Abstract
Rotavirus (RV) infection of the intestine is the major cause of severe dehydrating diarrhea in infants around the world. Although protective immunity against RV, especially acquired B and T cell responses, have been extensively studied, our understanding of RV immunity remains incomplete. In addition, the interaction between various protective immune mechanisms in the gut and specific enteric immune suppressor systems that normally exert a regulatory function on mucosal immunity has not been extensively investigated. Among the candidate suppressor systems, we hypothesized that CD4+ CD25+ Foxp3+ regulatory T (Treg) cells may play a role in modulating RV immunity since such cells are naturally present in large numbers in the intestine and function nonspecifically. Here we demonstrate that neonatal murine RV (EC) infection induces an expansion of the Treg cell population and the magnitude of the T cell mediated immune response is modulated by Treg cells. Accordingly, when natural Treg cells in neonatal mice were depleted before virus infection, both CD4+ and CD8+ T cell responses to RV, such as proliferation and IFN-γ secretion, were enhanced in mesenteric lymph nodes (MLNs) and the spleen. Interestingly, increased proliferation of CD19+ B cells from Treg cell depleted animals was also observed. Finally, we analyzed the in vivo effect of the Treg cell depletion on diarrheal disease, virus shedding and IgA RV-specific response. Treg cell depletion did not affect these functions. Our studies of immune modulatory Treg cells in the RV infection model may promote a better understanding of the basis for RV immunity as well as providing valuable clues for the development of more immunogenic RV vaccines.
Keywords: Rotavirus, Regulatory T cell, vaccines
Introduction
Rotavirus (RV) infection is the major cause of severe dehydrating diarrhea in infants around the world [1]. Rotaviruses are the cause of an estimated 600,000 annual deaths worldwide in young children [1, 2]. Previously, an effective first generation RV vaccine was withdrawn from the market due to its association with intussusception [3]. Recently, however, two safe and effective second generation vaccines were introduced into the US, Europe and Latin America [4, 5]. The affordability and efficacy of these vaccines in the poorest counties of the world is currently unknown and an in depth understanding of the safety of these vaccines for very rare side effects will require extensive post marketing studies. Previous studies in the murine model of RV infection suggested that mucosal immunoglobulin A (IgA) produced by local antibody secreting cells plays a primary role in protective immunity against RV following infection [6]. In addition, T cells also play an important role in antiviral immunity since CD4+ T cells contribute to the development of RV specific IgA by memory B cells [7] and RV specific CD8+ T cells accelerate the resolution of primary virus infection [8]. Although it seems that these various components of the acquired immune system eventually control primary RV infection and provide for protective immunity against RV re-infection, it is not yet clear whether the acquired responses elicited are maximal or whether they are being modulated by suppressor functions which are generally operative in the enteric immune system.
Viruses employ various strategies to escape from the host’s immune response [9]. One of these methods is the suppression of the host immune system [10]. Recently, the existence of immune cells capable of regulating the immune response has been demonstrated phenotypically and functionally and numerous studies have described the role of such cells [11–13]. Among those candidates, a subset of CD4+ T cells coexpressing CD25, the IL-2α chain receptor, was identified as natural regulatory T (Treg) cells [14] and the activity of the unique forkhead transcription factor, Foxp3, in Treg cells has been revealed to closely associate with prevention of gene activation of activated effector T cells [15]. Treg cells generally respond to various self antigens and regulate self-reactive autoimmune T cells [16, 17], but they can also respond to various infectious pathogens [18–24]. In the latter case, Treg cells suppress the activity of antigen specific effector cells having protective and beneficial effects to host [25], suggesting that such infectious pathogens may subvert or stimulate Treg cells to escape from the host antimicrobial immune response. For example, in several bacterial [21, 22], parasitic [18, 19] and viral [20, 23, 24] infections a regulatory function for natural Treg cells has been demonstrated. Additionally, these studies proposed that down regulation of natural Treg cell suppressor functions might result in more efficient control of infection and enhancement of vaccine effectiveness.
The intestinal immune system is normally activated since the intestine is constantly exposed to a wide array of foreign antigens including ingested food antigens and the endogenous microbial flora. However, immune responses in the gut are generally modulated as exemplified by the lack of response to most food and commensal bacterial antigens. It is now thought that Treg cells are one of the major immune suppressors acting in the intestine [26]. In addition, since peripheral conversion of non-Treg to Treg cells occurs in the lamina propria and gut-associated lymphoid tissue after antigen exposure [27], enteric microbial pathogens may be first encountered by natural as well as inducible Treg cells present in the intestine. In the current study, we hypothesize that RV replication could be enhanced by this novel cell type in the favor of its survival and we have carried out a series of studies to examine the role of Treg cells on RV replication and the host immune response to infection.
Materials and methods
Viruses
A stock of virulent wild-type murine RV (EC strain) was prepared as previously described [6]. Briefly, 5 to 6 days old suckling BALB/c mice were infected with RV (EC) and intestines were collected and homogenized. Centrifugation clarified intestinal homogenate was used as virus stock in this study. The infectivity of the murine strains was determined in vivo in suckling mice and expressed as a diarrhea dose 50 (DD50). Tissue culture adapted heterologous rhesus RV (RRV) was prepared as previous described [28] and stock virus titers were determined by plaque forming assay (PFU per milliliter) using the green monkey kidney MA104 cell line.
Mice and viral infection
BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and bred in the laboratory animal facility of Palo Alto Veterans Administration Health Care System. Suckling or adult mice were orally inoculated by gastric gavage with 104 DD50 of the murine RV strain EC. All animal studies were approved by the Stanford Institutional Animal Care Committee.
Antibodies and reagents
PC61 (anti-CD25) hybridoma was kindly provided by Dr. B.T. Rouse (University of Tennessee, Knoxville, TN) and antibody (Ab) was grown in 300 cm2 tissue culture flasks (BD Falcon™, Franklin Lakes, NJ). For all injections, an ammonium sulfate cut of PC61 monoclonal Ab was used. Antibodies purchased from eBioscience or BD PharMingen (San Diego, CA) were FITC, PE anti-CD4, APC anti-CD25 (PC61), PE anti-Foxp3, FITC, PE anti-CD8α, APC anti-CD19, PE anti-IFN-γ, purified Rat IgG1, FITC anti-Bromodeoxyuridine (BrdU), and ELISA Abs including anti-IFN-γ, anti-IL-10, anti-TGF-β, and their biotinylated counterpart Abs.
In vivo depletion of CD25 expressing cells
Four days old suckling or 6 weeks old adult BALB/c mice were intra-peritoneally (IP) given PC61 monoclonal Ab (50 µg/ neonate mouse, 200 µg/ adult mouse) or purified rat IgG1 Ab (for the control group) three doses over three days. At different time points (from day 3 to 35 post injection) mice were sacrificed and spleens and mesenteric lymph nodes (MLNs) were collected for determining the kinetics of depletion of CD25 expressing cells by FACS.
Confocal microscopy
For immunofluorescence staining, small intestines were frozen in optimum cutting temperature (OCT) compound (Sakura Finetechnical Co., Tokyo, Japan). Six-micrometer-thick sections were cut, air-dried, and fixed in cold solution (1:1 mixture of acetone and methanol) for 10 min at −20°C. Nonspecific binding was blocked with 3% BSA-PBS-0.05% Tween 20 for 2 h at 37°C or room temperature. For detection of Treg cells, the sections were incubated with a mixture of FITC anti-mouse CD4 (0.5 µg/ml) and PE anti-mouse Foxp3 (0.5 µg/ml) or isotype control PE Rat IgG2a (0.5 µg/ml) Ab in 1% BSA-PBS-0.05% Tween 20 for 2 h at room temperature. The Ab mixture also included Rnase A (1:500) and TOTO-3 iodide (Molecular Probe, Eugene, OR) for nucleus staining. Slides were mounted with Aqua Poly/Mount (Polysciences, Warrington, PA). The images were acquired with a Zeiss LSM 510 confocal microscope system (Carl Zeiss MicroImaging, Inc., Thornwood, NY) and images were analyzed using a LSM510 computer program.
Flow cytometric analysis
Cell preparation
Single cell suspensions were prepared from the spleen, MLNs, and small intestines. At the indicated time points after RV infection, mice were sacrificed by CO2 inhalation. Single cells from spleen and MLNs were isolated by mechanical disruption through a cell strainer with 40 µm nylon mesh. Red blood cells in the spleen suspension were lysed with a commercial lysing buffer (Sigma-Aldrich, St. Louis, MO). A single cell suspension was made in complete RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) supplemented with 100 U/ml Penicillin and 100 µg/ml of Streptomycin with 10% FCS. For isolation of lamina propria lymphocytes (LPLs), small intestines were pooled, longitudinally opened, and cut into 1-cm segments followed by incubation in RPMI 1640 containing 2% FCS, 100 U of penicillin/ml, 100 µg of streptomycin/ml, and 1mM of EDTA for 15 min at 37°C. The supernatant containing intraepithelial lymphocytes and epithelial cell debris were discarded and intestinal tissues were incubated with RPMI 1640 containing 2% collagenase type VIII (Sigma-Aldrich) for 30 min at 37°C. The cell supernatant containing LPLs and cell debris was centrifuged at 400 × g for 10 min at 4°C, and the precipitates were resuspended in RPMI 1640-2% FCS and passed through a cell strainer with 40 µm nylon mesh. The cell suspension was centrifuged and resuspended in 40% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ). A discontinuous Percoll gradient made by layering 5 ml of the cell suspension in 40% Percoll above 2 ml of 75% Percoll in a 15-ml conical tube was centrifuged at 600 × g for 30 min at room temperature. Lymphocytes were collected from the 40 to 75% interface and washed with complete RPMI medium.
Staining for flow cytometry
The single cell suspensions obtained from spleen, MLNs, and small intestines were stained for different cell surface molecules or intracellular molecules such as cytokines, Foxp3, and BrdU for FACS. In brief, single cells were first blocked with an unconjugated anti-CD32/CD16 mAb (BD Biosciences, San Jose, CA) for 15 min at 4°C in FACS buffer and stained by indicated fluorochrome-labeled Abs for an additional 30 min at 4°C. For intracellular staining, surface labeled cells were permeabilized with the Cytofix/Cytoperm buffer (BD Biosciences) and fluorochrome-labeled indicated antibodies were added for 30 min at 4°C. Finally, the cells were washed three times, and samples were acquired on a FACSCalibur. The data were analyzed using the CellQuest software, version 3.1 (BD Biosciences).
Cytokine quantification by ELISA
The supernatants from RV stimulated splenocytes and MLN cells or the lysates from spleens and MLNs were used for the measurement of IFN-γ or IL-10 and TGF-β, respectively by a standard sandwich ELISA protocol. For preparation of tissue lysates, tissue samples were collected, weighed, mixed with T-PER tissue protein extraction reagent (PIERCE, Rockford, IL), and homogenized. The lysates were then clarified by centrifugation at 12,000 rpm for 5 min at 4°C. The supernatant was collected and stored at −80°C until further use. The ELISA plates were coated with the indicated anti-mouse cytokine capture Ab (100 µl/well of the capture Ab at predetermined concentrations based on preliminary assays using standardized positive controls or as indicated by manufacturer, Purified anti-mouse IFN-γ, IL-10, TGF-β Ab, eBioscience) and incubated at 4°C overnight. The plates were washed with 0.05% Tween 20/PBS and blocked with 3% BSA/PBS for 2 h at 37°C. After washing, serially diluted samples were added to the plates and incubated at 4°C overnight. The plates were washed and then incubated with the appropriate biotinylated detection Abs (biotin-labeled anti-mouse IFN-γ, IL-10, TGF-β Ab, eBioscience) for 2 h at 37°C. Finally peroxidase conjugated streptavidin (Jackson ImmunoResearch Laboratory, West Grove, PA) was added. The color reaction was developed using TMB substrate reagent (Kirkegaard & Perry Laboratories, Gaithersburg, ML) and measured with an ELISA reader (Bio-Tek, Burlington, VT) at 450 nm.
Lymphoproliferation assay
For lymphoproliferation assay, splenocytes and MLN cells from mice were suspended in 10% RPMI-1640, and 106 cells in 1 ml were stimulated in vitro with mitomycin C treated syngeneic-enriched splenocytes pulsed with UV-inactivated RRV (MOI, 2 before UV inactivation). Alternatively, the same cells were stimulated with a mixture of RV specific H-2d restricted CD4+ and CD8+ T cell epitope peptides (VP6245–259 and VP6157–171, respectively), as described previously [29]. The cells were cultured in the presence of IL-2 (20 U/ml) at 37°C for 4 d in 96-well round-bottom plates. Finally, for the last 6 h, 10 µl of the 1 mM BrdU solution (BD Biosciences) was added to each ml of culture medium (final concentration of 10 µM). The cells were harvested and cells were processed for extra- and intracellular staining for FACS.
Polyclonal stimulation
Isolated splenocytes and MLN cells were cultured in anti-CD3 mAb (5.0 µg/ml) precoated 96-well culture plates in the presence or absence of IL-2 (20 U/ml) at 37°C for 4 d or 16 h. GolgiPlug containing brefeldin A (BD Biosciences) or BrdU solution was added for the last 6 h. Finally, the cells were processed for FACS staining or for BrdU incorporation assay as described earlier.
Mouse stool and serum collection
After RV infection, stool samples were collected from individual mice from day 1 to 10, measured and suspended as a 10% suspension (weight/volume, Tris, 10mM Nacl, 0.5 mM CaCl2 containing 5% FCS, 0.05% Tween 20, 0.02% sodium azide, 1% protease inhibitors). The stool samples were then clarified by centrifugation at 12,000 rpm for 5 min at 4°C. The supernatant was collected and stored at −80°C until further use. Blood samples were collected from each mouse on day 5, 12, 20 after RV infection, kept at 4°C overnight, then centrifuged at 3,000 rpm for 10 min at room temperature. The serum was collected and stored at −80°C until further use.
Detection of RV antigen
For detection of RV antigen in stool samples, a sandwich ELISA was conducted as described previously [6]. In brief, ELISA plates were coated with guinea pig anti-rhesus RV serum (1:5000 dilution in PBS) and incubated at 4°C overnight. The plates were washed with 0.05% Tween 20/PBS and blocked with 5% nonfat dry milk for 2 h at 37°C. Stool samples were serially diluted with 1% nonfat dry milk, added to the plate, and incubated at 4°C overnight. RV was detected with rabbit anti-RRV serum (1:5000 dilution in PBS), followed by HRP-conjugated goat anti-rabbit IgG (1:5000 dilution in PBS, Kirkegaard & Perry Laboratories). TMB substrate reagent (Kirkegaard & Perry Laboratories) was used for color development, and the reaction was stopped with 1N HCL. Plate was read at 450 nm using ELISA reader.
Detection of anti-RV IgA
RV specific IgA was detected by a standard ELISA. Briefly, plates were first coated with rabbit anti RV serum (1:7500 dilution in PBS) and incubated at 4°C overnight. The plates were washed with 0.05% Tween 20/PBS and blocked with 5% nonfat dry milk for 2 h at 37°C. After washing, plates were incubated with a 1:10 dilution of rhesus-RV stock at 4°C overnight. After washing, serially diluted 10% stool suspension was added to the plate and incubated at 37°C for 4h. Anti-RV IgA was detected with HRP-conjugated goat anti-mouse IgA (1:3000 dilution in 1% nonfat dry milk). Color reaction and plate reading were conducted as described for RV antigen detection method.
Statistical analysis
Results are expressed as mean ± standard deviation. Significance of differences between groups was evaluated with Student’s t test. Some data were generated from pooled samples of multiple animals due to insufficient cell numbers from individual animals. In this case the experiments were repeated and the data were analyzed using nonparametric Mann-Whitney test. P < 0.05 was considered significant.
Results
RV infection induces an expansion of the Treg cell population
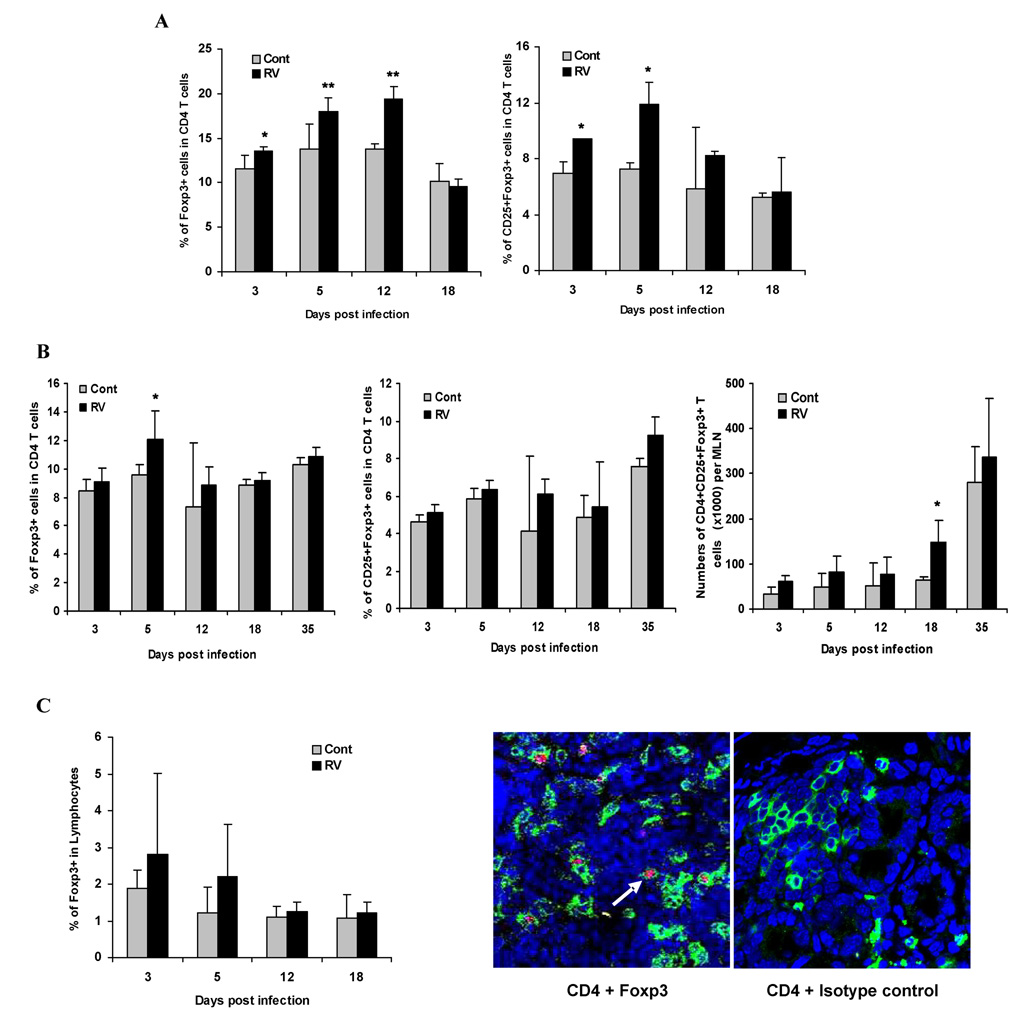
It has been previously shown that RV induced diarrhea occurs in neonatal mice before day 15 of life [7]. Since it was reported that thymic production of Treg cells begins on day 3 after birth [30], we determined whether neonatal RV infection influenced the ontogeny of Treg cell development. Five or six days old suckling BALB/c mice (n=4/time point) were orally inoculated with 104 DD50 of the murine RV strain EC and at selected time point after infection the number of Treg cells was measured in the spleen and MLNs. The percentage of Foxp3+ or CD25+Foxp3+ cells among CD4+ T cells in the spleen was significantly increased at early time points after virus infection (Fig. 1A, p<0.05). In addition, the absolute numbers of Treg cells in MLNs were also significantly increased compared to non-infected animals at 18 days post infection (Fig. 1B). To determine whether the population of Treg cells in the small intestine, the primary target organ of RV, was affected by RV infection, lymphocytes were harvested at regular intervals to measure Treg cell populations. The numbers of Treg cells among total LPLs in the small intestine of virus infected mice were slightly higher than in the noninfected group although there was wide variability between animals in the same group rendering these differences not significant (Fig. 1C). Confocal microscopic observation of the small bowel during infection revealed that most of the Treg cells were located in the lamina propria compartment of small intestine (1C) which was also the case in non-infected mice (data not shown).
Figure 1. In vivo expansion of Treg cells in lymphoid organs and the small intestine of mice after RV infection.
Five or six days old suckling BALB/c mice (n=4/time point) were orally inoculated with 104 DD50 of the murine RV strain EC and at selected time points after infection the number of Treg cells was measured in lymphoid organs and the small intestine. Lymphocytes from spleen (A), MLNs (B), and collagenase-digested intestinal samples (C) obtained from RV-infected and control mice of the same age were stained for CD4+CD25+Foxp3+ cells. The population of Foxp3+ Treg cells in each organ was increased after RV infection. Total cells (100,000) were gated on CD3+ and CD4+. The figure summarizes results of two independent experiments. *, p < 0.05, **, p < 0.01. For detection of Treg cell expressing Foxp3 in tissue (C), small intestinal sections were 5 incubated with a mixture of FITC-coupled monoclonal anti-mouse CD4 (0.5 µg/ml) and 6 PE anti-mouse Foxp3 (0.5 µg/ml) Ab or PE Rat IgG2a (0.5 µg/ml) Ab for isotype control. The white arrow indicates the cross-section of Treg cells expressing both CD4 (green) and intranuclear Foxp3 (red), located in the lamina propria of small intestine (original magnification, ×600).
In vivo depletion of Treg cells before RV infection enhances the RV specific immune response
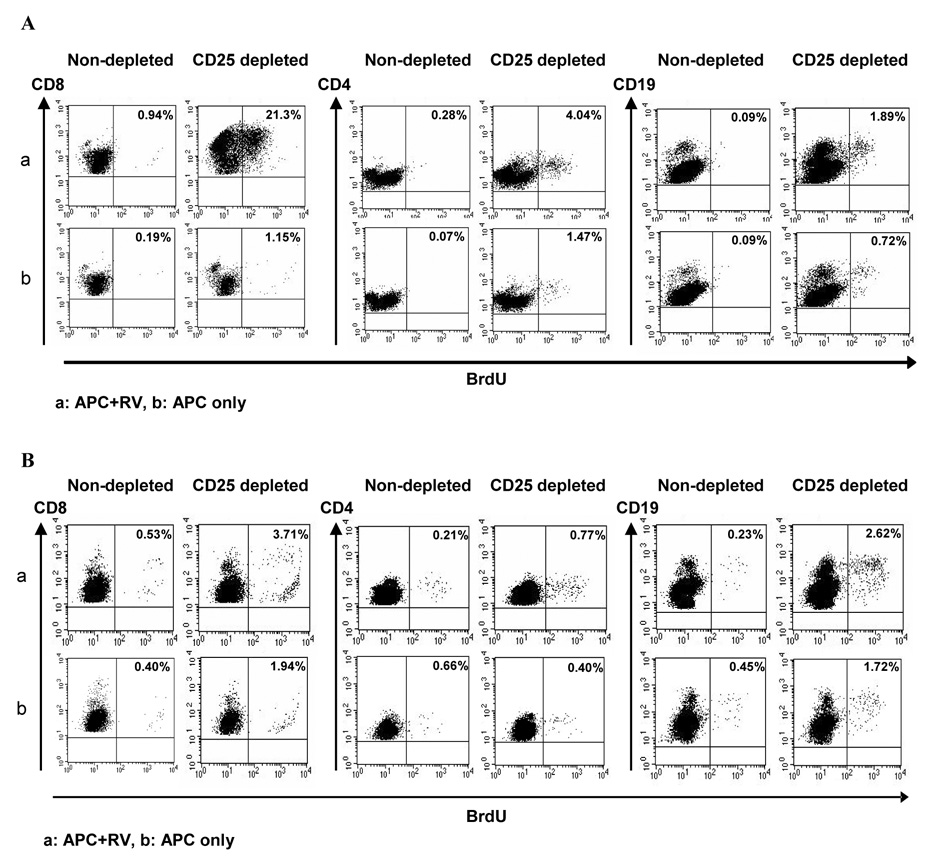
To study in vivo the role of CD4+CD25+ Treg cells in regulating the RV-specific immune response, four or five days old suckling BALB/c mice were treated daily for three days with 50 µg of anti-CD25 mAb PC61 IP. The kinetics of depletion in neonatal mice was studied from day 3 to 35 post treatment in the spleen and MLNs of anti-CD25 mAb treated mice. Total depletion of CD4+CD25+ Treg cells was observed by day 3 and this depletion was maintained through day 26 post injection, but CD4+CD25+ Treg cell levels had returned to approximately 40 ~ 60% of normal by day 35 post injection (Fig. 2). Treg cell depletion by anti-CD25 mAb was confirmed using immunostaining for Foxp3+ cells where a significant, but not complete, decrease in the number of Foxp3+ cells was noted (Fig. 2). At two days post final injection of anti-CD25 mAb, suckling mice (8 or 9 days old) were orally inoculated with 104 DD50 of the murine RV strain EC. The mice (n=4/group) were killed on day 7 and 21 post infection to examine in vivo the influence of the absence of Treg cells on antigen-specific immune responses to RV infection in MLNs and the spleen. At 21 days post infection, CD25-depletion resulted in greater proliferation of RV specific CD8+ and CD4+ T cells in both the spleens (Fig. 3A) and MLNs (Fig. 3B). In 3 independent experiments, the percentage of proliferated cells was 10 – 23 fold (CD8) or 1.7 – 14 fold (CD4) higher in the spleens, and 1.5 – 3.3 fold (CD8) or 3.7 – 11 fold (CD4) higher in the MLNs of CD25-depleted mice compared to the control mice (P<0.05, Mann-Whitney test based on 3 independent experiments). Interestingly, increased proliferation of CD19+ B cells from both spleen (1.4 – 6.7 fold) and MLNs (1.7 – 11 fold) of CD25-depleted animals was also observed (Fig. 3), indicating that CD25 depletion influences the proliferation of B cells as well as T cells. Similar results were obtained with the RV specific (VP6 derived) peptides stimulation although the proliferative responses were generally less than those of RV stimulation (data not shown). However, at 7 days post infection, proliferation of lymphocytes from both CD25-depleted and non-depleted mice were negligible and no difference was observed between the groups (data not shown).
Figure 2. Depletion of Treg cells in neonatal mice.
CD4+ splenocytes (A) or MLN cells (B) from BALB/c mice (one mouse/time point) that were treated daily for three days starting at 4 days of age with 50 µg of anti-CD25 mAb PC61 administered IP were stained and analyzed for CD25 or Foxp3 expression by FACS from day 3 to 35 post injection. Total cells (100,000) were gated on lymphocyte gate (forward scatter [FSC] versus side scatter [SSC]). The figure represents two independent experiments with same results. Treg cell depletion was also confirmed in the experiments in which cytokine responses were determined.
Figure 3. Enhancement of RV-specific immune response in Treg cells depleted mice.
In vitro proliferation of CD4, CD8, and CD19 cells from spleen and MLNs was measured by BrdU incorporation assay. 8 or 9 day old suckling mice (n=4/group) were infected with RV 2 days after final injection of anti-CD25 mAb. Mice were terminated 21 dpi to examine the in vitro proliferation capacity of virus specific immune cells. Isolated splenocytes (A) and MLN cells (B) from CD25-depleted or control non-depleted RV infected mice were pooled and stimulated with mitomycin treated naïve splenocytes (APC) pulsed with UV-inactivated RV (RRV) (a) or no antigen (b) for 4 days and BrdU solution was added for the last 6 h. Total cells (50,000) were gated on lymphocytes and the percentage of BrdU+ cells among the indicated lymphocyte subsets (CD8, CD4, CD19) is represented in the upper right quadrant. This figure represents one of three independent experiments.
To measure IFN-γ response in splenic or MLN cells, we infected CD25-depleted and non-depleted groups of mice (n=4/group) with the murine RV and sampled selected lymphoid tissue on day 12 and 20 post infection (Fig. 4). Splenocytes and MLN cells from CD25-depleted mice on day 20 post infection produced higher levels of IFN-γ compared to non-depleted mice when specifically stimulated with RRV (p<0.05, p<0.01). However, even without stimulation with RV antigen, IFN-γ secretion in both splenocytes and MLN cells was significantly elevated in CD25-depleted animals compared with non-depleted control mice on day 20 post infection. Taken together, these results demonstrate that both RV specific and non-specific immune responses are significantly enhanced in RV infected mice when CD25+ cells are depleted before RV infection.
Figure 4. Increased levels of IFN-γ from the lymphoid organs of CD25-depleted mice.
On indicated days post RV infection, splenocytes and MLN cells of mice (n=4/time point) were restimulated with naïve splenocytes pulsed with UV-inactivated RRV plus IL-2 or IL-2 alone for 5 d. Levels of IFN-γ were determined in the supernatants of cell cultures of CD25-depleted or control mice by an Ab capture ELISA, as outlined in Materials and Methods. The data are representative of three separate experiments with similar results. *, p < 0.05; **, p < 0.01.
Depletion of CD25+ cells enhances immune response following polyclonal stimulation
To determine whether depletion of CD25+ cells influences the immune response following polyclonal, as well as antigen specific stimulation, isolated splenocytes and MLN cells from CD25-depleted and non-depleted RV infected mice (n=3, 21 days post infection) were pooled and stimulated with anti-CD3 Ab followed by measurement of immune cell proliferation (Fig. 5A) and cytokine production (Fig. 5B). The percentage of proliferated CD8+ and CD4+ T cells from spleens of CD25-depleted mice was significantly higher in the CD25-depleted mice compared to that of control mice (P<0.05, Mann-Whitney test based on 2 independent experiments), although a similar difference was not seen in MLNs (data not shown). In both experiments, the percentage of proliferated CD8+ and CD4+ T cells from spleens was 1.3 – 1.5 fold (CD8) or 1.3 – 1 3.1 fold (CD4) higher in CD25-depleted mice compared to control mice. In addition, CD25 depletion resulted in a significant increase of IFN-γ producing CD8+ and CD4+ T cells in response to anti-CD3 stimulation (P<0.05, Mann-Whitney test based on 3 independent experiments). In all 3 experiments, the percentage of IFN-γ producing CD8+ or CD4+ T cells was 1.4 – 2.2 fold (CD8) or 1.7 – 2.8 fold (CD4) higher in the spleens of CD25-depleted mice, and 1.4 – 1.6 fold (CD8) or 2.0 – 3.3 fold (CD4) higher in MLN of CD25-depleted mice, compared to control mice. Thus RV infected, CD25- depleted mice generated a greater proliferation and INF-γ response than non-depleted control mice following polyclonal stimulation.
Figure 5. Enhanced lymphocyte proliferation and IFN-γ production following polyclonal stimulation of lymphocytes from RV infected mice after CD25 depletion.
Isolated splenocytes and MLN cells from CD25-depleted or control non-depleted RV infected mice (n=3, 21 dpi) were pooled and cultured in anti-CD3 mAb (5.0 µg/ml) precoated culture plates to analyze cell proliferation (4 day assay) and IFN-γ production (16 h assay). For the splenocyte proliferation assay (A), BrdU solution was added to the culture for the last 6 h and single cells were extracellularly stained with anti-mouse CD4 or CD8 PE Ab and then intracellularly stained with anti-mouse BrdU FITC Ab. Total cells (100,000) were gated on lymphocytes and the percentage of BrdU+ cells among the indicated lymphocyte subsets (CD8, CD4) is represented in the upper right quadrant. The dot plots represent one of two independent experiments. For the intracellular IFN-γ staining assay (B), single cells were extracellularly stained with anti-mouse CD4 or CD8 Ab and then intracellularly stained with anti-mouse IFN-γ PE Ab. For analysis, cells (100,000) were gated on CD4+ or CD8+ lymphocytes. Values shown in each plot reflect the percentage of splenocytes or MLNs cells expressing IFN-γ. The dot plots represent one of three independent experiments.
Decreased production of IL-10 after Treg cell depletion
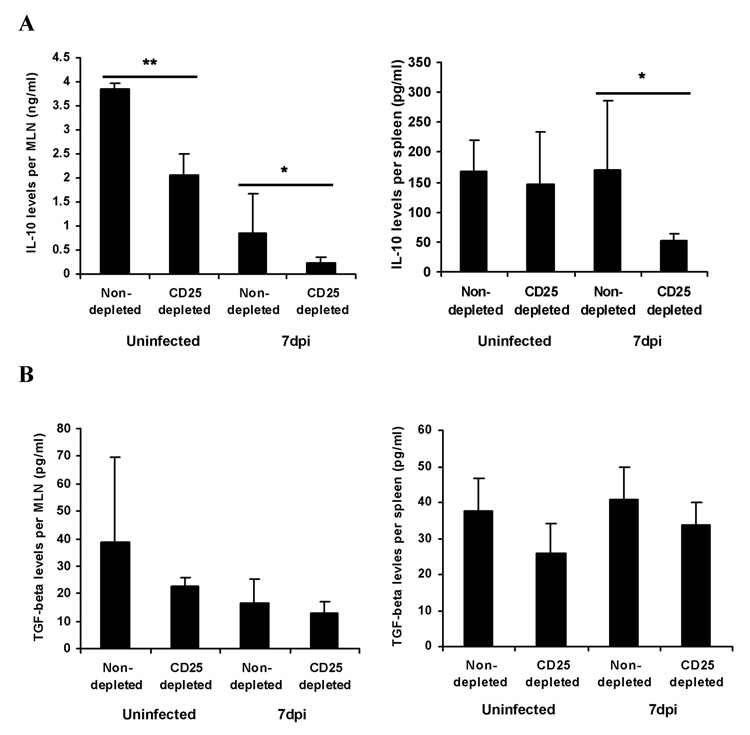
Since Treg cells produce IL-10 and TGF-β [31–34], major immunosuppressive cytokines, additional experiments were performed to compare IL-10 and TGF-β levels in lymphoid organs after RV infection of CD25-depleted and control mice. To measure the cytokine levels, MLNs and spleens were collected at 7 dpi, homogenized, and cytokine levels were measured in the collected supernatant by ELISA. IL-10 levels in MLNs from CD25-depleted, RV non-infected and infected animals were significantly decreased compared to non-depleted animals (Fig. 6A, p<0.05). IL-10 levels in the spleens of CD25-depleted RV infected animals were also significantly decreased compared to controls indicating that Treg cells significantly contribute to IL-10 production in the MLNs of RV infected mice. A similar pattern was observed when TGF-β levels were compared between the groups, but no significant differences were observed and the levels of TGF-β in MLNs and spleens were quite low (Fig. 6B).
Figure 6. Decreased levels of immunosuppressive cytokines in the lymphoid organs of CD25-depleted and RV infected mice.
At 7 dpi, MLNs and spleen from CD25-depleted or control RV and mock infected mice (n=6) were processed to measure the IL-10 (A) and TGF-β (B) protein levels. Levels of each protein were determined in the supernatant of each organ’s lysates by an Ab capture ELISA, as outlined in Materials and Methods. The figure summarizes results of two independent experiments *, p < 0.05; **, p < 0.01.
Depletion of Treg cells does not affect RV induced diarrhea, viral shedding, or the primary mucosal antibody response
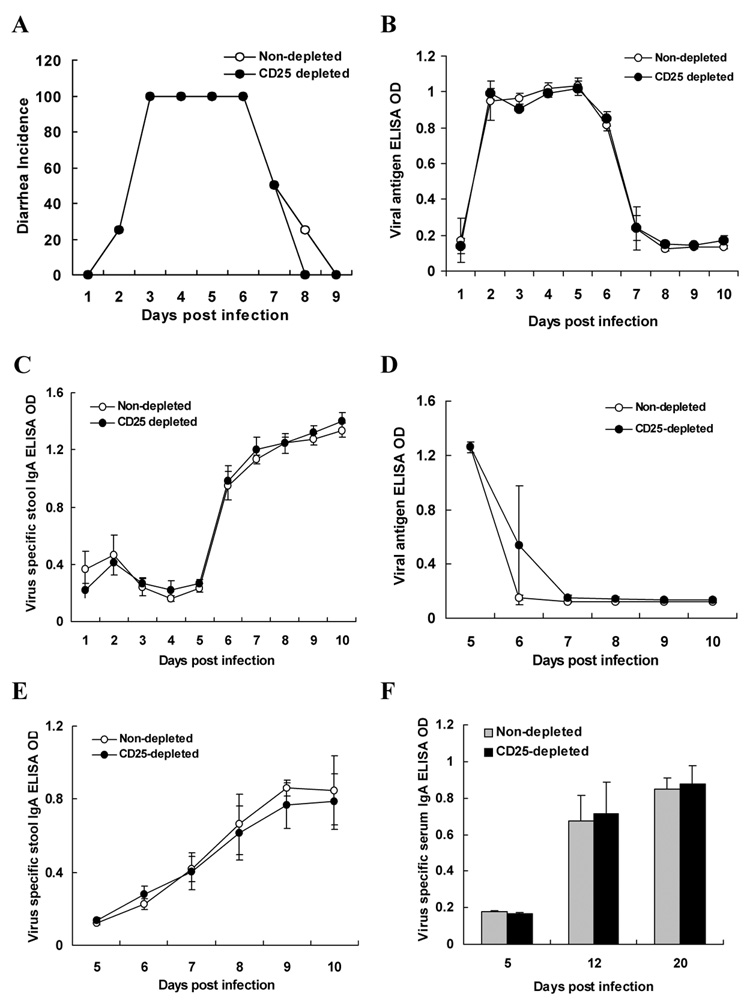
We next examined whether the enhancement of the RV specific immune response observed after Treg cells depletion affected RV induced clinical symptoms or the course of viral infection. Our data indicate that RV induced diarrhea was not affected by CD25-depletion (Fig. 7A). Furthermore, the levels of viral antigen shed in stool (Fig. 7B) and viral RNA copy levels in various organs as measured by real-time PCR (data not shown) were comparable in both groups. Analysis of the RV specific stool (Fig. 7C) and serum IgA (data not shown) at various time points post primary infection revealed no significant difference between CD25-depleted and non-depleted mice, indicating that depletion of Treg cells does not affect the replication of RV or the RV related primary mucosal humoral immune response in neonate mice. To further determine whether Treg cell depletion would have any effect on RV antigen shedding and antibody responses in adult mice, Treg cell depleted adult mice were infected with RV. Analysis of viral shedding (Fig. 7D), and RV specific stool (Fig. 7E) and serum IgA responses (Fig. 7F) revealed no difference between the groups.
Figure 7. Diarrhea incidence, fecal virus shedding, and RV-specific IgA in stool or serum of RV infected CD25-depleted or control mice.
7 days or 6 weeks old CD25-depleted or control mice (n=6) were orally infected with RV (EC strain). Diarrheal disease was observed daily after infection for EC infected mice and presented as the percentage of neonate mice with diarrhea (A). There was no statistical difference between the groups. Levels of RV antigen (B) and RV specific IgA (C) in feces of CD25-depleted or control neonate and RV antigen (D), RV specific IgA in stool (E) and serum (F) of adult mice were measured by ELISA, as described in Materials and Methods.
Discussion
To the best of our knowledge, the role of Treg cells in RV infection has not been studied previously. In the current study, we observed that neonatal murine RV infection induces an expansion of the Treg cell population and RV specific cellular immune responses are modified when Treg cells are depleted before infection. This modification includes enhanced CD4+ and CD8+ T cell responses to RV antigens and Th1-type responses such as proliferation and IFN-γ secretion. Interestingly, the absence of Treg cells also increased the proliferation of CD19+ B cells indicating that bystander effects of Treg cell depletion may indirectly influence B cell immune responses although this effect did not appear to translate into an increased mucosal antibody response. We hypothesized that the increase in the numbers of Treg cells seen during acute RV infection might be a mechanism for the virus to diminish the host antiviral response and hence increase viral replication. However, our study failed to demonstrate any effect of Treg cell depletion on either the degree of viral replication and shedding or the severity of disease. These findings indicate that the any immuno-suppressive effects that Treg cells normally mediate are not sufficient to modify primary RV infection and that the enhanced RV specific immune response seen following Treg cell depletion does not enhance the resolution of primary infection and viral shedding or shorten the length of disease.
The evidence that the Treg cells carry out immune-regulatory functions is well established [16–25]. Interest in Treg cells has mainly focused on autoimmune disease [16, 17]. In such instances, Treg cells regulate immune reactivity against self antigens and suppress the immunopathogenesis associated with colitis and spontaneous autoimmune diabetes [16, 35]. Thus, the activities of Treg cells are beneficial to the host in several autoimmune disease models. Of note, several recent reports have focused on the role of Treg cells in regulating immunity to tumor antigens [36, 37], alloantigens [38], and various infectious pathogens [18–24]. Furthermore, new evidence indicates that Treg cells may affect the immune response to vaccination against various pathogens [39, 40] and that Treg cells may enhance the virulence of some pathogens by restricting the host immune responses [41].
Our current study shows that the absence of Treg cells leads to an enhanced RV specific immune response during primary infection, but this enhanced response failed to reduce RV diarrhea or viral replication. We speculate that there are several reasons why depletion of Treg cells failed to effect primary RV infection despite its effect on RV specific cellular immunity.
First, as demonstrated previously [6], RV specific IgA secreting B cells are a major antiviral effector mechanism during primary infection. The B cell response consists of both T cell dependent and independent components [42]. Secretion of RV specific IgA in stool can be detected by day 6 post infection and viral antigen shedding in stool disappears around day 7 post infection. It is not clear why the enhanced B cell proliferation associated with Treg depletion did not correspond to an increase in fecal IgA except that this increased proliferation of CD19+ cells took place much later than the resolution of infection and the increase in RV-specific fecal IgA. Similarly, enhancement in T cell proliferation in the Treg cell depleted mice must take place more than 1 week after RV infection is initiated since differences in CD4+ and CD8+ T cell proliferation were not observed in the CD25-depleted group during the first week after RV infection (data not shown). Hence it seems unlikely that the immune potentiating effects of Treg cell depletion occur rapidly enough to play a role in the resolution of primary RV infection. Several published studies support the notion that Treg cells primarily modulate the immune response during chronic rather than acute infections [10, 43, 44].
Second, in vivo depletion of Treg cells with anti CD25 can result in loss of other immune cells, thereby potentially diminishing the magnitude of the depletion effect on the anti-RV immune responses. Several studies have shown that certain subtypes of immune effector cells express CD25 molecule, the alpha subunit of the IL-2 receptor, indicating that CD25 is not a unique phenotypic marker for Treg cells [45–50]. Recently a unique subtype of B cells expressing CD25 [45–47] has also been described. Interestingly, Brisslert et al. reported that CD25+ B cells expressed higher levels of IgA, IgG and IgM on their surface and were better than CD25− B cells as antigen-presenting cells since they triggered a stronger CD4+ T cell proliferation response [47]. In addition, monocyte-derived dendritic cells [48], CD8+ T cells [50], and lamina propria macrophages in the intestinal lesions in ulcerative colitis [49] also express CD25. Therefore, it is possible that our anti-CD25 treatment depleted other immune cell types in addition to Treg cells. It is not known whether such non-specific depletion of various effector cells influenced RV specific immune responses independently of the immune enhancing effects of CD4+ Treg cell depletion.
Third, depletion of CD25 expressing cells by CD25 depleting antibody treatment does not completely deplete all phenotypes of Foxp3 expressing regulatory cells such as CD8+Foxp3+ and CD4+CD25−Foxp3+ T cells. Our data (Fig. 2) supports this idea since the depletion of Foxp3 cells was not complete although depletion of CD25 expressing cells was complete. This observation suggests that CD25−Foxp3+ cells might continue to have immunosuppressive effects against RV related immunity. Further studies will be required to demonstrate the exclusive role of Treg cells in the RV infection based on a more specific way to deplete only Treg cells.
Although Treg cell depletion did not directly influence the extent of RV inducing diarrhea or virus replication, it is appropriate to assess whether this intervention affects the induction of long term immunity following primary infection. Our results show that depletion of Treg cells enhanced RV specific immune cell proliferation as well as IFN-γ production. Such enhanced immune responses were also increased by polyclonal stimulation when Treg cells were depleted. In addition, the levels of immunosuppressive cytokines such as IL-10 in lymphoid organs were significantly decreased when Treg cells were depleted. Interestingly, similar results were observed in MLNs of CD25-depleted and uninfected mice, indicating that the immune suppression mediated by Treg cells is both pathogen specific and non-specific. Hence, we predict that the efficiency of vaccination and the magnitude of immune responses against RV or other pathogens would be significantly enhanced if Treg cells were depleted or diminished during the period of vaccination. Of note, reduced IL-10 levels in MLNs of CD25-depleted as well as non-depleted RV infected mice were observed in our study. In contrast, increased levels of IL-10 in serum of RV infected children have been shown previously [51, 52]. We are currently investigating this interesting apparent contradiction with the hypothesis that the inhibitory effects of RV on IL-10 might be organ site specific.
Our findings are in general agreement with the previous finding that Treg cells negatively regulate effector T cell subsets. However, our data also showed that proliferation of CD19+ B cells was significantly increased following RV stimulation when Treg cells were depleted. Similar effects were observed when immune cells were stimulated with T cell epitope peptides specific for CD4+ (VP6245–259) and CD8+ (VP6157–171) T cells. Presumably, in the absence of Treg cells, more robustly proliferating T helper cells and/or enhanced secretion of cytokines such as IL-2 from these T cells indirectly mediates the enhanced activation of B cells. To our knowledge, there were very few reports regarding the regulatory effects of Treg cells on B cell function. Bystry et al. [53] and Lim et al. [54] observed that Treg cells could suppress LPS-induced B cell proliferation and Treg cells inhibited production of immunoglobulin from B cells, respectively. Recently, however, Zhao et al. demonstrated that Treg cells, in a cell-contact dependent manner, suppress B cell proliferation by directly inducing cell death [55]. In our study, depletion of Treg cells may have extended the life of B cells as well as T cells. A more detailed analysis of the mechanisms by which depletion of Treg cells influences the fate of RV specific B cells will be carried out in future studies.
Acknowledgments
We would like to thank Dr. Barry T. Rouse (University of Tennessee, TN) for kindly providing PC61 (anti-CD25) hybridoma and Emily M. Deal for her helpful comments. We also thank to Thanhhoa Nguyen for her technical assistance. This work was supported by a VA Merit Review Research Award, by grants RO1 AI 021362 and P30 DK 56339 from the NIH and by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-E00531).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerging infectious diseases. 2003 May;9(5):565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerging infectious diseases. 2006 Feb;12(2):304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen L, Viboud C, Elixhauser A, Taylor RJ, Kapikian AZ. More on RotaShield and intussusception: the role of age at the time of vaccination. The Journal of infectious diseases. 2005 Sep 1;192 Suppl 1:S36–S43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. The New England journal of medicine. 2006 Jan 5;354(1):11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. The New England journal of medicine. 2006 Jan 5;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 6.Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, Greenberg HB. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995 Feb 20;207(1):143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 7.Franco MA, Greenberg HB. Immunity to rotavirus infection in mice. The Journal of infectious diseases. 1999 May;179 Suppl 3:S466–S469. doi: 10.1086/314805. [DOI] [PubMed] [Google Scholar]
- 8.Franco MA, Tin C, Rott LS, VanCott JL, McGhee JR, Greenberg HB. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. Journal of virology. 1997 Jan;71(1):479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey S, Jameel S. Putting T cells to sleep: a new paradigm for immune evasion by persistent viruses. Journal of biosciences. 2006 Dec;31(5):497–501. doi: 10.1007/BF02708398. [DOI] [PubMed] [Google Scholar]
- 10.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature immunology. 2005 Apr;6(4):353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 11.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature reviews. 2003 Mar;3(3):253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Seminars in immunology. 2004 Apr;16(2):81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Zimring JC, Kapp JA. Identification and characterization of CD8+ suppressor T cells. Immunologic research. 2004;29(1–3):303–312. doi: 10.1385/IR:29:1-3:303. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug 1;155(3):1151–1164. [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Science. 5609. Vol. 299. New York, NY: 2003. Feb 14, Control of regulatory T cell development by the transcription factor Foxp3; pp. 1057–1061. [DOI] [PubMed] [Google Scholar]
- 16.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally-occurring CD4+ regulatory T cells contributes to the onset of autoimmune diabetes. Diabetes. 2007 Oct 10; doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 17.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nature clinical practice. 2007 Nov;3(11):619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 18.Aseffa A, Gumy A, Launois P, MacDonald HR, Louis JA, Tacchini-Cottier F. The early IL-4 response to Leishmania major and the resulting Th2 cell maturation steering progressive disease in BALB/c mice are subject to the control of regulatory CD4+CD25+ T cells. J Immunol. 2002 Sep 15;169(6):3232–3241. doi: 10.4049/jimmunol.169.6.3232. [DOI] [PubMed] [Google Scholar]
- 19.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002 Dec 5;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 20.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proceedings of the National Academy of Sciences of the United States of America. 2004 May 18;101(20):7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. The Journal of experimental medicine. 2002 Jan 21;195(2):221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavan S, Suri-Payer E, Holmgren J. Antigen-specific in vitro suppression of murine Helicobacter pylori-reactive immunopathological T cells by CD4CD25 regulatory T cells. Scandinavian journal of immunology. 2004 Jul-Aug;60(1–2):82–88. doi: 10.1111/j.0300-9475.2004.01447.x. [DOI] [PubMed] [Google Scholar]
- 23.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004 Apr 1;172(7):4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 24.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. The Journal of experimental medicine. 2003 Sep 15;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson SJ, Hasenkrug KJ. The role of virus-induced regulatory T cells in immunopathology. Springer seminars in immunopathology. 2006 Aug;28(1):51–62. doi: 10.1007/s00281-006-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. International immunology. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 27.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007 Aug 6;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino Y, Wyatt RG, Greenberg HB, Flores J, Kapikian AZ. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. The Journal of infectious diseases. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- 29.Jaimes MC, Feng N, Greenberg HB. Characterization of homologous and heterologous rotavirus-specific T-cell responses in infant and adult mice. Journal of virology. 2005 Apr;79(8):4568–4579. doi: 10.1128/JVI.79.8.4568-4579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. The Journal of experimental medicine. 1996 Aug 1;184(2):387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohyama M, Sugahara D, Sugiyama S, Yagita H, Okumura K, Hozumi N. Inducible costimulator-dependent IL-10 production by regulatory T cells specific for self-antigen. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 23;101(12):4192–4197. doi: 10.1073/pnas.0400214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005 Jan 11;102(2):419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004 Jan 15;172(2):834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. The Journal of experimental medicine. 2001 Sep 3;194(5):629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997 Oct 16;389(6652):737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 36.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer research. 2005 Mar 15;65(6):2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 37.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Current opinion in immunology. 2004 Apr;16(2):157–162. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Browning MB, Woodliff JE, Konkol MC, Pati NT, Ghosh S, Truitt RL, et al. The T cell activation marker CD150 can be used to identify alloantigen-activated CD4(+)25+ regulatory T cells. Cellular immunology. 2004 Feb;227(2):129–139. doi: 10.1016/j.cellimm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005 Aug 15;175(4):2517–2524. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- 40.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. Journal of virology. 2004 Dec;78(23):13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider-Schaulies S, Dittmer U. Silencing T cells or T-cell silencing: concepts in virus-induced immunosuppression. The Journal of general virology. 2006 Jun;87(Pt 6):1423–1438. doi: 10.1099/vir.0.81713-0. [DOI] [PubMed] [Google Scholar]
- 42.Franco MA, Greenberg HB. Immunity to rotavirus in T cell deficient mice. Virology. 1997 Nov 24;238(2):169–179. doi: 10.1006/viro.1997.8843. [DOI] [PubMed] [Google Scholar]
- 43.Peng G, Li S, Wu W, Sun Z, Chen Y, Chen Z. Circulating CD4(+) CD25(+) regulatory T cells correlate with chronic hepatitis B infection. Immunology. 2008 Jan;123(1):57–65. doi: 10.1111/j.1365-2567.2007.02691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proceedings of the National Academy of Sciences of the United States of America. 2001 Jul 31;98(16):9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brisslert M, Bokarewa M, Larsson P, Wing K, Collins LV, Tarkowski A. Phenotypic and functional characterization of human CD25+ B cells. Immunology. 2006 Apr;117(4):548–557. doi: 10.1111/j.1365-2567.2006.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amu S, Gjertsson I, Tarkowski A, Brisslert M. B-cell CD25 expression in murine primary and secondary lymphoid tissue. Scandinavian journal of immunology. 2006 Nov;64(5):482–492. doi: 10.1111/j.1365-3083.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 47.Amu S, Tarkowski A, Dorner T, Bokarewa M, Brisslert M. The human immunomodulatory CD25+ B cell population belongs to the memory B cell pool. Scandinavian journal of immunology. 2007 Jul;66(1):77–86. doi: 10.1111/j.1365-3083.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- 48.Velten FW, Rambow F, Metharom P, Goerdt S. Enhanced T-cell activation and T-cell-dependent IL-2 production by CD83+, CD25high, CD43high human monocyte-derived dendritic cells. Molecular immunology. 2007 Mar;44(7):1544–1550. doi: 10.1016/j.molimm.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Choy MY, Walker-Smith JA, Williams CB, MacDonald TT. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990 Dec;31(12):1365–1370. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bienvenu B, Martin B, Auffray C, Cordier C, Becourt C, Lucas B. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005 Jul 1;175(1):246–253. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 51.Azim T, Ahmad SM, Sefat EK, Sarker MS, Unicomb LE, De S, et al. Immune response of children who develop persistent diarrhea following rotavirus infection. Clinical and diagnostic laboratory immunology. 1999 Sep;6(5):690–695. doi: 10.1128/cdli.6.5.690-695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang B, Snipes-Magaldi L, Dennehy P, Keyserling H, Holman RC, Bresee J, et al. Cytokines as mediators for or effectors against rotavirus disease in children. Clinical and diagnostic laboratory immunology. 2003 Nov;10(6):995–1001. doi: 10.1128/CDLI.10.6.995-1001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nature immunology. 2001 Dec;2(12):1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 54.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005 Oct 1;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 55.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006 May 15;107(10):3925–3932. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]