Abstract
Introduction
Survival rates for gastrointestinal (GI) and bronchopulmonary (BP) neuroendocrine tumors (NETs) have not significantly altered (overall 67%, 5 yr survival) in thirty years (1973–2004) while the incidence has increased (~1,000%) in the same time-frame. No effective or specific antineoplastic agent(s) is available for treatment although somatostatin analogs inhibit tumor secretion. Given the coexistence of somatostatin and dopamine regulatory receptors on NET cells, we evaluated the antiproliferative efficacy as well as the signaling and transcriptional targets their ligands.
Methods
The cytotoxic effects of twelve somatostatin/dopamine compounds were evaluated in three NET cell lines and real-time PCR and ELISA studies performed to delineate antiproliferative signaling pathways.
Results
The atypical BP-NET, NCI-H720, was most sensitive to the sst5 analog BIM23206 (IC50:2.4pM) and showed similar sensitivity to lanreotide and the sst2 analog BIM23120. The typical BP-NET, NCI-H727, was most sensitive to BIM23120 (0.7nM) and to the pan somatostatin receptor analog (BIM23A779). The GI-NET, KRJ-I, was most sensitive to sst2,5 analogs lanreotide (1nM) and BIM23244 (7.4nM). Lanreotide activated ERK1/2 phosphorylation and p21WAF1/CIP1 transcription but inhibited Ki67 transcription. NCI-H720 was most sensitive to the sst2,5 and D2-selective compound BIM23A761 (4.2nM) as was NCI-H727 (5.5nM). KRJ-I did not respond to any chimeric analog. BIM23A761 activated JNK signaling and caused inhibition of Ki67 transcripts. P21WAF1/CIP1 transcription was activated only in NCI-H727 cells.
Conclusion
The different responses of each individual cell line suggests that NETs from different locations arising from different NE cells may require cell-specific antiproliferative agents based on the unique receptor profile of individual lesions.
Introduction
Neuroendocrine tumors (NET) – previously considered as “carcinoids” – account for 0.66% of all malignancies; however, it is noteworthy that the incidence has increased 3–10% per year over the last 30 years 1, 2. Although they predominantly occur in the gastrointestinal tract (67.5%), a substantial percentage is found in the bronchopulmonary system (25.3%) 3. A paucity are evident in the ovaries (1.01%), testes (0.07%) or thymus (0.38%) but they can occur in any location that contains cells of the diffuse neuroendocrine system 1. In rare circumstances (<10%), such tumors may present as metastatic disease from an unknown primary site 4. Of note is the high proportion (22.4%) of associated non-carcinoid malignancies that occur concurrently with NETs, especially of the small bowel 1.
It is also noteworthy that there has been no improvement in survival in the last 3 decades (SEER data base 1973–2004) 3, 5 and there exists no effective, safe and targeted therapy for the disease. The overall five-year survival rate for gastrointestinal NETs is 67.2% 5 while for bronchopulmonary NETs it is 50–88% 3, with a worse prognosis for atypical lesions that are defined by a higher mitotic count (2–10 mitoses per square millimeter in 10 high-power fields) and/or the presence of necrosis 6. Given the rising incidence of these lesions and the failure to improve outcome, there is a substantial need to identify more effective treatment modalities 1, 5.
Although the only curative therapy for NETs is surgery, more than 85% of NETs are metastatic at diagnosis hence ab initio, less than 15% of patients have any likelihood of having curative intervention 7. Currently available therapy (biotherapy, radiotherapy, surgical resection, and debulking) focuses on disease stabilization and symptom control. Tumor regression is very rare and estimated at 2–5% 7. The majority of contemporary therapy for NET disease is based on empirical data derived from experience with adenocarcinomas; few adequately powered prospective studies exist and it is unclear at a cellular and molecular level how the individual NETs studied differ from each other 5.
The most thoroughly investigated group of agents are the long-acting somatostatin analogs, which include lanreotide and octreotide. These compounds, which have been assessed in phase III clinical studies 8, are effective in controlling most symptoms associated with the hypersecretory activity of NET cells. Lanreotide is effective in the treatment of active postoperative acromegaly 9, but the antiproliferative role of both it and octreotide in gastrointestinal and bronchopulmonary NETs is limited. Direct mechanisms of somatostatin analog action require the activation of five receptors (sst1–5), belonging to the family of G-protein coupled receptors (GPCRs) and the induction of cell cycle arrest or apoptosis, mainly through the regulation of phosphotyrosine phosphatase and MAP kinase activities 10. The indirect mechanisms involve inhibition of tumor angiogenesis and secretion of factors which are required for tumor growth.
Dopamine regulates secretion and gene expression mediated by D2 receptors coupled to Gαi proteins, with a resultant inhibition of adenylyl cyclase, a decreased cAMP production, and suppression of activated phosphokinase A 11. Recently, chimeric molecules that possess potent, selective agonist activity for both somatostatin and dopamine receptors have been synthesized. These compounds have been shown to be more effective in suppressing growth hormone and prolactin secretion from cultured human growth hormone-secreting pituitary adenomas than either octreotide or mono-receptor ligands alone 11–13.These agents have not been evaluated against human NETs of the gastrointestinal and bronchopulmonary system.
We analyzed the effects of twelve novel somatostatin analogs and somatostatin/dopamine chimeric compounds (Table 1) on three human NET (carcinoid) cell lines (atypical bronchopulmonary NET: NCI-H720, a bronchial NET: NCI-H727, and a small intestinal NET: KRJ-I 14, 15) to investigate whether different NETs require cell-specific antiproliferative agents based upon the unique receptor profile of individual lesions. In addition, we examined the antiproliferative signaling mechanisms of somatostatin- and dopamine-mediated cell inhibition in each NET type.
Table 1.
Human somatostatin receptor subtype specificity (IC50) 39
| Compound | sst1 | sst2 | sst3 | sst4 | sst5 | D2 |
|---|---|---|---|---|---|---|
| Somatostatin-14 | 1.95 | 0.25 | 1.2 | 1.77 | 1.41 | - |
| Somatostatin-28 | 1.86 | 0.31 | 1.3 | 5.4 | 0.4 | - |
| BIM23023 | >1000 | 0.42 | 86.88 | >1000 | 4.18 | - |
| BIM23926 | 3.6 | >1000 | 1000 | 833 | 788 | - |
| BIM23120 | 1000 | 0.34 | 412 | 1000 | 213.5 | - |
| BIM23206 | >1000 | 166 | 1000 | >1000 | 2.4 | - |
| BIM23244 | >1000 | 0.29 | 133 | >1000 | 0.67 | - |
| Lanreotide | >1000 | 0.75 | 98 | >1000 | 12.7 | - |
| BIM23A719 | 40 | 1 | 5.6 | 1000 | 0.37 | - |
| BIM23A779 | 2.46 | 0.32 | 0.57 | 20.5 | 0.56 | - |
| BIM53061 | - | - | - | - | - | 114.7 |
| BIM23A758 | 486 | 0.1 | 324 | >1000 | 27 | 8 |
| BIM23A760 | 662 | 0.03 | 160 | >1000 | 42 | 15 |
| BIM23A761 | 602 | 0.128 | 196 | >1000 | 8.5 | 25 |
- not done
Materials and Methods
Culture conditions
KRJ-I and NCI-H720 cells were cultured as floating aggregates at 37°C with 5% CO2. KRJ-I cells were kept in Ham’s F12 medium (Gibco™) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich), penicillin 100U/ml and streptomycin (100 μg/ml) 15–17. For NCI-H720 a 1:1 solution of Ham’s F12 and Dulbecco’s minimal essential medium (DMES) supplemented with final concentrations of FBS (5%), insulin (0.005mg/ml), transferrin (0.01 mg/ml), sodium selenite (30nM), hydrocortisone (10nM), β-estradiol (10nM), HEPES medium (10mM) and L-glutamine (2mM) 14. The adhesive growing NCI-H727 cells were kept at 37°C in RPMI 1640 medium containing final concentrations of FBS (10%), L-Glutamine (2mM), sodium pyruvate (1mM) and glucose (2.5g/l) 14. For further processing, NCI-H727 cells were washed in PBS before Trypsin-EDTA was applied.
Proliferation studies
After being spun down, 5 minutes –1,500g, the pellets of the three cell lines were resuspended in each medium to a concentration of 5 × 105 cells/ml and seeded in 96 well plates at 100μl with 5 × 104 cells/well (2 plates/experimental condition). Lane 1 and 2 contained negative (medium only) and positive (pure cell suspension) controls 17. The BIM compounds were provided by IPSEN (Milford, MA). Drugs were diluted in PBS and applied every 24 hrs in final concentrations from 10−10 to 10−6.
Agent Profiles
The agent profiles and somatostatin and dopamine receptor subtype specificities (IC50) of each of the tested compounds are provided in Table 1. All compounds are stable in vitro and degradation is not an issue (MC – personal communication).
MTT assay
After 72 hours of incubation (37°C; 5% CO2) with each compound, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added to a final concentration of 0.5mg/ml per well followed by additional incubation for 3 hours 17, 18. The reaction was stopped and the formazan dye was solubilized by adding an equal volume (100μl) of acid-isopropanol (0.01N HCl in isopropanol). The optical density was read at 595 nm using a microplate reader (Bio-Rad 3500) 16, 17.
ELISA (CASE) Assay
The effects of somatostatin analogs, BIM23926 (sst1), BIM23120 (sst2), BIM23023, BIM23244 (both sst2,5) and BIM23206 (sst5) was measured on the ERK1/2 signaling pathway as described19. In separate studies, the effects of lanreotide on ERK1/2 signaling pathway and BIM23A761 on ERK/AKT/JNK and NFκB signaling pathways were evaluated 19. Cells were stimulated with IC50 concentrations (from proliferation studies – Table 2) for 30 mins. To assess the specificity of ERK pathway activation, cells were pre-incubated with PD98059 (30 μM) (an inhibitor of ERK1/2 phosphorylation) for 10 min prior to addition of lanreotide and BIM23A761. ERK, AKT, JNK and NFκB phosphorylation were measured using an ELISA (SuperArray CASE™) as per the manufacturer’s protocol. Briefly, stimulated cells were fixed (4% formaldehyde), and stained with either primary antibodies against the non-phosphorylated or phosphorylated forms of each protein (60 min, RT). After washing, and secondary antibody application (60 min, RT), cells were incubated with color developer (10 min, RT) and plates read at 450 nm. Thereafter, protein was assayed in each well (protein development – reading at 595nm). Results were calculated as antibody reading (at 450nm)/protein concentration (measured at 595nm) and normalized to unstimulated cells. Phosphorylated signal was compared to total non-phosphorylated signal.
Table 2.
Summary of effects of somatostatin analogs and somatostatin/dopamine chimeric compounds on NET cell line proliferation and ERK1/2 phosphorylation
| Compound | Action | H720 | ERKp | H727 | ERKp | KRJ-I | ERKp |
|---|---|---|---|---|---|---|---|
| BIM23023 | sst2/5 agonist | 0.23 Nm | Y | No effect | N | 0.28 μM | Y |
| BIM23926 | sst1 agonist | No effect | N | No effect | N | 8.5 nM | Y |
| BIM23120 | sst2 agonist | 41 pM | Y | 0.7nM | Y | No effect | N |
| BIM23206 | sst5 agonist | 2.4 pM | Y | No effect | N | No effect | N |
| BIM23244 | sst2/5 agonist | 0.11 nM | Y | No effect | N | 7.4 nM | Y |
| Lanreotide | sst2/5 agonist | 6.5 pM | Y | No effect | N | 1 nM | Y |
| BIM23A719 | pan sst agonist 1 | 6.2 nM | No effect | 0.53 nM | |||
| BIM23A779 | pan sst agonist 2 | No effect | 1.9pM | 0.35 μM | |||
| BIM53061 | Dopamine Agonist | 21 nM | No effect | 0.71 μM | |||
| BIM23A758 | sst/da agonist 1 | No effect | No effect | No effect | |||
| BIM23A760 | sst/da agonist 2 | 18.3 nM | No effect | No effect | |||
| BIM23A761 | sst/da agonist 3 | 4.2 nM | N | 5.5 nM | N | No effect | N |
Real-Time PCR
RNA was extracted from 2×106 NCI-H70, NCI-H727 and KRJ-I, cells in log phase growth (n=3) or from normal brain (n=3) (TRIZOL®, Invitrogen, USA), and cleaned (Qiagen RNeasy kit and DNeasy Tissue kit, Qiagen Inc., USA) to minimize contaminating genomic DNA. RNA (2μg) was converted to cDNA (High Capacity cDNA Archive Kit, Applied Biosystems) 17, 20, 21. Real time RT-PCR analysis was performed using Assays-on-Demand™ products and the ABI 7900 Sequence Detection System according to the manufacturer’s suggestions17, 20, 21. Cycling was performed under standard conditions (TaqMan® Universal PCR Master Mix Protocol) and data normalized using geNorm 22 and expression of the novel house-keeping genes, ALG9, TFCP2 and ZNF410 17, 21
1. Receptor profile
Levels of sst1–5 and D1–5 were measured in NCI-H720, NCI-H727 and KRJ-I cell lines. Normal brain was used as a control. Receptor probes were used to quantify presence of somatostatin and dopamine receptors in each cell line. Transcript levels <0.1 normalized geNorm were considered absent.
2. Cell cycle markers
Ki67 and p21WAF1/CIP1 transcriptions were measured in response to lanreotide and BIM23A761. NCI-H720, NCI-H727, and KRJ-I cells were stimulated with IC50 concentrations of lanreotide and BIM23A761 respectively. Cells were cultured for 24 hrs prior to RNA extraction. RNA was isolated using the standard PCR protocol 17, 20, 21.
Statistical Evaluation
All statistical analyses were performed using Prism 4 (GraphPad Software, San Diego, CA). Sigmoidal dose responses and non-linear regression analyses were calculated to identify half-maximal concentrations (IC50) for each drug. Alterations in signal transduction and transcriptional activation were assessed using 2-tailed paired t-tests.
Results
1. Receptor profile by PCR
Analysis of somatostatin and dopamine receptor levels using real-time PCR in each cell line demonstrated a different expression profile for each cell line.
NCI-H720
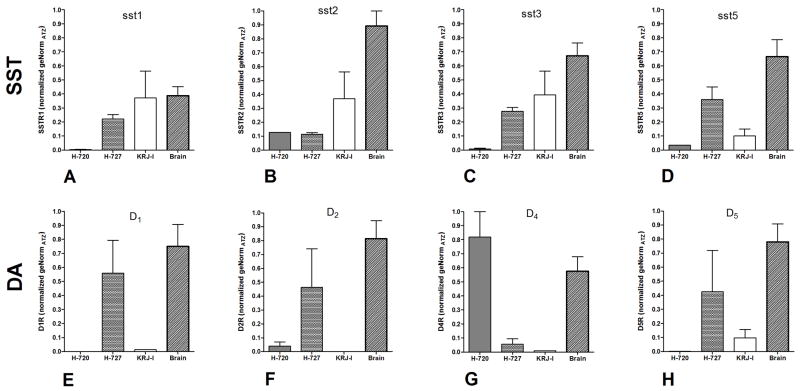
This cell line was derived from an “atypical” bronchopulmonary NET 14. It is specific for somatostatin receptors 2 and 5 as well as the dopamine receptors 2 and 4 (Figure 1). The sst/da receptor profile of NCI-H720 is thus designated as sst2,5/D2,4.
Figure 1. Somatostatin and dopamine receptor profiles in NCI-H720 (atypical), NCI-H727 (typical), and KRJ-I assessed by real-time PCR.
Sst1, sst3, sst5 receptors were found exclusively in NCI-H727 and KRJ-I cell lines (1A, 1C, 1D), sst2 were present in all cell lines (1B). D1, D2, D4 and D5 receptors were present in NCI-H727 cells (1E, 1F) while inhibitory D2 and D4 receptors were identified in NCI-H720 (1G). D5 receptors were observed in KRJ-I cells (1H). All sst and D receptors were identified in brain tissue (positive control). Mean±SEM; n=3.
NCI-H727 is cell line derived from a “typical” bronchial NET 14. It is positive for sst1–3,5 in addition to D1,2,5. Very low levels of D4 (<0.1 normalized geNorm) were also identified (Figure 1). The receptor profile of NCI-H727 is therefore sst1,2,3,5/D1,2,5.
KRJ-I is derived from a “typical” small intestinal NET 15. It is positive for sst1–3,5 as well as low levels of D5 (=0.1 normalized geNorm) (Figure 1). The receptor profile of KRJ-I is sst1,2,3,5/D5.
2. Antiproliferative effects of somatostatin and somatostatin/dopamine chimeras
NCI-H720
Proliferation of this cell line is inhibited by the sst5 agonist, BIM23206 (IC50 = 2.4pM) with a comparable sensitivity to Lanreotide (sst2,5 agonist – 6.5pM) and the sst2 agonist, BIM23120 (IC50 = 41pM) (Table 2). NCI-H720 cells were less responsive to the sst2,5 agonist, BIM23244 (IC50 = 0.11nM) and did not significantly respond to the sst1 agonist, BIM23926. These results are consistent with the somatostatin receptor profile of the NCI-H720 cells (Figures 1A, B, D) and with ERK1/2 phosphorylation induced by these somatostatin analogs (Table 2). Of the two pan somatostatin receptor agonists tested, NCI-H720 cells were most sensitive to BIM23A719 (pan somatostatin receptor agonist 1, IC50 = 6.2nM).
Cell proliferation was also inhibited by the dopamine agonist, BIM53061 (IC50 = 0.23nM) and both BIM23A760 (sst/da agonist 2, IC50 = 18.3nM) and BIM23A761 (sst/da agonist 3, IC50 = 4.2nM).
NCI-H727
The typical BP-NET was most sensitive to the sst2 agonist, BIM23120 (IC50 = 0.7nM) and to the pan somatostatin agonist 2, BIM23A779 (IC50 = 1.9pM) (Table 2). Neither sst1, sst2,5 nor any of the sst2, 5 agonists caused inhibition of proliferation despite expression of transcripts for these receptors (Figures 1A, B, D). These results are consistent with the absence of ERK1/2 phosphorylation by these agents (Table 2).
Cell proliferation was also inhibited by the sst2,5 and D2 selective compound BIM23A761 (IC50 = 5.5nM).
KRJ-I
This was most sensitive to lanreotide (IC50 = 1nM), BIM23244 (sst2,5 agonist, IC50 = 7.4nM) and BIM 23926 (sst1 agonist, IC50 = 8.5nM) but was not responsive to either a pure sst2 (BIM23120) or sst5 (BIM23206) agonist (Table 2). The cell proliferation inhibitory effects were consistent with ERK1/2 phosphorylation by these compounds; neither BIM23120 nor BIM23206 induced phosphorylation (Table 2). KRJ-I cells were sensitive to both pan somatostatin receptor agonists but were most sensitive to the type 1 agent (BIM23A719).
KRJ-I cell proliferation could not be effectively inhibited by either the dopamine agonists (IC50 = 0.7μM) or the sst/da agonist chimeras which reflects the absence of significant transcripts for the inhibitory dopamine receptor (type 2) on this cell line (Figure 1F).
3. Effects of Lanreotide and BIM23A761 on cell signaling pathways
NCI-H720
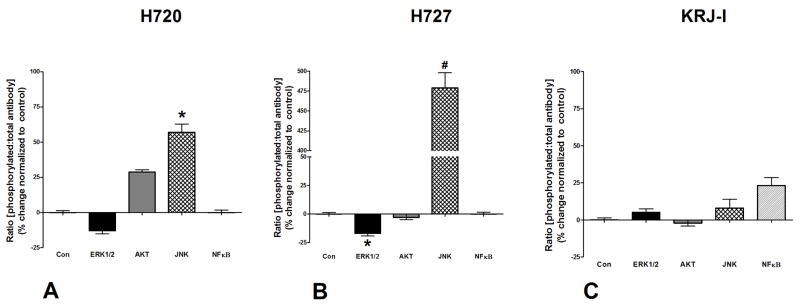
Lanreotide activated MAPK signaling (182±35%, p<0.01 vs. control) through phosphorylation of ERK1/2 which could be inhibited (65±17, p<0.01 vs. lanreotide) by the MEK inhibitor, PD98059 (Figure 2A). This is consistent with the inhibitory effects of Lanreotide on NCI-H720 cell proliferation. When cells were stimulated with the type 3 sst/da chimera, BIM23A761, ERK was not phosphorylated. In contrast, the JNK pathway was activated by this compound; pJNK levels were elevated (61±4%, p<0.05 vs. control: Figure 3A). Neither NFκB nor AKT were significantly altered by BIM23A761.
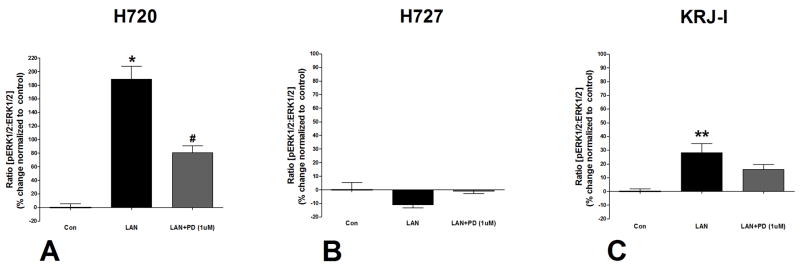
Figure 2. Effects of Lanreotide on ERK1/2 phosphorylation.
Lanreotide phosphorylated ERK1/2 in NCI-H720 and KRJ-I cell lines (4A, 4C). Lanreotide had no effect on ERK1/2 phosphorylation in NCI-H727 (4B). The effects of lanreotide were inhibited by the MEK inhibitor, PD98059 (1μM) (4A–C). *p<0.01 vs. control; **p<0.05 vs. control; #p<0.05 vs. lanreotide. Mean±SEM; n=4.
Figure 3. Effects of BIM23A761 on cell signaling pathways.
BIM23A761 inhibited ERK1/2 phosphorylation and activated JNK signaling in NCI-H720 and NCI-H727 cells (5A–B). BIM23A761 has no effect on signaling pathways in KRJ-I cells (5C). *p<0.05 vs. control; #p<0.05 vs. control. Mean±SEM; n=4.
NCI-H727
Lanreotide had no effect on ERK phosphorylation (Figure 2B) which is consistent with the observation that proliferation could not be inhibited (Table 2). The BIM23A761 sst/da chimera was associated, as in NCI-H720 cells, with significant activation of JNK phosphorylation (475±27%, p<0.01 vs. control: Figure 3B). While ERK1/2 signaling was inhibited (17±3, p<0.05 vs. control), neither the NFκB nor AKT signaling pathways were significantly activated.
KRJ-I
Lanreotide activated ERK1/2 signaling by 25±8% (p=0.05 vs. control) which could be inhibited by the MEK inhibitor, PD98059 (Figure 2C). BIM23A761 did not significantly alter phosphorylation of any of the signaling pathways which was consistent with lack of any demonstrable effect of this sst/da chimera on KRJ-I cell proliferation (Table 2).
Effects of lanreotide and BIM23A761 on downstream transcriptional targets
NCI-H720
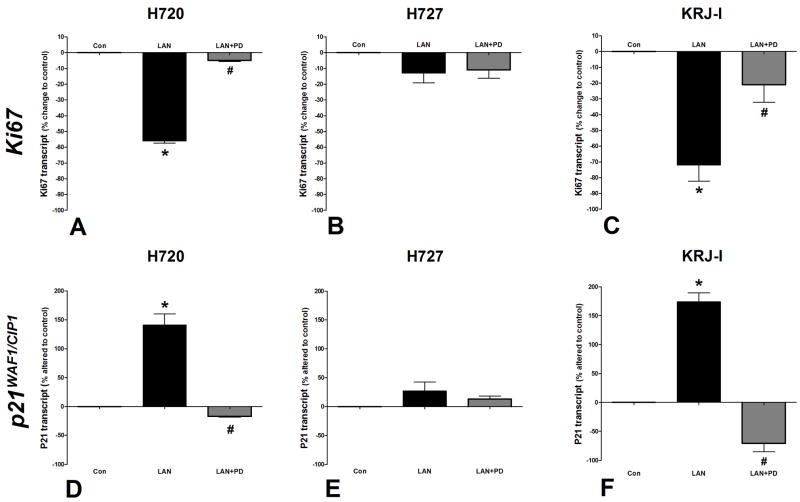
Real-time PCR analysis of cells incubated with lanreotide for 24 hr demonstrated that Ki67 transcript levels were significantly decreased (45±7%) compared to unstimulated cells (p<0.01: Figure 4A). This is consistent with the observation that lanreotide inhibits NCI-H720 proliferation. The effect was specific since it could be inhibited by PD98059 (6±2%, p<0.05 vs. lanreotide alone). Lanreotide significantly increased p21WAF1/CIP1 transcript levels compared to unstimulated cells (140±27%, p<0.01 vs. control) and this increase was reversible by PD98059 (p<0.05 vs. lanreotide alone: Figure 4D). These results are consistent with the anti-proliferative effects of this compound on NCI-H720.
Figure 4. Real-time PCR analysis of the effect of Lanreotide on Ki67 and p21WAF1/CIP1 transcripts.
Lanreotide significantly inhibited Ki67 transcription in all cell lines (2A-C). P21WAF1/CIP1 transcript was stimulated in all cell lines (2D-F). These effects were inhibited by PD98059 (1μM). *p<0.01 vs. control; #p<0.05 vs. lanreotide; **p<0.01 vs. lanreotide. Mean±SEM; n=4.
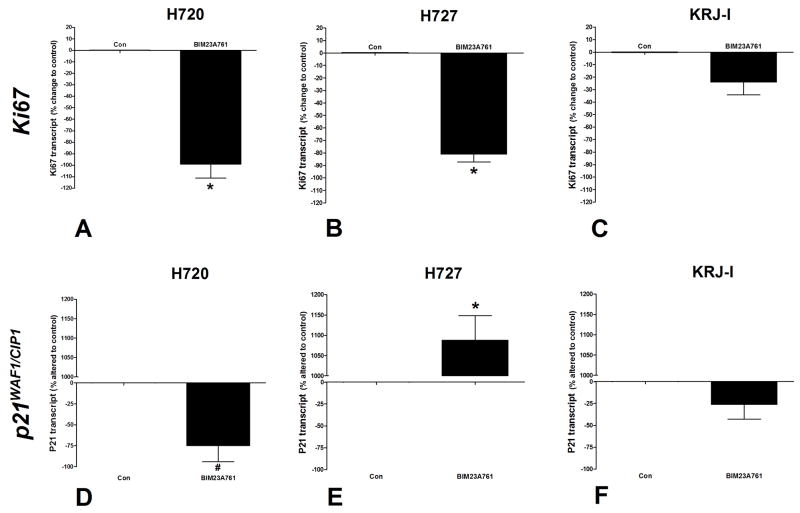
Real-Time PCR analysis of BIM23A761-stimulated cells demonstrated that Ki67 transcript levels were decreased 99±17% (p<0.01 vs. controls: Figure 5A) confirming that this compound inhibited NCI-H720 cell proliferation. In addition, and in contrast to the effects of lanreotide in these cells, p21WAF1/CIP1 transcript levels were significantly decreased (74±27, p<0.05 vs. control).
Figure 5. Real-time PCR analysis of the effects of BIM23A761 on Ki67 and p21WAF1/CIP1 transcripts.
BIM23A761 significantly inhibited Ki67 transcription in NCI-H720 and NCI-H727 cell lines (3A, 3B). Transcripts for p21WAF1/CIP1 were inhibited by BIM23A761 in NCI-H720 (3D) and stimulated in NCI-H727 cells (3E). No significant effects were identified in KRJ-I cells (3C, 3F). *p<0.01 vs. control; #p<0.05 vs. control. Mean±SEM; n=4.
NCI-H727
Real-Time PCR analysis of the effect of lanreotide on Ki67 demonstrated that this was not significantly decreased compared to control cells (Figure 4A), while p21WAF1/CIP1 transcripts were not significantly up-regulated (Figure 4E); results consistent with the absence of an inhibitory effect of lanreotide on NCI-H727 proliferation.
PCR assessment of BIM23A761 demonstrated significant (p<0.01) down regulation of Ki67 transcript levels (80±12% change to control: Figure 5B) while p21WAF1/CIP1 transcripts were elevated (1082±97%, p<0.01 vs. control).
KRJ-I
Real-Time PCR measurements of Ki67 transcript levels demonstrated that lanreotide down regulated Ki67 (73±12%; p<0.01 vs. control: Figure 4C) and this was inhibited by PD98059 (p<0.05 vs. lanreotide alone). Transcripts for p21WAF1/CIP1 were significantly elevated (175±21%, p<0.01 vs. control: Figure 4F), results consistent with the antiproliferative effects of this compound on KRJ-I.
In BIM23A761 treated KRJ-I cells, neither Ki67 nor p21WAF1/CIP1 transcript levels were significantly decreased (Figure 5C, 5F), consistent with observations that BIM23A761 did not inhibit KRJ-I cell proliferation.
Discussion
This study reveals that individual lung and gastrointestinal NET cell lines exhibit substantially different receptor expressions for somatostatin and dopamine receptors which are differently coupled to the ERK and JNK signaling pathways. In addition, somatostatin analogs and somatostatin/dopamine chimeric compounds are potent inhibitors of NET cell proliferation, while cell lines from the same organ (lung: typical versus atypical NET) were differently sensitive to receptor targeting. These molecular observations confirm the need to individualize therapeutic strategies based upon analysis of the receptor profile of a specific lesion as opposed to a global strategy based purely upon a histopathological characterization or classification. The differences in ERK signaling activation to specific somatostatin analogs suggests that defining the receptor profile alone may not be sufficient to predict NET responsiveness to these analogs. This is highlighted by the results with lanreotide, BIM23244 and BIM23023 which all are predicted to inhibit cell function through activation of sst2,5. In the current study, lanreotide was the most effective inhibitor in NCI-H720 and KRJ-I cells. In contrast, and despite expression of sst2,5, none of these compounds inhibited NCI-H727 cell proliferation. The absence of an effect in this cell line may suggest either abnormal receptor expression (cell membrane/protein level) as has been noted in acromegalic tumors 23 or receptor coupling resulting in non-functional and divergent signaling pathways as noted in rat somatotrophs 24. Because ERK1/2 phosphorylation studies demonstrated no responses to sst2,5 ligands in NCI-H727 cells but did show an effect in NCI-H720 and KRJ-I cells, we conclude that effective receptor signaling may be a prerequisite for appropriate inhibition of NET proliferation.
An examination of pan somatostatin receptor agonists demonstrated that the type 1 (BIM23A719) was most effective in NCI-H720 and KRJ-I cells (IC50 in NCI-H720 = 6.2 nM and IC50 in KRJ-I = 0.53 nM) while the type 2 agonist (BIM23A779) was most effective in NCI-H727 cells (IC50 NCI H727 = 1.9 pM). Of note is the fact that the pan somatostatin receptor agents were not as effective as individualized somatostatin agonists in the atypical BP NET (NCI-H720) as in the “typical” BP NET. The sensitivity of the latter to a pan somatostatin compound conforms to a previous study demonstrating that SOM230 was more effective than octreotide in inhibiting NCI-H727 proliferation25. This also confirms our observation that NCI-H727 cells are not effectively inhibited by a sst2,5 compound despite expressing these receptors25, 26.
Targeting the D2 receptor is an effective mechanism for suppressing NET secretion 11–13 but its antiproliferative effects are not well characterized, particularly on lung and gastrointestinal NETs. Targeting D2, which is coupled to Gai and through coupling to phosphodiesterase activity reduces intracellular cAMP 27, is associated with inhibition of MAPK signaling 28. Activation of inhibitory D2 receptors would therefore be predicted to inhibit cell proliferation. In the current studies, NCI-H720 cells which express the inhibitory dopamine receptors (D2,4R) exhibit a decrease in cell proliferation when exposed to BIM53061 (IC50 = 21nM). This compound also inhibits KRJ-I (μM concentrations) but not NCI-H727 cell proliferation. These differences could reflect expression of the stimulatory D1,5 receptors in these cell lines and suggests that a D2 agonist would probably be ineffective in neoplastic NE cells expressing multiple dopamine receptor subtypes.
Somatostatin/dopamine chimeras target multiple receptor complexes to effect growth inhibition 13. Proliferation of NCI-H720 was inhibited by both sst/da agonists 2 and 3 (IC50: 4.2–18 nM). These agents, however, were less effective (<100-fold) than targeting specific somatostatin receptors alone e.g. sst5 or the pan somatostatin receptor agonist 1 on NCI-H720 cells. Targeting the dopamine in addition to somatostatin receptors is not therefore additionally inhibitory in this cell line. In NCI-H727 cells, sst/da agonist 3 was an effective inhibitor of proliferation. The KRJ-I cell line was not effectively inhibited by any of the sst/da compounds.
Having determined that NET cells lines were differentially sensitive to somatostatin analogs and sst/da chimeras, we next evaluated the mechanisms (signaling pathways, downstream transcriptional targets) by which two agents, lanreotide (sst2,5) and BIM23A761 (sst/da agonist 3), inhibited cell proliferation. These agents were chosen because they were both potent (lanreotide: 6.5pM NCI-H720 and 1nM KRJ-I; BIM23A761: 4.2nM NCI-H720 and 5.5nM NCI-H727) and they significantly inhibited only two (lanreotide: H720 and KRJ-I; BIM23A761: NCI-H720 and NCI-H727) of the three cell lines; one cell line therefore could be used as a negative control.
Lanreotide stimulated ERK1/2 activity in both NCI-H720 and KRJ-I cells but not in NCI-H727 cells. This resulted in a decrease in transcripts for Ki67 and an elevation of the cell cycle inhibitor, p21WAF1/CIP1. Preincubation with the MEK inhibitor, PD98059, decreased these lanreotide-mediated alterations in p21WAF1/CIP1 expression. In studies in Chinese Hamster epithelial ovary CHO-K1cells, lanreotide-stimulated ERK activity led to accumulation of phosphorylated P27Kip1, inhibition of cyclinE-cdk2 kinase activity, accumulation of hypo-phosphorylated p105Rb and cell cycle arrest 29, 30. Over expression of P21WAF1/CIP1, a cyclin-dependent kinase (cdk) inhibitor, leads to G1 and G2 or S-phase arrest 31. Our findings indicate that the inhibitory effects of lanreotide on H720 and KRJ-I are associated with cell cycle arrest at least via p21WAF1/CIP1 transcriptional upregulation.
In NCI-H720, BIM23A761 did not phosphorylate ERK1/2, AKT or NFκB but activated JNK phosphorylation. JNK was also phosphorylated in BIM23A761-treated NCI-H727 cells but phosphorylation of ERK1/2 was inhibited. Although BIM23A761 caused a decrease in transcripts for Ki67 in both cell lines, an elevation of the cell cycle inhibitor, p21WAF1/CIP1 was only detected in NCI-H727 cells. In contrast, expression of this cdk inhibitor was significantly decreased in NCI-H720 cells. This seemingly discrepant observation raises the issue of the precise role of JNK signaling in apoptosis since JNK has been reported to function as both a pro- and anti-apoptotic agent 32. JNK induces phosphorylation of c-Jun at Ser63, a site important for c-Jun-dependent transcriptional activity 33 and the combination of c-Jun activation and p21WAF1/CIP1 suppression stimulates P53-dependant apoptosis 31. Our finding of suppressed levels of P21WAF1/CIP1 in NCI-H720 cells is consistent with a role for BIM23A761 in inhibiting cell proliferation via a P53-dependant apoptosis. In contrast, BIM23A761-mediated growth inhibition in NCI-H727 cells, like lanreotide in NCI-H720 and KRJ-I cells, is associated with P21WAF1/CIP1-mediated cell cycle inhibition through the retinoblastoma pathway 29, 30. It is thus likely that individual NETs will not share completely similar transduction mechanisms for proliferative regulation and that specific assessments will be necessary to identify and predict the most efficacious therapeutic agent.
Synthetic derivatives of somatostatin, such as octreotide, are in common clinical use as therapy for acromegaly and gastroenteropancreatic tumors 34. While effective in the inhibition of the secretion of bioactive products and the amelioration of symptomatology 35, they have been disappointing as antiproliferative agents in vivo 7. The reasons for this are not known but indicate that secretory regulation of NET cells is an intrinsic property, whereas the proliferative drive may comprise many agents (angiogenic factors, growth factors) and has a substantial extrinsic basis, likely from the tumor micro-milieu.
We have used an in vitro model to study the effects of selective somatostatin and dopamine compounds on NET cells. With the caveat that our studies cannot measure the effects of these compounds on cells, e.g. fibroblasts, endothelial cells, in the NET microenvironment that regulate NET proliferation and secretion5, 36, 37, we demonstrate that targeting specific receptor subtypes on tumor cells, with selective somatostatin agonists may be more advantageous in “atypical” BP-NETs than a single generic compound. In contrast, BP-NETs and GI-NETs with an extensive somatostatin receptor expression profile may be more responsive to a pan somatostatin analog 25. Since some NETs express somatostatin and D2 receptors, it is has been suggested that sst/da chimeric compounds may prove to be valuable in the management of NETs that exhibit both receptor profiles 38. Our results support this observation as somatostatin/dopamine chimeric compounds are as effective as pan somatostatin analogs in inhibiting NET cell proliferation. They do, however, appear to be less effective than single receptor targeted somatostatin agonist therapy for “atypical” BP NETs.
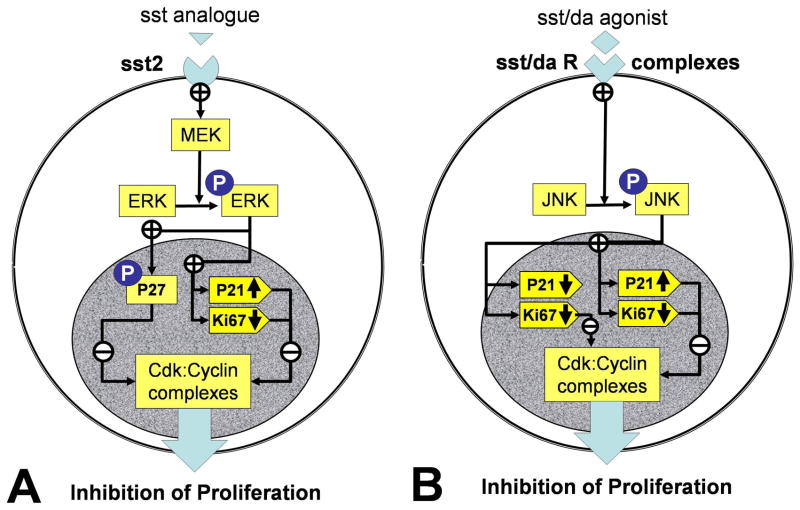
In conclusion, individual (site and cell specific) NETs respond differently to specific somatostatin analogs and sst/da chimeric compounds depending on the somatostatin and dopamine receptor profile coupling to signaling pathways. Antiproliferative effects are due to ERK1/2 phosphorylation and JNK signaling and activation of cdk inhibitors with a decrease in Ki67 (Figure 6). If a therapeutic strategy that embraces receptor targeting is utilized for NETs, it will require prior delineation of the specific receptor profile of the tumor and the selection of “receptor-tailored” pharmacotherapy to optimize effective treatment.
Figure 6. Somatostatin and sst/da chimera receptor signaling pathways in NET cell lines.
Somatostatin receptor activation (largely via sst2) results in MEK activation and downstream phosphorylation of ERK (6A). Activated ERK phosphorylates and activates the cdk inhibitor P27KIP1 and up-regulates P21WAF1/CIP1 while inhibiting transcription of the cyclin, Ki67. The combination of cdk inhibitor upregulation and a decrease in Ki67 results in growth arrest. Sst/da receptor activation (largely sst2/5/D2) results in phosphorylation of JNK (6B). Activated JNK up-regulates P21WAF1/CIP1 while inhibiting transcription of the cyclin, Ki67. This combination results in growth arrest. Depending on the sst/da receptor complex, p21WAF1/CIP1 transcription can also be decreased.
Acknowledgments
This work is supported in part by NIH R01-CA1185285 (IMM) and the Bruggeman Medical Foundation.
References
- 1.Modlin I, Lye K, Kidd M. A five-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the diffuse neuroendocrine system. Curr Opin Oncol. 2008;20(1):1–12. doi: 10.1097/CCO.0b013e3282f1c595. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson BI, Kidd M, Chan AK, Malfertheiner MV, IMM Broncho-Pulmonary Neuroendocrine Tumors. Cancer. 2008 doi: 10.1002/cncr.23542. in press. [DOI] [PubMed] [Google Scholar]
- 4.Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10(2):123–31. doi: 10.1634/theoncologist.10-2-123. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. The Current status of Gastroenteropancreatic Neuroendocrine Tumors. Lancet Oncology. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 6.Kosmidis PA. Treatment of carcinoid of the lung. Curr Opin Oncol. 2004;16(2):146–9. doi: 10.1097/00001622-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Modlin IM, Latich I, Kidd M, Zikusoka M, Eick G. Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol. 2006;4(5):526–47. doi: 10.1016/j.cgh.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 8.van der Hiel B, Stokkel MP, Chiti A, Lucignani G, Bajetta E, Pauwels EK, et al. Effective treatment of bone metastases from a neuroendocrine tumour of the pancreas with high activities of Indium-111-pentetreotide. Eur J Endocrinol. 2003;149(6):479–83. doi: 10.1530/eje.0.1490479. [DOI] [PubMed] [Google Scholar]
- 9.Lin JD, Lee ST, Weng HF. An open, phase III study of lanreotide (Somatuline PR) in the treatment of acromegaly. Endocr J. 1999;46(1):193–8. doi: 10.1507/endocrj.46.193. [DOI] [PubMed] [Google Scholar]
- 10.Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822–40. doi: 10.2741/2722. [DOI] [PubMed] [Google Scholar]
- 11.Gruszka A, Ren SG, Dong J, Culler MD, Melmed S. Regulation of growth hormone and prolactin gene expression and secretion by chimeric somatostatin-dopamine molecules. Endocrinology. 2007;148(12):6107–14. doi: 10.1210/en.2007-0378. [DOI] [PubMed] [Google Scholar]
- 12.Ren SG, Kim S, Taylor J, Dong J, Moreau JP, Culler MD, et al. Suppression of rat and human growth hormone and prolactin secretion by a novel somatostatin/dopaminergic chimeric ligand. J Clin Endocrinol Metab. 2003;88(11):5414–21. doi: 10.1210/jc.2003-030302. [DOI] [PubMed] [Google Scholar]
- 13.Jaquet P, Gunz G, Saveanu A, Dufour H, Taylor J, Dong J, et al. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol. 2005;153(1):135–41. doi: 10.1530/eje.1.01950. [DOI] [PubMed] [Google Scholar]
- 14.Phelps RM, Johnson BE, Ihde DC, Gazdar AF, Carbone DP, McClintock PR, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl. 1996;24:32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 15.Pfragner R, Wirnsberger G, Niederle B, Behmel A, Rinner I, Mandl A, et al. Establishment of a continuous cell line from a human carcinoid of the small intestine (KRJ-I): Characterization and effects of 5-azacytidine on proliferation. International Journal of Oncology. 1996;8:513–20. doi: 10.3892/ijo.8.3.513. [DOI] [PubMed] [Google Scholar]
- 16.Kidd M, Eick G, Modlin I, Pfragner R, Champaneria M, Murren J. Further delineation of the continuous human neoplastic enterochromaffin (EC) cell line, KRJ-I, and the inhibitory effects of Lanreotide and Rapamycin. Journal of Molecular Endocrinology. 2007;38:181–92. doi: 10.1677/jme.1.02037. [DOI] [PubMed] [Google Scholar]
- 17.Kidd M, Schally AV, Pfragner R, Malfertheiner MV, Modlin IM. Inhibition of proliferation of small intestinal and bronchopulmonary neuroendocrine cell lines by using peptide analogs targeting receptors. Cancer. 2008;112:1404–14. doi: 10.1002/cncr.23303. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Kidd M, Modlin I, Eick G, Camp R, Mane S. The role of CCN2/CTGF in the proliferation of Mastomys Enterochromaffin-like cells and gastric carcinoid development. Am J Physiol. 2007;292:G191–200. doi: 10.1152/ajpgi.00131.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor-beta type II receptor are absent in small bowel carcinoid tumors. Cancer. 2005;103(2):229–36. doi: 10.1002/cncr.20750. [DOI] [PubMed] [Google Scholar]
- 21.Kidd M, Nadler B, Mane SM, Eick GN, Malfertheiner MV, Champaneria MC, et al. GeneChip, geNorm and Gastrointestinal tumors: novel reference genes for real-time PCR. Physiol Genomics. 2007;30:363–70. doi: 10.1152/physiolgenomics.00251.2006. [DOI] [PubMed] [Google Scholar]
- 22.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casarini AP, Pinto EM, Jallad RS, Giorgi RR, Giannella-Neto D, Bronstein MD. Dissociation between tumor shrinkage and hormonal response during somatostatin analog treatment in an acromegalic patient: preferential expression of somatostatin receptor subtype 3. J Endocrinol Invest. 2006;29(9):826–30. doi: 10.1007/BF03347378. [DOI] [PubMed] [Google Scholar]
- 24.Cervia D, Zizzari P, Pavan B, Schuepbach E, Langenegger D, Hoyer D, et al. Biological activity of somatostatin receptors in GC rat tumour somatotrophs: evidence with sst1-sst5 receptor-selective nonpeptidyl agonists. Neuropharmacology. 2003;44(5):672–85. doi: 10.1016/s0028-3908(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 25.Ono K, Suzuki T, Miki Y, Taniyama Y, Nakamura Y, Noda Y, et al. Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res. 2007;27(4B):2231–9. [PubMed] [Google Scholar]
- 26.Moreno A, Akcakanat A, Munsell MF, Soni A, Yao JC, Meric-Bernstam F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15(1):257–66. doi: 10.1677/ERC-07-0202. [DOI] [PubMed] [Google Scholar]
- 27.Conti M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol Endocrinol. 2000;14(9):1317–27. doi: 10.1210/mend.14.9.0534. [DOI] [PubMed] [Google Scholar]
- 28.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89(1):73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 29.Pages P, Benali N, Saint-Laurent N, Esteve JP, Schally AV, Tkaczuk J, et al. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274(21):15186–93. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 30.Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. J Endocrinol Invest. 2005;28(11 Suppl):5–9. [PubMed] [Google Scholar]
- 31.Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1(8):639–49. [PubMed] [Google Scholar]
- 32.Lee YJ, Shukla SD. Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes. Eur J Pharmacol. 2005;508(1–3):31–45. doi: 10.1016/j.ejphar.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353(6345):670–4. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 34.Oberg K. Established clinical use of octreotide and lanreotide in oncology. Chemotherapy. 2001;47(Suppl 2):40–53. doi: 10.1159/000049160. [DOI] [PubMed] [Google Scholar]
- 35.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 36.Kidd M, Modlin IM, Pfragner R, Eick GN, Champaneria MC, Chan AK, et al. Small bowel carcinoid (enterochromaffin cell) neoplasia exhibits transforming growth factor-beta1-mediated regulatory abnormalities including up-regulation of C-Myc and MTA1. Cancer. 2007;109:2420–31. doi: 10.1002/cncr.22725. [DOI] [PubMed] [Google Scholar]
- 37.Kidd M, Modlin I, Shapiro M, Camp R, Mane S, Usinger W, et al. CTGF, intestinal stellate cells and carcinoid fibrogenesis. World J Gastroenterol. 2007;13(39):5208–16. doi: 10.3748/wjg.v13.i39.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Toole D, Saveanu A, Couvelard A, Gunz G, Enjalbert A, Jaquet P, et al. The analysis of quantitative expression of somatostatin and dopamine receptors in gastro-entero-pancreatic tumours opens new therapeutic strategies. Eur J Endocrinol. 2006;155(6):849–57. doi: 10.1530/eje.1.02307. [DOI] [PubMed] [Google Scholar]
- 39.Ferone D, Saveanu A, Culler MD, Arvigo M, Rebora A, Gatto F, et al. Novel chimeric somatostatin analogs: facts and perspectives. Eur J Endocrinol. 2007;156(Suppl 1):S23–8. doi: 10.1530/eje.1.02356. [DOI] [PubMed] [Google Scholar]