Abstract
Chronic N-Methyl-D-aspartate (NMDA) administration, a model of excitotoxicity, and chronic intracerebroventricular lipopolysaccharide infusion, a model of neuroinflammation, are reported to upregulate arachidonic acid incorporation and turnover in rat brain phospholipids as well as enzymes involved in arachidonic acid metabolism. This suggests cross-talk between signaling pathways of excitotoxicity and of neuroinflammation, involving arachidonic acid. To test whether chronic NMDA administrations to rats can upregulate brain markers of neuroinflammation, NMDA (25 mg/kg i.p.) or vehicle (1 ml saline/kg i.p.) was administered daily to adult male rats for 21 days. Protein and mRNA levels of cytokines and other inflammatory markers were measured in the frontal cortex using immunoblot and real-time PCR. Compared with chronic vehicle, chronic NMDA significantly increased protein and mRNA levels of interleukin-1beta, tumor necrosis factor alpha, glial fibrillary acidic protein and inducible nitric oxide synthase. Chronic NMDA receptor overactivation results in increased levels of neuroinflammatory markers in the rat frontal cortex, consistent with cross-talk between excitotoxicity and neuroinflammation. As both processes have been reported in a number of human brain diseases, NMDA receptor inhibitors might be of use in treating neuroinflammation in these diseases.
Keywords: Excitotoxicity, Neuroinflammation, Interleukin-1beta, Tumor necrosis factor alpha, Interleukin-10, Glial fibrillary acidic protein, Inducible nitric oxide synthase, Brain
Introduction
Glutamate is the major natural excitatory neurotransmitter in the mammalian central nervous system. N-Methyl-D-aspartate (NMDA) receptors are glutamate-gated calcium channels that play important roles in physiological and pathological conditions [1]. NMDA receptors are present on neurons, microglia, astrocytes, and oligodendrocytes in brain [2]. Binding to these receptors by glutamate or NMDA causes an influx of extracellular calcium into the cell, leading to activation of many calcium-dependent enzymes, including cytosolic phospholipase A2 (cPLA2) [3, 4], calmodulin kinase [5], protein kinase C [6], phospholipase C [7], and calcineurin [8], and also stimulates multiple secondary pathways.
Overactivity of NMDA receptors can result in excitotoxic neuronal death. Excitotoxicity has been implicated in the pathophysiology of various human brain disorders, including Alzheimer disease [9, 10], Parkinson disease [11], Huntington disease [12], schizophrenia [13], and bipolar disorder [14–16]. Studies have demonstrated increased concentrations of glutamate and reduced levels of NMDA receptor (NR) subunits in the brain of some of these patients. Thus, increased levels of glutamate and decreased levels of the NR-1 and NR-3A subunits have been noted in postmortem brain from bipolar patients [13, 17, 18], while NMDA receptor antagonists or inhibitors of NMDA release have shown some promise in treating Alzheimer disease (e.g. memantine) [19] and bipolar disorder (e.g. lamotrigine). A study also has reported increased NMDA-sensitive glutamate binding in the striatum of Alzheimer and Parkinson patients [20], further indicating a role for glutamate excitotoxicity in neurodegenerative diseases.
Several studies have suggested cross-talk between excess NMDA receptor function (associated with excitotoxicity) and neuroinflammation. Chronic administration of a sub-convulsant dose of NMDA to rats to produce excitotoxicity, as well as chronic intra-cerebroventricular infusion in rats of bacterial lipopolysaccharide (LPS) to produce neuroinflammation, have been reported to decrease brain protein levels of NR-1 or NR-3A, and to increase transcription of cytosolic phospholipase A2 (cPLA2) through increased binding of its transcription factor, activator protein (AP)-2 in the brain [4, 21–23]. Additionally, inflammatory cytokines like IL-1β have been reported to upregulate brain cPLA2 expression by activating AP-2 or NF-κB, transcription factors that are recognized on promoter regions of the cPLA2 gene [24–27]. Furthermore, the NMDA receptor antagonists, memantine and MK-801, were reported to protect against LPS-induced neuroinflammation in rats, and memantine attenuated cytotoxic effects of chronic LPS infusion on cholinergic neurons [28].
In this paper, we wished to see whether cross-talk between experimental NMDA-induced excitoxicity and neuroinflammation could be demonstrated in rat frontal cortex. We chose to measure brain protein and mRNA levels of two inflammatory cytokines, interleukin 1beta (IL-1β) and tumor necrosis factor alpha (TNFα). We also measured brain protein and mRNA levels of glial fibrillary acidic protein (GFAP), a marker of activated astrocytes in neuroinflammation, and of inducible nitric oxide synthase (iNOS), a marker of activated microglia [29]. Based on our prior studies, we administered saline i.p. or 25 mg/kg i.p. NMDA daily for 21 days, and prepared and dissected the rat brain 3 h after the last injection. We analyzed the frontal cortex because we had performed our prior studies with NMDA on the frontal cortex tissue [30, 31].
Materials and Methods
Animals
The study was conducted following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Publication no. 80–23), and was approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development Eunice Kennedy Shriver. Male CDF-344 rats (weighing 200–215 g, Charles River Laboratories; Wilmington, MA, USA) were randomly assigned to a control group (n = 10) that received vehicle (0.9% saline) once daily for 21 days, and to an NMDA group (n = 10) that received 25 mg/kg NMDA (Sigma Chemical Co., St Louis, MO, USA) once daily for 21 days. The NMDA dose was based on our earlier studies with NMDA in rats [4, 14, 32]. Three hours after the last saline or NMDA injection, a rat was anesthetized with CO2 and then decapitated. The brain was rapidly excised and the frontal cortex dissected, cut sagittally, frozen in 2-methylbutane at −50°C, and stored at −80°C until use.
Preparation of Cytosol Fractions
Cytosolic fractions were prepared from frontal cortex as previously described [33]. Tissue from control or chronic NMDA administered rats were homogenized in a homogenizing buffer containing 20 mM Tris–HCl (pH 7.4), 2 mM EGTA, 5 mM EDTA, 1.5 mM pepstatin, 2 mM leupeptin, 0.5 mM phenylmethylsulfonyl fluoride, 0.2 U/ml aprotinin, and 2 mM dithiothreitol, using a polytron homogenizer. The supernatant was centrifuged at 100,000g for 60 min at 4°C. The resulting supernatant was the cytosol fraction. Protein concentration of cytosolic fractions was determined by using Bio-Rad protein reagent (Bio-Rad, Hercules, CA).
Western Blot Analysis
Proteins from cytosolic extracts (65 μg) were separated on 10–20% SDS-polyacrylamide gels (PAGE) (Bio-Rad). Following SDS-PAGE, the proteins were electrophoretically transferred to a nitrocellulose membrane (Bio-Rad). Cytosolic blots were incubated with primary antibodies for IL-1β, TNFα, iNOS and GFAP (1:200) (Santa Cruz Biotech, Santa Cruz, CA) [34]. The blots then were incubated with appropriate HRP-conjugated secondary antibodies (Bio-Rad) and were visualized using a chemiluminescence reaction (Amersham, Piscataway, NJ) on X-ray film (XAR-5, Kodak, Rochester, NY). Optical densities of immunoblot bands were measured using Alpha Innotech Software (Alpha Innotech, San Leandro, CA) and were normalized to β-actin (Sigma) to correct for unequal loading. All experiments were carried out twice with up to 6 independent samples. Values are expressed as percent of control.
Total RNA Isolation and RT-PCR
Total RNA was isolated from frontal cortex of control and chronic NMDA-administered rats using an RNeasy lipid tissue mini kit (Qiagen, Valencia, CA, USA). Expression of IL-1β, TNFα, iNOS and GFAP was determined using specific primers and probes, purchased from TaqManR gene expression assays (Applied Biosystems). Data were expressed as the level of the target gene mRNA in the NMDA-administered animals normalized to the level of the endogenous control mRNA (β-globulin) and relative to the control rats (saline injected) (calibrator), as previously described [4]. All experiments were carried out in duplicate with six independent samples per group.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was calculated using a two-tailed, unpaired t-test with significance set at P < 05.
Results
As illustrated in Fig. 1, chronic NMDA administration for 21 days, compared with chronic saline, significantly increased protein levels of the inflammatory cytokines, IL-1β (142%; P < 0.01), TNFα (49%; P < 0.05), as well of GFAP (67%;P < 0.05) and iNOS (73%; P < 0.01) in the rat frontal cortex. As shown in Fig. 2, chronic NMDA administration for 21 days, compared with chronic saline, also significantly increased mRNA levels of the inflammatory cytokines, IL-1β (5-fold; P < 0.001), TNFα (1.7-fold; P < 0.05) as well of GFAP (1.6-fold; P < 0.05) and iNOS (2.18-fold; P < 0.05) in the rat frontal cortex.
Fig. 1.
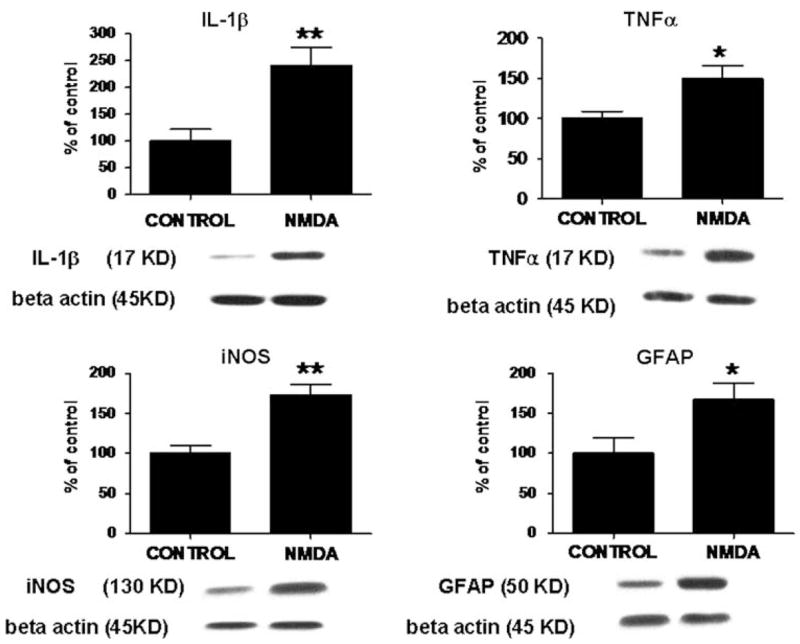
Protein levels of IL-1β, TNFα, iNOS and GFAP in frontal cortex of control rats (n = 6) and of chronic NMDA-treated rats (n = 6), measured using immunoblot. Optical densities of immunoblot bands were normalized to β-actin to correct for unequal loading. Values are expressed as percent of control (means ± SEM, *P < 0.05, **P < 0.01)
Fig. 2.
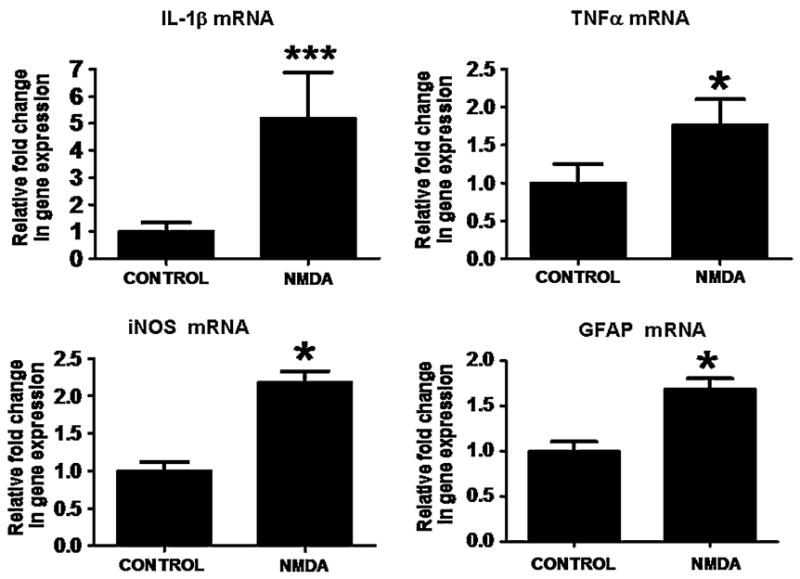
mRNA levels of IL-1β, TNFα, iNOS and GFAP in frontal cortex of control rats (n = 6) and of chronic NMDA-treated rats (n = 6), measured using RT-PCR. Data are expressed as mRNA level in frontal cortex of chronic NMDA administered rats, normalized to the endogenous level of β-globulin mRNA, and relative to the control (calibrator), using the ΔΔCT method (means ± SEM, *P < 0.05, ***P < 0.001)
Discussion
Excitotoxicity and neuroinflammation often have been considered to be two distinct neuropathological processes, although there is considerable evidence for cross-talk between the two (see Introduction). The present results support the existence of cross-talk, since protein and mRNA levels of IL-1β, TNFα, GFAP and iNOS were elevated in the frontal cortex of rats chronically administered a subconvulsant dose of NMDA to produce excitotoxicity. These products are inflammatory (IL-1β and TNFα) cytokines and markers of activated astrocytes (GFAP) and of inflammatory microglia (iNOS) microglia [29, 35].
Earlier studies have reported that single intraperitoneal injection of high doses of NMDA causes convulsions [36], neuronal damage [37], and upregulation of immediate early genes in rat and mouse brain [38]. NMDA administered systemically can act directly on the specific receptors located in the brain after passing through the blood–brain barrier [39]. The mechanisms by which chronic NMDA increased the mRNA levels of IL-1β, TNFα, GFAP and iNOS in the rat frontal cortex remain to be clarified. An earlier study reported that infusion into the rat cortex of the NMDA receptor agonist, cis-2,4-methanoglutamate, increased IL-1β expression [40]. In agreement, we found that chronic i.p. NMDA increased IL-1β protein and mRNA levels in the rat frontal cortex. Studies also have reported that chronic i.p. NMDA as well as chronic intra-cerebroventricular infusion of LPS upregulated cPLA2 protein and the level of one of its transcription factors, AP-2, in the rat brain [4, 22]. AP-2 binding sites also have been identified on the promoter region of genes of many cytokines, including IL-1β, TNFα, as well as of GFAP [41, 42]. Consequently, IL-1β, TNFα, and GFAP protein and mRNA may have been increased in our study because of increased AP-2 DNA binding activity.
The increased protein and mRNA levels of GFAP, a marker of astrocyte activation, are a characteristic brain response to diverse insults. Our results are consistent with an earlier report that chronic NMDA administration to rats increased GFAP protein and mRNA in the cortex [43].
Excitotoxicity in chronic NMDA administered rats was evidenced by increased protein and mRNA levels of iNOS since iNOS is considered a marker of excitotoxicity [44]. A previous study showed that LPS infusion also increased cPLA2 and iNOS expression, and that iNOS expression was attenuated by cPLA2 inhibition in glial cells [45]. Because iNOS can be upregulated by PGE2, a downstream product of the arachidonic acid that is released from phospholipid by cPLA2, increased cPLA2 activity caused by chronic NMDA may have contributed to the elevated iNOS mRNA in the rat frontal cortex [4].
We previously reported that this chronic NMDA regimen decreased rat frontal cortex NR-1 and NR-3A subunit densities, and increased cPLA2 protein, phosphorylation, activity and mRNA levels, without changing expression of secretory sPLA2 or DNA-binding activity of NF-κB [4]. Furthermore, using equations from our in vivo fatty acid model, we also reported that, compared to controls, chronic NMDA increased the rate of incorporation of plasma unesterified arachidonic acid into brain phospholipids as well as the turnover rate of arachidonic acid within brain phospholipids. These effects were absent 3 h after a single NMDA injection [46], thus were due only to the chronic regimen. Chronic ventricular LPS infusion similarly increased cPLA2 protein and mRNA levels in rat brain [16, 23]. Thus, in rat models of excitotoxicity (chronic NMDA administration) and of neuroinflammation (chronic intracerebroventricular LPS infusion), an overlap also exists with regard to an upregulated AA cascade, involving cPLA2 expression and AA recycling within brain phospholipids.
Together, this and prior papers indicate that excitotoxicity and neuroinflammatory pathways have marked interactions [47]. Both have been implicated in the pathophysiology of many human neurodegenerative and psychiatric diseases [18, 48]. Developing drugs that effectively attenuate NMDA receptor function, such as memantine and lamotrigine, thus might prove useful in suppressing excitotoxicity as well as neuroinflammation in those conditions.
In summary, our results showing upregulated protein and mRNA levels of IL-1β, TNFα, iNOS and GFAP in rat frontal cortex following chronic NMDA administration support the hypothesis of cross-talk between excitotoxicity and neuroinflammation. In order to extend our conclusions and elucidate mechanisms of cross-talk, it would be useful to determine the regulation of neuroinflammatory markers whose mRNA levels were elevated by chronic NMDA, and also to determine expression of potentially relevant transcription factors, such as NF-κB p50 and p65 DNA-binding activities.
Acknowledgments
This work was entirely supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Abbreviations
- NMDA
N-Methyl-D-aspartate
- IL-1β
Interleukin-1beta
- TNFα
Tumor necrosis factor alpha
- GFAP
Glial fibrillary acid protein
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- NR
N-Methyl-D-aspartate receptor
- AP-2
Activator protein-2
- NF-κB
Nuclear factor-kappa B
References
- 1.Wenthold RJ, Sans N, Standley S, Prybylowski K, Petralia RS. Early events in the trafficking of N-methyl-D-aspartate (NMDA) receptors. Biochem Soc Trans. 2003;31:885–888. doi: 10.1042/bst0310885. [DOI] [PubMed] [Google Scholar]
- 2.Verkhratsky A, Kirchhoff F. NMDA receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- 3.Weichel O, Hilgert M, Chatterjee SS, Lehr M, Klein J. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A2 activation and phospholipid breakdown in rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:609–615. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- 4.Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A(2) and its transcription factor, activator protein-2. J Neurochem. 2007;102(6):1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- 5.Fukunaga K, Soderling TR, Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem. 1992;267:22527–22533. [PubMed] [Google Scholar]
- 6.Giordano G, Sanchez-Perez AM, Burgal M, Montoliu C, Costa LG, Felipo V. Chronic exposure to ammonia induces isoform-selective alterations in the intracellular distribution and NMDA receptor-mediated translocation of protein kinase C in cerebellar neurons in culture. J Neurochem. 2005;92:143–157. doi: 10.1111/j.1471-4159.2004.02852.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurd JW, Bissoon N. The N-methyl-D-aspartate receptor subunits NR2A and NR2B bind to the SH2 domains of phospholipase C-gamma. J Neurochem. 1997;69:623–630. doi: 10.1046/j.1471-4159.1997.69020623.x. [DOI] [PubMed] [Google Scholar]
- 8.Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang M, Li J, Tiu SC, Zhang L, Wang M, Yew DT. N-methyl-D-aspartate receptor and apoptosis in Alzheimer’s disease and multiinfarct dementia. J Neurosci Res. 2005;81:269–274. doi: 10.1002/jnr.20558. [DOI] [PubMed] [Google Scholar]
- 10.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 11.Hallett PJ, Dunah AW, Ravenscroft P, Zhou S, Bezard E, Crossman AR, et al. Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Neuropharmacology. 2005;48:503–516. doi: 10.1016/j.neuropharm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Young AB, Greenamyre JT, Hollingsworth Z, Albin R, D’Amato C, Shoulson I, et al. NMDA receptor losses in putamen from patients with Huntington’s disease. Science. 1988;241:981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- 13.Mueller HT, Meador-Woodruff JH. NR3A NMDA receptor subunit mRNA expression in schizophrenia, depression and bipolar disorder. Schizophr Res. 2004;71:361–370. doi: 10.1016/j.schres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- 15.Basselin M, Villacreses NE, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration reduces N-methyl-D-aspartate receptor-initiated signaling via arachidonic acid in rat brain. Biol Psychiatry. 2007;62:934–943. doi: 10.1016/j.biopsych.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007;102:761–772. doi: 10.1111/j.1471-4159.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62(11):1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Nudmamud-Thanoi S, Reynolds GP. The NR1 subunit of the glutamate/NMDA receptor in the superior temporal cortex in schizophrenia and affective disorders. Neurosci Lett. 2004;372:173–177. doi: 10.1016/j.neulet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Rands GS. Memantine as a neuroprotective treatment in schizophrenia. Br J Psychiatry. 2005;186:77–78. doi: 10.1192/bjp.186.1.77-a. [DOI] [PubMed] [Google Scholar]
- 20.Ulas J, Weihmuller FB, Brunner LC, Joyce JN, Marshall JF, Cotman CW. Selective increase of NMDA-sensitive glutamate binding in the striatum of Parkinson’s disease, Alzheimer’s disease, and mixed Parkinson’s disease/Alzheimer’s disease patients: an autoradiographic study. J Neurosci. 1994;14:6317–6324. doi: 10.1523/JNEUROSCI.14-11-06317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J Neuroinflammation. 2004;1:12. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HR, Islam Z, Pestka JJ. Kinetics of lipopolysaccharide-induced transcription factor activation/inactivation and relation to proinflammatory gene expression in the murine spleen. Toxicol Appl Pharmacol. 2003;187:147–161. doi: 10.1016/s0041-008x(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, et al. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- 24.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302(Pt 3):723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suyama K, Kabuyama Y, Suzuki S, Kawasaki Y, Suzuki J, Suzuki H, et al. Induction of transcription factor AP-2 by cytokines and prostaglandins in cultured mesangial cells. Am J Nephrol. 2001;21:307–314. doi: 10.1159/000046266. [DOI] [PubMed] [Google Scholar]
- 26.Thommesen L, Sjursen W, Gasvik K, Hanssen W, Brekke OL, Skattebol L, et al. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- 27.Morri H, Ozaki M, Watanabe Y. 5′-flanking region surrounding a human cytosolic phospholipase A2 gene. Biochem Biophys Res Commun. 1994;205:6–11. doi: 10.1006/bbrc.1994.2621. [DOI] [PubMed] [Google Scholar]
- 28.Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84:1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 30.Rao JS, Bazinet RP, Rapoport SI, Lee HJ. Chronic administration of carbamazepine down-regulates AP-2 DNA-binding activity and AP-2alpha protein expression in rat frontal cortex. Biol Psychiatry. 2007;61:154–161. doi: 10.1016/j.biopsych.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Rao JS, Rapoport SI, Bosetti F. Decrease in the AP-2 DNA-binding activity and in the protein expression of AP-2 alpha and AP-2 beta in frontal cortex of rats treated with lithium for 6 weeks. Neuropsychopharmacology. 2005;30:2006–2013. doi: 10.1038/sj.npp.1300740. [DOI] [PubMed] [Google Scholar]
- 32.Ormandy GC, Song L, Jope RS. Analysis of the convulsant-potentiating effects of lithium in rats. Exp Neurol. 1991;111:356–361. doi: 10.1016/0014-4886(91)90103-j. [DOI] [PubMed] [Google Scholar]
- 33.Dwivedi Y, Rizavi HS, Rao JS, Pandey GN. Modifications in the phosphoinositide signaling pathway by adrenal glucocorticoids in rat brain: focus on phosphoinositide-specific phospholipase C and inositol 1, 4, 5-trisphosphate. J Pharmacol Exp Ther. 2000;295:244–254. [PubMed] [Google Scholar]
- 34.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 35.Sheng WS, Hu S, Kravitz FH, Peterson PK, Chao CC. Tumor necrosis factor alpha upregulates human microglial cell production of interleukin-10 in vitro. Clin Diagn Lab Immunol. 1995;2:604–608. doi: 10.1128/cdli.2.5.604-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoepp DD, Gamble AY, Salhoff CR, Johnson BG, Ornstein PL. Excitatory amino acid-induced convulsions in neonatal rats mediated by distinct receptor subtypes. Eur J Pharmacol. 1990;182:421–427. doi: 10.1016/0014-2999(90)90039-9. [DOI] [PubMed] [Google Scholar]
- 37.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 38.Chung KC, Shin SW, Yoo M, Lee MY, Lee HW, Choe BK, et al. A systemic administration of NMDA induces immediate early gene pip92 in the hippocampus. J Neurochem. 2000;75:9–17. doi: 10.1046/j.1471-4159.2000.0750009.x. [DOI] [PubMed] [Google Scholar]
- 39.Brace H, Latimer M, Winn P. Neurotoxicity, blood-brain barrier breakdown, demyelination and remyelination associated with NMDA-induced lesions of the rat lateral hypothalamus. Brain Res Bull. 1997;43:447–455. doi: 10.1016/s0361-9230(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 40.Pearson VL, Rothwell NJ, Toulmond S. Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- 41.Miura M, Tamura T, Mikoshiba K. Cell-specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis- and trans-acting promoter elements for astrocyte-specific expression. J Neurochem. 1990;55:1180–1188. doi: 10.1111/j.1471-4159.1990.tb03123.x. [DOI] [PubMed] [Google Scholar]
- 42.Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- 43.Burtrum D, Silverstein FS. Excitotoxic injury stimulates glial fibrillary acidic protein mRNA expression in perinatal rat brain. Exp Neurol. 1993;121:127–132. doi: 10.1006/exnr.1993.1078. [DOI] [PubMed] [Google Scholar]
- 44.Acarin L, Peluffo H, Gonzalez B, Castellano B. Expression of inducible nitric oxide synthase and cyclooxygenase-2 after excitotoxic damage to the immature rat brain. J Neurosci Res. 2002;68:745–754. doi: 10.1002/jnr.10261. [DOI] [PubMed] [Google Scholar]
- 45.Won JS, Im YB, Khan M, Singh AK, Singh I. Involvement of phospholipase A2 and lipoxygenase in lipopolysaccharide-induced inducible nitric oxide synthase expression in glial cells. Glia. 2005;51:13–21. doi: 10.1002/glia.20178. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008;49:162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 48.Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]


