Abstract
Acute administration of γ-aminobutyric acid (GABA)-B receptor agonists decreases nicotine, cocaine, ethanol, and heroin self-administration, and also decreases food-maintained responding and suppresses locomotor activity at high doses. GABAB receptor positive modulators may represent potentially improved therapeutic compounds because of their fewer side-effects than receptor agonists. The present study investigated the effects of administration of the GABAB receptor positive modulators 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine (BHF177), and co-administration of the GABAB receptor positive modulator N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) with the GABAB receptor agonist (3-amino-2[S]-hydroxypropyl)-methylphosphinic acid (CGP44532) on nicotine- and food-maintained responding under fixed-ratio 5 (FR5) and progressive-ratio schedules of reinforcement. Furthermore, the effects of BHF177 and CGP44532 on nicotine-induced enhancement of brain reward function were evaluated. The results indicated that administration of CGP7930 decreased nicotine self-administration under an FR5 schedule. Administration of either GS39783 or CGP44532 selectively decreased nicotine self-administration, while co-administration of these compounds had additive effects. BHF177 administration selectively decreased nicotine-, but not food-, maintained responding under FR5 and progressive-ratio schedules. The nicotine-induced enhancement of brain reward function was blocked by BHF177 or CGP44532, although the highest doses of both compounds, particularly CGP44532, decreased brain reward function when administered alone, suggesting an additive, rather than interactive, effect. Overall, the present results indicate that GABAB receptor positive modulators, similarly to GABAB receptor agonists, attenuated the reinforcing and reward-enhancing effects of nicotine, perhaps with higher selectivity than GABAB receptor agonists. Thus, GABAB receptor positive modulators may be useful anti-smoking medications.
Introduction
Nicotine dependence in the form of cigarette smoking results in 5 million deaths per year globally, and this number is expected to double by the year 2025 (Proctor, 2004). The currently available anti-smoking therapies are not particularly effective (Henningfield et al., 2005; Jorenby et al., 2006), therefore novel anti-smoking medications are highly desirable. Compulsive nicotine use is maintained by multiple motivational forces, including the rewarding effects of acutely administered nicotine (for review, Markou and Paterson, 2008). Nicotine self-administration provides a measure of the positive reinforcing effects of nicotine (Corrigall, 1999). Nicotine also enhances the rewarding effects of non-drug reinforcers (Chaudhri et al., 2006), including electrical stimulation of brain reward regions (Harrison et al., 2002; Kenny and Markou, 2006). These reward-enhancing effects of nicotine, in addition to the primary rewarding effects of nicotine, have been suggested to contribute to the maintenance of nicotine dependence (Chaudhri et al., 2006; Kenny and Markou, 2006; Markou and Paterson, 2008).
The rewarding effects of nicotine are hypothesized to be mediated by increased glutamate neurotransmission in the ventral tegmental area that in turn increases dopamine transmission in the nucleus accumbens and other forebrain structures (Mansvelder and McGehee, 2002). The close anatomical relationship of γ-aminobutyric acid (GABA), glutamate, and dopamine neurons provides the basis for a major inhibitory role of GABA in the modulation of central reward processes, including those induced by nicotine (Paterson et al., 2005a). Specifically, GABAB receptors are located on dopaminergic and glutamatergic neurons in the ventral tegmental area, mediating inhibitory input from the nucleus accumbens and the ventral pallidum. Inhibitory GABAergic interneurons also are located within the ventral tegmental area (for review see, Kalivas et al., 1992). Systemic administration of the GABAB receptor agonist baclofen decreases the firing rate of ventral tegmental area dopaminergic neurons. Furthermore, GABAB receptor activation reduces extracellular glutamate and dopamine in the ventral tegmental area and nucleus accumbens, respectively (for review see, Bowery, 2006).
Consistent with the inhibitory role of GABAB receptors in the mesolimbic circuitry, administration of either one of two GABAB receptor agonists, CGP44532 or baclofen (Froestl et al., 1995), attenuated the reinforcing effects of nicotine (Corrigall et al., 2000; Fattore et al., 2002; Paterson et al., 2004) and other drugs of abuse, such as cocaine, heroin, and alcohol, in rats (for review, Cousins et al., 2002). CGP44532 also attenuated cocaine-induced enhancement of brain reward function (Dobrovitsky et al., 2002). Nonetheless, GABAB receptor agonists have undesirable side-effects, including disruption of performance on the rotarod test, a measure of locomotor impairment (Cryan et al., 2004), and decreased responding for non-drug rewards such as food and electrical brain stimulation (Macey et al., 2001; Paterson et al., 2005b; Slattery et al., 2005).
Positive allosteric GABAB modulators increase both the potency and efficacy of GABA in activating GABAB receptors. Positive GABAB modulator compounds, such as GS39783, are devoid of substantial intrinsic agonistic activity in the absence of GABA (Urwyler et al., 2003). Thus, positive modulators offer more physiological means to activate receptors in vivo than agonists, as the modulators do not perturb receptor signaling on their own, but potentiate the effect of GABA only where and when it is endogenously released to activate GABAB receptors. Allosteric modulators bind to a distinct side from the agonist binding pocket, therefore it is possible to co-apply positive modulators with agonists, to potentiate the effects of the agonists. The positive allosteric GABAB modulator GS39783, in contrast to the agonist baclofen, did not impair performance when administered on its own in the rotarod test (Cryan et al., 2004). GS39783 selectively decreased cocaine- (Filip et al., 2007) or ethanol- (Liang et al., 2006), but not food- or water-, maintained responding.
The present studies assessed the effects of GABAB receptor positive modulators on both the reinforcing and reward-enhancing effects of nicotine in rats. Specifically, we determined the effects of two different GABAB receptor positive modulators, CGP7930 (Urwyler et al., 2001) and BHF177 (Guery et al., 2007), on nicotine- and food-maintained responding under fixed-ratio and progressive-ratio (BHF177 only) schedules of reinforcement. The effects of co-administration of the GABAB receptor positive modulator GS39783 (Urwyler et al., 2003) and the GABAB receptor agonist CGP44532 (Froestl et al., 1995) on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement also were assessed. Finally, the effects of GABAB receptor activation, via administration of either the GABAB receptor agonist CGP44532 or the GABAB receptor positive modulator BHF177, on nicotine-induced enhancement of brain reward function were evaluated.
Methods
Subjects
Male Wistar rats (Charles River, Raleigh, NC) weighing 300−350 g upon arrival in the laboratory were group housed (two per cage) in a temperature- and humidity-controlled vivarium on a 12 h reverse light-dark cycle with unrestricted access to water except during testing. Rats were food-restricted to 12−20 g/day throughout the experiments (see below), with the exception of the surgical recovery and initial habituation periods. All behavioral testing occurred during the dark phase of the light-dark cycle. For 1 week after arrival in the vivarium, animals were allowed to habituate to their new environment and were handled twice during this period. All subjects, animal facilities, and experimental protocols were in accordance with National Institutes of Health and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the institute's Animal Research Committee.
Drugs
(−)Nicotine hydrogen tartrate was purchased from Sigma (St. Louis, MO) and dissolved in saline (pH adjusted to 7.0 ± 0.5 with sodium hydroxide). The solution then was filtered through a 0.22 μm syringe filter (Fisher Scientific, Pittsburgh, PA) for sterilization purposes. Nicotine doses are reported as free base concentrations. All GABAB receptor ligands are reported as salt concentrations. CGP44532 (Froestl et al., 1995), CGP7930 (Urwyler et al., 2001), GS39783 (Urwyler et al., 2003), and BHF177 (compound #27 in Guery et al., 2007) were synthesized and provided by Novartis Pharma AG (Basil, Switzerland). The most recently synthesized compound, BHF177, is structurally different but has a potency (1.7 μM, Guery et al., 2007) similar to the previously published positive modulators CGP7930 and GS39783 (Urwyler et al., 2001, 2003). BHF177 crosses the blood-brain barrier. Administration of 10 mg/kg, p.o., to mice led to brain concentrations of BHF177 close to its EC50 value 1 h after administration (C. Gentsch, Novartis; personal communication). CGP44532 was dissolved in 0.9% saline and administered subcutaneously in a volume of 1 ml/kg, with a pretreatment time of 30 min. CGP7930, GS39783, and BHF177 were suspended in 0.5% methylcellulose and administered either orally in a volume of 2 ml/kg with a pretreatment time of 1 h (GS39783 and BHF177 in Experiments 2, 3, and 4) or intraperitoneally in a volume of 2 ml/kg with a pretreatment time of 15 min (CGP7930 in Experiment 1) or 30 min (BHF177 in Experiment 5). Different routes of administration were used in these experiments. The first nicotine self-administration study assessed the effects of CGP7930 administered intraperitoneally. With this study, we discovered the relatively poor solubility of this class of compounds and thus, we decided to use the per os administration in all subsequent nicotine and food self-administration studies. In all ICSS experiments, we chose to use the intraperitoneal route of administration for direct comparisons with previous studies assessing the effects of GABAB receptor agonists and antagonists on performance in the ICSS task (Macey et al., 2001).
Apparati
Intravenous nicotine self-administratio apparati
Intravenous nicotine self-administration and food-maintained responding took place in 12 Plexiglas experimental chambers (25 × 31 × 24 cm; Med Associates, St. Albans, VT), each housed in a sound-attenuated box (San Diego Instruments, San Diego, CA). One wall of the chamber contained two levers, measuring approximately 3 cm in width and located approximately 3 cm above the metal grid floor of the chamber. All data collection and test session functions were controlled by computers and Med PC IV software (Med Associates, St. Albans, VT).
Intracranial self-stimulation apparati
The experimental apparatus consisted of 16 Plexiglas chambers measuring 30.5 × 30 × 17 cm (Med Associates, St. Albans, VT), each housed in a sound-attenuating box (San Diego Instruments, San Diego, CA). Each chamber contained a metal wheel manipulandum (5 cm wide), centered in a side-wall, that required approximately 0.2 N force to rotate it a quarter turn. Brain stimulation was delivered by constant current stimulators (Stimtech 1200, San Diego Instruments, San Diego, CA). Subjects were connected to the stimulation circuit with bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (model SL2C, Plastics One, Roanoke, VA). The stimulation parameters, data collection, and all test session functions were controlled by a computer.
Surgeries
Intravenous catheterization surgery
Rats were anesthetized with an isoflurane/oxygen mixture (1−1.5% isoflurane) and prepared with a catheter inserted into the right jugular vein. Catheters were constructed from a 14 cm piece of silastic tubing (0.3 mm inside diameter × 0.63 mm outside diameter, Dow Corning) attached to a 22 gauge stainless steel guide cannula (Plastics One, Roanoke, VA), a molded base (Teets Denture Material, Co-Oral-Lite Mfg. Co., Diamond Springs, CA), and mesh (Small Parts Inc., Miami Lakes, FL). The mesh anchored the catheter base underneath the skin at the level of the scapulae. Animals were given 7 days to recover from surgery prior to being trained to lever press for 45 mg food pellets (described below). All rats received 7 days of 20 mg/day of the antibiotic Timentin after surgery. In addition, animals received a 0.1 ml infusion of heparinized saline (33.3 U/ml) before and after each self-administration session.
ICSS electrode placement surgery
Subjects were anesthetized with an isoflurane/oxygen vapor mixture (1.0−1.5%) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Stainless steel bipolar electrodes (model MS303/2, Plastics One, Roanoke, VA), cut to a length of 11 mm, were implanted in the posterior lateral hypothalamus (AP: −0.5 mm from bregma, ML: ±1.7 mm, DV: −8.3 mm from dura, with the incisor bar 5 mm above the interaural line; Pellegrino et al. 1979). Dental acrylic was applied around the base of the electrode, and four stainless steel screws were inserted into the skull to fix the assembly permanently.
Behavioral Procedures
Food training and testing
Approximately 1 week after preparation with intravenous catheters, rats were food-restricted (5 g/day) for 48 h prior to starting food training. After the initiation of food training, animals received 12 g rat chow per day, at least 1 h after the end of the food training session. During the initial 30 min session, the animals received 45 mg food pellets at fixed intervals (one pellet every 12 s for 5 min, then a 5 min break; this sequence was repeated until a total number of 75 pellets was delivered) with no requirement to lever press. After this session and on subsequent days, rats were allowed to press a lever to receive the food pellets on a fixed-ratio 1 time-out 1 s (FR1TO1 s) schedule of reinforcement. Only one lever was extended into the operant testing chamber during the initial food training period. The schedule was gradually changed according to the sequence FR1TO1 s, FR2TO10 s, FR5TO20 s, with sessions lasting 30 min. Animals moved through the sequence only after the successful acquisition of the previous schedule (defined as earning 50 pellets within the 30 min session). After successful acquisition of food-maintained responding, all rats were maintained on 20−26 g rat chow per day, given at least 1 h after testing.
An identical training procedure was implemented in the subjects that were used to assess the effects of the test compounds on food-maintained responding. After successful acquisition of food-maintained responding, these rats were allowed to continue responding for food (45 mg Noyes food pellet) on an FR5TO20 s schedule during 1 h test sessions 5 days per week. During these sessions, two levers (active and inactive) were present in the box. This testing procedure was identical in all parameters to the nicotine self-administration procedure (see below).
Nicotine self-administration under a fixed-ratio schedule of reinforcement
After the successful completion of food training, rats that participated in the self-administration experiments were allowed to self-administer nicotine (0.03 mg/kg/infusion; base) by switching the delivery of a food pellet with the delivery of a nicotine infusion. Two levers were present in the operant testing chamber, only one of which (the lever previously paired with food delivery) was paired with the delivery of a nicotine infusion (the active lever). Responses on the other lever were recorded but had no consequences (the inactive lever). Responding on the active lever resulted in the delivery of the nicotine solution in a volume of 0.1 ml infused over 1 s (0.03 mg/kg/inj base; Razel syringe pump model A, Razel Scientific Instruments, Stamford, CT). The delivery of an infusion was paired with a cue light, located above the active lever, that was lit simultaneously with the initiation of the nicotine infusion and remained illuminated throughout the 20 s time-out period, during which responding was recorded but not reinforced. The delivery of an infusion was earned by responding five times on the active lever (FR5TO20 s). Rats were considered to have acquired stable nicotine self-administration when they pressed the active lever more than twice the number of times they pressed the inactive lever and received a minimum of six infusions per 1 h session, with less than 20% variation in the number of infusions earned per session over 3 consecutive sessions. Rats were allowed to self-administer nicotine 5 days per week.
Progressive-ratio responding for nicotine or food reinforcers
For assessment of the effects of the positive modulators on responding under the progressive-ratio schedule of reinforcement, after successful acquisition of the FR5TO20 s schedule, the rats that participated in these experiments were tested on a progressive-ratio schedule of reinforcement, with sessions lasting 4−6 h. All animals reached breakpoints during the 6 h sessions (most commonly within 4 h). The breakpoint was defined as the highest ratio completed prior to the first 1 h period during which no injections or pellets were earned. The number of responses required to earn a nicotine infusion or food pellet on the progressive-ratio was determined by the exponential progression: [5e(0.25*(infusion number+3)-5], with the first two values replaced by 5 and 10 (modified from Richardson and Roberts, 1996), so that the response requirements for successive reinforcers were 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, etc. The same criteria were used to determine acquisition of stable responding under the FR5 and progressive-ratio schedules, with the exception that under the progressive-ratio schedule, the minimum number of infusions required per session was five rather than six, based on the animals' performance in the task.
ICSS procedure
The discrete-trial current-threshold procedure was a modification of a task initially developed by Kornetsky and Esposito (1979). The rats first were trained to turn the wheel manipulandum on an FR1 schedule of reinforcement. Each quarter turn of the wheel resulted in the delivery of a 500 ms train of 0.1 ms cathodal square-wave pulses at a frequency of 100 Hz. After the successful acquisition of responding for stimulation on this FR1 schedule, defined as 100 reinforcements within 10 min, the rats were trained gradually on the discrete-trial current-threshold procedure.
Each trial began with the delivery of a noncontingent electrical stimulus, followed by a 7.5 s response window within which the subject could make a response to receive a second contingent stimulus identical to the initial noncontingent stimulus. A response during this time window was labeled a positive response, while the lack of a response was labeled a negative response. During a 2 s period immediately after a positive response, additional responses had no consequence. The intertrial interval that followed either a positive response or the end of the response window in the case of a negative response had an average duration of 10 s (ranging from 7.5 s to 12.5 s). Responses that occurred during the intertrial interval were labeled time-out responses and resulted in a further 12.5 s delay of the onset of the next trial. During training on the discrete-trial procedure, the duration of the intertrial interval and delay periods induced by time-out responses were gradually increased until animals performed consistently for a fixed stimulation intensity at standard test parameters. The animals were subsequently tested on the current-intensity threshold procedure in which stimulation intensities were varied according to the classical psychophysical method of limits. A test session consisted of four alternating series of descending and ascending current intensities starting with a descending series. Blocks of three trials were presented to the subject at a given stimulation intensity, and the current intensity was changed by steps of 5 μA between blocks of trials. The initial stimulus intensity was set at approximately 40 μA above the baseline current-intensity threshold for each animal. Each test session typically lasted 30−40 min and provided two dependent variables for behavioral assessment: threshold and response latency.
Threshold
The current-threshold for each descending series was defined as the stimulus intensity between a successful completion of a set of trials (positive responses during two or more of the three trials) and the stimulus intensity for the first set of trials, of two consecutive sets, during which the animal failed to respond positively on two or more of the three trials. The current-threshold for each ascending series was defined as the stimulus intensity between a current intensity where the animal failed to respond positively on two or more of the three trials and the first set of trials, of two consecutive sets, during which the animal responded positively on two or more of the three trials. The mean of the four series' thresholds was defined as the threshold for the session.
Response latency
The time interval between the beginning of the noncontingent stimulus and a positive response was recorded as the response latency. The response latency for each session was defined as the mean response latency on all trials during which a positive response occurred.
Experimental Procedures
Experiment 1: Effects of the GABAB receptor positive modulator CGP7930 on nicotine self-administration under a fixed-ratio schedule of reinforcement
After successful acquisition of stable nicotine self-administration (see above), CGP7930 (0, 5, 10, and 30 mg/kg, IP, n = 6) was administered according to a within-subjects Latin-square design. CGP7930 was administered only after rats had exhibited stable nicotine self-administration across 3 consecutive days, with at least 6 days elapsing between each drug administration. CGP7930 was the first GABAB receptor positive modulator whose effects on nicotine self-administration were assessed. Due to the fact that larger effects on nicotine self-administration were observed with GABAB receptor positive modulators assessed subsequently (i.e., GS39783, BHF177), we determined that it was most informative to assess the effects of these latter two compounds on responding for food and on the nicotine self-administration under the progressive ratio schedule of reinforcement.
Experiment 2: Effects of the GABAB receptor positive modulator BHF177 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
After successful acquisition of stable nicotine- (n = 11) or food- (n = 7) maintained responding, the effects of BHF177 (0, 10, 20, and 40 mg/kg, PO) were assessed according to a within-subjects Latin-square design in naive rats. BHF177 was administered only after rats had exhibited stable nicotine- or food-maintained responding during 3 consecutive days, with at least 6 days elapsing between each drug administration.
Experiment 3: Effects of the GABAB receptor positive modulator BHF177 on nicotine- and food-maintained responding under a progressive-ratio schedule of reinforcement
After successful acquisition of stable nicotine- (n = 7) or food- (n = 10) maintained responding, the effects of BHF177 (0, 10, 20, and 40 mg/kg, PO) were assessed according to a within-subjects Latin-square design in naive rats. BHF177 was administered only after rats had exhibited stable responding across 3 consecutive days, with at least 6 days elapsing between each drug administration.
Experiment 4: Effects of co-administration of the GABAB receptor positive modulator GS39783 and the GABAB receptor agonist CGP44532 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
After successful acquisition of stable operant responding, rats were assigned to different groups (nicotine or saline groups), counter-balanced for initial rates of nicotine- or food-maintained responding and body-weights. The groups were used to test the effects of CGP44532 (0 mg/kg, n = 12; 0.125 mg/kg, n = 10; 0.25 mg/kg, n = 8; SC; between-subjects design) administered in combination with various doses of GS39783 (0, 10, 20, and 40 mg/kg, PO) according to a within-subjects Latin-square design. The dose of CGP44532 was a between-subjects factor, and the dose of GS39783 was a within-subjects factor. Drug testing was performed only after rats had exhibited stable nicotine- or food-maintained responding across 3 consecutive days, with at least 6 days elapsing between each drug administration.
Experiment 5: Effects of GABAB receptor activation on nicotine-induced facilitation of brain reward function
After establishing stable ICSS thresholds, rats were assigned to two groups, counter-balanced for ICSS threshold values and body-weights. The effects of co-administration of nicotine (0, 0.4 mg/kg, SC) and either BHF177 (0, 3.75, 7.5, 15, and 30 mg/kg, IP, n = 12) or CGP44532 (0, 0.125, 0.25, 0.375, and 0.5 mg/kg, SC, n = 11) were assessed according to a within-subjects Latin-square design. Drug combinations were administered only when rats exhibited stable ICSS thresholds over 2 consecutive days, and at least 2 days elapsed between each drug administration.
Data Analyses
Intravenous self-administration and food-maintained responding data obtained under a fixed-ratio schedule were expressed as percentage of baseline number of rewards earned, where baseline was defined as the mean number of rewards earned over the 3 days prior to each drug testing session. Intravenous self-administration and food-maintained responding data obtained under a progressive-ratio schedule were expressed as absolute number of reinforcers earned and final ratio attained. ICSS data were expressed as percentage of baseline, where baseline was defined as the mean ICSS thresholds or latencies obtained over the 2 days prior to each drug testing session. Data were analyzed with appropriate one- and two-way mixed-factor analyses of variance (ANOVAs). Where indicated by significant main or interaction effects, oneor two-way follow-up ANOVAs and/or Newman-Keuls post hoc tests were conducted. The level of significance was set at 0.05. Statistical analyses were conducted using the Statistical Package for the Social Sciences v.15.0.
Results
See Table 1 for the mean (± SEM) baseline number of nicotine infusions or food pellets earned during the baseline testing days preceding each drug testing session for Experiments 1−4. One-way ANOVAs on these pre-drug baseline values showed no statistically significant differences in any of the experiments, and thus demonstrated stable responding throughout the experiments under baseline conditions. Table 1 also shows mean body weights obtained immediately prior to the initiation of drug testing.
Table 1.
Baseline absolute numbers of reinforcers (nicotine or food) earned are expressed as the mean ± SEM of baseline values obtained prior to each drug testing day. Body weight data are the weights of the subjects prior to the initiation of any drug testing.
| Experimental group | Group size | Reinforcer | Mean number of rewards earned | Body weight (g) |
|---|---|---|---|---|
| CGP7930 (fixed-ratio) | n = 6 | nicotine | 15 ± 2 | 330 ± 3.7 |
| 0 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 12 | nicotine | 14.9 ± 1 | 426 ± 9.4 |
| 0 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 10 | food | 125.8 ± 7.5 | 395.8 ± 8.3 |
| 0.125 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 10 | nicotine | 12.2 ± 1.6 | 440.4 ± 13 |
| 0.125 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 10 | food | 117.2 ± 6.1 | 403.8 ± 3.2 |
| 0.25 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 8 | nicotine | 13 ± 1.2 | 438.4 ± 12.4 |
| 0.25 mg/kg CGP44532 + GS39783 (fixed-ratio) | n = 10 | food | 121.7 ± 7 | 401.9 ± 8.9 |
| BHF177 (fixed-ratio) | n = 11 | nicotine | 13.7 ± 1.03 | 332.8 ± 4.2 |
| BHF177 (fixed-ratio) | n = 7 | food | 134.7 ± 6.8 | 386.6 ± 12.5 |
| BHF177 (progressive-ratio) | n = 7 | nicotine | 6.3 ± 1.2 | 447.4 ± 8.2 |
| BHF177 (progressive-ratio) | n = 10 | food | 9 ± 0.7 | 405 ± 5.8 |
Experiment 1: Effects of the GABAB receptor positive modulator CGP7930 on nicotine self-administration under a fixed-ratio schedule of reinforcement
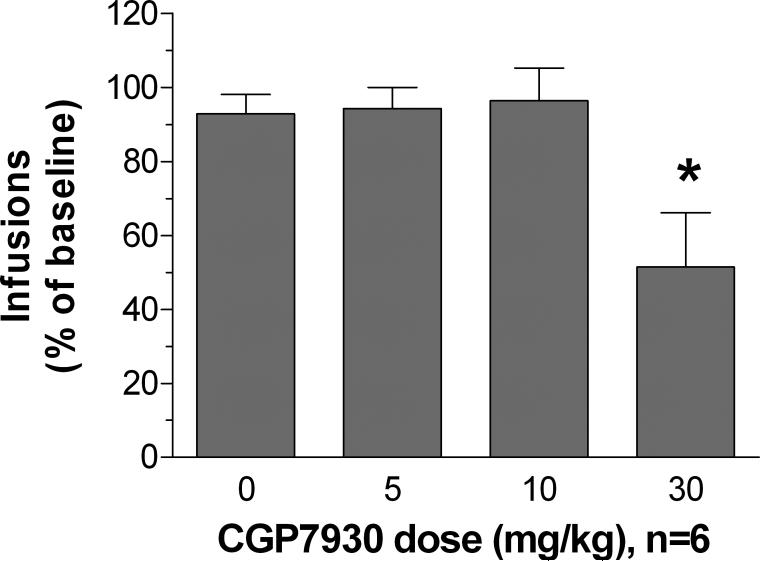
A one-way ANOVA indicated that CGP7930 decreased nicotine self-administration [F(3,15) = 5.83, P < 0.05]. Post hoc comparisons attributed this effect to a decrease in nicotine infusions earned after administration of 30 mg/kg CGP7930 compared with infusions earned after vehicle administration (*P < 0.01; Figure 1). No effect of CGP7930 was observed on inactive lever presses.
Figure 1. Effects of the GABAB receptor positive modulator CGP7930 on nicotine self-administration under a fixed-ratio schedule of reinforcement.
Data are expressed as a percentage of number of rewards earned under baseline conditions (mean ± SEM). The asterisk (*P < 0.05) indicates a significant difference from the vehicle-treated condition.
Experiment 2: Effects of the GABAB receptor positive modulator BHF177 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
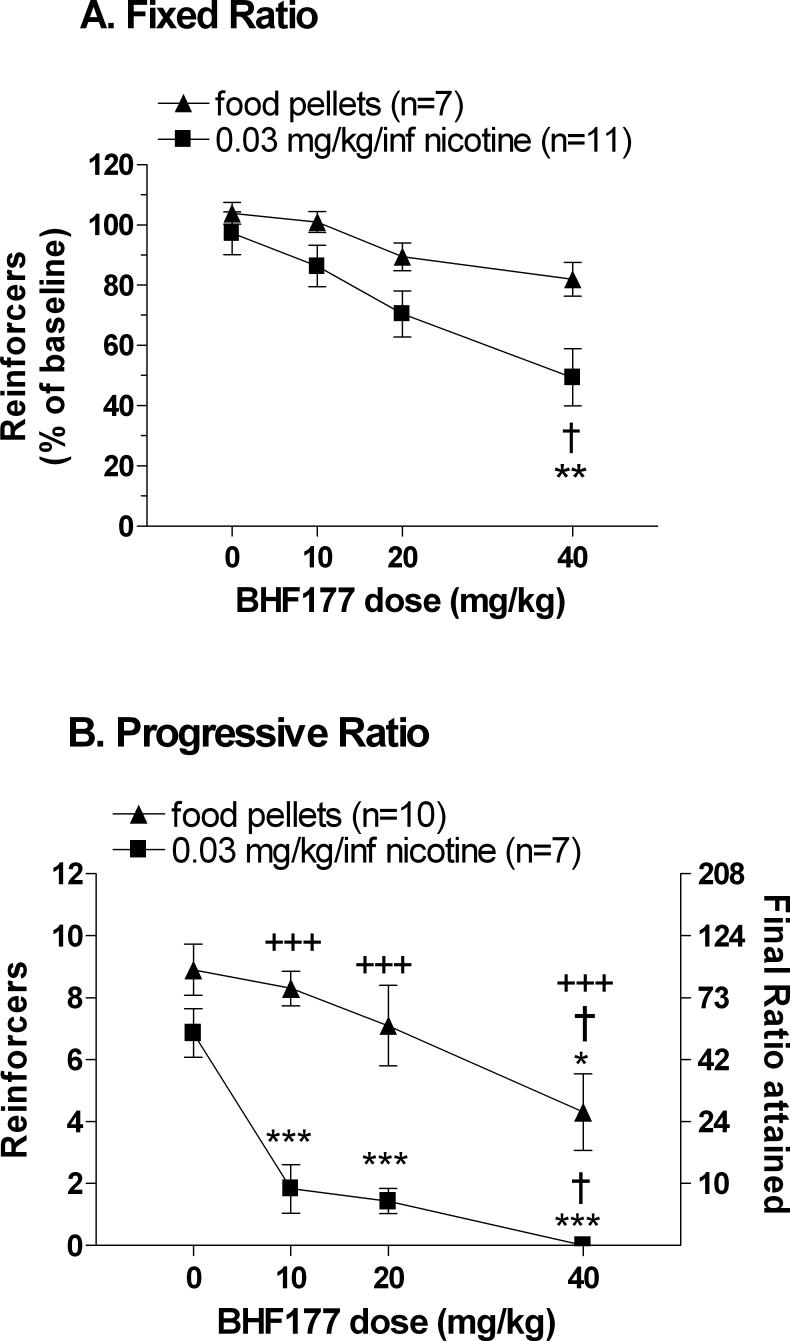
A two-way ANOVA indicated that BHF177 decreased responding for both nicotine and food [main effect of BHF177 Dose: F(3,48) = 9.85, P < 0.001] and revealed a main effect of Reinforcer [F(1,16) = 11.86, P < 0.01]. No statistically significant BHF177 Dose × Reinforcer interaction was observed. Pre-planned comparisons indicated that nicotine self-administration was significantly decreased after administration of 40 mg/kg BHF177 compared with nicotine self-administration after vehicle administration (P < 0.01) and compared with food-maintained responding after administration of 40 mg/kg BHF177 (P < 0.01; Figure 2). Analysis of inactive lever data revealed only a significant main effect of Reinforcer that likely was attributable to the higher number of active lever presses in the food-responding group compared with the nicotine-responding group [F(1,16) = 706.82, P < 0.001].
Figure 2. Effects of the GABAB receptor positive modulator BHF177 on nicotine- and food-maintained responding under fixed- and progressive-ratio schedules of reinforcement.
The graph shows nicotine- and food-maintained responding expressed as a percentage of baseline number of rewards earned (mean ± SEM) (A) and as number of reinforcers earned (left ordinal axis) and corresponding final ratio attained (i.e., breakpoints, right ordinal axis) (B). Asterisks and dagger signs indicate significant differences compared with nicotine- or food-maintained responding after administration of either vehicle (*P < 0.05, **P < 0.01, ***P < 0.001) or 10 mg/kg BHF177 (†P < 0.05), respectively. Plus signs (+++P < 0.001) indicate a significant difference between nicotine- and food-maintained responding within specific doses of BHF177.
Experiment 3: Effects of the GABAB receptor positive modulator BHF177 on nicotine- and food-maintained responding under a progressive-ratio schedule of reinforcement
A two-way ANOVA indicated that BHF177 decreased breakpoints for both reinforcers (main effect of BHF177 Dose: F(3,65) = 14.981, P < 0.01) and that breakpoints varied as a function of Reinforcer [main effect of Reinforcer: F(3,65) = 28.780, P < 0.001]. No statistically significant BHF177 Dose × Reinforcer interaction was observed. Follow-up one-way ANOVAs on the data from each reinforcer showed that BHF177 decreased breakpoints for nicotine [F(3,24) = 18.967, P < 0.001] and food pellets [F(3,36) = 3.951, P < 0.01]. Post hoc comparisons indicated that breakpoints for food were significantly decreased after administration of 40 mg/kg BHF177 compared with breakpoints after vehicle (P < 0.05) or 10 mg/kg BHF177 (P < 0.05). Importantly, breakpoints for nicotine were significantly decreased after administration of any of the three doses of BHF177 compared with breakpoints after vehicle administration (P < 0.001). Furthermore, 40 mg/kg BHF177 decreased breakpoints for nicotine compared with breakpoints after 10 mg/kg BHF177 (P < 0.05). Finally, nicotine maintained higher breakpoints compared with food for each BHF177 dose tested (P < 0.001; Figure 2). Analysis of inactive lever data revealed only a significant main effect of Reinforcer that may have been attributable to the higher breakpoints for food compared with the breakpoints for nicotine [F(1,39) = 5.720, P < 0.004].
Experiment 4: Effects of co-administration of the GABAB receptor positive modulator GS39783 and the GABAB receptor agonist CGP44532 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement
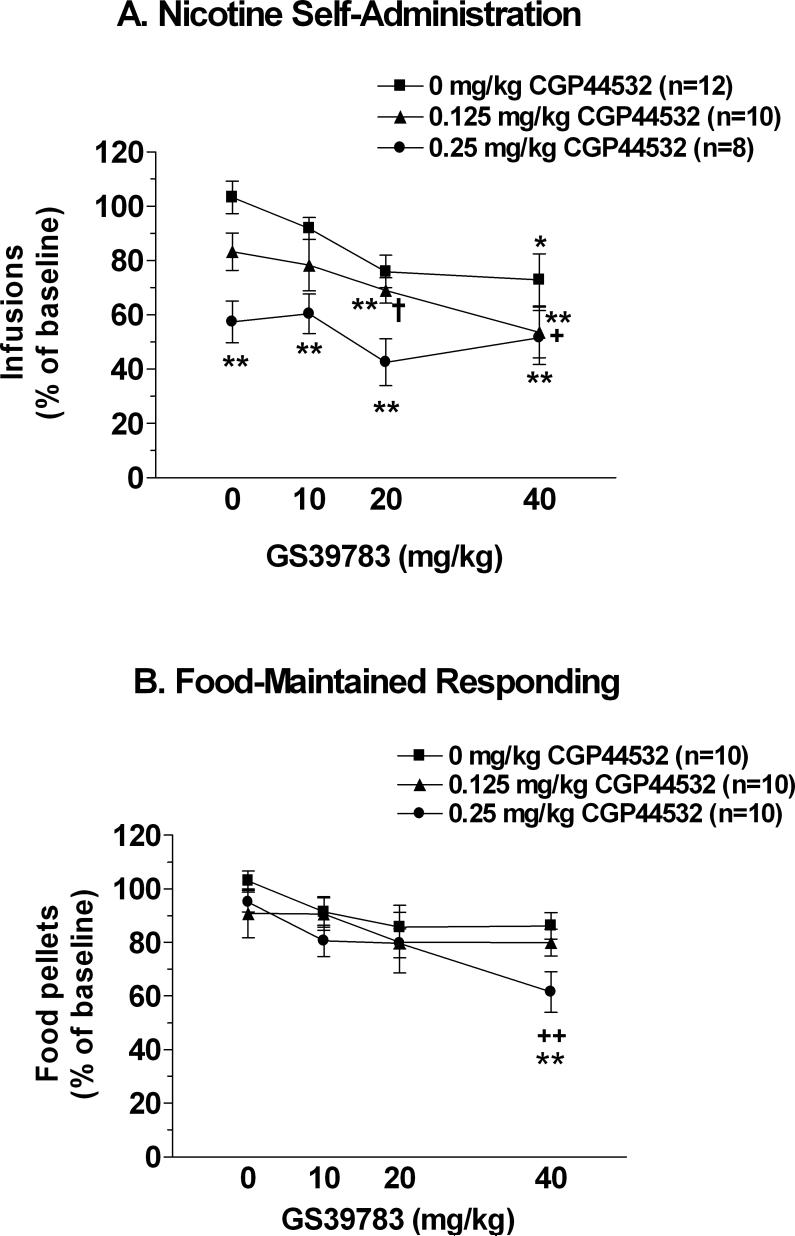
A three-way ANOVA indicated that administration of either the GABAB receptor positive modulator GS39783 [F(3,162) = 15.16, P < 0.001] or the GABAB receptor agonist CGP44532 [F(2,54) = 10.41, P < 0.001] decreased responding for nicotine and food. Although no significant Reinforcer × CGP44532 or Reinforcer × GS39783 interaction effects were observed, a main effect of Reinforcer was found [F(1,54) = 14.49, P < 0.001], due to lower responding in the nicotine- vs. food-maintained groups across treatments. Pre-planned comparisons indicated that co-administration of 40 mg/kg GS39783 + vehicle significantly decreased nicotine self-administration compared with vehicle + vehicle (P < 0.05). Co-administration of only the higher dose of CGP44532 (0.25 mg/kg) + vehicle decreased nicotine self-administration. Nicotine self-administration was significantly decreased after co-administration of 0.125 mg/kg CGP44532 + 20 or 40 mg/kg GS39783 compared with nicotine self-administration after vehicle + vehicle treatment (P < 0.01), and after 0.125 mg/kg CGP44532 + 40 mg/kg GS39783 compared with 0.125 mg/kg CGP44532 + vehicle co-administration (P < 0.05). Finally, co-administration of 0.25 mg/kg of the GABAB receptor agonist CGP44532 plus any of the doses of the GABAB receptor positive modulator GS39783 decreased nicotine self-administration compared with vehicle + vehicle treatment (P < 0.01). Furthermore, co-administration of 0.25 mg/kg CGP44532 + 20 mg/kg GS39783 selectively decreased nicotine- but not food-maintained responding (P < 0.01). Co-administration of 0.25 mg/kg CGP44532 + 40 mg/kg GS39783 selectively decreased food-maintained responding compared with vehicle + vehicle treatment (P < 0.01) and compared with 0.25 mg/kg CGP44532 + vehicle co-administration (P < 0.01; Figure 3). Analysis of inactive lever data indicated that inactive lever presses were higher in the nicotine self-administration groups across treatments [main effect of Reinforcer: F(1,54) = 4.7, P < 0.05] and were decreased by administration of the GABAB receptor agonist CGP44532 [Reinforcer × CGP44532: F(2,54) = 3.72, P < 0.05]. No other main or interaction effects were found.
Figure 3. Effects of co-administration of the GABAB receptor positive modulator GS39783 and the GABAB receptor agonist CGP44532 on nicotine- and food-maintained responding under a fixed-ratio schedule of reinforcement.
Data are expressed as a percentage of rewards earned under baseline conditions (mean ± SEM). Asterisks (*P < 0.05, **P < 0.01) indicate significantly decreased responding compared with the vehicle/vehicle-treated group. Plus signs (+P < 0.05, ++P < 0.01) indicate significantly decreased responding after administration of 40 vs. 0 mg/kg GS39783 within CGP44532 dose groups. The dagger sign (†P < 0.05) indicates significantly decreased responding for nicotine compared with food after administration of 0.25 mg/kg CGP44532 + 20 mg/kg GS39783.
Experiment 5: Effects of GABAB receptor activation on nicotine-induced enhancement of brain reward function
BHF177 + nicotine
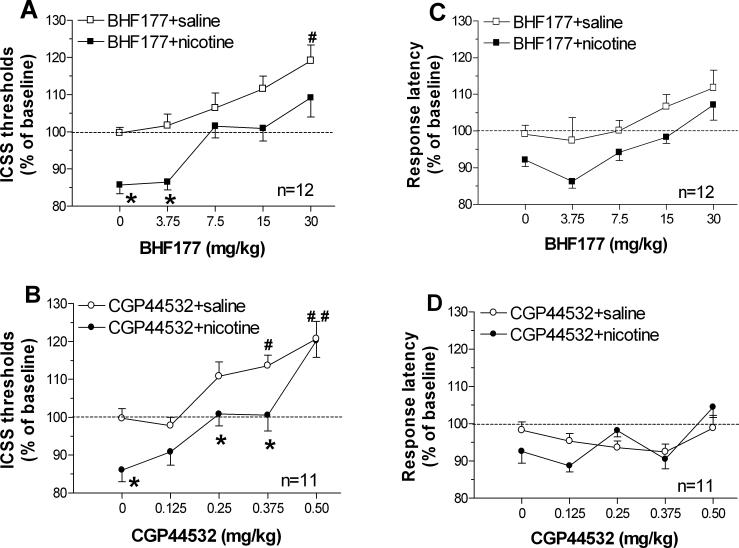
Mean ± SEM of absolute ICSS threshold and response latency values on the days prior to each drug administration were 110.9 ± 10.6 μA and 3.2 ± 0.1 s, respectively. One-way ANOVAs on these preinjection threshold and latency values indicated no statistically significant effects, demonstrating the stability of baseline ICSS thresholds and latencies during the experiment. A two-way ANOVA revealed significant main effects of BHF177 [F(4,110) = 14.9, P < 0.001] and nicotine [F(1,110) = 27.98, P < 0.001] but no significant interaction effect. Pre-planned comparisons indicated that nicotine significantly lowered ICSS thresholds when co-administered with vehicle or 3.75 mg/kg BHF177 (P < 0.05 and P < 0.01, respectively) compared with thresholds after saline administration. By contrast, thresholds were significantly elevated after administration of 30 mg/kg BHF177 compared with vehicle in the absence of nicotine (P < 0.05; Figure 4A). A two-way ANOVA on response latency data indicated that administration of BHF177 [F(4,110) = 8.17, P < 0.01] and nicotine [F(1,110) = 22.64, P < 0.01] increased and decreased response latencies, respectively, but no significant BHF177 × Nicotine interaction was observed.
Figure 4. Effects of GABAB receptor activation on nicotine-induced facilitation of brain reward function.
Effects of the GABAB receptor positive modulator BHF177 on nicotine-induced lowering of brain reward thresholds (A) and response latency (B). Effects of the GABAB receptor agonist CGP44532 on nicotine-induced lowering of brain reward thresholds (C) and response latency (D). Data are expressed as a percentage of baseline ICSS threshold and response latency (mean ± SEM) before each drug session. Asterisks (*P < 0.05, **P < 0.01) indicate significant differences between saline- and nicotine-treated subjects within the same dose of GABAB receptor agonist or positive modulator. Dagger signs (†P < 0.05, ††P < 0.01) indicate significant differences in thresholds compared with values after vehicle administration in the absence of nicotine.
CGP44532 + nicotine
Mean ± SEM absolute ICSS threshold and response latency values on the days prior to each drug administration were 112.4 ± 12.8 μA and 3.3 ± 0.2 s, respectively. ANOVAs on these preinjection baseline data indicated that thresholds were stable throughout the course of the experiment under baseline conditions (i.e., in the absence of drug treatments). However, a one-way ANOVA on preinjection response latency baselines indicated a significant effect that was attributable to increasing preinjection response latencies as testing progressed [F(9,90) = 5.33, P < 0.01]. However, taking into consideration the slight drift in latencies that occurred over the course of this experiment, it is unlikely that this small drift affected the evaluation of the drug effects as drug effects were expressed as percent of the immediate preceding pre-drug baseline.
A two-way ANOVA indicated significant main effects of CGP44532 [F(4,100) = 22.35, P < 0.001] and nicotine [F(1,100) = 17.31, P < 0.001] but no interaction effect. Pre-planned comparisons indicated that nicotine significantly lowered thresholds when co-administered with vehicle, 0.25 mg/kg, or 0.375 mg/kg CGP44532. By contrast, thresholds were significantly elevated by administration of 0.375 or 0.5 mg/kg CGP44532 compared with thresholds after vehicle treatment in the absence of nicotine (P < 0.05 and P < 0.01, respectively; Figure 4B). A two-way ANOVA on response latency data indicated that administration of either CGP44532 or nicotine had no effect on response latencies, and no significant CGP44532 × Nicotine interaction was observed.
Discussion
The present studies showed that administration of the GABAB receptor allosteric positive modulators CGP7930, GS39783, or BHF177 attenuated the reinforcing effects of nicotine. Administration of GS39783 increased the attenuating effect of a sub-effective dose of the GABAB receptor agonist CGP44532 on nicotine reinforcement, thereby selectively decreasing nicotine- but not food-maintained responding. In addition, administration of BHF177 attenuated the motivation to seek nicotine, assessed via a progressive-ratio schedule of reinforcement, while having limited effects on food-maintained responding under either fixed- or progressive-ratio schedules of reinforcement. Furthermore, administration of either BHF177 or the GABAB receptor agonist CGP44532 blocked the reward-enhancing effects of noncontingent nicotine, although these effects are likely to be nonspecific because these compounds elevated thresholds on their own (indicated by the lack of statistically significant interactions).
Data obtained from fixed-ratio schedules of reinforcement provide a measure of drug intake and reinforcer efficacy. Nicotine self-administration exhibits a relatively flat dose-response curve under a fixed-ratio schedule of reinforcement that is shifted downward after administration of nicotinic acetylcholine receptor antagonists. This decrease in nicotine self-administration is seen at all unit doses of nicotine tested (e.g., Donny et al., 1999; Watkins et al., 1999; but see Fattore et al., 2002). Therefore, the decreased nicotine self-administration observed after pretreatment with any one of the three GABAB receptor positive modulators in the present studies can be unambiguously interpreted as a decrease in the reinforcing effects of nicotine. Although the effects of the GABAB receptor positive modulator CGP7930 were not assessed on responding for a non-nicotine reinforcer, administration of either GS39783 or BHF177 decreased nicotine intake at doses that had no effect on responding for food under a fixed-ratio schedule. Administration of the GABAB receptor agonist CGP44532 selectively decreased nicotine vs. food self-administration on the fixed-ratio schedule of reinforcement, and co-administration of both GS39783 and CGP44532 had additive effects in decreasing nicotine self-administration. Only co-administration of the two highest doses of the two compounds (40 mg/kg of GS39783 + 0.25 mg/kg of CGP44532) decreased food-maintained responding in addition to decreasing nicotine self-administration.
Data obtained from progressive-ratio schedules of reinforcement provide a measure of the motivation to obtain a reinforcer (Arnold and Roberts, 1997). The present studies indicated that administration of intermediate doses of the GABAB receptor positive modulator BHF177 attenuated the motivational properties of nicotine without decreasing breakpoints for food. Nonetheless, the highest dose of BHF177 (40 mg/kg) significantly decreased breakpoints for both nicotine and food. The same dose of BHF177 selectively decreased nicotine but not food-maintained responding under a fixed-ratio schedule of reinforcement, perhaps indicating greater sensitivity of the motivational effects of nicotine to enhanced GABAergic tone, although this difference also may be explained by the increased response requirements under the progressive-compared to the fixed-ratio schedule.
The selective nature of the effects of GABAB receptor positive modulators contrasts with the nonselective suppressant effects of the GABAB receptor agonist compounds baclofen or CGP44532 on nicotine or cocaine self-administration compared with food-maintained responding when administered either acutely (Roberts et al., 1996; Paterson et al., 2004) or chronically (Paterson et al., 2005b). Nevertheless, the GABAB receptor agonist CGP44532 also selectively decreased breakpoints for cocaine but not food under a progressive-ratio schedule of reinforcement (Brebner et al., 1999). Corrigall and colleagues (2000) suggested that the different degrees of selectivity for cocaine- or nicotine- vs. food-maintained responding may be related to greater recruitment of GABAergic negative feedback from the nucleus accumbens to the ventral tegmental area after cocaine vs. nicotine self-administration.
Interestingly, the nonselective effects of the highest BHF177 dose on the motivational properties of nicotine also were seen clearly in the ICSS procedure, where 30 mg/kg BHF177 significantly elevated ICSS thresholds when administered alone. The intermediate doses of BHF177 (7.5 and 15 mg/kg) blocked the ICSS threshold-lowering effect of noncontingent nicotine while nonsignificantly elevating brain reward thresholds. These doses of BHF177 are in the same range as the doses of BHF177 (10 and 20 mg/kg) that selectively decreased nicotine- but not food-maintained responding under fixed- and progressive-ratio schedules of reinforcement. As indicated above, the ICSS threshold-lowering effect of acute nicotine is hypothesized to reflect the reward-enhancing effect of nicotine (Harrison et al., 2002; Kenny and Markou, 2006). Thus, administration of the GABAB positive receptor modulator BHF177 attenuated the reinforcing, reward-enhancing, and motivational properties of nicotine. In contrast to the effects of BHF177, only the highest dose of CGP44532 blocked the threshold-lowering effect of nicotine at a dose that significantly impaired brain reward function when administered alone (0.5 mg/kg). A lower dose of CGP44532 (0.25 mg/kg) that elevated ICSS thresholds when administered alone but did not block the reward-enhancing effect of nicotine, decreased nicotine self-administration under the fixed-ratio schedule.
As indicated previously, GABAB receptor agonists exert unwanted locomotor side-effects, in contrast to the GABAB receptor positive modulators that are mostly free of such undesirable side-effects (Cryan et al., 2004). Consistent with a lack of locomotor suppression by the GABAB receptor positive modulator GS39783 (Cryan et al., 2004), the present self-administration studies found no effect of administration of any of the positive modulators on inactive lever responding, unlike the GABAB receptor agonist CGP44532. In the ICSS experiments, although BHF177 was found to increase response latencies, CGP44532 had no such effect. Finally, the selective effects of GS39783 and BHF177 on nicotine vs. food-maintained responding suggest the absence of motor-suppressant effects because behaviors emitted at higher rates (such as food-maintained responding in the present studies) tend to be more sensitive to locomotor-suppressant effects of drug pretreatments. Taken together, these results clearly indicate that the effects of GABAB receptor positive modulators on the behaviors reported here are highly unlikely to be attributable to motor suppressant effects of these compounds.
The behavioral effects of the GABAB receptor positive modulators in the present study are consistent with GS39783-induced blockade of the rewarding effects of nicotine measured with the conditioned place preference procedure in rats (Mombereau et al., 2007). Administration of GS39783 also prevented nicotine-induced accumulation of δFosB in the nucleus accumbens (Mombereau et al., 2007). Beyond nicotine, the GABAB receptor positive modulators GS39783 and CGP7930 decreased ethanol intake in alcohol-preferring rats (Orru et al., 2005), with no effect on responding for water in the case of CGP7930 (Liang et al., 2006). Interestingly, co-administration of subeffective doses of the GABAB receptor agonist baclofen and CGP7930 decreased ethanol self-administration (Liang et al., 2006), similar to the effects of co-administration of CGP44532 and GS39783 in the present study. Previously, the GABAB receptor positive modulators GS39783 and CGP7930 decreased cocaine self-administration under both fixed- and progressive-ratio schedules (Smith et al., 2004). More recently, CGP7930 selectively decreased cocaine, but not food, self-administration (Filip et al., 2007) and attenuated cocaine- and cue-induced reinstatement of cocaine-seeking behavior, but not food-induced reinstatement of food-seeking behavior (Filip and Frankowska, 2007). Furthermore, consistent with the present data, the GABAB receptor positive modulator GS39783 selectively attenuated cocaine-induced lowering of ICSS thresholds, unlike baclofen that blocked cocaine-induced reward facilitation at doses that elevated ICSS thresholds when administered alone (Slattery et al., 2005). Finally, GS39783 partially attenuated cocaine-induced δFosB induction in the dorsal striatum and blocked cocaine-induced CREB (cyclic adenosine monophosphate [cAMP] response-element binding protein) activation and DARPP-32 (dopamine and cAMP-regulated phosphoprotein with relative molecular weight of 32 kDa) accumulation in the nucleus accumbens in the mouse (Lhuillier et al., 2006). Thus, GABAB receptor allosteric positive modulators attenuate the reinforcing and motivational properties of ethanol, cocaine, cocaine-associated conditioned reinforcers, and the molecular correlates of psychostimulant exposure.
In summary, accumulating evidence suggests that GABAB receptor allosteric positive modulators may be useful in attenuating the reinforcing, motivational, and reward-facilitating effects of nicotine (present studies) and in preventing nicotine-induced δFosB accumulation (Mombereau et al., 2007). Thus, the currently available data indicate that GABAB receptor positive modulators deserve further investigation as potential treatments for nicotine dependence.
Acknowledgements
The authors would like to thank Mr. Michael Arends for editorial assistance, and Mrs. Gina Stouffer, Mrs. Jessica Benedict, and Mr. Bryant Silbaugh for technical assistance.
This work was supported by NIH grant U01 MH69062 to AM. NEP was supported by a Peter McManus research grant. This work was presented in abstract form at the Society for Neuroscience 2007 Annual Meeting in San Diego, California, abstract numbers 10.7 and 273.16.
Abbreviations
- GABA
γ-aminobutyric acid
- FR
fixed-ratio
- TO
time-out
- ICSS
intracranial self-stimulation
- CGP7930
2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol
- BHF177
N-[(1R,2R,4S)-bicyclo[2.2.1]hept-2-yl]-2-methyl-5-[4-(trifluoromethyl)phenyl]-4-pyrimidinamine
- GS39783
N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine
- CGP44532
(3-amino-2[S]-hydroxypropyl)-methylphosphinic acid
- ANOVA
analysis of variance
Footnotes
Neil E.Paterson and Styliani Vlachou contributed equally to this work.
References
- Arnold JM, Roberts DCS. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor: a site for therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Brebner K, Froestl W, Andrews M, Phelan R, Roberts DCS. The GABAB agonist CGP 44532 decreases cocaine self-administration in rats: demonstration using a progressive ratio and a discrete trials procedure. Neuropharmacology. 1999;38:1797–1804. doi: 10.1016/s0028-3908(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL, Chow BL, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and γ-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology (Berl) 2000;149:107–114. doi: 10.1007/s002139900355. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Roberts DC, de Wit H. GABAB receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N'-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- Dobrovitsky V, Pimentel P, Duarte A, Froestl W, Stellar JR, Trzcinska M. CGP 44532, a GABAB receptor agonist, is hedonically neutral and reduces cocaine-induced enhancement of reward. Neuropharmacology. 2002;42:626–632. doi: 10.1016/s0028-3908(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta MC, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M. Effects of GABAB receptor agents on cocaine priming, discrete contextual cue and food induced relapses. Eur J Pharmacol. 2007;571:166–173. doi: 10.1016/j.ejphar.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Przegalinski E. Effects of GABAB receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol. 2007;574:148–157. doi: 10.1016/j.ejphar.2007.07.048. [DOI] [PubMed] [Google Scholar]
- Froestl W, Mickel SJ, Hall RG, von Sprecher G, Strub D, Baumann PA, Brugger F, Gentsch C, Jaekel J, Olpe HR, et al. Phosphinic acid analogues of GABA: 1. New potent and selective GABAB agonists. J Med Chem. 1995;38:3297–3312. doi: 10.1021/jm00017a015. [DOI] [PubMed] [Google Scholar]
- Guery S, Floersheim P, Kaupmann K, Froestl W. Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABAB receptors. Bioorg Med Chem Lett. 2007;17:6206–6211. doi: 10.1016/j.bmcl.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322−283, 325. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Striplin CD, Steketee JD, Klitenick MA, Duffy P. Cellular mechanisms of behavioral sensitization to drugs of abuse. Ann NY Acad Sci. 1992;654:128–135. doi: 10.1111/j.1749-6632.1992.tb25961.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABAB receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2006;32:388–398. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–639. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Froestl W, Koob GF, Markou A. Both GABAB receptor agonist and antagonists decreased brain stimulation reward in the rat. Neuropharmacology. 2001;40:676–685. doi: 10.1016/s0028-3908(00)00204-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. Multiple motivational forces underlying nicotine dependence. In: Caggiula AR, Bevins R, editors. The Motivational Impact of Nicotine and Its Role in Tobacco Use. Springer; Berlin: 2008. (series title: Nebraska Symposium Motivation) in press. [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan JF. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens ΔFosB accumulation. J Pharmacol Exp Ther. 2007;321:172–177. doi: 10.1124/jpet.106.116228. [DOI] [PubMed] [Google Scholar]
- Orru A, Lai P, Lobina C, Maccioni P, Piras P, Scanu L, Froestl W, Gessa GL, Carai MA, Colombo G. Reducing effect of the positive allosteric modulators of the GABAB receptor, CGP7930 and GS39783, on alcohol intake in alcohol-preferring rats. Eur J Pharmacol. 2005;525:105–111. doi: 10.1016/j.ejphar.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Bruijnzeel AW, Kenny PJ, Wright CD, Froestl W, Markou A. Prolonged nicotine exposure does not alter GABAB receptor-mediated regulation of brain reward function. Neuropharmacology. 2005a;49:953–962. doi: 10.1016/j.neuropharm.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology (Berl) 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-induced reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005b;30:119–128. doi: 10.1038/sj.npp.1300524. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. 2nd ed Plenum Press; New York: 1979. [Google Scholar]
- Proctor RN. The global smoking epidemic: a history and status report. Clin Lung Cancer. 2004;5:371–376. doi: 10.3816/CLC.2004.n.016. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Markou A, Froestl W, Cryan JF. The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology. 2005;30:2065–2072. doi: 10.1038/sj.npp.1300734. [DOI] [PubMed] [Google Scholar]
- Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DCS. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berl) 2004;173:105–111. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, Bettler B, Kaupmann K. Positive allosteric modulation of native and recombinant γ-aminobutyric acidB receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol. 2001;60:963–971. [PubMed] [Google Scholar]
- Urwyler S, Pozza MF, Lingenhoehl K, Mosbacher J, Lampert C, Froestl W, Koller M, Kaupmann K. N,N'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of γ-aminobutyric acidB receptor function. J Pharmacol Exp Ther. 2003;307:322–330. doi: 10.1124/jpet.103.053074. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]