Abstract
The primary mode of prevention of adult disease from Streptococcus pneumoniae is vaccination with anti-capsular polysaccharide vaccine; however, its effects are less in the targeted older population than in young persons. Few studies have examined the mechanism behind this limited effectiveness. We have measured antibody concentrations and opsonization titers for multiple serotypes amongst both old adults and young, healthy controls. To avoid specificity problems associated with pneumococcal antibody ELISA, we absorbed the serum samples with c-polysaccharide and capsular polysaccharide of 22F type. Antibody concentrations were found to be similar for six out of the seven tested serotypes, while opsonization titers were significantly higher in six out of seven serotypes in the younger population. Antibody potency, as measured by the ratio of opsonization titer to antibody concentration, was found to be significantly higher for the younger subjects for all serotypes. We conclude that, while all ages of adults make similar concentrations of antibodies in response to pneumococcal vaccine, the effectiveness of those antibodies is significantly reduced in the older adult population.
Keywords: Streptococcus pneumoniae, vaccine, efficacy
1. Introduction
Streptococcus pneumoniae infection remains a major cause of morbidity and mortality amongst older adults [1], and it is associated with a substantial loss of independence [2]. Prevention through vaccination has been stressed as a mechanism to reduce disease burden [1,3]. Unfortunately, the effectiveness of the currently approved 23-valent pneumococcal polysaccharide vaccine (PPV23) becomes reduced as a person’s age increases above 75 years of age [4,5]. The original studies [6,7] that led to vaccine approval were performed on younger, healthier adults compared to the population currently recommended to receive pneumococcal vaccine.
It has been hypothesized that this decrement in vaccine protective efficacy might be due to an impaired immune response to vaccination in older adults. It has been previously reported that older adults have a less effective antibody response to PPV23 than do their younger counterparts [8–10]. Because anti-pneumococcal antibodies provide protection by opsonizing the bacteria, these studies investigated not only antibody production but also the opsonic potential of the immune sera from older adults compared to younger adults. However, due to difficulties with the assays, opsonic capacities were measured for a limited number of serotypes and led to variable results [8,10]. Also, none of these studies used the currently accepted, third generation enzyme linked immunosorbent assay (ELISA) [11,12] for quantitation of antibody response. Older-generation ELISAs were limited because they measured some antibodies that are not capsule-specific [13], limiting the reliability of reported antibody titers. It is therefore difficult to definitively determine with the available data if older adults’ poor vaccine immune response is due to less production of antibody or if the poor response is due to poor effectiveness of the produced antibodies.
To our knowledge, this is the first study to compare PPV23 antibody response between younger and older adults using the third-generation ELISA[11]. We will also examine the functionality of those serotype-specific antibodies produced using a multiplexed opsonization assay. The multiplexed assay allows us to measure opsonization of multiple serotypes across a large number of samples in an efficient manner. This type of large-scale testing of opsonization has not been feasible until recent advances in the opsonization assay were developed [14], thus the existing literature for studies on this scale is limited [8,15]. Our goal is to determine whether older adults actually produce fewer antibodies and to examine if those antibodies produced have reduced functionality.
2. Materials and Methods
Study preparations
Licensed pneumococcal polysaccharide vaccine (PPV23) was 23-valent vaccine from either Wyeth (PNU-IMMUNE® 23, Wyeth-Lederle Lab., Pearl River NY) or Merck (Pneumovax®, Merck, Whitehouse Station NJ) and contained 25 mcg of each serotype polysaccharide per 0.5 mL dose.
Clinical trial design
Older adult subjects were taken from the control-group subset of a previously reported randomized, double-blind, placebo-controlled multicenter trial of protein conjugated pneumococcal vaccine in community-dwelling elderly adults [16]. This group included subjects 65 years of age or older (median age 72 years). Subjects were excluded if they were high-risk (i.e. immunocompromised, splenectomized, or with advanced renal, hematologic or hepatic disease) or if they had received PPV23 within the last 5 years. This control-group subset received the usual dose of 0.5 mL of PPV23. Sera used in this analysis were drawn 28 days post vaccination.
Younger adult subjects were healthy volunteers, who were 45 years of age or younger. These subjects were recruited from laboratory research personnel from large university medical centers. Most subjects performed clinical duties and were not exposed to pneumococci in their work. All subjects had not previously received PPV23 and received 0.5 mL of PPV23 and serum samples were drawn approximately 28 days post vaccination.
Laboratory analysis
Enzyme Linked Immunoassay for IgG anti-PS antibody
The amount of IgG anti-capsular PS antibody was determined by a "sandwich" type 3rd generation pneumococcal antibody ELISA [11] using fresh solutions prepared with water from a Milli-Q UF water purification system (Millipore, Bedford MA) to minimize background signals. Briefly, the wells of medium binding microtiter plates were coated at 37°C with pre-determined concentration (usually 1–10 µg/ml) of capsular PS (ATCC, Rockville, MD) for 5 hr in PBS with 0.02% NaN3. Next, the plates were washed with TBS containing 0.1% Brij-35 (Sigma, St. Louis, MO). All serum samples (20 µl) were pre-absorbed with 5 µg C-PS (Statens Serum Institut, Copenhagen, Denmark) and 10 µg 22F-PS in a total volume of 1 ml of PBS containing 0.05% Tween-20 for 30 min at room temperature. Serum pool 89-SF was absorbed only with C-PS and used as the standard (Food and Drug Administration, Bethesda, MD). The pre-absorbed serum samples and 89-SF were serially diluted and aliquots were added to the PS coated microtiter wells. The plates were incubated for 2 hrs at room temperature and washed as described above. Next, alkaline phosphatase-conjugated goat antibody specific for human IgG (Biosource, Camarillo, CA) in PBS with 0.02% NaN3 was added for 2 hr at room temperature. The amount of enzyme conjugate immobilized to each well was determined with the addition of the p-nitrophenyl phosphate substrate (Sigma) in diethanolamine buffer and incubation at room temperature for 2 hr. The reactions were stopped with NaOH, and the optical density at 405 nm and 690 nm was measured using an ELISA microplate reader. The amount of antibody was determined by comparing the optical densities for each of the diluted serum samples to the optical density curve constructed with the standard sample (89-SF) at multiple dilutions.
Multiplexed quadruple-serotype opsonophagocytic killing assay (MOPA4)
MOPA4 was performed as described previously [14]. Briefly, opsonization assay target bacteria were prepared for 7 serotypes (serotypes 4, 6B, 7F, 9V, 14, 18C, 19F, and 23F). Each target bacteria has been made to be resistant to one of 4 antibiotics (optochin, streptomycin, spectinomycin, and trimethoprim) but susceptible to the other three. Frozen aliquots of target pneumococci were thawed, washed twice with opsonization buffer B {Hanks’ balanced salt solution (HBSS) with Mg/Ca, 0.1% gelatin, and 10% fetal bovine serum} by centrifugation (12,000 × g, 2 minutes), and diluted to the proper bacterial density (~2 × 105 cfu/ml of each serotype). Equal volumes of 4 bacterial suspensions that were chosen to be analyzed together, were pooled. All serum samples were incubated at 56°C for 30 minutes before serial dilutions in Opsonization Buffer B. Serially diluted serum (20 µl/well) was mixed with 10 µl of bacterial suspension in each well of round bottom 96-well plates (Corning Inc., Corning, NY). After 30 minute incubation at RT with shaking (mini orbital shaker, Bellco Biotechnolgy, Vineland, NJ) at 700 rpm, 10 µl of 3–4 week-old-rabbit complement (PelFreeze Biologicals, Rogers, Arkansas) and 40 µl of HL60 cells (4 × 105 cells) were added to each well. HL60 cells were differentiated to granulocytes by culturing in RPMI-1640 with 10% fetal bovine serum and 1 % L-glutamine and 0.8% dimethylformamide at a starting density of 4 × 105 cells/ml for 5–6 days. Plates were incubated in a tissue culture incubator (37°C, 5% CO2) with shaking at 700 rpm. After a 45 minute incubation, plates were placed on ice for 10–15 minutes and an aliquot of the final reaction mixture (10 µl) was spotted onto 4 different THY agar plates (Todd-Hewitt broth with 0.5 % yeast extract and 1.5% agar). When the fluid was absorbed into the agar, an equal volume of an overlay agar (THY with 0.75% agar and 25 mg/L of 2,3,5-tripheynyltetrazolium chloride) containing one of the four antibiotics was applied to each THY agar plate. After an overnight incubation at 37°C, the number of bacterial colonies in the agar plates was enumerated. Opsonization titers were defined as the exact serum dilution that kills 50% of bacteria, which was estimated by interpolating results. A detailed protocol is posted on a website (www.vaccine.uab.edu). To reduce analytical variations, all the samples were analyzed using identical reagent lots and within a short period of time.
Statistical Analysis
Antibody concentrations, opsonization titers, and the ratio of opsonization titers to antibody concentrations (as a measure of antibody potency) were graphically represented using reverse cumulative distribution curves (RCDC). Antibody potency was defined as the opsonization titer divided by the antibody concentration for each serotype. Geometric mean concentrations (GMC) for ELISA, geometric mean titers (GMT) for opsonization, and geometric mean potency (GMP) for antibody potency were calculated for each serotype for both age groups. A two-tailed Mann-Whitney U test was used to analyze differences between groups because data were non-normally distributed. Statistical analysis was conducted using NCSS and PASS (Number Cruncher Statistical Systems, Kaysville, Utah).
3. Results
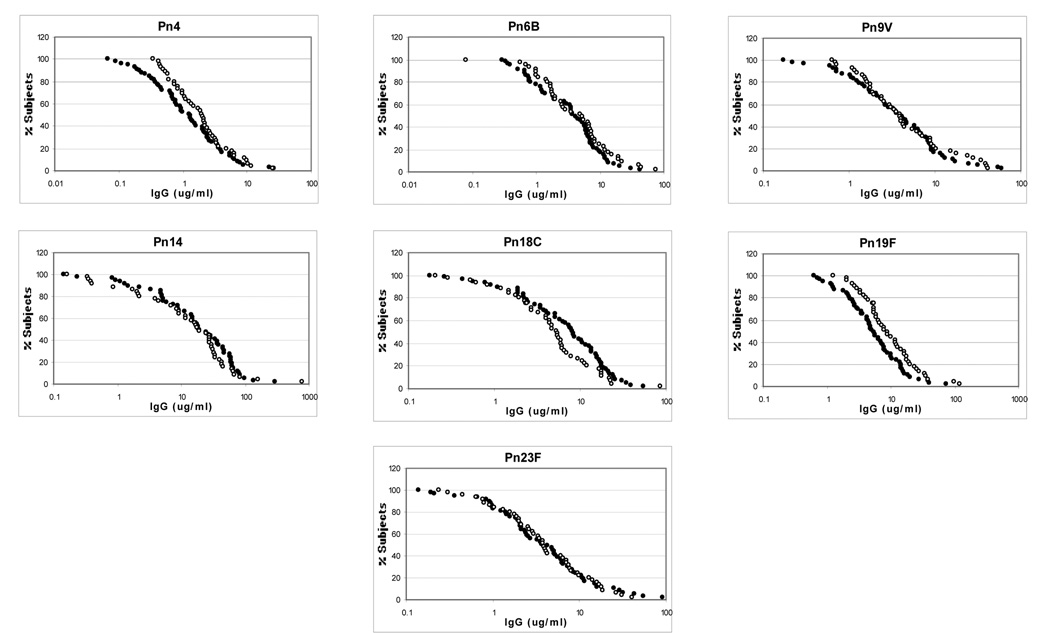
A total of 102 subjects were analyzed, 44 in the younger adults group and 58 in the older adults group. The RCDCs for serotype specific antibody concentrations are shown in Figure 1, and a summary of the mean concentrations, 95% confidence intervals (CI), and calculation of statistical differences are shown in Table 1. Antibody concentrations were similar for six out of the seven serotypes, with serotype 19F revealing higher antibody concentrations in the younger adult group as compared to the older adult group (GMC 9.14 µg/mL [CI 6.79-12.31] vs. 5.24 µg/mL [CI 3.99-6.88], p = 0.01).
Figure 1.
Reverse cumulative distribution curves for antibody concentration
Open circle = young adults
Closed circle = older adults
Table 1.
Geometric Means for Antibody Concentration, Opsonization Titers, and Antibody Potency
| Serotype | Age | IgG conc (ug/ml) |
Opsonic titer (OT) |
Antibody potency (OT/[IgG]) |
Antibody needed for 1:8 Ops. Index (ng/ml)f | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GMCa | 95% CId | Sige | GMTb | 95% CId | Sige | GMPc | 95% CId | Sige | |||
| 4 | Young | 1.84 | 1.35–2.51 | NSg | 1735 | 1074–2804 | p<0.01 | 969 | 612–1536 | p=0.02 | 7.7 |
| Old | 1.15 | 0.82–1.63 | 453 | 250–821 | 393 | 233–665 | 16.6 | ||||
| 6B | Young | 4.04 | 2.71–6.01 | NS | 1707 | 1046–2787 | p<0.01 | 405 | 247–665 | p<0.01 | 21.0 |
| Old | 3.16 | 2.29–4.35 | 377 | 188–756 | 119 | 67–214 | 43.6 | ||||
| 9V | Young | 4.25 | 3.01–6.01 | NS | 3957 | 1921–8151 | p<0.001 | 917 | 464–1810 | p<0.0001 | 7.2 |
| Old | 3.56 | 2.58–4.94 | 411 | 203–832 | 115 | 61–217 | 45.3 | ||||
| 14 | Young | 11.45 | 6.65–19.70 | NS | 3715 | 1888–7309 | p<0.01 | 343 | 177–665 | p<0.0001 | 30.6 |
| Old | 15.53 | 10.29–23.43 | 1250 | 717–2179 | 80 | 49–131 | 92.9 | ||||
| 18C | Young | 4.46 | 3.09–6.42 | NS | 1344 | 721–2505 | p=0.09 | 310 | 174–550 | p=0.02 | 21.5 |
| Old | 6.33 | 4.57–8.77 | 933 | 500–1740 | 149 | 100–221 | 36.6 | ||||
| 19F | Young | 9.14 | 6.79–12.31 | p=0.01 | 1254 | 707–2223 | p<0.01 | 135 | 81–227 | p=0.03 | 45.0 |
| Old | 5.24 | 3.99–6.88 | 266 | 133–532 | 51 | 28–93 | 89.9 | ||||
| 23F | Young | 3.79 | 2.63–5.47 | NS | 766 | 377–1554 | p<0.001 | 204 | 119–349 | p<0.0001 | 41.3 |
| Old | 3.69 | 2.59–5.26 | 147 | 76–285 | 40 | 25–64 | 160.0 | ||||
GMC = Geometric mean concentration
GMT = Geometric mean titer
GMP = Geometric mean potency
CI = Confidence interval
Sig = Statistical significance
Amount of antibody needed for 1:8 opsonization titer was obtained by dividing the antibody concentration (ng/ml) by the opsonization index and multiplying by 8. The median value was shown for each group.
NS = Not significant
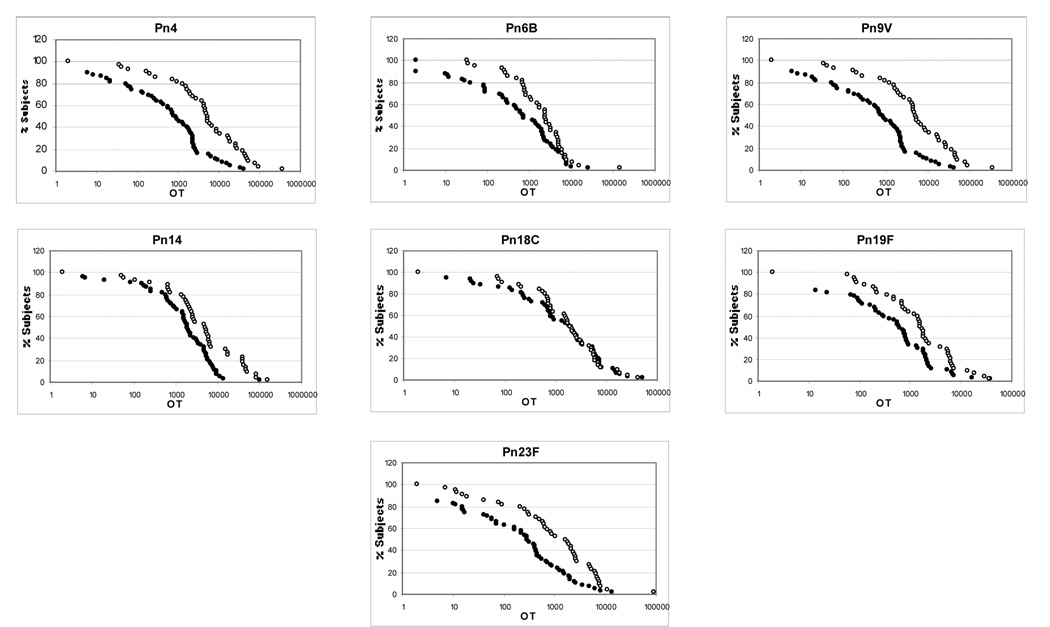
Opsonization titers were determined for each subject for each serotype. The RCDCs for each serotype specific opsonization titer are demonstrated in Figure 2. Numerical results are summarized in Table 1. Opsonization titers were significantly higher in the younger adult group in six of the seven serotypes, with serotype 18C opsonization titers being similar between the two groups (1343 [CI 721-2505] vs. 933 [CI 500-1740], p = 0.66).
Figure 2.
Reverse cumulative distribution curves for opsonization titers
Open circle = young adults
Closed circle = older adults
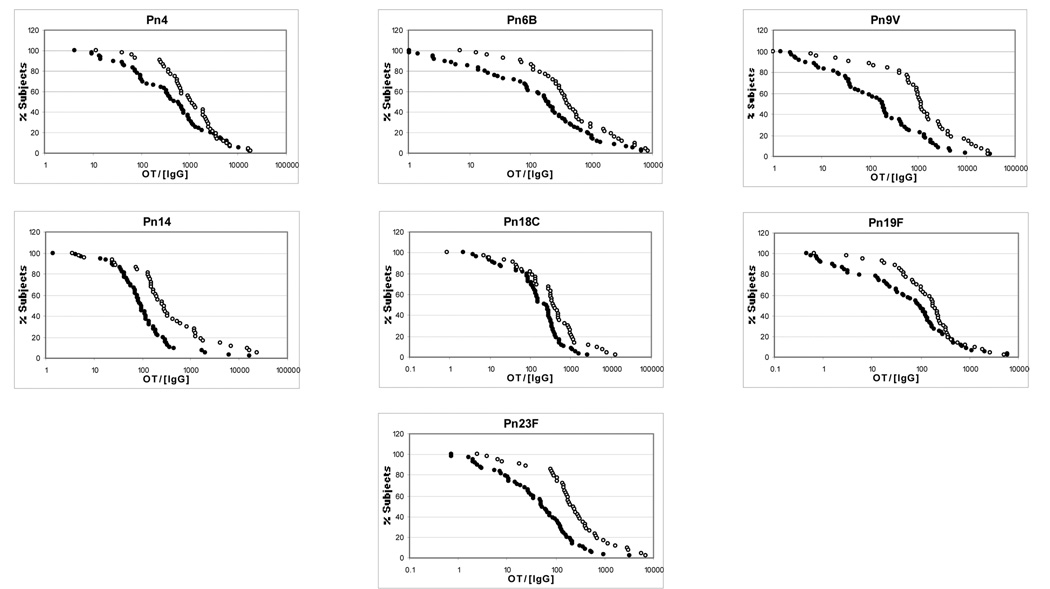
Measurements of antibody potency were made by dividing the opsonization titer by the antibody concentration for each serotype. Antibody potency RCDCs are shown in Figure 3 and the data is numerically shown in Table 1. Antibody potency was significantly higher in younger subjects for all seven serotypes (p<0.05 for all serotypes).
Figure 3.
Reverse cumulative distribution curves for antibody potency
Open circle = young adults
Closed circle = older adult
4. Discussion
To examine the functionality of the antibodies present after a PPV23 vaccination, we have correlated antibody concentration with opsonization indices as a measure of antibody potency for a large number of samples for many different serotypes. To our knowledge, this is the first report comparing the functionality of antibody response to PPV23 between younger and older adults using the third-generation ELISA assay method. Our data demonstrate that older adults (65 years and older) make similar quantities of anti-capsular pneumococcal antibodies as do younger adults (45 years old and younger), but that those antibodies are less functional and do not opsonize bacteria as efficiently. These findings are consistent with clinical data that have demonstrated limited vaccine efficacy amongst the older adult population [4,17–20]. It suggests that the antibodies produced in old adults may not have the same potency as those elicited in younger adults.
Our study has some limitations. First, subjects were administered vaccine produced by two different manufacturers. It is possible that the vaccines from the different manufacturers could stimulate different amounts of antibody or more functional antibody, and potentially alter our results. However, comparison of antibody responses between the two different vaccines revealed no differences in antibody titers (data not shown). Additionally, subjects were recruited for the younger group at a separate time from the older adults as they were not part of the same original study. Though the dates of collection were different, all samples were analyzed together to minimize technical differences in data generation. However, these differences are likely of limited importance because our conclusion is based on the differences between the two independent measures (ELISA vs. opsonization assay) of antibody response.
Our findings significantly extend the findings of previous studies. For instance, prior studies by Rubins [9,10] also showed inconsistent production of antibodies after vaccination. However, Rubins et al. only examined opsonization titers for one serotype, limiting the ability to make generalized conclusions based on that result. Romero-Steiner et al. have also reported that old adults produce less functional antibodies than do young adults [8]. However, the most significant limitation to all of these studies is that they employed the second generation ELISA, which could have over estimated antibody concentrations for old adults and may have reduced apparent functionality of antibodies. In addition, the Romero-Steiner study had a limited control group (n=12), potentially reducing the ability to make generalizations based on its findings. Our data, based on a large number of samples and serotypes, clearly demonstrate that even with similar antibody concentrations, there is significant functional limitation of those antibodies as evidenced by the universally reduced antibody potency.
We believe this definitive demonstration of low opsonic capacity of post-vaccination sera from old adults is critically important. It demonstrates that opsonization assay results are a more reliable predictor of the immune status than ELISA results. This conclusion would be expected since anti-capsule antibodies provide protection by opsonizing pneumococci for phagocytes. This conclusion is also supported by clinical experience as well. For instance, a conjugate vaccine induces young children to produce antibodies that are cross-reactive to serotype 19A by ELISA, but no cross-reactivity is seen when measured by an opsonization assay [21]. Dramatic increases in 19A prevalence since the introduction of the conjugate vaccine [22,23] clearly demonstrate that the opsonization assay is a more reliable indicator of immunity than is ELISA. In the studies of HIV infected adults and children [24,25], the two assays produced different results and opsonization assay results are now widely accepted as the true measure of immune status. Since opsonization assay is now quite easy to perform and has become largely standardized [26], we believe that the opsonization assay is an important analytical tool for studying pneumococcal vaccines among old adults.
There are several possible explanations for the poor antibody potency observed in the older adult subjects. It has been shown that older adults express a different antibody V region repertoire of both the light chains [27] and heavy chains [28] in response to pneumococcal vaccination. Whether these changes are functionally significant has not been well established, though this possibility certainly warrants further investigation. It has also been shown that there are significant changes in antibody glycosylation with aging [29–31] with a greater proportion of agalactosyl glycoforms of IgG produced in older subjects. Deglycosylation has further been shown to lead to a decrease in antibody binding to antigen [32], though this effect has not been specifically demonstrated for antibodies to pneumococcal capsule. Further, it has been shown that there are distinct populations of antibody-producing B cells, B-1 cells, that change with aging [33]. These B-1 cells, which may also exist in humans [34], produce antibodies that may enhance the specific B-cell immune response to an antigen challenge. Because the number of B-1 cells decrease with age, it is plausible that the reduction in antibodies produced by these cells could result in production of less effective anti-capsular antibodies.
We have demonstrated that while older adults tend to produce similar quantities of antibody in response to PPV23, the quality of those antibodies is different than those of younger subjects. We have extended the findings of prior studies which were limited by the use of older, less accurate procedural methods. Future work should be aimed at identifying the cause of this reduction in antibody function so that future vaccines can be designed to produce more effective antibodies in this at-risk population.
Acknowledgements
Supported in part by NIH grants 2T32HL07553, Basic Mechanisms in Lung Disease and AI-69695. We thank Ms. Patti Hall for her superb technical support and Drs. J. Treanor and M. Shelly for their continued interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 1997;46(RR8):1–24. [PubMed] [Google Scholar]
- 2.Chi RC, Jackson LA, Neuzil KM. Characteristics and outcomes of older adults with community-acquired pneumococcal bacteremia. J Am Geriatr Soc. 2006;54(1):115–120. doi: 10.1111/j.1532-5415.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- 3.Pneumococcal vaccines. WHO position paper. Wkly Epidemiol Rec. 1999;74(23):177–183. [PubMed] [Google Scholar]
- 4.Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 5.Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal Vaccination for Patients with COPD: Current Practice and Future Directions. Chest. 2008 doi: 10.1378/chest.07-0996. [DOI] [PubMed] [Google Scholar]
- 6.Austrian R, Douglas RM, Schiffman G, et al. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- 7.Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. Jama. 1977;238(24):2613–2616. [PubMed] [Google Scholar]
- 8.Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 9.Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999;67(11):5979–5984. doi: 10.1128/iai.67.11.5979-5984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubins JB, Puri AK, Loch J, et al. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178(2):431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 11.Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol. 2003;10(4):514–519. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Concepcion NF, Frasch CE. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–272. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Sun Y, Frasch CE, Concepcion N, Nahm MH. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin Diagn Lab Immunol. 1999;6:519–524. doi: 10.1128/cdli.6.4.519-524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13(9):1004–1009. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson LA, Neuzil KM, Nahm MH, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2007;25(20):4029–4037. doi: 10.1016/j.vaccine.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 16.Shelly MA, Schiff G, Smith V, et al. ISPPD-4. Finland: Helsinki; 2004. Evaluation of 9-valent CRM197 conjugated pneumococcal polysaccharide vaccine in elderly adults; p. 231. [Google Scholar]
- 17.Simberkoff MS, Cross AP, Al-Ibrahim M, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;315(21):1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 18.Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med. 1997;103(4):281–290. doi: 10.1016/s0002-9343(97)00149-6. [DOI] [PubMed] [Google Scholar]
- 19.Ortqvist A, Hedlund J, Burman LA, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351:399–403. doi: 10.1016/s0140-6736(97)07358-3. [DOI] [PubMed] [Google Scholar]
- 20.Honkanen PO, Keistinen T, Miettinen L, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine. 1999;17(20–21):2493–2500. doi: 10.1016/s0264-410x(99)00069-9. [DOI] [PubMed] [Google Scholar]
- 21.Yu X, Gray B, Chang SJ, Ward JI, Edwards KM, Nahm MH. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis. 1999;180:1569–1576. doi: 10.1086/315096. [DOI] [PubMed] [Google Scholar]
- 22.Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumoniae-- Massachusetts, 2001–2006. MMWR. 2007;56(41):1077–1080. [PubMed] [Google Scholar]
- 23.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 24.Madhi SA, Kuwanda L, Cutland C, Holm A, Kayhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J. 2005;24(5):410–416. doi: 10.1097/01.inf.0000160942.84169.14. [DOI] [PubMed] [Google Scholar]
- 25.Tarrago D, Casal J, Ruiz-Contreras J, et al. Assessment of antibody response elicited by a 7-valent pneumococcal conjugate vaccine in pediatric human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2005;12(1):165–170. doi: 10.1128/CDLI.12.1.165-170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nahm MH, Romero-Steiner S. Functional assays for pneumococcal antibody. In: Siber GR, Klugman KP, Makela PH, editors. Pneumococcal Vaccines. The impact of conjugate vaccine. Washington, D.C: ASM Press; 2008. pp. 213–226. [Google Scholar]
- 27.Smithson SL, Kolibab K, Shriner AK, Srivastava N, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable light chain repertoire. Infect Immun. 2005;73(11):7477–7484. doi: 10.1128/IAI.73.11.7477-7484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun. 2005;73(11):7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj J. 1997;14(3):401–405. doi: 10.1023/a:1018582930906. [DOI] [PubMed] [Google Scholar]
- 30.Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj J. 1998;15(7):683–689. doi: 10.1023/a:1006936431276. [DOI] [PubMed] [Google Scholar]
- 31.Kobata A. Glycobiology in the field of aging research--introduction to glycogerontology. Biochimie. 2003;85(1–2):13–24. doi: 10.1016/s0300-9084(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 32.Wright A, Tao M, Kabat EA, Morrison SL. Antibody variable region glycosylation: position effects on antigen binding and carbohydrate structure. EMBO.J. 1991;10:2717–2723. doi: 10.1002/j.1460-2075.1991.tb07819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohler H, Bayry J, Nicoletti A, Kaveri SV. Natural autoantibodies as tools to predict the outcome of immune response? Scand J Immunol. 2003;58(3):285–289. doi: 10.1046/j.1365-3083.2003.01314.x. [DOI] [PubMed] [Google Scholar]
- 34.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7(3):213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]