Abstract
Objectives
To evaluate a novel series of quinoxaline 1,4-di-N-oxides for in vitro activity against Mycobacterium tuberculosis and for efficacy in a mouse model of tuberculosis (TB).
Methods
Ketone and amide derivatives of quinoxaline 1,4-di-N-oxide were evaluated in in vitro and in vivo tests including: (i) activity against M. tuberculosis resistant to currently used antitubercular drugs including multidrug-resistant strains (MDR-TB resistant to isoniazid and rifampicin); (ii) activity against non-replicating persistent (NRP) bacteria; (iii) MBC; (iv) maximum tolerated dose, oral bioavailability and in vivo efficacy in mice; and (v) potential for cross-resistance with another bioreduced drug, PA-824.
Results
Ten compounds were tested on single drug-resistant M. tuberculosis. In general, all compounds were active with ratios of MICs against resistant and non-resistant strains of ≤4.00. One compound, 5, was orally active in a murine model of TB, bactericidal, active against NRP bacteria and active on MDR-TB and poly drug-resistant clinical isolates (resistant to 3–5 antitubercular drugs).
Conclusions
Quinoxaline 1,4-di-N-oxides represent a new class of orally active antitubercular drugs. They are likely bioreduced to an active metabolite, but the pathway of bacterial activation was different from PA-824, a bioreducible nitroimidazole in clinical trials. Compound 5 was bactericidal and active on NRP organisms indicating that activation occurred in both growing and non-replicating bacteria leading to cell death. The presence of NRP bacteria is believed to be a major factor responsible for the prolonged nature of antitubercular therapy. If the bactericidal activity and activity on non-replicating bacteria in vitro translate to in vivo conditions, quinoxaline 1,4-di-N-oxides may offer a path to shortened therapy.
Keywords: antitubercular drugs, resistance, in vivo efficacy
Introduction
Tuberculosis (TB) is a contagious disease with high mortality worldwide. It is estimated that 1.7 million deaths resulted from TB in 2006, and there are an estimated 8 million new cases each year. Moreover, up to 50 million people are infected with drug-resistant forms of TB.1 Although drug-resistant TB has existed since the introduction of the anti-TB chemotherapy, the global magnitude of drug-resistant TB was not adequately studied until recently.2 The magnitude and extent of drug-resistant strains have increased concern that TB may once again become an incurable disease and emphasized the need for new drugs to treat this infection. The recent appreciation of the widespread existence of extensively drug-resistant tuberculosis (XDR-TB) has further heightened the awareness of the need for new anti-tubercular agents with novel modes of action and full activity on poly drug-resistant strains (resistant to 3–5 antitubercular drugs), including multidrug resistance (MDR; resistant to isoniazid and rifampicin) and XDR-TB.3–9
Our group has previously reported on the synthesis and biological evaluation of a large number of quinoxaline and quinoxaline 1,4-di-N-oxide derivatives.10–15 Various 2-acetyl, 2-benzoyl and 2-carboxamide quinoxaline derivatives have been evaluated as anti-Mycobacterium tuberculosis agents.16,17 Specific analogues showed good in vitro parameters in cytotoxicity assays and in a TB-infected macrophage model. We later confirmed the antimicrobial activity of this class of compounds, and report here on the in vitro activity of ketone and amide derivatives of quinoxaline 1,4-di-N-oxide against different strains of drug-resistant M. tuberculosis and in the rapid in vivo mouse efficacy model conducted as part of the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF).
Materials and methods
Synthesis of compounds
The methods for the synthesis of quinoxaline-2-acetyl, 2-benzoyl and 2-carboxamide 1,4-di-N-oxide derivatives were reported elsewhere.16,17 A list of compounds, their structures and significant values from the earlier assays are presented in Table 1 and Figure 1. The criteria used by the TAACF for moving a compound on to in vivo testing includes a selectivity index, defined as the ratio of the measured IC50 in VERO cells to the MIC, of >10. In the same way, the concentrations which cause a 90% reduction in residual mycobacterial growth in the macrophage assay should be lower than 16 × the MIC.16,17
Table 1.
Structures and previous in vitro activity against M. tuberculosis
| Compound | R6 | R7 | R2 | MIC (mg/L) | SIa | IC90/MICb | TAACF # |
|---|---|---|---|---|---|---|---|
| 1 | H | H | CH3 | 3.13 | >20.0 | 0.87 | 149520 |
| 2 | H | CH3 | CH3 | 3.13 | >20.0 | 0.80 | 151989 |
| 3 | H | OCH3 | CH3 | 1.56 | 37.82 | 4.29 | 118850 |
| 4 | H | F | CH3 | 3.13 | >20.0 | 0.79 | 150568 |
| 5 | H | Cl | CH3 | 0.78 | 20.13 | 3.13 | 118845 |
| 6 | CH3 | CH3 | CH3 | 6.25 | >10.0 | 0.44 | 148142 |
| 7 | H | H | Ph | 6.25 | >10.0 | ND | 150355 |
| 8 | H | H | NH-Ph | 3.13 | >20.0 | 0.89 | 150354 |
| 9 | H | H | NH-Ph-(o)CH3 | 6.25 | >10.0 | 0.42 | 150356 |
| 10 | H | Cl | NH-Ph-(o)CH3 | 6.25 | >10.0 | 0.14 | 151986 |
aSI is the selectivity index calculated as IC50 (concentration inhibiting growth of VERO cells in culture by 50% following 72 h of exposure and assessed using the CellTiter 96® Non-Radioactive Cell Proliferation Assay reagent from Promega) divided by the MIC.
bIC90/MIC is a measure of the activity against intracellular M. tuberculosis taken up by mouse bone marrow macrophages (concentration required to inhibit growth of intracellular M. tuberculosis by 90%) divided by the MIC.
Figure 1.
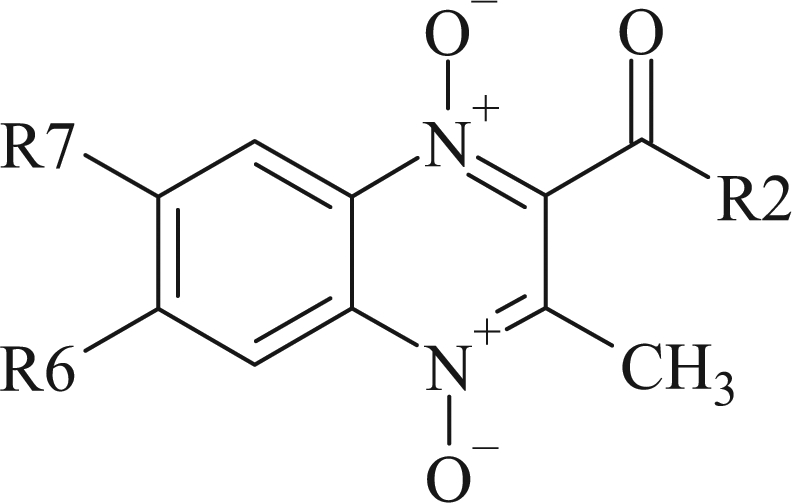
Quinoxaline 1,4-di-N-oxide core structure.
Determination of MICs against single drug-resistant M. tuberculosis and MBC
MICs were determined in the microplate Alamar Blue assay (MABA)18,19 for strains of single drug-resistant (SDR) M. tuberculosis (each strain resistant to a single TB drug): isoniazid (ATCC 35822), rifampicin (ATCC 35838), thiacetazone (ATCC 35829), ethambutol (ATCC 35837), p-aminosalicylic acid (ATCC 35821), ethionamide (ATCC 35839) and ciprofloxacin (laboratory stock collection) as well as the drug-susceptible H37Rv strain (ATCC 35801). Generally, MICs for SDR strains should not be >10× the MIC for non-resistant strains to continue compound evaluation.
The MBC was then determined for M. tuberculosis H37Rv strain by subculturing onto drug-free solid medium and enumeration of Cfu after drug exposure in Middlebrook 7H9 medium supplemented with drug concentrations equivalent to and higher than the previously determined MICs against the respective strains. Samples were incubated for 7 days at 37°C and then plated for change in cfu. Cfu was read after 10 days of incubation and followed for any changes in cfu for a total of 21 days. The MBC was the lowest concentration of drug that killed >99% of the bacterial population present when the drugs were added.
Potential for cross-resistance with PA-824
PA-824 is a nitroimidazole agent in clinical trials for treating TB. Mycobacterium bovis strains resistant to PA-824 were obtained from Dr Lacy Daniels (Texas A&M College of Pharmacy, Kingsville, TX, USA). M. bovis strains were used because: (i) these represented the best genetically and biochemically characterized mycobacterial strains resistant to PA-824; and (ii) M. bovis is 99.9% identical to M. tuberculosis at the genetic level.20 MICs were determined using microbroth dilution. PA-824 was obtained from the Global Alliance for Tuberculosis Drug Development (New York, NY, USA).
Determination of MICs against four poly drug-resistant and MDR-TB strains
MDR-TB strains obtained from Dr R. C. Chan (Department of Microbiology, Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, New Territories, Hong Kong, China)21 were cultured in 7H9 broth supplemented with ADC (Middlebrook ADC Enrichment containing bovine albumin fraction V, glucose and catalase) until an OD600 of 0.6–0.8 was obtained, and then frozen in aliquots at –80°C until required. Bacterial suspensions were prepared to reach an inoculum of 105 cfu per well in a total volume of 100 µL 7H9 medium. Drugs were dissolved in 100% DMSO to a 50× stock concentration. A 1:2 dilution series of both compounds were made in a separate 96-well plate using DMSO as the diluent. Two microlitres of the drugs were transferred to the appropriate wells of the microtitre plate. Only the inner 60 wells of the 96-well plate were used in the assay; the surrounding wells were filled with water to maintain humidity. Microtitre plates were incubated at 37°C for at least 14 days and observed every 3–4 days to determine changes in growth. Growth of the bacteria was scored at different days by a visual read and by a terminal spectrophotometer reading at OD600.
Maximum tolerated dose (MTD) and oral bioavailability
All experiments using mice were approved by the Animal Care and Use Committee at Colorado State University (approval 06-221A-02, expiration date 17 October 2008) and under the Animal Welfare Assurance number A3572-01. C57BL/6 female mice were orally administered (by gavage) a single dose of drug at 100, 300 and 500 mg/kg, using three mice per dose. Mice were observed post-administration at 4 and 6 h, and then twice daily for the duration of the study (1 week). Oral bioavailability was determined by bioassay as described.22
Rapid in vivo screen
Eight- to 10-week-old female specific pathogen-free C57BL/6-Ifngtm1ts mice [gamma interferon gene-disrupted (GKO) mice] were purchased from Jackson Laboratories, Bar Harbor, ME, USA.23 The mice were infected via low-dose aerosol exposure to M. tuberculosis Erdman using a Middlebrook aerosol generation device (Glas-Col Inc., Terre Haute, IN, USA), and the short-course mouse model was performed as described previously.24 Briefly this involves a delivery of ∼50–100 cfu to each mouse and this is confirmed 1 day post-infection by sacrificing three mice to verify the uptake of 50–100 cfu of bacteria per mouse. Treatment is initiated 15–18 days post-infection for nine daily treatments of one single oral dose (at 300 mg/kg). Bacterial load is determined the day after the nineth daily dose of drug in the lungs and spleens of the mice by serial dilution of the tissue homogenates on nutrient Middlebrook 7H11 agar plates (GIBCO-BRL, Gaithersburg, MD, USA). The plates were incubated at 37°C in ambient air for 4 weeks prior to the counting of viable M. tuberculosis colonies (cfu). The viable bacterial numbers were converted to logarithms, which were then evaluated by multiple-comparison analysis of variance by a one-way Dunnett test (SigmaStat software program). Differences were considered significant at the 95% level of confidence. Negative control mice remained untreated. An isoniazid control group, administered via oral gavage at 25 mg/kg/day, was included in each study. Each treatment group consisted of five mice. Five infected mice were killed at the start of treatment as pre-treatment controls. Drugs were administered daily by oral gavage.
Dose–response in the rapid in vivo screen
The in vivo activity of compound 5 (TAACF 118845) was examined in the rapid screening model as a repeat of the efficacy testing and to determine the minimal effective dose. Doses of 25, 100 and 300 mg/kg were tested using the same methods as in the initial in vivo test.
Statistical analysis
The viable counts were converted to logarithms, which were then evaluated by a one-way ANOVA followed by a multiple comparison analysis of variance by a one-way Tukey test (SigmaStat software program). Differences were considered significant at the 95% level of confidence.
Activity against non-replicating persistent M. tuberculosis
The activity of compound 5 against non-replicating M. tuberculosis was determined by measuring inhibitory activity under anaerobic conditions against M. tuberculosis adapted to low oxygen.25 This Low Oxygen Recovery Assay (LORA) quantifies antibacterial activity by measuring the subsequent ability of a recombinant, containing a plasmid with an acetamidase promoter driving a bacterial luciferase gene, to produce a luminescent signal when placed back into an environment with ambient oxygen. The activity against the same reporter strain is also tested under aerobic conditions as follows. The microplate cultures with added drug were placed in an incubator under ambient gaseous conditions (5% CO2-enriched air) for 7 days and 100 µL culture was transferred to white 96-well microtitre plates to determine the luminescence.
Results and discussion
The most potent compounds from our previous studies were subjected to the following set of tests: determination of MIC against different SDR strains of M. tuberculosis, MBC, oral bioavailability, MTD and in vivo efficacy in mice.
Table 2 shows the MIC values obtained against SDR strains of M. tuberculosis, including those resistant to isoniazid, rifampicin, thiacetazone, ethambutol, ciprofloxacin, kanamycin, ethionamide and p-aminosalicylic acid. MIC was also retested against a susceptible strain. In general, all the compounds showed good MIC values against resistant strains. Results showed that the most moderate activity was observed against the ciprofloxacin-resistant strain, and MICs ranged between 6.25 and 12.5 mg/L, although compound 9 revealed the poorest activity against an isoniazid-resistant strain, with an MIC value of 100 mg/L. The susceptibilities of rifampicin, thiacetazone, ethambutol and p-aminosalicylic acid-resistant strains can be considered comparable to those of H37Rv, as was indicated by the ratios of MICs against resistant and non-resistant strains (Table 3), which were generally ∼1. This indicates that there is a little, if any, cross-resistance with the current anti-TB drugs thereby supporting a novel mechanism of action. These results are promising for the development of new effective compounds against the growing number of drug-resistant strains. Only compound 9 showed resistance with the isoniazid-resistant strain, with a ratio >31.9. The reason for this finding is unknown.
Table 2.
Determination of MIC against strains of SDR M. tuberculosis
| Compound | MIC (mg/L)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| H37Rv | INH-R | RIF-R | TAC-R | EMB-R | CIP-R | KAN-R | ETA-R | PAS-R | |
| 1 | 3.13 | 6.25 | 3.13 | 3.13 | 3.13 | 6.25 | 3.13 | 6.25 | ND |
| 2 | 3.13 | 6.25 | 3.13 | ND | 3.13 | 12.5 | 6.25 | ND | ND |
| 3 | ≤1.56 | 6.25 | 3.13 | ND | ≤1.56 | 12.5 | 6.25 | ND | ND |
| 4 | ≤1.56 | NDb | ≤1.56 | 0.2 | ND | 3.13 | ND | 3.13 | ≤1.56 |
| 5 | 0.78 | 1.56 | ≤0.39 | ND | ≤0.39 | 1.56 | 0.78 | ND | ND |
| 6 | ≤3.13 | 6.25 | ≤3.13 | 6.25 | ≤3.13 | 12.5 | 6.25 | 6.25 | ND |
| 7 | ≤3.13 | ND | ≤3.13 | 0.2 | 0.39 | 6.25 | ND | 6.25 | ≤3.13 |
| 8 | 3.13 | 6.25 | 3.13 | 3.13 | 3.13 | 6.25 | 3.13 | 3.13 | ND |
| 9 | 3.13 | 100 | ≤3.13 | ≤3.13 | ≤3.13 | 6.25 | 6.25 | 6.25 | ND |
| 10 | 6.25 | 6.25 | ≤3.13 | ≤3.13 | ND | 6.25 | ND | 6.25 | ND |
aINH-R, isoniazid-resistant strain; RIF-R, rifampicin-resistant strain; TAC-R, thiacetazone-resistant strain; EMB-R, ethambutol-resistant strain; CIP-R, ciprofloxacin-resistant strain; KAN-R, kanamycin-resistant strain; ETA-R, ethionamide-resistant strain; PAS-R, p-aminosalicylic-acid-resistant strain.
bND, no data available.
Table 3.
Ratios of MICs against resistant and non-resistant strains
| Compound | Resistant strain MIC: H37Rv MICa |
|||||||
|---|---|---|---|---|---|---|---|---|
| INH-R | RIF-R | TAC-R | EMB-R | CIP-R | KAN-R | ETA-R | PAS-R | |
| 1 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 2.00 | ND |
| 2 | 2.00 | 1.00 | ND | 1.00 | 4.00 | 2.00 | ND | ND |
| 3 | ≥4.00 | ≥2.00 | ND | ND | ≥8.00 | ≥4.00 | ND | ND |
| 4 | NDb | ND | ≥0.13 | ND | ≥2.00 | ND | ≥2.00 | ND |
| 5 | 2.00 | ≤0.50 | ND | ≤0.50 | 2.00 | 1.00 | ND | ND |
| 6 | ≥2.00 | ND | ≥2.00 | ND | ≥4.00 | ≥2.0 | ≥2.0 | ND |
| 7 | ND | ND | ≥0.06 | ≥0.13 | ≥2.00 | ND | ≥2.00 | ND |
| 8 | 2.00 | 1.00 | 1.00 | 1.00 | 2.00 | 1.00 | 1.00 | ND |
| 9 | 31.9 | ≤1.00 | ≤1.00 | ≤1.00 | 2.00 | 2.00 | 2.00 | ND |
| 10 | 1.00 | ≤0.5 | ≤0.5 | ND | 1.00 | ND | 1.00 | ND |
aINH-R, isoniazid-resistant strain; RIF-R, rifampicin-resistant strain; TAC-R, thiacetazone-resistant strain; EMB-R, ethambutol-resistant strain; CIP-R, ciprofloxacin-resistant strain; KAN-R, kanamycin-resistant strain; ETA-R, ethionamide-resistant strain; PAS-R, p-aminosalicylic-acid-resistant strain. Ratios of MICs against resistant and non-resistant strains with values of 1–4 indicate activity against the resistant strain within experimental error.
bND, no data available, or endpoints not attained in Table 2 data.
Compound 5 was also active against MDR-TB strains, including strains with resistance to additional TB drugs and quinolones (Table 4). The activity on the drug-susceptible strain and the four MDR-TB strains varied only 2-fold, which is within the variation of MIC determinations. One of the strains tested was resistant to isoniazid, rifampicin, streptomycin, ethambutol and pyrazinamide, indicating that quinoxaline 1,4-di-N-oxides will maintain activity on MDR and poly drug-resistant strains. Such activity is particularly important in light of the recent reports of XDR strains of TB.3–5,7–9,26 Overall, compound 5 was tested and found to be active on M. tuberculosis H37Rv, M. tuberculosis strain Erdman, M. bovis strain BCG Montreal (also a human pathogen), four independent clinical isolates of M. tuberculosis from China with multiple resistance phenotypes and eight SDR strains of M. tuberculosis (Table 2).
Table 4.
Activity of compound 5 and moxifloxacin control (mg/L) on drug-resistant clinical isolates of M. tuberculosis
| Straina |
|||||
|---|---|---|---|---|---|
| M10 | M13 | M14 | M70 | H37Rv | |
| DNA gyrase A mutation | none | Asp-94→Gly | none | Asp-94→Gly | none |
| INHb | R | R | R | R | S |
| RIF | R | S | R | R | S |
| STR | R | R | R | S | S |
| ETH | R | S | S | R | S |
| PZA | R | S | R | R | S |
| Moxifloxacin MIC (mg/L) | 0.5 | 2 | 0.25 | 2 | 0.2 |
| Compd. 5 MIC (mg/L) | 1.25 | 1.25 | 0.625 | 1.25 | 0.625 |
aStrains M10, M13, M14 and M70 were obtained from Dr A. Cheng, Department of Microbiology, Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, New Territories, Hong Kong, China.21
bINH, isoniazid; RIF, rifampicin; STR, streptomycin; ETH, ethionamide; PZA, pyrazinamide.
Compound 5 is likely activated via bioreduction in bacteria, similar to the reduction observed for other substituted N-oxides.27 Since PA-824, a nitroimidazole in clinical trials for treating TB,28 is bioreduced to an active intermediate,29–31 we tested the activity of compound 5 against an isogenic set of M. bovis strains with defined resistance to PA-824 (Table 5). PA-824 is bioreduced to an active form by a pathway involving the deazaflavin F420 cofactor-dependent glucose dehydrogenase (Fdg1)29 and other cellular factors including molybdopterin, a cofactor for many oxido-reductase enzymes. Thus, loss of function of Fdg1 or loss of the ability to synthesize the F420 cofactor leads to resistance to PA-824 (Table 5). Compound 5 was active on all PA-824-resistant M. bovis strains tested, thus showing the lack of cross-resistance and supporting a different pathway of drug activation.
Table 5.
Activity of compound 5 (µM) in the LORA and on M. bovis strains with defined resistance to PA-824, and M. tuberculosis strain H37Rv with and without a reporter gene
| Compound | PA-824 | Compd. 5 | Niclosamide |
|---|---|---|---|
| Structure | 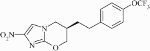 |
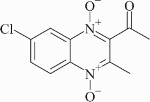 |
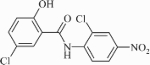 |
| LORA (RLU)a | 1.40 (4.85)b | 0.42 (1.65) | 0.32 (0.99) |
| Aerobic (RLU)c | 0.10 (0.35) | 0.31 (1.21) | 0.29 (0.87) |
| MTB H37Rvd | 0.032 (0.11) | 0.44 (1.74) | 0.64 (1.97) |
| BCGe WT | 0.035 (0.12) | 0.41 (1.63) | 1.02 (3.13) |
| fgd (F420-dependent) | >37.0 (>128) | 0.81 (3.19) | 0.53 (1.61) |
| moaA (molybdopterin synthesis) | >37.0 (>128) | 0.50 (1.97) | 0.61 (1.86) |
| fbi C (F420 synthesis) | >37.0 (>128) | 0.47 (1.84) | 0.51 (1.57) |
| moaD (molybdopterin synthesis) | >37.0 (>128) | 0.98 (3.87) | 0.62 (1.90) |
| pil 8f (Rv2627) | >37.0 (>128) | 0.75 (2.96) | 0.61 (1.87) |
| Comment | active in vivo; some activity in LORA | active in vivo; active in LORA | inactive in vivo; active in LORA |
aLORA (RLU): low oxygen recovery assay using relative light units.
bValues are in mg/L with micromolar values given in parentheses.
cAerobic (RLU): the same strain used in the LORA (MTB H37Rv containing a plasmid with an acetamidase promoter driving a bacterial luciferase gene) but incubated aerobically (5% CO2 enriched air) for 7 days and 100 µL culture transferred to white 96-well microtitre plates for determination of luminescence.
dM. tuberculosis strain Rv measured in MABA.
eBCG: M. bovis strain BCG Montreal measured in MABA as were all other M. bovis strains.
fpil 8: Rv2627, function unknown.
The antitubercular activity of compound 5 was concurrently tested against M. tuberculosis strain H37Rv using MABA and strain Rv containing the luciferase reporter, both under aerobic conditions; activity was comparable in both cases (Table 5). Niclosamide was included as another control compound that is structurally different from PA-824 and activated by a different pathway. Table 5 also shows that compound 5 is equally active on growing bacteria and non-replicating persistent (NRP) bacteria adapted to low oxygen in the LORA test. In this test, activity was 1.65 and 1.21 µM (0.42 and 0.31 mg/L) against growing and NRP bacteria, respectively. This is another unique and important property of quinoxaline 1,4-di-N-oxides that may translate to a faster sterilization of infected tissues. The long continuation phase for the treatment of TB is believed to be in part due to the presence of non-replicating organisms that persist even in the presence of antitubercular drugs. PA-824 is an experimental nitroimidazole that is in Phase I clinical trials. PA-824 is bioreduced by M. tuberculosis to an active component but unlike compound 5, PA-824 is only about 1/10 as active against NRP bacteria compared with aerobically growing cells.
The MBCs of several compounds against H37Rv were determined (Table 6). A compound is generally considered to be bactericidal if the ratio of MIC to MBC is ≤4;32 so, compounds 1, 5 and 8 could be considered to be bactericidal due to the low ratios obtained. On the other hand, compounds 3, 6 and 9, which showed higher MBC/MIC ratios for H37Rv, may be less bactericidal.
Table 6.
MBCs against H37Rv and SDR strainsa
| MTB H37Rv |
INH-resistant |
RIF-resistant |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | MIC (mg/L) | MBC (mg/L) | MBC/MIC | MIC (mg/L) | MBC (mg/L) | MBC/MIC | MIC (mg/L) | MBC (mg/L) | MBC/MIC |
| 1 | 3.13 | 6.25 | 2.00 | 3.13 | 3.13 | 1.0 | 3.13 | 3.13 | 1.0 |
| 3 | ≤1.56b | 12.5 | ≥8.00 | 6.25 | 6.25 | 1.0 | 3.13 | 3.13 | 1.0 |
| 5 | 0.78 | 0.78 | 1.00 | 1.56 | 0.78 | 0.5 | ≤0.39 | 0.78 | ≥2.0 |
| 6 | ≤3.13 | 12.5 | ≥4.0 | <3.13 | 12.5 | ≥4.0 | 6.25 | 6.25 | 1.0 |
| 8 | 3.13 | 6.25 | 2.0 | 6.25 | 6.25 | 1.0 | 3.13 | 3.13 | 1.0 |
| 9 | ≤3.13 | 12.5 | ≥4.0 | 100 | 50 | 0.5 | ≤3.13 | 6.25 | ≥2.0 |
aINH, isoniazid; RIF, rifampicin.
bOn occasion, the MIC dilution series was not diluted down far enough to capture the MIC in a given test, thus giving limits of the differential.
Compound 5 was chosen for evaluation in in vivo assays. The MTD of compound 5 was determined by using an escalating dose of drug given to mice by oral gavage. No adverse effects or reactions were observed at a dose of 500 mg/kg in this test of acute, single dose toxicity. Compound 5 was orally bioavailable as assessed in the bioassay method22 with an estimated blood level of ∼5 mg/L at 30 min post-oral dosing of mice with 200 mg/kg.
Preliminary in vivo evaluation of compound 5 was made at a dose of 300 mg/kg in infected GKO C57BL/6 mice.24 This compound afforded significant reductions of 2.7 and 2.82 log10 cfu in the lung and spleen tissues, respectively, versus the untreated controls. Compound 5 was bactericidal in vivo because the cfu present in the lung and spleen at the start of therapy (day 15) are lowered by greater than 2 logs (i.e. 99% killing) following 9 days of treatment. Visual inspection showed little lung pathology with a few small granulomas. Spleens appeared visibly normal and mice appeared normal and active. The efficacy of the compound was statistically better than controls (P < 0.05) and equivalent to the efficacy of isoniazid (P < 0.05). The protection shown by compound 5 is similar to clinically available compounds.33 In this same in vivo run, isoniazid at 25 mg/kg/day gave a reduction in cfu of 3.1 and 4.37 log10 in the lungs and spleen, respectively, and was bactericidal.
In a second experiment, the dose–response of compound 5 in vivo was determined using the GKO mouse model at doses of 25, 100 and 300 mg/kg (Table 7). Compound 5 was active in the lung and spleen at 100 and 300 mg/kg (P < 0.001), respectively. At 25 mg/kg, it was active in the spleen (P < 0.05) but not statistically active in the lung. Activity at 300 mg/kg dosing was striking in that it lowered the cfu by 4.04 and 5.19 log cfu, respectively, in the lung and spleen. Bactericidal activity was detected at the higher doses of 100 and 300 mg/kg (Table 7). No clear toxicity was apparent in the first in vivo experiment, while in the dose–response experiment, some toxicity was observed at the highest dose; two mice in the 300 mg/kg group died during treatment, which was truncated to 7 days instead of the usual 9 days. No cfu was recovered from the lungs of two of the surviving mice or from the spleen of one of the surviving mice dosed at 300 mg/kg, indicating that the organs may have been sterilized. Preliminary studies indicate that both in vitro (cytotoxicity) and in vivo toxicity can be separated from the antitubercular activity.
Table 7.
Activity of compound 5 (TAACF 118845) in the mouse low-dose aerosol model
| Sample (dose mg/kg) | Organ | Log cfu/organ (SD) | Log cfu decrease versus controls | Comments | |
|---|---|---|---|---|---|
| Exp. 1 | |||||
| untreated—d15 | lung | 6.81 (±0.09) | normal | ||
| spleen | 5.37 (±0.18) | normal | |||
| untreated—d24 | lung | 7.57 (±0.11) | normal | ||
| spleen | 6.57 (±0.17) | normal | |||
| Isoniazid (25) | lung | 4.47 (±0.12) | 3.10 | normal | |
| spleen | 2.20 (±0.45) | 4.37 | normal | ||
| 118845 (300) | lung | 4.88 (±0.21) | 2.70 | normal | |
| spleen | 3.75 (±0.18) | 2.82 | normal | ||
| Exp. 2 | |||||
| untreated—d15 | lung | 6.62 (±0.14) | normal | ||
| spleen | 4.42 (±0.52) | normal | |||
| untreated—d24 | lung | 7.76 (±0.10) | normal | ||
| spleen | 6.86 (±0.16) | normal | |||
| Isoniazid (25) | lung | 4.43 (±0.06) | 3.33 | normal | |
| spleen | 1.82 (±0.31) | 5.04 | 1 mouse w/o cfu | ||
| 118845 (25) | lung | 6.97 (±0.17) | 0.79 | normal | |
| spleen | 6.43 (±0.15) | 0.43 | normal | ||
| 118845 (100) | lung | 6.15 (±0.13) | 1.61 | normal | |
| spleen | 5.62 (±0.17) | 1.24 | normal | ||
| 118845 (300) | lung | 1.82 (±0.95) | 4.04 | toxicitya | |
| spleen | 1.67 (±0.55) | 5.19 | toxicityb |
d15, day 15 post-infection; d24, day 24 post-infection.
aData from only three mice. Two deaths due to apparent drug toxicity. Only one mouse with cfu, other two mice culture negative. Therapy stopped at 7 days. Slightly lethargic, hunched posture.
bData from only three mice. Two deaths due to apparent drug toxicity. Two mice with cfu, other mouse culture negative. Therapy stopped at 7 days. Slightly lethargic, hunched posture.
In conclusion, an extended evaluation of the in vitro and in vivo antimycobacterial activities of quinoxaline 1,4-di-N-oxide derivatives was performed. All of them displayed good inhibitory activity against resistant strains and only compound 9 showed a significant resistance in an isoniazid-resistant strain. Compounds 1, 5 and 8 can be considered to be bactericidal due to the low MBC/MIC ratios. Furthermore, compound 5 showed strong in vivo activity comparable to clinically used TB drugs, although a relatively high dose of compounds was required to obtain equivalent reductions in lung cfu. Overall, these data also suggest the importance of the chlorine group in position 7 of the benzene moiety. The activity of compound 5 is unique in that it is active on: (i) SDR strains; (ii) poly drug-resistant clinical isolates, including MDR-TB; and (iii) NRP mycobacteria. This latter activity may prove important for attaining cures in a shorter amount of time, since the presence of NRP bacteria is believed to be a major factor responsible for the prolonged nature of antitubercular therapy. Additional studies are planned to further assess the in vivo efficacy of compound 5 alone and in combination with other clinically used and antitubercular drugs in the standard mouse model of TB.
Funding
This research was funded in part by contracts N01-AI-95364 (Southern Research Institute/University of Illinois at Chicago) and N01-AI-95385 (Colorado State University) from the U.S. National Institute of Allergy and Infectious Diseases (NIAID). R. V. is indebted to the Navarra Government for a grant.
Transparency declarations
R. C. G. is a Program Officer with the National Institutes of Health, Bethesda, MD, an agency that funded portions of this work via research and development contracts. As a coauthor he participated in the design, execution and analysis of the data presented. J. A. M., A. J. L., S. G. F. and S.-H. C. receive funding via contracts from the U.S. National Institute of Allergy and Infectious Diseases (NIAID). Other than routine consultations with the Project Officer, there are no transparency issues to declare. The remaining authors have none to declare.
Acknowledgements
Antimycobacterial data were provided by the TAACF through research and development contracts. Portions of these data were presented in abstract form at the Gordon Conference on Tuberculosis and Drug Development (Oxford, UK, August 2007) and the 38th Union World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease, Cape Town, South Africa, November 2007.
References
- 1.Fact Sheet No. 104. World Health Organization; www.who.int/mediacentre/factsheets/fs104. (10 May 2008, date last accessed) [Google Scholar]
- 2.Espinal MA. The global situation of MDR-TB. Tuberculosis. 2003;83:44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Benatar SR. Extensively drug resistant tuberculosis—problem will get worse in South Africa unless poverty is alleviated. Br Med J. 2006;333:705. doi: 10.1136/bmj.333.7570.705-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahle UR. Extensively drug resistant tuberculosis—beware patients lost to follow-up. Br Med J. 2006;333:705. doi: 10.1136/bmj.333.7570.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 6.Goldman RC, Plumley KV, Laughon BE. The evolution of extensively drug resistant tuberculosis (XDR-TB): history, status and issues for global control. Infect Disord Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD, Wilkinson R. Extensively drug resistant tuberculosis—a serious wake-up call for global health. Br Med J. 2006;333:559–60. doi: 10.1136/bmj.38971.587222.AB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manissero D, Fernandez de la Hoz K. Extensive drug-resistant TB: a threat for Europe? Eurosurveillance. 2006;11:E060928. doi: 10.2807/esw.11.39.03056-en. [DOI] [PubMed] [Google Scholar]
- 9.Shah NS, Pratt R, Althomsons S, et al. Extensively drug-resistant tuberculosis—United States, 1993–2006 (Reprinted from MMWR 2007; 56: 250–3) J Am Med Assoc. 2007;297:1871–3. [Google Scholar]
- 10.Jaso A, Zarranz B, Aldana I, et al. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J Med Chem. 2005;48:2019–25. doi: 10.1021/jm049952w. [DOI] [PubMed] [Google Scholar]
- 11.Montoya ME, Sainz Y, Ortega MA, et al. Synthesis and antituberculosis activity of some new 2-quinoxalinecarbonitriles. Farmaco. 1998;53:570–3. doi: 10.1016/s0014-827x(98)00067-6. [DOI] [PubMed] [Google Scholar]
- 12.Ortega MA, Montoya ME, Jaso A, et al. Antimycobacterial activity of new quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide derivatives. Pharmazie. 2001;56:205–7. [PubMed] [Google Scholar]
- 13.Ortega MA, Sainz Y, Montoya ME, et al. Synthesis and antituberculosis activity of new 2-quinoxalinecarbonitrile 1,4-di-N-oxides. Pharmazie. 1999;54:24–5. [PubMed] [Google Scholar]
- 14.Ortega MA, Sainz Y, Montoya ME, et al. Anti-Mycobacterium tuberculosis agents derived from quinoxaline-2-carbonitrile and quinoxaline-2-carbonitrile 1,4-di-N-oxide. Arzneimittel-Forschung. 2002;52:113–9. doi: 10.1055/s-0031-1299866. [DOI] [PubMed] [Google Scholar]
- 15.Sainz Y, Montoya ME, Martinez-Crespo FJ, et al. New quinoxaline 1,4-di-N-oxides for treatment of tuberculosis. Arzneimittel-Forschung. 1999;49:55–9. doi: 10.1055/s-0031-1300359. [DOI] [PubMed] [Google Scholar]
- 16.Jaso A, Zarranz B, Aldana I, et al. Synthesis of new 2-acetyl and 2-benzoyl quinoxaline 1,4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Eur J Med Chem. 2003;38:791–800. doi: 10.1016/s0223-5234(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 17.Zarranz B, Jaso A, Aldana I, et al. Synthesis and antimycobacterial activity of new quinoxaline-2-carboxamide 1,4-di-N-oxide derivatives. Bioorg Med Chem. 2003;11:2149–56. doi: 10.1016/s0968-0896(03)00119-6. [DOI] [PubMed] [Google Scholar]
- 18.Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–9. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–6. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnier T, Eiglmeier K, Camus JC, et al. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA. 2003;100:7877–82. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng AFB, Yew WW, Chan EWC, et al. Multiplex PCR amplimer conformation analysis for rapid detection of gyrA mutations in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2004;48:596–601. doi: 10.1128/AAC.48.2.596-601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruppo V, Johnson CM, Marietta KS, et al. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50:1245–50. doi: 10.1128/AAC.50.4.1245-1250.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper AM, Dalton DK, Stewart TA, et al. Disseminated tuberculosis in interferon-gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenaerts AJM, Gruppo V, Brooks JV, et al. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob Agents Chemother. 2003;47:783–5. doi: 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SH, Warit S, Wan BJ, et al. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2007;51:1380–5. doi: 10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman RC, Plumley KV, Laughon BE. The evolution of extensively drug resistant tuberculosis (XDR-TB): history, status and issues for global control. Infect Disord Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]
- 27.Suter W, Rosselet A, Knusel F. Mode of action of quindoxin and substituted quinoxaline-di-N-oxides on Escherichia coli. Antimicrob Agents Chemother. 1978;13:770–83. doi: 10.1128/aac.13.5.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurenzi M, Ginsberg A, Spigelman M. Challenges associated with current and future TB treatment. Infect Disord Drug Targets. 2007;7:105–19. doi: 10.2174/187152607781001817. [DOI] [PubMed] [Google Scholar]
- 29.Choi KP, Bair TB, Bae YM, et al. Use of transposon Tn5367 mutagenesis and a nitroimidazopyran-based selection system to demonstrate a requirement for fbiA and fbiB in coenzyme F-420 biosynthesis by Mycobacterium bovis BCG. J Bacteriol. 2001;183:7058–66. doi: 10.1128/JB.183.24.7058-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi KP, Kendrick N, Daniels L. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F-420 and FO biosynthesis. J Bacteriol. 2002;184:2420–8. doi: 10.1128/JB.184.9.2420-2428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manjunatha UH, Boshoff H, Dowd CS, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:431–6. doi: 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White LE, Suling WJ, Ross LJ, et al. 2-Alkoxycarbonylaminopyridines: inhibitors of Mycobacterium tuberculosis FtsZ. J Antimicrob Chemother. 2002;50:111–4. doi: 10.1093/jac/dkf075. [DOI] [PubMed] [Google Scholar]
- 33.Lenaerts AJ, Johnson CM, Marrieta KS, et al. Significant increases in the levels of liver enzymes in mice treated with anti-tuberculosis drugs. Int J Antimicrob Agents. 2005;26:152–8. doi: 10.1016/j.ijantimicag.2005.04.011. [DOI] [PubMed] [Google Scholar]


