Abstract
Adhesion fibroblasts exhibit higher TGF-β1 and type I collagen expression as compared to normal peritoneal broblasts. Furthermore, exposure of normal peritoneal fibroblasts to hypoxia results in an irreversible increase in TGF-β1 and type I collagen. We postulated that the mechanism by which hypoxia induced the adhesion phenotype is through the production of superoxide either directly or through the formation of peroxynitrite. To test this hypothesis, normal peritoneal and adhesion fibroblasts were treated with superoxide dismutase (SOD), a superoxide scavenger, and xanthine/xanthine oxidase, a superoxide-generating system, under normoxic and hypoxic conditions. Also, cells were treated with peroxynitrite. TGF-β1 and type I collagen expression was determined before and after all treatments using real-time RT/PCR. Hypoxia treatment resulted in a time-dependent increase in TGF-β1 and type I collagen mRNA levels in both normal peritoneal and adhesion fibroblasts. Similarly, treatment with xanthine oxidase, to endogenously generate superoxide, resulted in higher mRNA levels of TGF-β1 and type I collagen in both normal peritoneal and adhesion fibroblasts. In contrast, treatment with SOD, to scavenge endogenous superoxide, resulted in a decrease in TGF-β1 and type I collagen expression in adhesion fibroblasts to levels seen in normal peritoneal fibroblasts; no effect on the expression of these molecules was seen in normal peritoneal fibroblasts. Exposure to hypoxia in the presence of SOD had no effect on mRNA levels of TGF-β1 and type I collagen in either normal peritoneal or adhesion fibroblasts. Peroxynitrite treatment alone significantly induced both adhesion phenotype markers. In conclusion, hypoxia, through the production of superoxide, causes normal peritoneal fibroblasts to acquire the adhesion phenotype. Scavenging superoxide, even in the presence of hypoxia, prevented the development of the adhesion phenotype. These findings further support the central role of free radicals in the development of adhesions.
Keywords: Adhesions, Type I collagen, Fibroblasts, Superoxide dismutase, TGF-β1
Introduction
Postoperative adhesions are a significant source of impaired organ functioning, decreased fertility, bowel obstruction, difficult reoperation, and possibly pain [1,2]. The processes that result in either normal peritoneal tissue repair or the development of adhesions include the migration, proliferation, and/or differentiation of several cell types, among them inflammatory, immune, mesothelial, and fibroblast cells [3]. The mechanism by which injury to the peritoneum triggers the inflammatory response, subsequently leading to postoperative adhesion development, remains poorly understood.
Previously, we have thoroughly characterized differences between fibroblasts isolated from normal peritoneum and adhesion tissues of the same patient(s) and have identified substantial phenotypic differences between these two cell types [3–5]. Several molecular markers for the adhesion phenotype have been identified; fibronectin, type I collagen, vascular endothelial growth factor, transforming growth factor (TGF)-β1, alpha smooth muscle actin (α-SM actin), cyclooxygenase 2, tissue plasminogen activator/plasminogen activator inhibitor (tPA/PAI-1), and matrix metalloproteinase-1/tissue inhibitor of metalloproteinase (MMP-1/TIMP-1) ratios [3,6].
Hypoxia, resulting from tissue injury, has been suggested to play an important role in wound healing, and may therefore be a critical factor in the development of postoperative adhesions [4,7]. Hypoxia is known to trigger the expression of TGF-β1, which consequently increases the expression of extracellular matrix proteins, including type I collagen [4]. Type I collagen synthesis has been shown to be crucially dependent on the availability of molecular oxygen in tissue culture, animal, and human wound healing experiments [8,9]. Moreover, exposure of normal peritoneal fibroblasts to hypoxia irreversibly induces TGF-β1 and type I collagen to levels seen in adhesion fibroblasts [4,10]. However, the mechanism by which hypoxia induces the adhesion phenotype is not yet fully understood. Additionally, hypoxia is known to acutely promote superoxide (O2.−) generation from disparate intracellular sources that include xanthine dehydrogenase oxidase [11], mitochondrial electron transport chain [12], and NAD[P]H oxidase [13]. In biological systems, superoxide dismutase (SOD) protects against the deleterious actions of this radical by catalyzing its dismutation to hydrogen peroxide plus oxygen, [14]. Whereas SOD breaks down O2.−, xanthine oxidase synthesizes O2.−. Xanthine oxidase appears to be one of the major superoxide-producing enzymes [14].
In this study, we sought to determine the mechanisms by which hypoxia induces the expression of TGF-β1 and type I collagen in normal peritoneal fibroblasts. Since we and others have previously reported a positive correlation between mRNA and protein levels for both TGFβ-1 and type I collagen, we will utilize the real-time RT/PCR to absolutely quantify mRNA copy numbers for each molecule [6,15,16]. We hypothesize that hypoxia exerts its effects through O2.− production. To address our hypothesis, we investigated the effects of generating and/or scavenging O2.− on the development of the adhesion phenotype. Subsequently, we examined whether scavenging O2.− would prevent the development of the adhesion phenotype in normal peritoneal fibroblasts when exposed to hypoxia. The results of this study will contribute to understanding the contribution of free radicals to the pathogenesis of postoperative adhesions.
Materials and methods
Source and culture of human fibroblasts
Normal parietal peritoneal tissue from the anterior abdominal wall lateral to midline incision and adhesion tissue were excised from patients undergoing laparotomy for pelvic pain, at the initiation of the surgery following entry into the abdominal cavity as previously described [17,18]. Normal peritoneum was at minimum 3 inches from any adhesions. Subjects did not have an active pelvic or abdominal infection and were not pregnant. All patients gave informed written consent to tissue collection, which was conducted under a protocol approved by the Wayne State University Institutional Review Board. Harvested tissue samples from five women were immediately placed in standard media (DMEM medium containing 10% fetal bovine serum, 2% penicillin, and streptomycin). Tissues were cut into small pieces in a sterile culture dish and transferred into another fresh T-25 flask with 3 ml of dispase solution (2.4 U/ml; GIBCO BRL, Life Technologies, Inc.). The flasks were incubated overnight at 37°C in an environ-shaker (LAB-LINE Instruments, Inc.). The samples were then centrifuged for 5 min at 1400 ×g, transferred into a fresh T-25 flask with prewarmed DMEM medium, and placed in 37°C incubator (95% air and 5% CO2); outgrowth of fibroblasts generally took 2 weeks.
Once confluence was reached, the cells were transferred to 90-mm2 tissue culture dishes and cultured in standard media with 10% FBS. Thereafter, the confluent dishes were subcultured by trypsinization (1:3 split ratios). Studies were conducted using passage 3–5 cells to maintain comparability.
Treatment of normal peritoneal and adhesion fibroblasts with hypoxia
All hypoxic experiments were performed in an airtight modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA). The chamber was deoxygenated by a positive infusion of 2% O2 in a CO2-nitrogen gas mixture. Cultures were placed in a standard humidified tissue incubator. There were no statistically significant differences in viability by crystal violet or trypan blue exclusion (data not shown). Parallel cultures were placed in normoxia for all time points. Cells were harvested after 3, 6, 12, and 24 h. All experiments were performed in triplicate from fibroblasts cultured from 5 different patients.
Treatment of human adhesion fibroblasts with superoxide dismutase
Cells (2×106) were treated with increasing concentrations of superoxide dismutase (Sigma-Aldrich, St. Louis, MO) 0, 5, 10, 15, and 20 units/ml for 24 h. All experiments were performed in triplicate from fibroblasts cultured from 5 different patients.
Treatment of human normal peritoneal fibroblasts with xanthine/xanthine oxidase
Fibroblast cells (2×106) were cultured in serum-free DMEM containing increasing concentrations of xanthine (0–2.4 μM). Xanthine oxidase (1 mU/ml) was added to each reaction and incubated for 24 h to generate superoxide. Under the reaction conditions, the amount of superoxide generated was measured to be 0, 0.1, 0.5, 1.0,1.5, and 2.0 μM/min, which is within the range of the amount of superoxide generated in normal peritoneal and adhesion fibroblasts exposed to 3, 6,12, and 24 h hypoxia, respectively. The rate of superoxide production in the reactions was determined by measuring the rate of SOD-inhibitable cytochrome c reduction using the spectrophotmetric method as previously described [19]. Phosphate buffer (2 mM, pH 7.0) containing 1.2 mM hypoxanthine, catalase (1300 U/ml), and 50 μM cytochrome c was incubated at room temperature. To test for linearity of superoxide production different amounts of xanthine oxidase were added to the reaction and the reduction of cytochrome c was monitored at 550 nm using UV/visible Cary spectrophotometer. Replica reactions that contained superoxide dismutase (100 U/ml) were run to calculate the SOD-inhibitable portion of cytochrome c reduction. When 0.3 U/ml of xanthine oxidase and 1.2 mM hypoxanthine were used the rate of SOD-inhibitable superoxide production displayed a linear relationship to xanthine oxidase concentration. The rate of superoxide production was then calculated from the slope of linear absorbance increase over time.
Treatment of normal peritoneal and adhesion fibroblasts with peroxynitrite
Cells (5×106) were treated with 0.5 mM peroxynitrite for 10 min. All experiments were performed in triplicate from fibroblasts cultured from 5 different patients.
Treatment of normal peritoneal and adhesion fibroblasts with superoxide dismutase under normal and hypoxic conditions
All hypoxic experiments were performed in an airtight modular incubator chamber as described above with and without superoxide dismutase (20 U/ml) for 24 h. There were no statistically significant differences in viability by crystal violet or trypan blue exclusion (data not shown). Parallel cultures were placed in normoxia for all time points. Cells were harvested after 24 h. All experiments were performed in triplicate from fibroblasts cultured from 5 different patients.
Real-time reverse transcription polymerase chain reaction (RT-PCR) for TGF-β1 and type I collagen
RNA isolation
Total RNA was extracted from human normal peritoneal fibroblasts and adhesion fibroblasts using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the protocol provided by the manufacturer.
Reverse transcription
A 20 μl cDNA reaction volume was prepared using the QuantiTect reverse transcription kit (Qiagen), as described by the manufacturer’s protocol.
Real-time RT-PCR primer design and controls
Optimal oligonucleotide primer pairs for real-time RT-PCR amplification of reverse-transcribed cDNA were selected with the aid of the software program, Beacon Designer (Premier Biosoft Int., Palo Alto, CA). Human oligonucleotide primers, which amplify variable portions of the protein coding regions, were used. β-Actin primers which amplified a 559-bp fragment are as follows: sense primer 5-AAGCAGGAGTATGAC-GAGTCCG-3, and antisense primer 5′-GCCTTCATACATCTCAAGTTGG-3′. TGF-β1 primers which amplified a 332-bp fragment are as follows: sense primer 5-AAC AAT TCC TGG CGA TAC-3, and antisense primer 5-AAG CAA TAG TTGGTGTCC-3. Type I collagen primers which amplified a 335-bp fragment are as follows: sense primer 5-TGTCTT ATG GCTATG ATG AG-3, and antisense primer 5-ATCCAAACC ACT GAAACC-3.
Real-time RT-PCR was performed with the QuantiTect SYBR Green RT-PCR kit (Qiagen) and a Cepheid 1.2f Detection System (Cepheid, Sunnyvale, CA). Each reaction was 25-μl consisting of 12.5 μl of 2 X QuantiTect SYBR Green RT-PCR master mix, 1 μl of cDNA template, and 0.2 μmol/L each of target-specific primer that was designed to amplify a part of the gene of interest. To quantify each target transcript, a standard curve was constructed using a 10-fold dilution series of β-actin standard plasmid (Invitrogen Corp, Carlsbad, CA). The PCR conditions for TGF-β1 and collagen I were programmed for each primer as follows: an initial cycle was performed at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min for collagen I and 56°C for 1 min for TGF-β1, and then a final cycle at 72°C for 7 min to allow completion of product synthesis. A control containing all the reaction components except for the template was included in all experiments. The amount of mRNAs was then normalized to β-actin, a housekeeping gene.
Statistical analysis
To compare results of cell treatments using each patient as their own control, repeated measures of testing were conducted. To determine the level of significance, repeated measures of testing were conducted evaluating confidence intervals for alpha levels of 0.05, 0.01, 0.005, and 0.001. All analyses were conducted using SPSS v 11.0. Results were considered significant when P<0.05. Figures demonstrate raw data of mRNA copies per microgram of RNA expressed for each marker in normal peritoneal and adhesion, and treated and untreated fibroblasts.
Results
Hypoxia treatment resulted in a time-dependent increase inTGF-β1 and type I collagen mRNA levels in both normal peritoneal and adhesion fibroblasts
Adhesion fibroblasts exhibited increased basal mRNA levels for TGF-β1 and type I collagen (9.80×103 and 6.90×103 mRNA copies/μg RNA) as compared to normal peritoneal fibroblasts (4.50×103 and 3.80×103 mRNA copies/μg RNA), respectively (Figs. 1A and B), confirming our previous reported studies [4]. Exposure of normal peritoneal and adhesion fibroblasts for various time points of hypoxia at 3, 6, 12, and 24 h resulted in a gradual increase in TGF-β1 at 3 h from 4.98×103 and 1.08×104 to 1.00×104 and 1.44×104 mRNA copies/μg RNA at 24 h in normal peritoneal and adhesion fibroblasts, respectively (Fig. 1A). This gradual increase was also seen for the same noted above time points in type I collagen at 3 h from 4.50×103 and 7.34×103 to 9.89×103 and 1.27×104 mRNA copies/μg RNA at 24 h in normal peritoneal and adhesion fibroblasts, respectively (Fig. 1B). All P values were <0.05.
Fig. 1.

Real-time RT/PCR for TGF-β1 and type I collagen was utilized using RNA isolated from normal peritoneal and adhesion fibroblasts treated with hypoxia (2% O2) at 0, 3, 6, 12, and 24 h. All experiments were performed in triplicate using RNA isolated from normal peritoneal and adhesion fibroblasts established from 5 different patients. (* indicates significant change compared to control.)
Endogenously generated superoxide enhances the adhesion phenotype by increasing TGF-β1 and type I collagen mRNA levels in both normal peritoneal and adhesion fibroblasts
Normal peritoneal and adhesion fibroblasts treated with xanthine/xanthine oxidase, a O2.− generating system, exhibited a dose–response increase (0.0, 0.5, 1.0, 1.5, and 2 μM/min) in TGF-β1 and type I collagen mRNA levels in both cell lines (Figs. 2A and B). TGF-β1 mRNA levels increased from 4.50×103 and 9.80×103 at 0.0 μM/min to 1.08×104 and 1.44×104 mRNA copies/μg RNA at 2.0 μmol/min in normal peritoneal and adhesion fibroblasts, respectively (Fig. 2A). Similarly, type I collagen mRNA levels increased from 3.80×103 and 6.90×103 at 0.0 μM/min to 1.19×104 and 1.49×104 mRNA copies/μg RNA at 2.0 μM/min in normal peritoneal and adhesion fibroblasts, respectively (Fig. 2B). There was no difference in the mRNA levels of TGF-β1 and type I collagen when 1 U/ml of catalase (one unit of catalase will decompose 1.0 μmol of hydrogen peroxide to oxygen and water per minute at pH 7.0 at 25°C at a substrate concentration of 50 mM hydrogen peroxide) was added to the reaction, confirming that the effects seen were solely due to O2.− and not hydrogen peroxide. All P values were <0.05.
Fig. 2.

Real-time RT/PCR for TGF-β1 and type I collagen was utilized using RNA isolated from normal peritoneal and adhesion fibroblasts treated with xanthine/xanthine oxidase which generated superoxide at the rate of 0.0, 0.5, 1.0, 1.5, and 2.0 μM/min. All experiments were performed in triplicate using RNA isolated from normal peritoneal and adhesion fibroblasts established from 5 different patients. (* indicates significant change compared to control.)
Scavenging superoxide restores both TGF-β1 and type I collagen mRNA levels in adhesion fibroblasts to levels observed in normal peritoneal fibroblasts
Normal peritoneal and adhesion fibroblasts treated with super-oxide dismutase, a O2.− scavenging enzyme, exhibited a dose–response decrease (0, 5, 10, 15, and 20 units/ml) in TGF-β1 and type I collagen mRNA levels in adhesion fibroblasts while not effecting normal peritoneal fibroblasts (Figs. 3A and B). TGF-β1 mRNA levels decreased from 9.80×103 copies/μg RNA at 0 units/ml to 3.24×103 mRNA copies/μg RNA at 20 units/ml in adhesion fibroblasts (Fig. 3A). Similarly, type I collagen mRNA levels decreased from 6.90×103 copies/μg RNA at 0 unit/ml to 2.56×103 mRNA copies/μg RNA at 20 units/ml in adhesion fibroblasts (Fig. 3B). All P values were <0.05.
Fig. 3.

Real-time RT/PCR for TGF-β1 and type I collagen was utilized using RNA isolated from normal peritoneal and adhesion fibroblasts treated with 0, 5, 10, 15, and 20 units/ml of superoxide dismutase. All experiments were performed in triplicate using RNA isolated from normal peritoneal and adhesion fibroblasts established from 5 different patients. (* indicates significant change compared to control.)
Scavenging superoxide during hypoxia exposure protects against the development of the adhesion phenotype
Normal peritoneal and adhesion fibroblasts treated with SOD (20 units/ml) combined with hypoxia (24 h) showed no significant change in the TGF-β1 or type I collagen mRNA levels (Fig. 4). All P values were <0.05.
Fig. 4.
Real-time RT/PCR for TGF-β1 and type I collagen was utilized using RNA isolated from normal peritoneal and adhesion fibroblasts treated with 20 units/ml superoxide dismutase under normal and hypoxic (2% O2) conditions. All experiments were performed in triplicate using RNA isolated from normal peritoneal and adhesion fibroblasts established from 5 different patients. (* indicates significant change compared to control.)
Peroxynitrite treatment increased the adhesion phenotype markers, TGF-β1 and type I collagen
Normal peritoneal and adhesion fibroblasts treated with a previously established dose (0.5 mM) and time (10 min) of peroxynitrite showed a significant increase in TGF-β1 and type I collagen mRNA levels in normal peritoneal but not in adhesion fibroblasts (Figs. 5A and B). TGF-β1 mRNA levels increased from 4.50×103 and 9.80×103 to 8.75×103 and 1.11×104 mRNA copies/μg RNA after peroxynitrite treatment, in normal peritoneal and adhesion fibroblasts, respectively (Fig. 5A). Similarly, type I collagen mRNA levels increased from 3.80×103 and 6.90×103 to 6.69×103 and 7.16×103 mRNA copies/μg RNA after peroxynitrite treatment, in normal peritoneal and adhesion fibroblasts, respectively (Fig. 5B). All P values were <0.05.
Fig. 5.
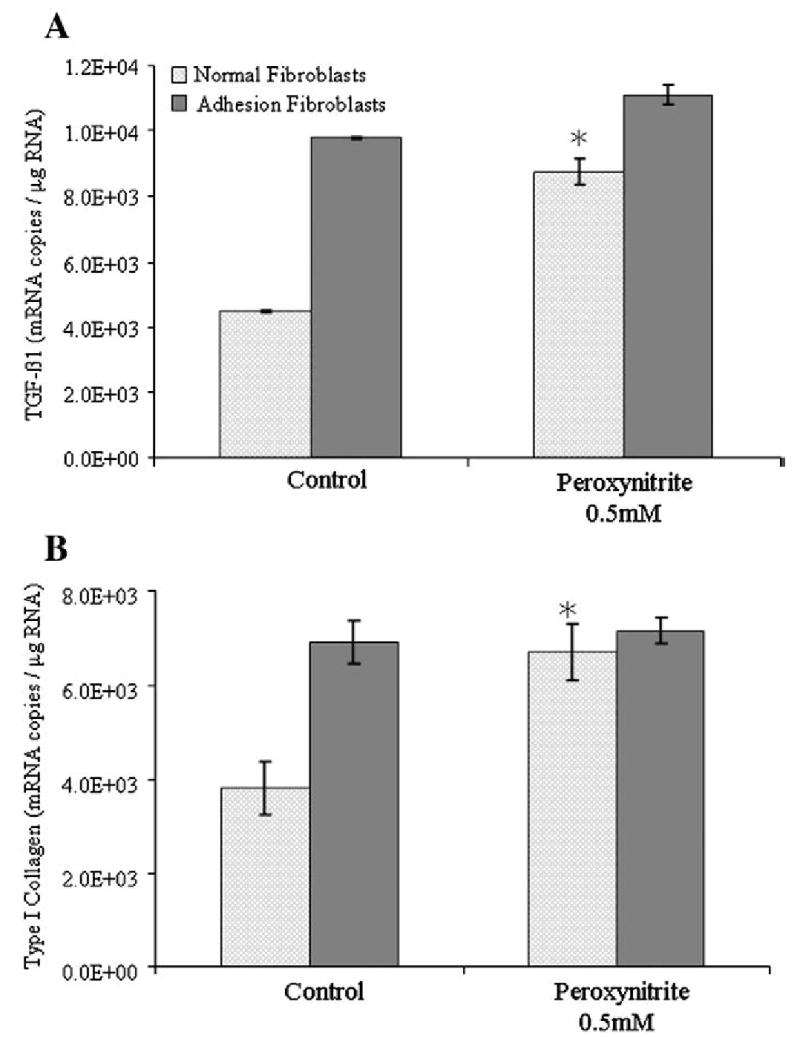
Real-time RT/PCR for TGF-β1 and type I collagen was utilized using RNA isolated from normal peritoneal and adhesion fibroblasts treated with 0.5 mM peroxynitrite for 10 min. All experiments were performed in triplicate using RNA isolated from normal peritoneal and adhesion fibroblasts established from 5 different patients. (* indicates significant change compared to control.)
Discussion
Several phenotypic changes in adhesion fibroblasts as compared to normal peritoneal fibroblasts have been previously identified [6]: specifically, the marked increase in TGF-β1 and type I collagen expression in response to hypoxia [4]. Adhesion fibroblasts are myofibroblasts, defined as transiently activated fibroblasts exhibiting features intermediate between those of smooth muscle cells and fibroblasts, including the expression of α-SM actin [20,21] and a depleted antioxidant system [22]. In normal wound healing, as the wound resolves, the cellularity decreases and the myofibroblasts disappear by apoptosis [23]. However, in several pathological cases, including fibrosis, myofibroblastic differentiation persists and causes excessive scarring [24,25]. In fact, postoperative adhesions, like fibrosis, reflect a pathologic excess of biologic events involved in normal tissue repair, and myofibroblasts can be considered responsible for postoperative adhesions as they are the primary source of the increased extracellular matrix protein expression, as well as a major source of fibrogenic growth factors, such as TGF-β1 [26–30]. This cytokine controls crucial cellular end points, including cell proliferation, differentiation, apoptosis, tissue morphogenesis, and wound healing [31–33]. Moreover, it has been described as a direct inductor of the myofibroblastic differentiation by controlling α-SM actin expression both in vivo and in vitro [34].
The mechanism by which hypoxia induces the adhesion phenotype remains poorly understood. In this study we hypothesize that hypoxia, as a marker of tissue injury, induces the production of high levels of O2.−, which triggers the production of TGF-β1, the major cytokine responsible for the induction of extracellular matrix (ECM) proteins in normal peritoneal fibroblasts. The results from the current study support this hypothesis as a similar increase in TGF-β1 and type I collagen was observed when peritoneal fibroblasts were treated with a O2.− generating system. This is further supported by the fact that when O2.− was scavenged, there was in a significant decrease in TGF-β1 and type I collagen in adhesion fibroblasts to levels seen in normal peritoneal fibroblasts. Moreover, O2.− has been shown to upregulate TGF-β1 in macrophages [35,36]. Consistent with our findings, the in vivo effect of hypoxia through the production of O2.− has been suggested to play a major role in TGF-β1-stimulated collagen production [37]. Reactive oxygen species (ROS) are involved in TGF-β-stimulated collagen production in murine embryo fibroblasts (NIH3T3), and the effect of glutathione depletion on TGF-β-stimulated collagen production may be mediated by facilitating ROS signaling [37].
We have evidence to believe that hypoxia may also exert its effect through a mechanism that involves peroxynitrite (OONO−) formation [38,39]. Reactive oxygen and nitrogen intermediates control the synthesis of cytokines and growth factors in several in vitro models [40]. For instance, they modulate the expression and/or release of monocyte chemoattractant protein-1 [41,42], tumor necrosis factor-α, interleukin (IL)-1 [43,44], IL-8 [45,46], platelet-derived growth factor [47,48], and TGF-β1 [49]. We demonstrated that both O2.− and ONOO− enhance TGF-β1 expression in normal peritoneal fibroblasts; although, ONOO− failed to induce TGF-β1 in adhesion fibroblasts. Adhesion fibroblasts exhibited a significantly lower level of nitric oxide (NO) and higher protein nitration as compared to normal peritoneal fibroblasts, although there was no difference in the iNOS expression level between the two cell lines [17,50,51]. This strongly indicates that adhesion fibroblasts use NO to form ONOO−, and consequently their basal ONOO− levels are higher than normal peritoneal fibroblasts. Therefore, consistent with our data, treatment with ONOO− will have a greater impact on normal peritoneal fibroblasts and little or no effect on adhesion fibroblasts. Our finding demonstrates that ONOO− leads to increased levels of TGF-β1 in normal peritoneal fibroblasts. However, ONOO− modulates the DNA-binding activity of the transcription factor AP-1 in lymphocytes [52] and AP-1 is thought to be involved in TGF-β1 gene expression [53].
There is a growing body of evidences that SOD could be a potential antifibrotic drug, as proved by clinical results on radiation-induced fibrosis [54,55] and its therapeutic effect could be related to a down-regulation of TGF-β1 expression and activity [27,56]. Superoxide dismutases exhibit potent anti-inflammatory properties [57], and all isoforms (SOD1, SOD2, and SOD3) induced 70% regression of a well-established fibrotic tissue and its replacement by regenerated normal tissue [54]. The molecular mechanisms underlying this regression have not yet been elucidated, although several hypotheses have been proposed. The reduction of the fibrotic block implies the degradation of the extracellular matrix and the elimination of myofibroblasts. Superoxide dismutase-induced decrease in cellularity could be mediated by two distinct or concomitant pathways (Fig. 6). Thus, treatment with SOD might affect the homeostasis of myofibroblasts by inducing cell death or the phenotypic reversion of myofibroblasts into normal fibroblasts. To evaluate the antifibrotic and/or antioxidant potential of SOD, we conducted experiments on primary cultures of fibroblasts isolated from normal peritoneal and adhesion tissues under normal and hypoxic conditions, specifically investigating SOD’s action on TGF-β1 and type I collagen expression. Our results clearly indicate that hypoxia generated O2.− is a key player in the formation of the adhesion phenotype. This became evident when normal peritoneal fibroblasts were treated with SOD under hypoxic conditions and no change in adhesion markers was seen. Moreover, adhesion fibroblasts did not have a further increase in these markers as previously seen after hypoxia treatment alone [4].
Fig. 6.

Proposed in vivo hypoxia model which describes the different pathways involved in the development of the adhesion phenotype.
In conclusion, a working model showing potential mechanisms involved in the in vivo effect of hypoxia on the development of the adhesion phenotype was constructed (Fig. 6). In this model, hypoxia-generated O2.− exerts its effect directly by enhancing the expression of TGF-β1, which consequently leads to elevated levels of type I collagen, a hallmark of the adhesion phenotype. Alternatively, this effect can be mediated through the formation of ONOO−, which directly affects TGF-β1 and type I collagen expression, or through protein nitration, the main function of ONOO−, of other yet to be determined molecules involved in the development of the adhesion phenotype. Additionally, our model clearly emphasizes a dual role for SOD and other antioxidants, one as a protector against the development of the adhesion phenotype in normal peritoneal fibroblasts on exposure to hypoxia, and the other as a treatment targeting the elimination of the adhesion phenotype.
Acknowledgments
This study was supported in part by NIH Grant NIH 1RO1 GM069941 to G.M. Saed.
Abbreviations
- α-SM actin
alpha smooth muscle actin
- NO
nitric oxide
- RT-PCR
reverse transcription polymerase chain reaction
- SOD
superoxide dismutase
- TGF
transforming growth factor
References
- 1.Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7:567–576. doi: 10.1093/humupd/7.6.567. [DOI] [PubMed] [Google Scholar]
- 2.Diamond MP, Daniell JF, Feste J, Surrey MW, McLaughlin DS, Friedman S, Vaughn WK, Martin DC. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil Steril. 1987;47:864–866. doi: 10.1016/s0015-0282(16)59181-x. [DOI] [PubMed] [Google Scholar]
- 3.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–768. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 4.Saed GM, Diamond MP. Hypoxia-induced irreversible up-regulation of type I collagen and transforming growth factor-beta1 in human peritoneal fibroblasts. Fertil Steril. 2002;78:144–147. doi: 10.1016/s0015-0282(02)03146-1. [DOI] [PubMed] [Google Scholar]
- 5.Saed GM, Diamond MP. Apoptosis and proliferation of human peritoneal fibroblasts in response to hypoxia. Fertil Steril. 2002;78:137–143. doi: 10.1016/s0015-0282(02)03145-x. [DOI] [PubMed] [Google Scholar]
- 6.Saed GM, Diamond MP. Molecular characterization of postoperative adhesions: the adhesion phenotype. J Am Assoc Gynecol Laparosc. 2004;11:307–314. doi: 10.1016/s1074-3804(05)60041-2. [DOI] [PubMed] [Google Scholar]
- 7.Saed GM, Zhang W, Diamond MP. Effect of hypoxia on stimulatory effect of TGF-beta 1 on MMP-2 and MMP-9 activities in mouse fibroblasts. J Soc Gynecol Invest. 2000;7:348–354. [PubMed] [Google Scholar]
- 8.Gleadle JM, Ratcliffe PJ. Hypoxia and the regulation of gene expression. Mol Med Today. 1998;4:122–129. doi: 10.1016/s1357-4310(97)01198-2. [DOI] [PubMed] [Google Scholar]
- 9.Sogawa K. [Molecular mechanism of hypoxic response in animals] Tanpakushitsu Kakusan Koso. 1999;44:2472–2477. [PubMed] [Google Scholar]
- 10.Alpay Z, Ozgonenel M, Savasan S, Buck S, Saed GM, Diamond MP. Altered in vitro immune response to hypoxia-treated normal peritoneal fibroblasts. Fertil Steril. 2007;87:426–429. doi: 10.1016/j.fertnstert.2006.07.1495. [DOI] [PubMed] [Google Scholar]
- 11.Terada LS, Guidot DM, Leff JA, Willingham IR, Hanley ME, Piermattei D, Repine JE. Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proc Natl Acad Sci U S A. 1992;89:3362–3366. doi: 10.1073/pnas.89.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson TL, Gores GJ, Nieminen AL, Herman B, Lemasters JJ. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993;264:C961–C967. doi: 10.1152/ajpcell.1993.264.4.C961. [DOI] [PubMed] [Google Scholar]
- 13.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 14.Fridovich I. The biology of oxygen radicals. Science (New York) 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 15.Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair Regen. 1999;7:504–510. doi: 10.1046/j.1524-475x.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- 16.Chegini N, Kotseos K, Zhao Y, Bennett B, McLean FW, Diamond MP, Holmdahl L, Burns J. Differential expression of TGF-beta1 and TGF-beta3 in serosal tissues of human intraperitoneal organs and peritoneal adhesions. Hum Reprod. 2001;16:1291–1300. doi: 10.1093/humrep/16.6.1291. [DOI] [PubMed] [Google Scholar]
- 17.Saed GM, Abu-Soud HM, Diamond MP. Role of nitric oxide in apoptosis of human peritoneal and adhesion fibroblasts after hypoxia. Fertil Steril. 2004;82(Suppl 3):1198–1205. doi: 10.1016/j.fertnstert.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Muschel RJ, Bernhard EJ, Garza L, McKenna WG, Koch CJ. Induction of apoptosis at different oxygen tensions: evidence that oxygen radicals do not mediate apoptotic signaling. Cancer Res. 1995;55:995–998. [PubMed] [Google Scholar]
- 19.Abu-Soud HM, Ichimori K, Nakazawa H, Stuehr DJ. Regulation of inducible nitric oxide synthase by self-generated NO. Biochemistry. 2001;40:6876–6881. doi: 10.1021/bi010066m. [DOI] [PubMed] [Google Scholar]
- 20.Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994;190:851–853. doi: 10.1016/S0344-0338(11)80988-X. [DOI] [PubMed] [Google Scholar]
- 21.Powell DW. Myofibroblasts: paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000;111:271–292. discussion 292-273. [PMC free article] [PubMed] [Google Scholar]
- 22.Delanian S, Martin M, Bravard A, Luccioni C, Lefaix JL. Abnormal phenotype of cultured fibroblasts in human skin with chronic radiotherapy damage. Radiother Oncol. 1998;47:255–261. doi: 10.1016/s0167-8140(97)00195-3. [DOI] [PubMed] [Google Scholar]
- 23.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 24.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995;19:471–476. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- 26.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 27.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 28.Saed GM, Kruger M, Diamond MP. Expression of transforming growth factor-beta and extracellular matrix by human peritoneal mesothelial cells and by fibroblasts from normal peritoneum and adhesions: effect of Tisseel. Wound Repair Regen. 2004;12:557–564. doi: 10.1111/j.1067-1927.2004.012508.x. [DOI] [PubMed] [Google Scholar]
- 29.Diamond MP, El-Hammady E, Wang R, Saed G. Regulation of matrix metalloproteinase-1 and tissue inhibitor of matrix metalloproteinase-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Fertil Steril. 2004;81:185–190. doi: 10.1016/j.fertnstert.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Diamond MP, El-Hammady E, Wang R, Kruger M, Saed G. Regulation of expression of tissue plasminogen activator and plasminogen activator inhibitor-1 by dichloroacetic acid in human fibroblasts from normal peritoneum and adhesions. Am J Obstet Gynecol. 2004;190:926–934. doi: 10.1016/j.ajog.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–374. [PubMed] [Google Scholar]
- 32.Alevizopoulos A, Mermod N. Transforming growth factor-beta: the breaking open of a black box. Bioessays. 1997;19:581–591. doi: 10.1002/bies.950190709. [DOI] [PubMed] [Google Scholar]
- 33.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 34.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2*-, TGF-beta, and VEGF production by macrophages. Free Radic Res. 2007;41:8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- 36.Patel B, Khaliq A, Jarvis-Evans J, McLeod D, Mackness M, Boulton M. Oxygen regulation of TGF-beta 1 mRNA in human hepatoma (Hep G2) cells. Biochem Mol Biol Int. 1994;34:639–644. [PubMed] [Google Scholar]
- 37.Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-beta-stimulated collagen production in fibroblasts. Am J Physiol. 2004;286:L121–L128. doi: 10.1152/ajplung.00231.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cudd A, Fridovich I. Electrostatic interactions in the reaction mechanism of bovine erythrocyte superoxide dismutase. J Biol Chem. 1982;257:11443–11447. [PubMed] [Google Scholar]
- 39.Rigo A, Viglino P, Rotilio G, Tomat R. Effect of ionic strength on the activity of bovine superoxide dismutase. FEBS Lett. 1975;50:86–88. doi: 10.1016/0014-5793(75)81047-7. [DOI] [PubMed] [Google Scholar]
- 40.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 41.Satriano JA, Shuldiner M, Hora K, Xing Y, Shan Z, Schlondorff D. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest. 1993;92:1564–1571. doi: 10.1172/JCI116737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. [DOI] [PubMed] [Google Scholar]
- 43.Schenk H, Vogt M, Droge W, Schulze-Osthoff K. Thioredoxin as a potent costimulus of cytokine expression. J Immunol. 1996;156:765–771. [PubMed] [Google Scholar]
- 44.Hill JR, Corbett JA, Kwon G, Marshall CA, McDaniel ML. Nitric oxide regulates interleukin 1 bioactivity released from murine macrophages. J Biol Chem. 1996;271:22672–22678. doi: 10.1074/jbc.271.37.22672. [DOI] [PubMed] [Google Scholar]
- 45.DeForge LE, Preston AM, Takeuchi E, Kenney J, Boxer LA, Remick DG. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268:25568–25576. [PubMed] [Google Scholar]
- 46.Andrew PJ, Harant H, Lindley IJ. Nitric oxide regulates IL-8 expression in melanoma cells at the transcriptional level. Biochem Biophys Res Commun. 1995;214:949–956. doi: 10.1006/bbrc.1995.2378. [DOI] [PubMed] [Google Scholar]
- 47.Pacchiarini L, Tua A, Grignani G. In vitro effect of reduced glutathione on platelet function. Haematologica. 1996;81:497–502. [PubMed] [Google Scholar]
- 48.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92:99–104. doi: 10.1172/JCI116604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellocq A, Azoulay E, Marullo S, Flahault A, Fouqueray B, Philippe C, Cadranel J, Baud L. Reactive oxygen and nitrogen intermediates increase transforming growth factor-beta1 release from human epithelial alveolar cells through two different mechanisms. Am J Respir Cell Mol Biol. 1999;21:128–136. doi: 10.1165/ajrcmb.21.1.3379. [DOI] [PubMed] [Google Scholar]
- 50.Jiang ZL, Zhu X, Diamond MP, Abu-Soud HM, Saed GM. Nitric oxide synthase isoforms expression in fibroblasts isolated from human normal peritoneum and adhesion tissues. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.07.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saed GM, Zhao M, Diamond MP, Abu-Soud HM. Regulation of inducible nitric oxide synthase in post-operative adhesions. Hum Reprod. 2006;21:1605–1611. doi: 10.1093/humrep/dei500. [DOI] [PubMed] [Google Scholar]
- 52.Jozsef L, Filep JG. Selenium-containing compounds attenuate peroxynitrite-mediated NF-kappaB and AP-1 activation and interleukin-8 gene and protein expression in human leukocytes. Free Radic Biol Med. 2003;35:1018–1027. doi: 10.1016/s0891-5849(03)00439-8. [DOI] [PubMed] [Google Scholar]
- 53.Weigert C, Sauer U, Brodbeck K, Pfeiffer A, Haring HU, Schleicher ED. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-beta1 promoter in mesangial cells. J Am Soc Nephrol. 2000;11:2007–2016. doi: 10.1681/ASN.V11112007. [DOI] [PubMed] [Google Scholar]
- 54.Lefaix JL, Delanian S, Leplat JJ, Tricaud Y, Martin M, Nimrod A, Baillet F, Daburon F. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35:305–312. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 55.Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 56.Martin M, Lefaix JL, Pinton P, Crechet F, Daburon F. Temporal modulation of TGF-beta 1 and beta-actin gene expression in pig skin and muscular fibrosis after ionizing radiation. Radiat Res. 1993;134:63–70. [PubMed] [Google Scholar]
- 57.Michelson AM, Puget K. [Medical aspects of superoxide dismutases] C R Seances Soc Biol Fil. 1979;173:380–393. [PubMed] [Google Scholar]



