ and BALB/cAnNHsd skin infection models. A, Weight loss and (B) abscess area for BALB/cJ mice infected with LAC and LACΔpvl. C, Weight loss and (D) abscess area for BALB/cAnNHsd mice infected with LAC and LACΔpvl. Results are the mean ± SE of 20 BALB/cJ or BALB/cAnNHsd mice infected with each S. aureus strain. *P < .05 versus LAC wild-type, unpaired t-test. E, Average skin abscess area per day. Each day is shown as a symbol and mice were monitored for 14 days (each symbol represents the average abscess size of 20 mice). F, Representative abscesses in BALB/cAnNHsd mice 4 days (day of largest abscess size) after infection with LAC (left panel) or LACΔpvl (right panel). G, Bacteria recovered from abscesses. Left panel, CFUs recovered from abscesses of 15 BALB/cJ or 14–15 BALB/cAnNHsd 4 days after infection with LAC or LACΔpvl as indicated. No bacteria were cultured from mice injected with DPBS (2 mice each of BALB/cJ and BALB/cAnNHsd, data not shown). Right panel, results are the abscess areas of the mice from which CFUs were recovered (see left panel). Statistics were performed using an unpaired t-test to compare wild-type LAC and LACΔpvl on identical days.
We performed a separate set of experiments using identical infection conditions to determine if PVL enhances S. aureus survival in mouse abscesses (Fig. 1G). S. aureus recovery from lesions of infected mice 4 days post-inoculation was similar between groups of animals challenged with LAC or LACΔpvl (Fig. 1G, left panel). In these experiments the average abscess size in BALB/cAnNHsd mice infected with LACΔpvl was significantly greater than that of the wild-type strain (P = 0.002, Fig. 1G, right panel). These data confirm and extend previous observations documenting that PVL does not play a unique role in the pathogenesis of staphylococcal skin abscess formation in mice.
We next evaluated the contribution of PVL to invasive disease by comparing virulence of LAC and LACΔpvl in a BALB/c mouse model of S. aureus pneumonia. BALB/cJ or BALB/cAnNHsd were infected with 4–6×107 CFUs of LAC or LACΔpvl, according to the protocol described by Labandeira-Rey et al. [12]. Infection with either strain resulted in an average weight loss of 1.5 grams at 2 days post-infection (Fig. 2A and 2B). The observation that weight loss was comparable in mice infected with LAC or LACΔpvl is at variance with the report of Labandeira-Rey et al., which used animals infected with the RN6390 laboratory strain and its engineered PVL-positive variants. Further, there was no significant difference in S. aureus numbers recovered from the right lung of infected animals 2 days post-inoculation between LAC and LACΔpvl strains in either BALB/cJ (Fig. 2C) or BALB/cAnNHsd (Fig. 2D) mice. The bacterial load in the lungs under these conditions was low; most animals harbored fewer than 100 staphylococci per lung 2 days post-infection. There was a trend towards increased lung recovery of LACΔpvl, albeit the difference was not statistically significant. Finally, we evaluated the histopathologic features of pneumonia caused by LAC and LACΔpvl. Infection with either strain resulted in an equal accumulation of cellular infiltrates in the alveolar space (Fig. 2E), in contrast to the observations of Labandeira-Rey. et al. [12], in which strains expressing PVL contributed to more significant inflammatory lesions.
Figure 2.
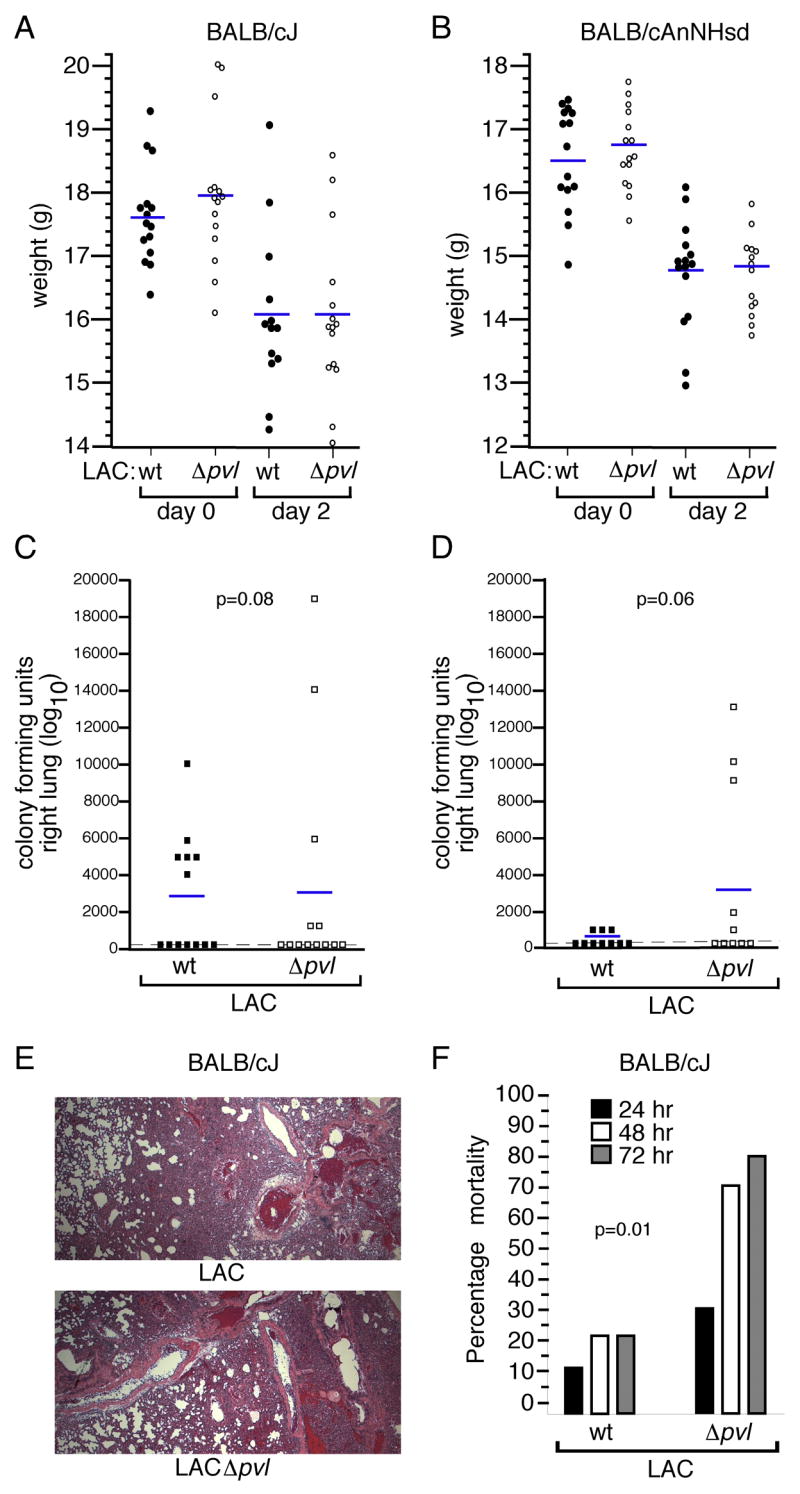
PVL expression correlates with decreased virulence in murine pneumonia. Weight loss data for BALB/cJ (A) and BALB/cAnNHsd (B) mice infected with either the LAC or LACΔpvl strains (4–6 × 107 CFU per animal). Weights were recorded at the time of infection (day 0) and 2 days post-inoculation. C, Right lung colony forming unit recovery two days post-infection in BALB/cJ mice and (D) BALB/cAnNHsd mice inoculated with 4–6 × 107 CFU S. aureus LAC or LACΔpvl. Statistical assessments were made for animal weights and CFU recovery data using the two-tailed Student’s t-test. E, Histopathologic appearance of representative thin lung sections from BALB/cJ mice infected with either LAC (upper panel) or LACΔpvl (lower panel). Tissue samples were derived from the left lungs of the animals described in B and C, and stained with hematoxylin and eosin prior to light microscopic evaluation. F, Acute lethal disease caused by the LAC and LACΔpvl strains when BALB/cJ mice were infected via intranasal route with 3–4 × 108 S. aureus. Mortality was recorded daily for 3 days. Statistical significance was calculated using the Fisher’s exact test.
Infection of BALB/c mice with 4–6×107 CFUs, as described above, did not result in appreciable animal mortality. Within the first 36 hours post-inoculation, animals appeared mildly ill with decreased mobility and ruffled fur; however, these clinical signs resolved by 48-hours. To more rigorously examine the contribution of PVL to severe S. aureus pneumonia, we infected animals with 3–4×108 CFUs of S. aureus, an inoculum demonstrated previously to result in acute lethal disease in a C57BL/6J model [13, 14]. Twenty percent of BALB/cJ mice infected with this inoculum of LAC succumbed to infection over 3 days (Fig. 2F). By comparison, LACΔpvl caused a more severe form of disease, with 80% animal mortality following infection (Fig. 2F). These data are in contrast to those from C57BL/6J mice, where LAC and LACΔpvl isolates are equally virulent, causing 80–100% mortality by 72 hours [13]. This finding indicates the BALB/c mouse strain is relatively resistant to S. aureus pneumonia in comparison to the C57BL/6J strain. Further, we observed no animal mortality in infected BALB/cAnNHSd mice (data not shown), suggesting that subtle differences in disease susceptibility exist even within the BALB/c background. Perhaps more interesting is finding that the LACΔpvl isogenic mutant strain was significantly more virulent than the parental strain.
Current views in the staphylococcal field hold that expression of PVL by CA-MRSA contributes to the aggressive nature of disease manifested by these isolates. Recent efforts to investigate the role of PVL in the pathogenesis of S. aureus infection of animals yielded conflicting results, especially as they pertain to S. aureus pneumonia, perhaps owing to experimental variation in animal model systems or staphylococcal strains investigated [11–13]. The data presented herein appraise the role of PVL in mouse models of staphylococcal skin abscess formation and pneumonia using a clinical isolate (LAC) representative of the most prevalent CA-MRSA genotype (USA300) and its isogenic PVL deletion mutant, revealing that PVL is not uniquely required for the pathogenesis of either disease. This conclusion is corroborated further by data acquired with mice from multiple genetic backgrounds. What, then, is the function of PVL in S. aureus infections? The significant increases in staphylococcal abscess size and pneumonia-related mortality in PVL deficient strains documented herein suggest that PVL may prime the host inflammatory response to facilitate pathogen recognition and clearance. A study of the effects of sublytic concentrations of PVL on neutrophils provides some support for this notion, as PVL was able to induce granulocyte production of inflammatory and chemotactic mediators that enhance immune responses [15]. Moreover, PVL has been shown to stimulate granulopoiesis in mice and rabbits. As granulocytes are pivotal in host immunity to S. aureus, it is interesting to speculate based on these observations that the secretion of PVL in the context of some infections may alter the innate immune response, favoring the host. Future experimentation will need to be conducted to critically assess this possibility.
Acknowledgments
The authors thank Kevin R. Braughton (NIAID), Adeline R. Whitney (NIAID), and Shirley Bond (University of Chicago) for technical assistance. This work was supported by the Intramural Research Program of the NIAID, NIH, and by Grant AI52747 from the NIAID, Division of Microbiology and Infectious Diseases to O.S. J.B.W. is an NICHD Fellow of the Pediatric Scientist Development Program (NICHD Grant Award K12-HD00850).
This work was supported by the Intramural Research Program of the NIAID, NIH, as well as by Grant AI52747 from the NIAID, Division of Microbiology and Infectious Diseases to O.S. J.B.W. is an NICHD Fellow of the Pediatric Scientist Development Program (NICHD Grant Award K12-HD00850). The authors declare no competing interests.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–7. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 4.Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–9. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba T, Takeuchi F, Kuroda M, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–27. doi: 10.1016/s0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 6.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 7.Miles G, Movileanu L, Bayley H. Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci. 2002;11:894–902. doi: 10.1110/ps.4360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 9.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 11.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 12.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–3. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 13.Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nature Medicine. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 14.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface Proteins and Exotoxins are Required for the Pathogenesis of Staphylococcus aureus Pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konig B, Prevost G, Piemont Y, Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–13. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]


