Abstract
The present study examined the role of cGMP in the regulation of α1-adrenoceptor-mediated pharmacomechanical coupling in the uterine artery of near-term pregnant sheep. The cell permeable cGMP analogue 8-bromo-cGMP produced a dose-dependent relaxation of the uterine artery, and shifted norepinephrine (NE) dose-response curve to the right with a decreased maximal contraction. Accordingly, 8-bromo-cGMP significantly decreased the potency and the maximal response of NE-induced inositol 1,4,5-trisphosphate (IP3) synthesis in the uterine artery. In addition, 8-bromo-cGMP significantly reduced the binding affinity of IP3 to the IP3 receptor. The density of IP3 receptors was not affected. Simultaneous measurement of intracellular Ca2+ concentrations ([Ca2+]i) and tensions in the same tissue indicated that 8-bromo-cGMP decreased NE-induced contractions by 92%, but only blocked 44% of [Ca2+]i. In accordance, 8-bromo-cGMP significantly decreased tension generation for a given [Ca2+]i (g/Rf340/380: 24.87 ± 3.43 vs. 3.10 ± 0.35). In the absence of extracellular Ca2+, NE produced a transient increase in [Ca2+]i and contraction, which were inhibited by 8-bromo-cGMP by 47% and 76%, respectively. In contrast to NE-induced responses, 8-bromo-cGMP had no significant effects on KCl-induced [Ca2+]i and contractions. The results indicate that cGMP suppresses α1-adrenoceptor-mediated pharmacomechanical coupling in the uterine artery by inhibiting IP3 synthesis and Ca2+ release from intracellular stores, as well as inhibiting the agonist-mediated Ca2+ sensitization of myofilaments, which is likely to play an important role in the adaptation of uterine artery contractility during pregnancy.
Introduction
The uterine circulation during pregnancy functions as a low-resistance shunt to accommodate the large increase of uteroplacental blood flow required for normal fetal development. The mechanisms in maintaining the low uterine vascular tone in pregnancy are complex and are not fully understood. A number of studies in humans and animals have demonstrated that endothelial nitric oxide plays an important role in maintaining low vascular resistance of the uterine circulation in pregnancy (Conrad et al., 1993; Sladek et al., 1997; Nelson et al., 2000; Yallampalli et al., 2002; Bird et al., 2003). Nitric oxide through the activation of guanylate cyclase increases cGMP, and elevated plasma and urinary cGMP levels are found in both human and sheep pregnancy (Kopp et al., 1977; Magness et al., 1997; Sladek et al., 1997).
Whereas it is clear that cGMP is the mediator of NO-dependent vasorelaxation, it is unknown whether and to what extent cGMP regulates vasoconstrictor-mediated contractions of the uterine artery in pregnancy. In vivo studies in pregnant sheep have demonstrated attenuated vasoconstriction of the uterine artery to norepinephrine and angiotensin II (Naden and Rosenfeld, 1981; Magness and Rosenfeld, 1986). Yet the cellular mechanisms are not fully understood. cGMP activates cGMP-dependent protein kinase and decreases intracellular free calcium concentrations leading to an inhibition of smooth muscle contraction (Lincoln and Cornwell, 1991). Although the specific substrate proteins for cGMP-dependent protein kinase are not fully understood at the present, several smooth muscle cell functions are affected by cGMP and their modification appears to account for the effect of cGMP on the vascular reactivity (Lincoln et al., 1994). Among these, the cGMP-dependent inhibition of inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release was described (Murthy et al., 1993) and was correlated with the phosphorylation of IP3 receptors induced by agents known to increase intracellular cGMP concentrations (Komalavilas and Lincoln, 1994).
The present study was designed to determine the role of cGMP in the regulation of α1-adrenoceptor-mediated pharmacomechanical coupling in pregnant uterine arteries and test the hypothesis that cGMP played an important role in the adaptation of uterine artery contractility in pregnancy. We examined the effect of 8-bromo-cGMP, a hydrolysis-resistant membrane permeable cGMP analogue, on the IP3 signaling pathway in the uterine artery. The time- and dose-dependent IP3 synthesis induced by norepinephrine was measured. To correlate directly IP3 synthesis to tension development and to determine tissue sensitivity to IP3, we developed a method to measure α1-adrenoceptor-mediated contractile tension and IP3 production simultaneously in the same tissue. Additionally, we characterized IP3 receptors in the uterine artery and determined the effect of 8-bromo-cGMP on IP3 binding affinity to the IP3 receptor and the receptor density. We went further to determine the effect of 8-bromo-cGMP on α1-adrenoceptor-induced Ca2+ mobilization and Ca2+ sensitization of myofilaments in the uterine artery.
Methods
Tissue Preparation
Pregnant sheep (∼140 days gestation) were anesthetized with thiamy (10 mg/kg) administered via the external left jugular vein. The ewes were then intubated and anesthesia was maintained on 1.5% to 2.0% halothane in oxygen throughout surgery. An incision in the abdomen was made and the uterus exposed. The uterine arteries were isolated and removed without stretching and placed into a modified Krebs solution (pH 7.4) of the following composition (in mM): 115.21 NaCl, 4.7 KCl, 1.80 Ca Cl2, 1.16 MgSO4, 1.18 KH2PO4, 22.14 NaHCO3, and 7.88 dextrose. EDTA (0.03 mM) was added to suppress oxidation of amines. The Krebs solution was oxygenated with a mixture of oxygen-carbon dioxide (95:5%). After removal of the tissues, animals were killed with T-61 (euthanasia solution, Hoechst-Roussel, Somervile, NJ, USA). All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of Arterial Tension
Fourth branches of the main uterine arteries were separated from the surrounding tissue, and were cut into 2-mm ring segments. To determine the role of endothelium in 8-bromo-cGMP's effect, the endothelium was removed from some arterial rings by gentle rotation of the tissue on an appropriately sized, rough-surfaced blunt hypodermic needle as described previously (Hu et al., 1996). Validation of the endothelium removal was demonstrated by the elimination of endothelium-dependent relaxation induced by the calcium ionophore A23187. Isometric tensions of arterial rings were measured in Krebs' solution in tissue baths at 37 °C as described previously (Hu et al., 1996). After 40 min of equilibration in the tissue bath, each ring was stretched to the optimal resting tension as determined by the tension developed in response to three exposures of KCl (120 mM) added at different stretch levels. Concentration-response curves were obtained by cumulative additions of the agonist in approximate one-half log increments. EC50 values for the agonist in each experiment were taken as the molar concentration at which the contraction-response curve intersected 50% of the maximum response, and were expressed as pD2 (-logEC50) values.
Measurement of IP3
The accumulation of IP3 was measured by the competitive ligand binding radioreceptor assay (Hu et al., 1999). The tissues were equilibrated in Krebs' solution at 37 °C for 30 min. Following treatments, tissue reactions were terminated by flash freezing tissues in liquid N2. The tissues were then homogenized in ice-cold 16.7% trichloroacetic acid (TCA). The homogenate was centrifuged at 1,500g for 10 min at 4 °C. The supernatant was extracted with water-saturated diethyl ether to remove TCA, and the pellet was saved for protein determination with a protein assay kit (Bio-Rad). IP3 in the supernatant was determined using a radioreceptor assay kit from DuPont-NEN (Boston, MA). Values were expressed as pmol IP3/mg protein.
Characterization of IP3 Receptors
Saturation binding of [3H]IP3 (Dupont-NEN, Boston, MA) was performed by a rapid filtration method as described previously (Hu et al., 1999). Uterine arteries were minced and suspended in 10 volume of the buffer (composition 20 mM Tris/HCl 20, 20 mM NaCl, 100 mM KCl, 1 mM EDTA, and 0.02% NaN3, pH 7.7). Tissues were then homogenized using a Polytron tissue homogenizer at setting 3.5 in two bursts of 15 s each. The homogenate was centrifuged at 100,000g for 30 min. Pellets were re-homogenized in half the original volume of the buffer at setting 3.5 for 15 s, and recentrifuged under the same conditions. The supernatant was discarded and the pellet was re-suspended in the same buffer with pH 8.5. Radioligand binding assays were carried out in a final volume of 200 μl consisting of 175 μl membranes, 20 μl radioligand, and 5 μl buffer or unlabeled IP3 (Research Biomedicals Inc., Natick, MA). Saturation binding experiments used concentrations of [3H] IP3 from 0.15 to 80 nM and nonspecific binding was determined by the addition of 12 μM cold IP3. Equilibrium binding was carried out at 4 °C for 15 min. All determinations were performed in triplicate. Bound and free radioligand was separated by a rapid filtration of the membrane suspension over polyethylenimine (0.5%)-pretreated filters (GF/B: Whatman Inc., Clifton, NJ) with a Brandel cell harvester. Filters were rinsed with two 5-ml aliquots of the ice-cold buffer, and counted for radioactivity at 45% efficiency in Packard 1900CA Tri-Carb liquid scintillation analyzer (Packard Instrument Co., Downers Grove, IL). Protein was determined with a protein assay kit (Bio-Rad).
Simultaneous Measurement of [Ca2+]i and Tension
Simultaneous measurement of tension and free intracellular calcium concentrations ([Ca2+]i) in the uterine artery smooth muscle was obtained as described previously by us (Zhang and Xiao, 1998). The tissues were attached to an isometric force transducer in a 5-ml tissue bath mounted on a CAF-110 intracellular Ca2+ analyzer (Jasco, Tokyo, Japan), and were equilibrated in Krebs' buffer under a resting tension of 0.5 g for 40 min. The tissues were then loaded with 5 μM fura 2-acetoxymethyl ester (fura 2-AM) for 2 h in the presence of 0.02% Cremophor EL at 25 °C. After loading, the tissues were washed with Krebs solution at 37 °C for 30 min to allow for hydrolysis of fura 2 ester groups by endogenous esterase. The tension and fura 2 fluorescence were measured simultaneously at 37 °C in the same tissue. During the stimulation with an agonist, the tissues were illuminated alternatively (125 Hz) at excitation wavelengths of 340 and 380 nm, respectively, by means of two monochromators in the light path of a 75-w Xe lamp. Fluorescence emission from the tissue was measured at 510 nm by a photomultiplier. The fluorescence intensity at each excitation wavelength (F340 and F380, respectively) and the ratio of these two fluorescence values (Rf340/380) were recorded with a time constant of 250 ms and stored with the force signal on a computer.
Data Analysis
Saturation binding and concentration-response curves were analyzed by computer-assisted nonlinear regression to fit the data and to determine the dissociation constant (KD), receptor density (Bmax), and pD2 (-log EC50) using GraphPad Prism (GraphPad Software, San Diego, CA). Results were expressed as means ± SEM and the differences were evaluated for statistical significance (P < 0.05) by analysis of variance.
Results
Effects of 8-bromo-cGMP on NE-Induced Contractions
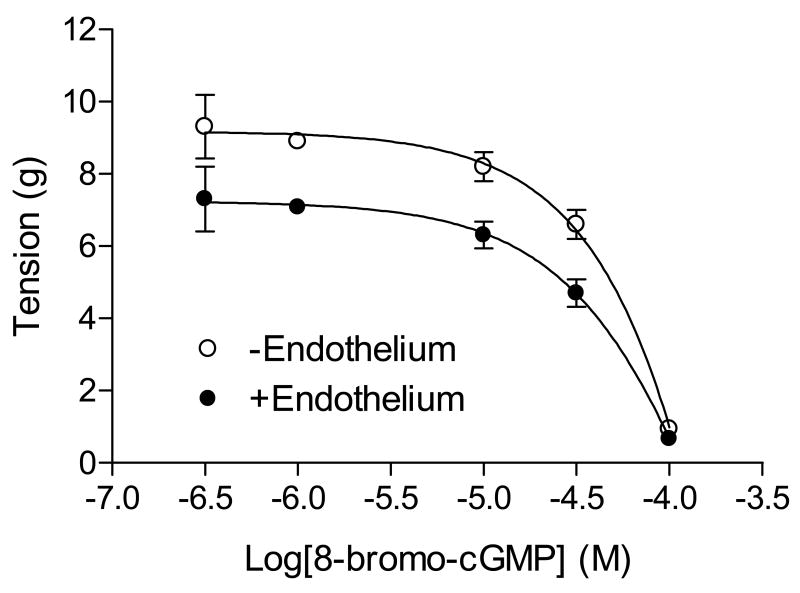
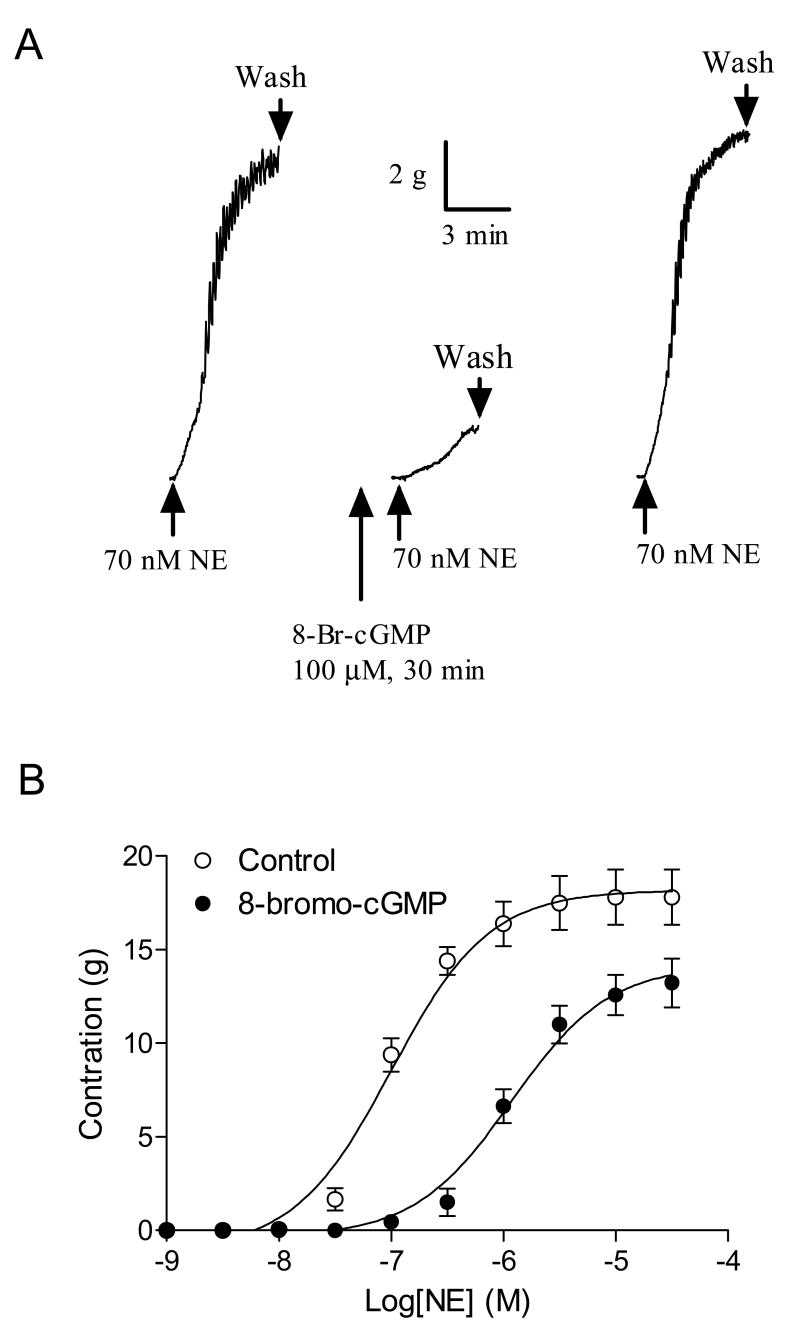
8-Bromo-cGMP produced a dose-dependent relaxation of the uterine artery pre-contracted with NE, which was not significantly affected by the endothelium removal (Fig. 1). Additionally, pretreatment of the uterine artery with 8-bromo-cGMP significantly inhibited the NE-induced contractions (Fig. 2). The inhibitory effect was completely reversible (Fig. 2A). 8-bromo-cGMP shifted the NE dose-response curve to the right (Fig. 2B) and significantly decreased the contractile sensitivity (pD2) from 7.00 ± 0.08 to 5.93 ± 0.09 (P < 0.05) and the maximal response from 18.2 ± 0.5 g to 14.2 ± 0.6 g (P < 0.05), respectively.
Figure 1. 8-Bromo-cGMP-mediated relaxation of the uterine artery.
Concentration-dependent relaxations induced by 8-bromo-cGMP were obtained with NE (0.1 μM)-pre-contracted uterine arteries with intact or denuded endothelium. Data are mean ± SEM of the tissues from five animals.
Figure 2. 8-Bromo-cGMP-mediated inhibition of NE-induced contractions of the uterine artery.
Panel A: Typical recordings of effect of 8-bromo-cGMP on the tension-induced by NE in the uterine artery. Panel B: Cumulative concentration-response curves to NE were obtained in the uterine artery in the absence or presence of 8-bromo-cGMP (100 μM, pretreatment for 30 min). Data are means ± SEM of the tissues from five animals.
Effect of 8-bromo-cGMP on NE-Induced IP3 Synthesis
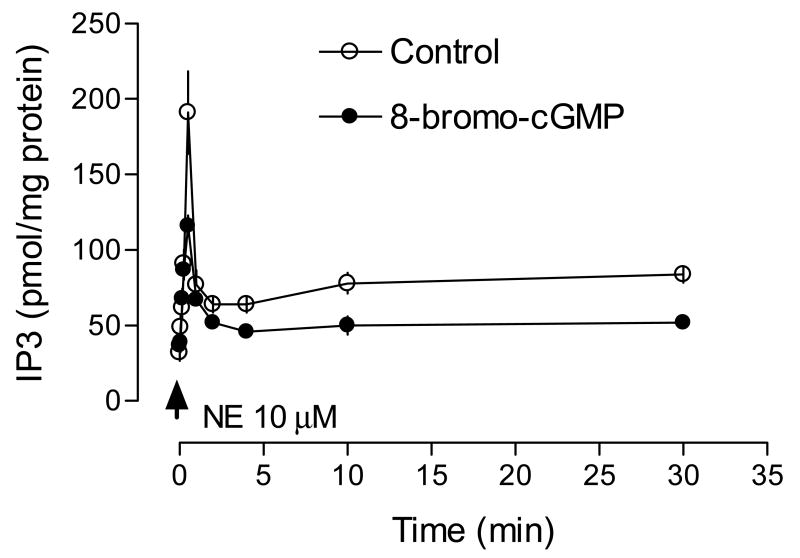
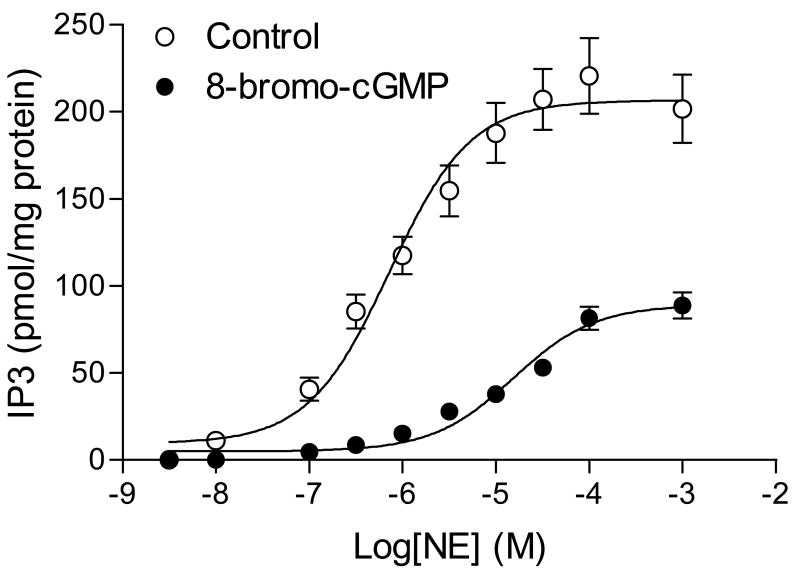
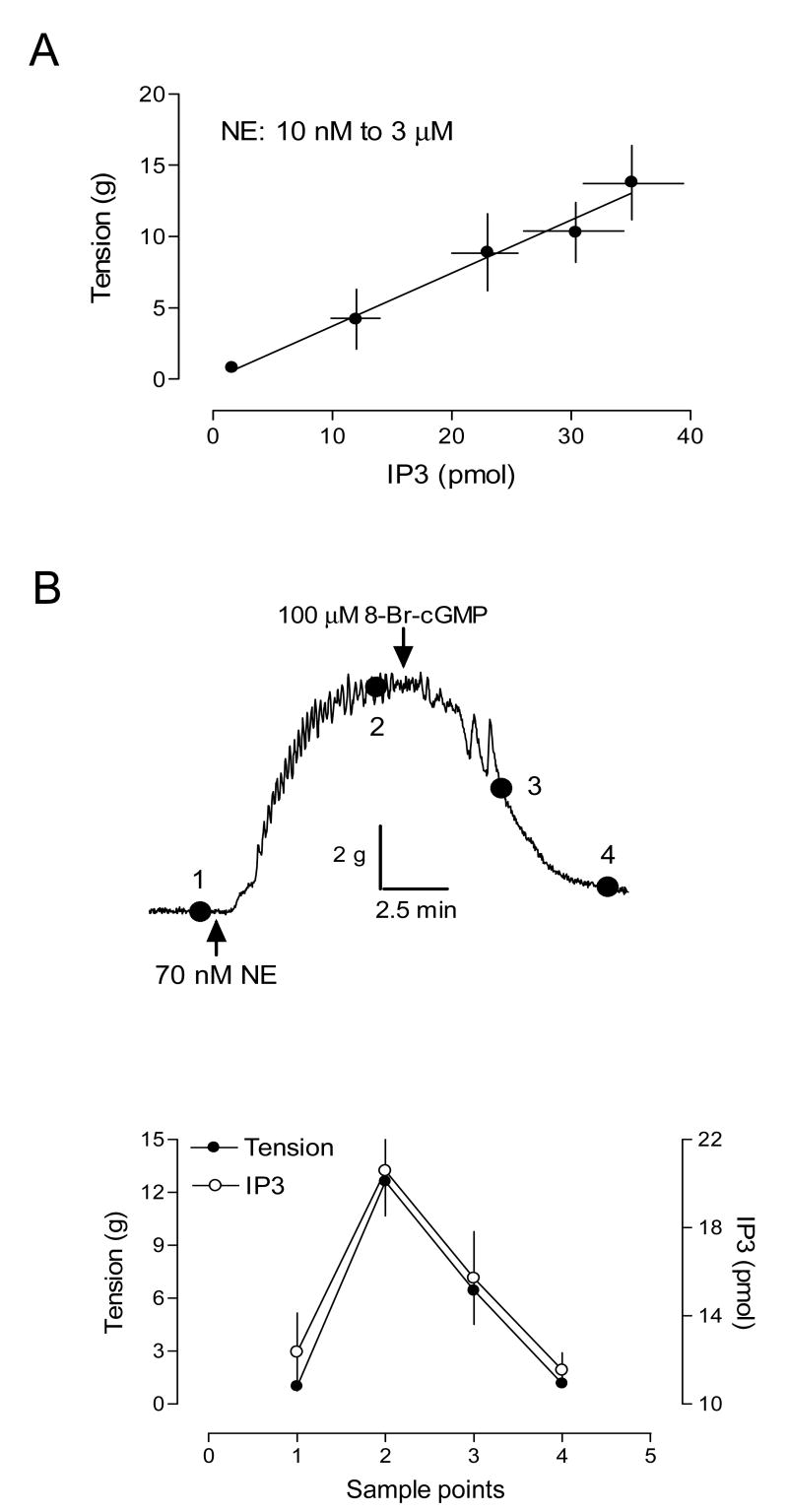
The time course of NE-stimulated IP3 synthesis in the uterine artery is shown in Fig. 3. NE stimulated a rapid increase in IP3, which reached the peak at 30 s. At 1 min, IP3 declined to a steady state level above the basal, which lasted at least for 30 min. 8-Bromo-cGMP did not alter the time course, but significantly decreased the peak level of IP3 elicited by NE (79 ± 4.6 vs. 159 ± 22.8 pmol/mg protein, P < 0.05). The elevated steady level was also decreased by 8-bromo-cGMP (Fig. 3). Fig. 4 shows the concentration-dependent response of IP3 synthesis induced by NE. 8-Bromo-cGMP caused about 20-fold rightward shift of the concentration-response curve and decreased the pD2 from 6.14 ± 0.10 to 4.81 ± 0.12 (P < 0.05). The maximal formation of IP3 induced by NE was also decreased from 206.6 ± 6.3 to 89.1 ± 5.1 pmol/mg protein (P < 0.05). To determine the correlation of agonist-induced IP3 synthesis and tension development in the uterine artery, we measured contractions and IP3 productions simultaneously in the same tissue. As shown in Fig. 5A, there was a tight correlation between NE-induced contractions and IP3 synthesis in the uterine artery. Simultaneous measurement of IP3 synthesis and contractions in the same tissue also indicated that 8-bromo-cGMP-mediated inhibition of NE-induced contractions was correlated with decreases in IP3 production in the same tissue (Fig. 5B).
Figure 3. Effect of 8-bromo-cGMP on the time course of NE-induced IP3 synthesis in the uterine artery.
Uterine arteries were stimulated with 10 μM NE for various times in the absence or presence of 8-bromo-cGMP (100 μM, pretreatment for 30 min). IP3 was measured by a competitive ligand binding radioreceptor assay as described in the Methods. Data are means ± SEM of the tissues from five animals.
Figure 4. 8-Bromo-cGMP-mediated inhibition of NE-stimulated IP3 synthesis in the uterine artery.
Uterine arteries were stimulated with various concentrations of NE for 30 s in the absence or presence of 8-bromo-cGMP (100 μM, pretreatment for 30 min). IP3 was measured by a competitive ligand binding radioreceptor assay as described in the Methods. Data are means ± SEM of the tissues from five animals.
Figure 5. Effect of 8-bromo-cGMP on NE-stimulated tension and IP3 synthesis in the uterine artery.
Panel A. NE-induced IP3 synthesis and tension development measured simultaneously in the same tissue. Panel B. Uterine arteries were stimulated with NE in the organ baths. After the contraction reached the plateau, 8-bromo-cGMP was added. Contractile tension and IP3 were measured simultaneously in the same tissue at four different stages as shown in the trace. Tensions were recorded at the indicated sample points and the tissues were snap-frozen in liquid N2 at corresponding points for measurement of the IP3 as described in Methods. Data are means ± SEM of the tissues from five animals.
Effect of 8-bromo-cGMP on the IP3 Receptor
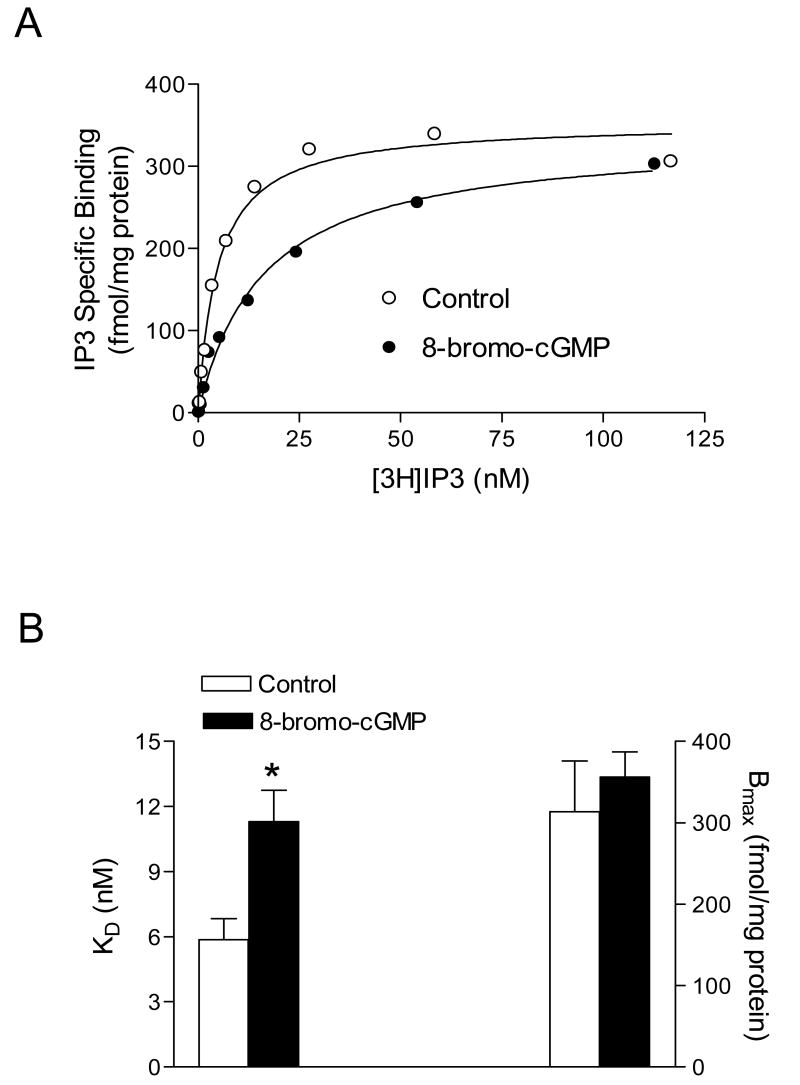
Coupling of IP3 to force generation in smooth muscle includes the binding of IP3 to IP3 receptors leading to release of Ca2+ from intracellular stores. Our previous studies have characterized the IP3 receptors in the uterine artery using a radioligand binding method (Hu et al., 1999). As shown in Fig. 6A, binding of [3H]IP3 to tissue membranes was specific and saturable. 8-Bromo-cGMP did not significantly affect the receptor density (Bmax: 313.6 ± 62.2 vs. 356.2 ± 30.9 fmol/mg protein, P > 0.05), but significantly increased the dissociation constant (KD: 11.3 ± 1.44 vs. 5.87 ± 0.97 nM, P < 0.05) of IP3 to its receptors (Fig. 6).
Figure 6. Effect of 8-bromo-cGMP on the IP3 receptor in the uterine artery.
Uterine arteries were treated with 100 μM 8-bromo-cGMP for 30 min. Saturation binding of [3H]IP3 to membranes prepared from the uterine arteries was conducted as described in Methods. Panel A shows the specific binding curves for [3H]IP3. Data points represent means of triplicate determinations in a single experiment. Panel B shows IP3 binding dissociation constant (KD) and IP3 receptor density (Bmax) determined in the saturation binding. Data are means ± SEM of the tissues from five animals. * P < 0.05
Effect of 8-bromo-cGMP on Ca2+ Homeostasis
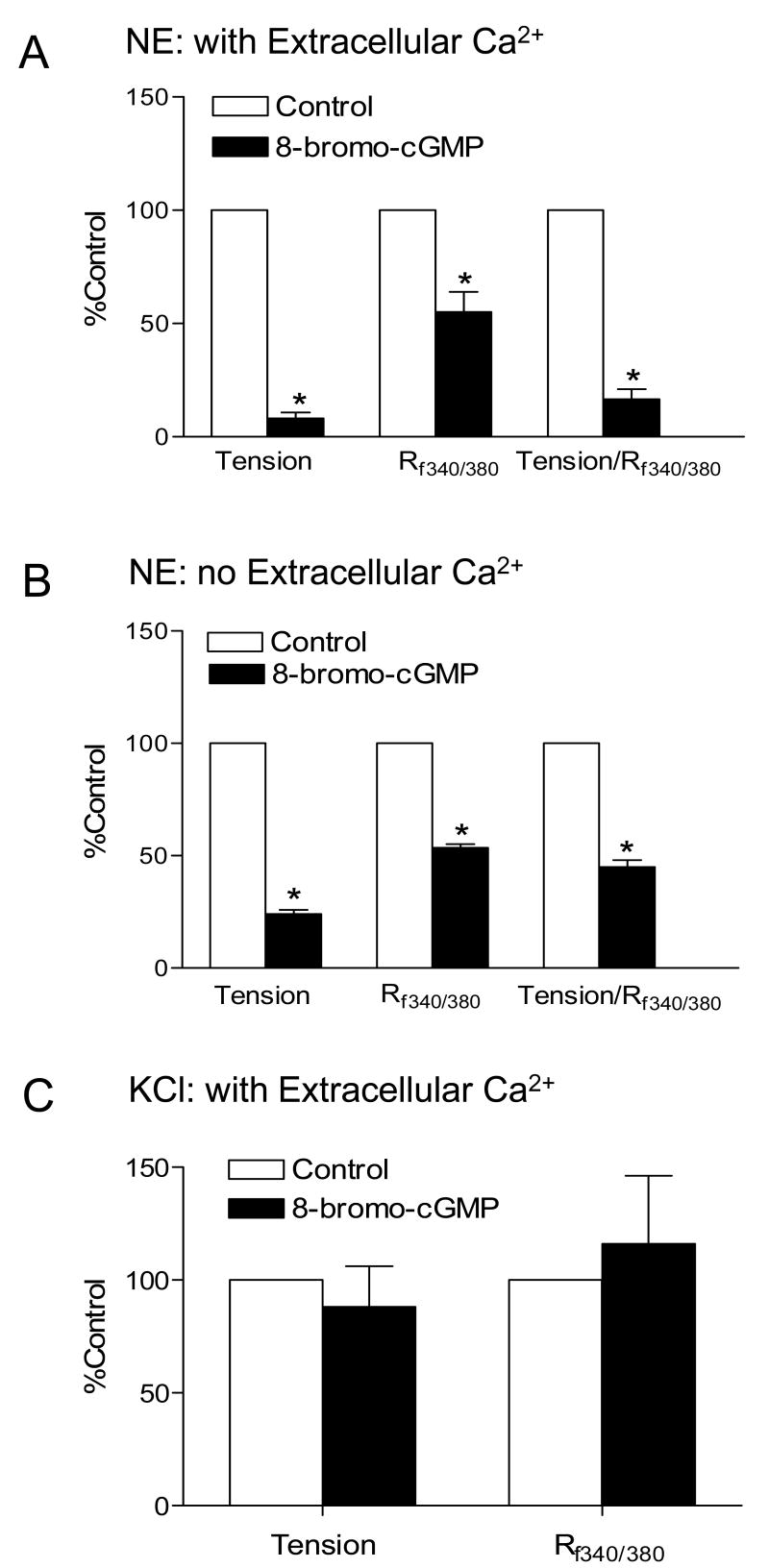
To examine the effect of 8-bromo-cGMP on NE-mediated Ca2+ homeostasis in the uterine artery, NE-induced contractions and [Ca2+]i were measured simultaneously in the same tissue as described in the Methods. As shown in Fig. 7A, 8-bromo-cGMP significantly decreased NE-stimulated increases in [Ca2+]i (Rf340/380: 0.046 ± 0.011 vs. 0.082 ± 0.013, P < 0.05) and contraction (0.143 ± 0.022 vs. 2.04 ± 0.38 g, P < 0.05) in the same tissue. The reduction of tension was significantly greater than that of [Ca2+]i. In accordance, the contractile tension of the uterine artery at a given amount of [Ca2+]i induced by NE was significantly decreased by 8-bromo-cGMP (g/R340/380: 3.10 ± 0.35 vs. 24.87 ± 3.43, P < 0.05). In contrast to NE-induced responses, 8-bromo-cGMP had no significant effect on either the tension (1.37 ± 0.29 vs. 1.57 ± 0.30 g, P > 0.05) or [Ca2+]i (Rf340/380: 0.07 ± 0.022 vs. 0.058 ± 0.017, P > 0.05) induced by KCl (Fig. 7C).
Figure 7. Effect of 8-bromo-cGMP on NE- and KCl-stimulated [Ca2+]i and contractions in the uterine artery.
NE (3 μM)- and KCl (60 mM)-induced [Ca2+]i (Rf340/380, ratio of F340 to F380) and contractions were measured simultaneously in the same tissue in the absence or presence of 8-bromo-cGMP (100 μM, pretreatment for 30 min). Panel A. NE-induced response in the presence of extracellular Ca2+; Panel B. NE-induced response in the absence of extracellular Ca2+. Panel C. KCl-induced response in the presence of extracellular Ca2+. Data are normalized to control and are means ± SEM of tissues from 5-7 animals, * P < 0.05.
To determine the role of 8-bromo-cGMP on NE-induced Ca2+ release from intracellular stores, NE-stimulated [Ca2+]i and contractions were determined in the absence of extracellular Ca2+. In contrast to the sustained increases in [Ca2+]i and tension development observed in the presence of extracellular Ca2+, NE produced a transient increase in both [Ca2+]i and tension in the uterine artery in the absence of extracellular Ca2+. In agreement with the findings in the presence of extracellular Ca2+, 8-bromo-cGMP significantly decreased the NE-induced tension by 76% and [Ca2+]i by 47%, and accordingly the NE-mediated Ca2+ sensitization (g/Rf340/380) by 45%, in the absence of extracellular Ca2+ (Fig. 7B).
Discussion
In the present study, we have demonstrated that KCl-induced Ca2+ mobilization and contractions in the uterine artery from near-term pregnant sheep are not altered by 8-bromo-cGMP. Similar findings were obtained in rabbit aorta in which sodium nitroprusside did not affect KCl-evoked contractions (Karaki et al. 1984, 1986). Consistently, KCl-stimulated [Ca2+]i and contractions were not significantly different between uterine arteries isolated from non-pregnant and pregnant sheep (Xiao and Zhang, 2004), albeit uterine cGMP levels were significantly increased during pregnancy in sheep (Magness et al., 1996; Rosenfeld et al., 1996; Sladek et al., 1997). These findings suggest that cGMP has minimum role in the regulation of L-type Ca2+ channels and electromechanical coupling of uterine artery contractions. Although K+ channels activation is a main mechanism induced by cGMP, in KCl-induced contractions these channels may not be activated.
In contrast to the lack of effect on KCl-induced contractions, 8-bromo-cGMP attenuated α1-adrenoceptor-mediated contractions by inhibiting both Ca2+ mobilization and the Ca2+ sensitivity of contractile myofilaments in the uterine artery. cGMP decreased NE-induced Ca2+ release from intracellular stores by inhibiting NE-mediated IP3 synthesis and IP3 binding affinity to the IP3 receptor. There are two major components in G protein-coupled receptor-mediated pharmacomechanical coupling: 1) agonist-induced increase in intracellular free Ca2+ concentration ([Ca2+]i); and 2) agonist-induced increase in the Ca2+ sensitivity of contractile myofilaments. We have demonstrated that NE contracts the uterine artery by acting on α1-adrenoceptors (Zhang et al., 1995b). Activation of α1-adrenoceptors stimulated a rapid increase of IP3 which correlated well with the contractile responses in the uterine artery (Zhang et al., 1995b). Release of intracellular Ca2+ from the sarcoplasmic reticulum by IP3 is a major mechanism of pharmacomechanical coupling in smooth muscle (Somlyo and Somlyo, 1994; Zhang et al., 1995a). In the present study, we demonstrated that 8-bromo-cGMP significantly inhibited NE-induced contractions of the uterine artery and shifted NE dose-response curve to the right. The finding of excellent correlation between 8-bromo-cGMP-induced decreases in NE-mediated tension development and IP3 production measured simultaneously in the same tissue strongly suggests that reduced IP3 synthesis plays an important role in cGMP-mediated inhibition of NE-induced contractions in the uterine artery. Whereas 8-bromo-cGMP did not affect the time course of NE-induce IP3 synthesis, it significantly decreased the potency and the maximal response of NE-induced IP3 production in the uterine artery. In agreement, it has been shown previously in cultured vascular smooth muscle cells that cGMP decreases vasopressin-mediated IP3 formation by inhibiting phospholipase C and/or receptor/phospholipase C coupling (Hirata et al., 1990).
Given that intracellular responses to IP3 are mediated by the IP3 receptor, the density of IP3 receptors and IP3 binding affinity to the IP3 receptor are key determinants in explaining coupling between IP3 and intracellular Ca2+ release. In the present study, we demonstrated that 8-bromo-cGMP significantly decreased IP3 binding affinity without changing the IP3 receptor density in the uterine artery. It has been demonstrated that cGMP, through activation of cGMP-dependent protein kinase (cGK), induces phosphorylation of the IP3 receptor in vascular smooth muscle and decreases the IP3 binding affinity (Komalavilas and Lincoln, 1994). However, whether phosphorylation of the purified IP3 receptor in constituted systems potentiates or inhibits its ability to release Ca2+ from intracellular stores remains controversial (Supattapone et al., 1988; Nakade et al., 1994). It has been demonstrated in intact megakaryocytes that activation of cGK inhibits Ca2+ release from the IP3-sensitive stores (Tertyshnikova et al., 1998). In agreement with this finding, the present study demonstrated that 8-bromo-cGMP inhibited NE-induced transient increase in [Ca2+]i in the absence of extracellular Ca2+, suggesting that activation of cGK in the uterine artery decreased Ca2+ release from intracellular stores. To our knowledge, the present study is the first to demonstrate cGK-mediated inhibition of Ca2+ release from the IP3-sensitive stores in the intact vessel. It has been shown in microsomal smooth muscle membranes that the cGK pathway regulates IP3-mediated Ca2+ release by the phosphorylation of not only IP3 receptors but also a regulatory protein named IP3 receptor-associated cGMP kianse substrate (IRAG) (Schlossmann et al., 2000). Additionally, in cultured vascular smooth muscle cells, 8-bromo-cGMP inhibited IP3-stimulated Ca2+ release induced by angiotensin II (Saito et al., 1993). These findings indicate that the effect of cGMP may not be specific to NE but also to other vasoconstrictors coupled to the IP3 pathway and suggest an important role of cGMP in the attenuated vasoconstriction of the uterine artery to both NE and angiotensin II in pregnancy.
The simultaneous measurement of [Ca2+]i with tension in the same intact tissue allowed us to determine directly the precise relationship between [Ca2+]i and tension development in the uterine artery and thus to estimate Ca2+ sensitivity of myofilaments with unimpaired excitation-contraction coupling processes and retained regulatory targets for second messenger pathways. In present study, the finding that addition of 8-bromo-cGMP nearly completely inhibited NE-induced contractions but decreased only 44% of the increased [Ca2+]i, suggests that not only does cGMP regulate Ca2+ mobilization, but it also inhibits Ca2+ sensitivity of myofilaments in the uterine artery. This is consistent with the previous findings that sodium nitroprusside produced a greater inhibition on agonist-induced contractions than its inhibition on intracellular Ca2+ concentrations in vascular smooth muscle (Karaki et al., 1986; McDaniel et al., 1992; Xiao et al., 2001b). In vascular smooth muscle, the sensitivity of contractile apparatus to Ca2+ is modulated by two major pathways: thick filament regulatory pathway and thin filament regulatory pathway (Horowitz et al., 1996). The thin filament regulatory pathway involves regulatory proteins caldesmon and calponin. The thick filament regulatory pathway is mediated by myosin light chain phosphatase that dephosphorylates myosin light chain. It has been demonstrated that cGMP-mediated vascular relaxation is characterized by both a reduction of intracellular Ca2+ concentrations and by an activation of myosin light chain phosphatase leading to a decrease in the sensitivity of contractile apparatus to intracellular Ca2+(Lincoln and Cornwell, 1993; Lee et al., 1997; Pfeifer et al., 1998; Sauzeau et al., 2000).
In summary, the present study demonstrates that cGMP inhibits α1-adrenoceptor-mediated pharmacomechanical coupling and contractions in the uterine artery of near-term pregnant sheep by suppressing both Ca2+ mobilization and the Ca2+ sensitivity of myofilaments (Fig. 8). From the physiological perspective, the uterine circulation during pregnancy functions as a low-resistance shunt to accommodate the large increase of uteroplacental blood flow, required for normal fetal development. In addition to growth and remodeling of vessels, the decreased uterine artery resistance is accomplished by increased endothelial nitric oxide release, decreased myogenic response, and a reversible sympathetic denervation of the uterine artery. Although decreased sympathetic innervation may sensitize postsynaptic α1-adrenoceptor signaling pathways, the present finding of the inhibitory effect of cGMP on α1-adrenoceptor-mediated contractions in the pregnant uterine artery reveals another important mechanism in maintaining the low uterine vascular tone in pregnancy given that uterine cGMP levels are elevated during pregnancy. Whereas it is not the goal of the present study, a comparison of cGMP mechanism in the regulation of α1-adrenoceptor-mediated signaling pathway between uterine arteries from non-pregnant and pregnant animals presents an intriguing area for the future investigation.
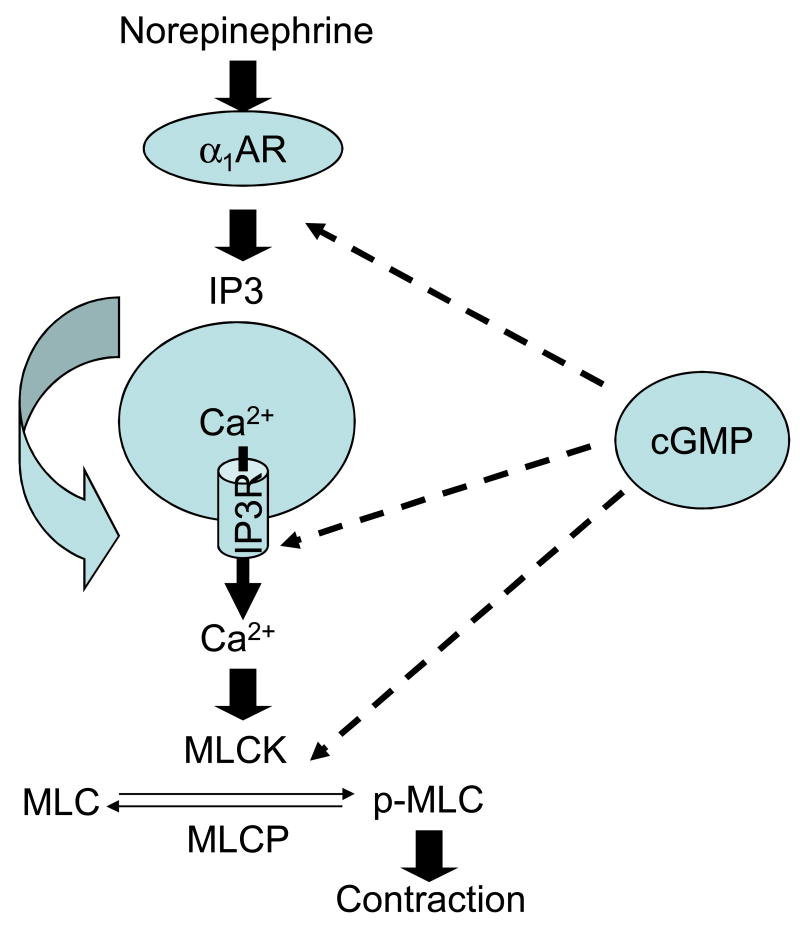
Figure 8. cGMP-mediated inhibition of α1-adrenoceptor-mediated pharmacomechanical coupling in the uterine artery.
cGMP inhibits α1-adrenoceptor-mediated IP3 synthesis and reduces IP3 binding affinity to the IP3 receptor, leading to a decreased Ca2+ release from Ca2+ stores. Additionally, cGMP inhibits the Ca2+ sensitivity of myofilaments possibly by activating myosin light chain phosphotase activity. α1AR, α1-adrenoceptor; IP3, inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate receptor; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphotase; MLC, myosin light chain; p-MLC, phosphorylated myosin light chain.
Acknowledgments
Source of financial support: This work was supported in part by NIH grants HL89012 (LZ) and HD31226 (LZ) and by Loma Linda University School of Medicine.
List of non-standard abbreviations
- NE
norepinephrine
- IP3
inositol 1,4,5-trisphosphate
- [Ca2+]i
intracellular free Ca2+ concentrations
References
- Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy induced increases in uterine artery endothelial function. Am J Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Charvez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993;7:566–571. [PubMed] [Google Scholar]
- Conrad KP, Vernier KA. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol. 1989;257:R847–R853. doi: 10.1152/ajpregu.1989.257.4.R847. [DOI] [PubMed] [Google Scholar]
- Hirata M, Kohse KP, Chang CH, Ikebe T, Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990;265:1268–1273. [PubMed] [Google Scholar]
- Horowitz A, Menice CB, Laporte R, Morgan KG. Mechanisms of smooth muscle contraction. Physiol Rev. 1996;76:967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- Hu XQ, Longo LD, Gilbert RD, Zhang L. Effects of long-term high altitude hypoxemia on α1-adrenergic receptors in the ovine uterine artery: functional and binding studies. Am J Physiol. 1996;270:H1001–H1007. doi: 10.1152/ajpheart.1996.270.3.H1001. [DOI] [PubMed] [Google Scholar]
- Hu XQ, Yang SM, Pearce WJ, Longo LD, Zhang L. Effect of chronic hypoxia on alpha-1 adrenoceptor-mediated inositol 1,4,5-trisphosphate signaling in ovine uterine artery. J Pharmacol Exp Ther. 1999;288:977–983. [PubMed] [Google Scholar]
- Karaki H, Nakagawa H, Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea pig taenia coli. Br J Pharmacol. 1984;81:393–397. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H, Murakami K, Urakawa N. Mechanism of inhibitory action of sodium nitroprusside in vascular smooth muscle of rabbit aorta. Arch Int Pharmacodyn. 1986;280:230–236. [PubMed] [Google Scholar]
- Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994;269:8701–8707. [PubMed] [Google Scholar]
- Kopp L, Paradiz G, Tucci JR. Urinary excretion of cyclic 3′,5′-adenosine monophosphate and cyclic 3′,5′-guanosine monophosphate during and after pregnancy. J Clin Endocrinol Metab. 1977;44:590–594. doi: 10.1210/jcem-44-3-590. [DOI] [PubMed] [Google Scholar]
- Lee MR, Li L, Kitazawa T. Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem. 1997;272:5063–5068. doi: 10.1074/jbc.272.8.5063. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991;28:129–37. doi: 10.1159/000158852. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Komalavilas P, Cornwell TL. Pleiotropic regulation of vascular smooth muscle tone by cyclic GMP-dependent protein kinase. Hypertension. 1994;23:1141–1147. doi: 10.1161/01.hyp.23.6.1141. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR. Systemic and uterine responses to α-adrenergic stimulation in pregnant and nonpregnant sheep. Am J Obstet Gynecol. 1986;155:897–904. doi: 10.1016/s0002-9378(86)80047-3. [DOI] [PubMed] [Google Scholar]
- Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. pregnancy effects on NO synthase expression. Am J Physiol. 1997;272:H1730–H1740. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- McDaniel NL, Chen XL, Singer HA, Murphy RA, Rembold CM. Nitrovasodilators relax arterial smooth muscle by decreasing [Ca2+]i and uncoupling stress from myosin phosphorylation. Am J Physiol. 1992;263:C461–C467. doi: 10.1152/ajpcell.1992.263.2.C461. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993;264:G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest. 1981;68:469–474. doi: 10.1172/JCI110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S, Rhee SK, Hamanaka H, Mikoshiba K. Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J Biol Chem. 1994;269:6735–6742. [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87:406–411. doi: 10.1161/01.res.87.5.406. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Himeiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 1998;17:3045–3051. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Hori MT, Fittingoff M, Hino T, Tuck ML. Insulin attenuates agonist-mediated calcium mobilization in cultured rat vascular smooth muscle cells. J Clin Invest. 1993;92:1161–1167. doi: 10.1172/JCI116685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loriand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol. 1997;272:R441–R463. doi: 10.1152/ajpregu.1997.272.2.R441. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S, Danoff SK, Theibert A, Joseph SK, Steiner J, Snyder SH. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci USA. 1988;85:8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teryshnikoca S, Yan X, Fein A. cGMP inhibits IP3-induced Ca2+ release in intact rat megakaryocytes via cGMP- and cAMP-dependent protein kinases. J Physiol. 1998;512:89–96. doi: 10.1111/j.1469-7793.1998.089bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao DL, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca2+ Am J Physiol. 2001;281:H183–H190. doi: 10.1152/ajpheart.2001.281.1.H183. [DOI] [PubMed] [Google Scholar]
- Xiao DL, Zhang L. Calcium homeostasis and contractions of the uterine artery: Effect of pregnancy and chronic hypoxia. Biol Reprod. 2004;70:1171–1177. doi: 10.1095/biolreprod.103.024943. [DOI] [PubMed] [Google Scholar]
- Yallampalli C, Chauhan M, Thota CS, Kondapaka S, Wimalawansa SJ. Calcitonin gene-related peptide in pregnancy and its emerging receptor heterogeneity. Trends Endocrinol Metab. 2002;13:263–269. doi: 10.1016/s1043-2760(02)00563-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bradley ME, Buxton ILO. Inositol polyphosphate binding sites and their likely role in calcium regulation in smooth muscle. Int J Biochem Cell Biol. 1995a;27:1231–1248. doi: 10.1016/1357-2725(95)00111-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Pearce WJ, Longo LD. Noradrenaline-mediated contractions of ovine uterine artery: Role of inositol 1,4,5-trisphosphate. Eur J Pharmacol (Mol Pharmacol Section) 1995b;289:375–382. doi: 10.1016/0922-4106(95)90116-7. [DOI] [PubMed] [Google Scholar]