Abstract
Purpose
The purpose is to determine absorption mechanism of five bioactive prenylated flavonoids (baohuoside I, icariin, epimedine A, B, and C) present in heat-processed Epimedium koreanum Nakai (Yin Yanghuo).
Methods
Transport of five prenylated flavonoids present in heat-processed herbs were studied in the human intestinal Caco-2 model and the perfused rat intestinal model.
Results
In the perfused rat intestinal model, prenylated flavonoids with a monoglucosidic bond (e.g., icariin) was rapidly hydrolyzed into corresponding metabolites (e.g., baohuoside I). In the Caco-2 model, apical to basolateral permeability of a monoglycoside baohuoside I (1.46 ×10−6 cm/sec) was more than 2 folds greater than four prenylated flavonoids with 2 or more sugar moieties (<0.6×10−6 cm/sec). The slow apical to basolateral transport of baohuoside I was the result of efflux. This efflux was carrier-mediated and active since its transport was vectorial, concentration- and temperature-dependent with activation energies greater than 15 kcal/mol. Efflux of baohuoside I was significantly suppressed by inhibitors of BCRP and MRP2, whereas efflux of icariin was significantly inhibited only by p-glycoprotein inhibitor verapamil. Because YHH is often heat-processed for better efficacy, we determined and found the optimal condition for increasing contents of more bioavailable flavonoids (i.e., baohuoside I) to be 160–170°C for 5–7 min.
Conclusions
Poor bioavailability of prenylated flavonoids results from their poor intrinsic permeation and transporter-mediated efflux. Heat processing parameters may be optimized to preserve the herb’s bioavailable flavonoids, which help retain and improve its efficacy during processing.
Keywords: flavonoids, Caco-2, efflux, inhibitor, prenylated, Epimedium, heat-processing
INTRODUCTION
Prenylated flavonoids (e.g., icariin in Fig. 1) are widely distributed in the plant world and have a variety of biological effects including anticancer and anti-aging (e.g., alleviating aging-related degenerative diseases) (1–3). Prenylated flavonoids also serve as lead compounds to the development of novel estrogen receptor agonists and antagonists (4). Several prenylated flavonoids (e.g., 8-prenylated-naringenin) are also widely consumed because they are present in hops that are used to make beer (5,6).
Fig. 1.
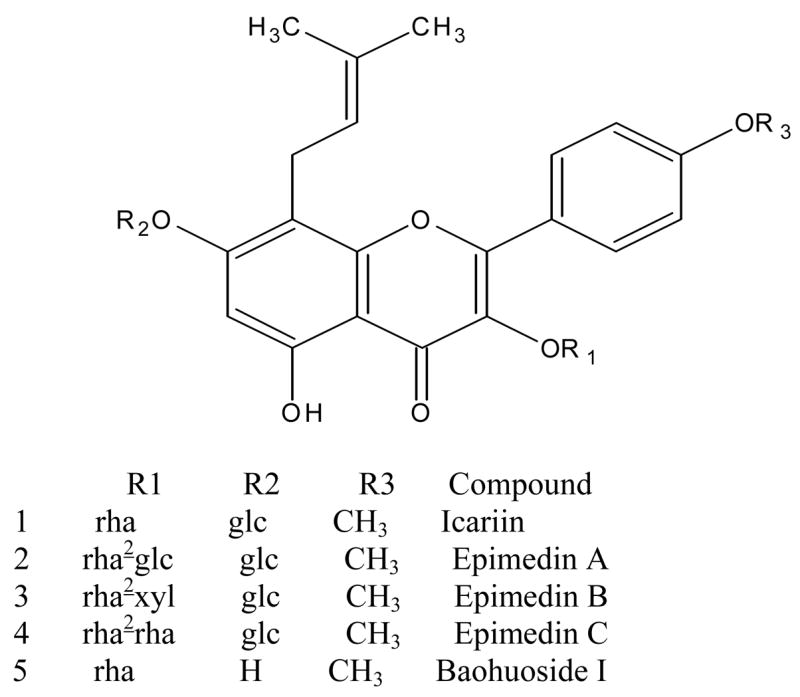
Chemical structures of five prenylated flavonoids isolated from Epimedium koreamum Nakai or Yin Yanghuo: icariin, epimedin A, epimedin B, epimedin C, and baohuoside I. The symbol “glc” refers to glucose, “rha” to rhamnose, and “xyl” to xylose.
Prenylated (specifically isopentenyl) flavonoids (Fig. 1) are also an important class of active ingredients in an important Chinese medicinal herb Yin Yanghuo (YYH) or Horny Goat Weed (Epimedium koreanum Nakai). YYH has been used for more than 2000 years, ever since it was first recorded in an ancient Chinese medicinal book titled “Shennong’s Classic of Materia Medica of China.” YYH remains to be widely used today because it has been shown to have a variety of biological activities including anti-cancer (7–9). Usually, YYH is processed by heating with sheep oil to increase efficacy, but it is not clear if the heat-processing process can be optimized to increase the efficacy of YYH. In any rate, quality of this herb is controlled by measuring the contents of total prenylated flavonoids and/or icariin presented in the herbs after heat processing, according to Chinese Pharmacopeia (10). Four isopentenyl-flavonoids that are diglycosides or triglycosides: icariin, epimedin A, epimedin B, and epimedin C, are the main flavonoids present in YYH, all with an isopentenyl group at 8-position and a glucose group at 7-O position and additional sugar moieties at 3-O position (Fig. 1). Another important prenylated flavonoid in YYH is baohuoside I (Fig. 1), which does not have glucose at the 7-OH position.
Despite these claimed biological activities, prenylated flavonoids have not been tested rigorously for their absorption and metabolism in appropriate models. They are expected to have poor bioavailability since most of the flavonoids have poor bioavailabilities (11). Similarly, bioavailability of these flavonoids in herbs such as YYH, which are dried by heating for the purpose of activity preservation and enhancement, had not been rigorously studied. Therefore, the purpose of this study was to determine the absorptive characteristics of baohuoside I and its four prenylated flavonoid analogs that are di- or tri-glycosides. Here, we used Caco-2 cell culture model and rat intestinal perfusion model to study the absorption mechanisms of prenylated flavonoids, because these models are routinely used to investigate drug absorption and are recognized by FDA as viable models of human intestinal absorption. These two models were also chosen because they have been used previously for the study of flavonoid glycoside, and it was found that the rat intestine expresses large quantities of glucosidases and can rapidly hydrolyze mono-glucosides (12–15). In contrast, Caco-2 cells have very poor expression of glucosidases, which are often not measurable in a 4 hour experiments (15).
MATERIALS AND METHODS
Materials
Cloned Caco-2 TC7 cells were a kind gift from Dr. Moniqué Rousset of INSERM U178, (Villejuit, France). Icariin (purity > 98%) was purchased from National Institute for the Control of Pharmaceutical and Biological Products (China). Epimedin A, epimedin B, epimedin C, and baohuoside I (all purity>98%) were provided by the Laboratory of Pharmaceutical Preparation (Jiangsu Provincial Academy of Chinese Medicine, China). MK-571 was purchased from Cayman Chemical Company (Ann Arbor, MI). Dipyridamole, verapamil, leukotriene C4 and Hanks’ balanced salt solution (HBSS; powder form) were purchased from Sigma-Aldrich (St. Louis, MO). Yin Yanghuo or Epimedium koreamum Nakai was purchased from a drug store in Nanjing (China) and was identified to be the correct species by Professor Dekang Wu, a pharmacognosy researcher at Nanjing University of Chinese Medicine (Nanjing, China). All other materials (typically analytical grade or better) were used as received.
Cell Culture
The Caco-2 TC7 cell line was developed in the laboratories of Dr. Moniqué Rousset of INSERM U178, (Villejuit, France) and is generally similar to wild-type Caco-2 cells (16). However, it is more stable during passage since it is a cloned cell line. The culture conditions for growing Caco-2 cells have been described previously (14,17,18). To seed the cells, we used 3 μm porous polycarbonate cell culture inserts from Nunc (Catalog number 137435, distributed by VWR International), which has a surface area of 4.2 cm2. The seeding density (100,000 cells/cm2), growth media (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum), and quality control criteria were all implemented according to previously published reports (14,17,18). Caco-2 TC7 cells were maintained at 37°C at 90% humidity, and 5% CO2. The culture media was changed every other day. The monolayers were ready for experiments from 19 to 22 days after seeding.
Transport Experiments in the Caco-2 Cell Culture Model
Experiments in triplicate were performed in pH 7.4 HBSS. The protocol for performing cell culture experiments was described previously (14,17,18). Briefly, the cell monolayers were washed three times with 37°C HBSS (pH 7.4). The transepithelial electrical resistance (TEER) values of cell monolayers were measured, and those values less than 425 ohms×cm2 (after subtracting the blank) were discarded. The monolayers were incubated with the buffer for 1 hr, and the incubation medium was then aspirated. Afterward, a solution containing the compound of interest was loaded on to the apical or basolateral (donor) side. The amounts of transported flavonoids in the receiver media were followed as a function of time using ultra-performance liquid chromatography or UPLC (methods to follow). Four donor samples (400μl) and four receiver samples (400μl) were taken at different times (typically 1 hr), followed by the addition of 400μl of fresh donor solution to the donor side or 400μl of fresh buffer to the receiver side. When comparing the permeability of five different compounds, each compound was used at the same concentration (10μM) and the samples were taken at 0, 1, 2, 3, and 4 hr after incubation. For studying the effects of temperature and inhibitors on transport and/or accumulation of baohuoside I, a concentration of 20μM was used and the samples were taken at 0, 2, 3, 4, and 5 hr to ensure that we are able to measure the receiver concentrations of baohuoside I accurately at lower temperature and in the presence of effective inhibitors. Similarly, for studying the effects of concentration on the transport of baohuoside I, samples were also taken at 0, 2, 3, 4, and 5 hr to ensure analytical accuracy at lower concentration (e.g., 5 μM). Lastly, transport inhibitors were loaded only at the donor side when they were used.
To each transport sample (400 μl), 100μl of acetonitrile containing 100μM of testosterone was added as an internal standard and preservative, and the resulting mixture was vortexed for 30 sec and then centrifuged at 15,000 rpm for 15 min, and the supernatant was analyzed by UPLC (see below).
Cellular Accumulation Studies in the Cell Monolayers
After the cell monolayers were incubated with prenylated flavonoids for a predetermined period of time (e.g., 4 or 5 hr), the mature monolayers were gently washed three times with ice-cold saline immediately after the incubation buffer was removed. Subsequently, the monolayers were cut out together with the porous polycarbonate membranes, put in 1 ml HBSS buffer (pH 7.4), and sonicated in an ice-cold water bath for 30 min using Aquasonic 150D sonicator (VWR Scientific, Bristol, CT) at the maximum power (135 average watts). The cellular lysates were then centrifuged at 15,000 rpm for 15 min so the supernatant can be injected into UPLC.
Animals
Male Sprague-Dawley rats (150–170 days old) weighing between 350 and 400 g were from Harlan Laboratory (Indianapolis, Indiana). The rats were fed with Teklad F6 rodent diet (W) from Harlan Laboratories (Indianapolis, Indiana). The rats were fasted overnight before the day of the experiment.
Animal Surgery
The procedures were approved by University of Houston’s Institutional Animal Care and Uses Committee. The intestinal surgical procedures were the same as our previous publication (19), in that we perfused the whole small intestine as one segment (from duodenum to ileum).
One Site Single-Pass Rat Intestinal Perfusion Experiment
A single-pass perfusion method was used by perfusing the small intestine as one whole segment (from duodenum to ileum). To keep the temperature of the perfusate constant, the inlet cannulae was insulated and kept warm by a 37°C circulating water bath. A flow rate of 0.382 ml/min was used, and the perfusate samples were collected every 30 min. The outlet concentrations of test compounds in the perfusate were determined by UPLC.
Heat Processing Temperature Change and Prenylated Flavonoid Contents in the Herb
Air-dried leaves of raw herb (Epimedium koreamum Nakai) were collected and ground to powders (about 40 mesh), which were then heated at different temperatures (160°C, 170°C, 180°C, 190°C, 200°C, and 210°C) for different lengths of time (0, 1, 3, 5, 7, 10, 15, 20, and 30min). Afterwards, prenylated flavonoids were extracted out according to a validated method from Chinese Pharmacopeia. Briefly, the powders were cooled to room temperature, and 0.2g of which was placed into conical flask. After 20 ml of 50% alcohol was added to the powder, it was vortexed and then sonicated for 1hr at room temperature. The supernatant was then filtered through a 0.2 um filter, and the filtrate was analyzed by HPLC (method below) for prenylated flavonoid contents.
UPLC Analysis of Transport Samples and Perfusion Samples
We used the same UPLC method to detect the metabolites of the perfused prenylated flavonoids and to determine the concentration of these flavonoids in the transport samples obtained from using the Caco-2 model. The conditions for UPLC analysis of flavonoids of transport samples and perfusion samples were as follows: system, Waters Acquity UPLC with photodiode array detector and Empower software; column, Acquity UPLC BEH C18, 1.7μm, 2.1×50mm (Waters, Milford, MA); mobile phase A, 90% 5μM ammonium acetate plus formic acid (pH 3.0), 10% acetonitrile; mobile phase B, 90% acetonitrile, 10%water; gradient, 0 to 0.3min, 5% B, 0.3 to 2.9min, 5% to 35% B, 2.9 to 3.8 min, 35% to 100% B; flow rate, 0.75ml/min; wavelength 268 nm; injection volume, 10μl. The retention times for epimedin A, epimedin B, epimedin C, icariin, baohuoside I were 1.95, 1.99, 2.06, 2.10, 3.23min, respectively. The UPLC chromatograms of perfusate samples were shown in Fig. 2. In general, these methods are selective and reproducible with day to day variability less than 3%. The accuracy and precision were greater than 98%. The tested linear response ranges for all flavonoids except for icariin were 0.3125 μM to 40 μM, whereas the tested linear response range was 0.3125 μM to 100 μM.
Fig. 2.
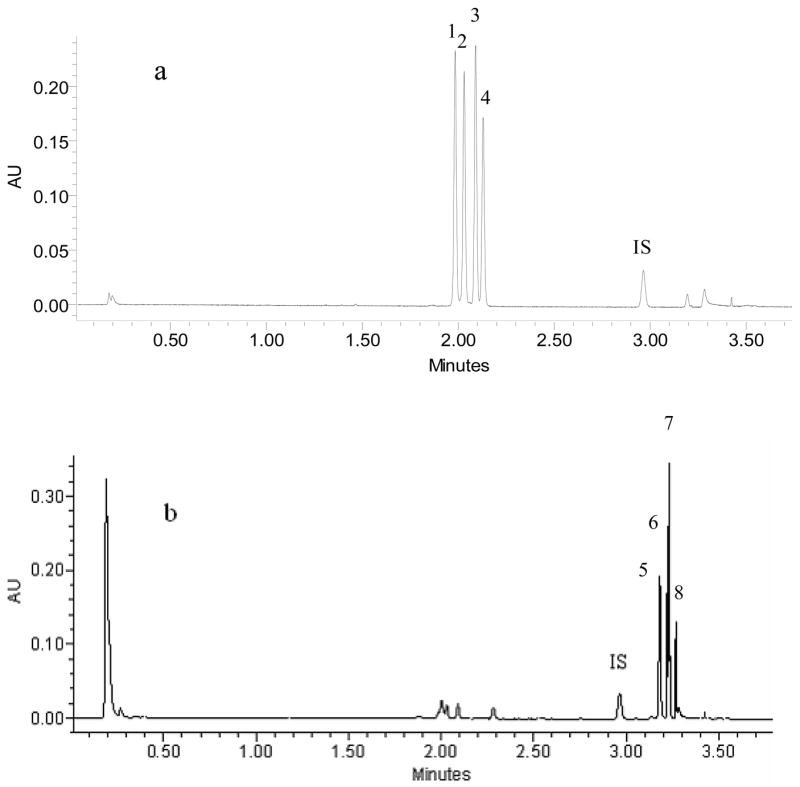
UPLC elution profiles of four 7-O-glucose-prenylflavonoids and their intestinal metabolites. In Fig. 2a. the UPLC profile represents a perfusate that contains 10μM each of epimedin A, epimedin B, epimedin C and icariin, which were labeled in the UPLC profile as peak number 1, 2, 3, and 4, respectively. In Fig. 2b. the profile is a representative perfusate samples after the perfusate present in Fig. 2a has undergone 2 hr single-pass rat intestinal perfusion as described in the method. The rat intestinal perfusate sample has 4 corresponding metabolites of 1, 2, 3 and 4, and only baohuoside I (peak number 8) is identified whereas the other three peaks are unidentified glucosidase metabolites of 1, 2 and 3. Testosterone was used as an internal standard (IS) in both panels.
HPLC Analysis of Prenylated Flavonoids in Heat-Processed YYH
HPLC was used to analyze the flavonoid contents of herbs subjected to heat processing, because this is close to the method used in Chinese Pharmacopeia. The actual conditions were as follows: Agilent 1100 with diode array detector running the ChemStation software; column, ZORBAX SB-C18, 5μm, 250×4.6 mm; mobile phase A (MPA), 100% acetonitrile; mobile phase B, water; gradient, 0 to 15min, 10% to 25% MPA, 15 to 40min, 25% MPA, 40to 63min, 25% to 71% MPA; wavelength, 268nm; flow rate, 1ml/min; and injection volume, 10μl. The retention times for epimedin A, epimedin B, epimedin C, icariin, and baohuoside I were 26.6, 28.3, 30.3, 33.1, and 56.6min, respectively (chromatogram not shown). The HPLC method has similar linear response range, accuracy and precision. Recoveries of prenylated flavonoids were greater than 99% (range 99.6–101.2%).
Data Analysis
Rate of transport is obtained from amount transported versus time curve using linear regression. The permeability of a compound is calculated using the following equation (Eq. 1):
| (1) |
Where V is the volume of the receiver (typical volume is 2.5 ml), S is the surface area of the cell monolayer (typical surface area is 4.2 cm2), C is the initial concentration, is the rate of concentration change in the receiver side, and is the rate of drug transport. The rate of drug transport is obtained by linear regression analysis (a Microsoft Excel function).
A modified Arrhenius equation (eq.2) was used to calculate the activation energy required for carrier-mediated transport:
| (2) |
Where Ea is the activation energy (kcal/mol), R is the gas constant (0.001987 kcal/mol·K), T (K) is the absolute temperature, and A is the Arrhenius constant.
Statistical Analysis
One-Way ANOVA with Tamhane’s post hoc analysis was used to analyze data for multiple comparisons when the variance was shown to be unequal because this method is less likely to generate type II errors. One-Way ANOVA with Tukey’s post hoc analysis method not used here to avoid type II errors. Unpaired Student’s t test (Microsoft Excel) was used to analyze the data when there were only two groups in the experiments. The prior level of significance was set at 5%, or p<0.05.
RESULTS
Metabolism of Prenylated Flavonoids in Rat Intestine
We found that 10μM icariin and three epimedin-7-0-glucosides (10μM epimedin A, 10μM epimedin B, and 10μM epimedin C) perfused through the rat small intestine were rapidly metabolized. As the time progressed, the amounts of epimedin-7-0-glucosides decreased, whereas the amounts of corresponding metabolites increased till most of the parent compounds disappeared and transformed to their corresponding metabolites, as shown by four major metabolite peaks in the chromatogram (Fig. 2). One of those four metabolites was isolated and identified as baohuoside I using an authentic standard of the compound (Fig. 2). Baohuoside I, which remains a flavonoid glycoside, has one less glucose than icariin and is a β-glucosidase-catalyzed hydrolytic metabolite of icariin (Fig. 1). Intestinal perfusion of pure baohuoside I did not produce any new metabolite (not shown), confirming an earlier observation that only mono-glucoside bond is sensitive to the rat intestinal glycosidase (20).
Absence of Metabolism of Prenylated Flavonoids in Caco-2 Cell Monolayers
We did not detect any metabolites of epimedin A, epimedin B, epimedin C, icariin or baohuoside I using the Caco-2 cells even after 5 hr of incubation. This is consistent with previous studies that showed low expression of β-glucosidase in these cells (21–23). Because of this lack of metabolism, we chose this cell culture model to determine the transport mechanisms of above five prenylated flavonoid glycosides.
Vectorial Transport of Prenylated Flavonoids in Caco-2 Cell Monolayers
Display of significant vectorial transport (i.e., much higher apical to basolateral vs. basolateral to apical or vice versa) is a reliable preliminary indicator that a transporter is involved in the transepithelial transport of a compound (15,18,23). Among the prenylated flavonoids studied, monoglycoside baohuoside I (10μM) was significantly (p<0.05) better transported than its diglycoside counter part icariin (10μM) or triglycoside analogs of icariin; epimedin A, epimedin B, and epimedin C (each @ 10μM) (Table 1). The absorptive transport rates of baohuoside I were the fastest, followed by icariin, epimedin C, epimedin A, and epimedin B. And as expected, apical to basolateral (or absorptive) permeabilities of epimedin A, epimedin B, epimedin C, and icariin were found to be much lower than baohuoside I. The absorptive permeability (Papp) of baohuoside I (Papp=1.46×10−6 cm/s) was at least 2 (range: 2~6) fold higher than its 7-O-glucoside analogs, which had permeability values ranging from 0.276×10−6 to 0.591×10−6 cm/s. Overall, these prenylated flavonoids, because they are glycosides, were not highly permeable since the absorptive permeability of the most permeable baohuoside I was even slightly smaller than that of paracellular transport marker mannitol (Papp=1.7×10−6 cm/s) (16).
Table 1.
Permeabilities (P) and Efflux Ratios of Prenylated Flavonoids⋆
| Compound(10μM) | PAB(cm/s) | PBA(cm/s) | Efflux Ratio | ||
|---|---|---|---|---|---|
| Mean·10−6 | SD·10−7 | Mean·10−6 | SD·10−7 | PBA/PAB | |
| Epimedin A | 0.322 | 0.39 | 1.59** | 1.94 | 4.94 |
| Epimedin B | 0.276 | 0.20 | 1.33* | 1.90 | 4.82 |
| Epimedin C | 0.423 | 0.025 | 1.20** | 1.05 | 2.85 |
| Icariin | 0.591 | 0.091 | 2.36*** | 0.71 | 3.99 |
| Baohuoside I | 1.460 | 2.02 | 14.30*** | 1.25 | 9.84 |
Experiments were performed in triplicate at 37°C, pH 7.4 HBSS. Donor and receiver volumes were 2.5 ml. The sample taken time was 0, 1, 2, 3, 4 hr after incubation. Absorptive permeability was expressed as PAB, whereas secretory permeability was expressed as PBA. Data are expressed as mean ± SD (N=3). One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically. The statistically significant difference (as shown by the “*” symbol) is between the secretory permeabilities and absorptive permeabilities of the prenylated flavonoids. The level of significance is signified by
p<0.001,
p<0.01, and
p<0.05.
In contrast, basolateral to apical (or secretory) transport permeabilities of all five prenylated flavonoids were much faster than their absorptive transport permeabilities (Table 1). There were also significant differences in secretory permeabilities among the five flavonoids, and once again secretory permeability of baohuoside I was again much higher (p<0.05) than the other four flavonoids. The rank order of the permeability values of the five flavonoids were baohuoside I> icariin> epimedin A > epimedin B > epimedin C. Because baohuoside I, which is a monoglycoside derived from icariin (via the action of glucosidases in the rat intestine), had substantially higher (≈10 times, p<0.05) secretory permeability than absorptive permeability (p<0.001), further studies were performed to determine the mechanisms by which baohuoside I is transported across the Caco-2 cell monolayers.
Concentration-Dependent Transport
The results showed that an increase in apical concentrations of baohuoside I from 5 to 20μM increased (p<0.05) the absorptive permeability (PAB), but at 40μM, the PAB value decreased (Fig. 3). In contrast, the secretory permeability (PBA) values decreased significantly (p<0.05) as the concentration increased (Fig. 3). As a consequence, the efflux ratio (defined as the ratio of the secretory permeability to the absorptive permeability or PBA/PAB) decreased from ~16.6 to ~4.7 as the concentration of baohuoside I increased from 5 to 40μM.
Fig. 3.
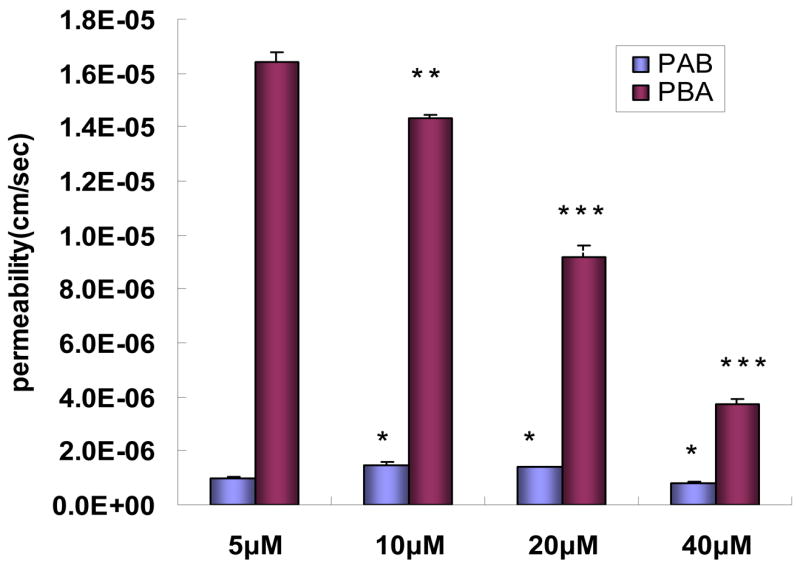
Permeabilities of baohuoside I at different concentrations (from 5μM to 40μM). The experiments were performed at 37°C. Five samples were taken time at 0, 2, 3, 4, 5 hr after the start of the experiments. The amounts transported were then plotted against sampling time (2, 3, 4, and 5 hr), which always generated linear curves, the slopes of which are rates of drug transport. The rates of transport were then used to calculate permeabilities using equation (1) and the calculated permeabilities are plotted here as bars. Each bar represents the average of three determinations and the error bars are the standard deviation of the means. The “*” symbol indicates a statistically significant difference between permeability at 5μM (control) and that at a higher concentration. The number of “*” symbol indicates the level of significance with ***p<0.001, **p<0.01 and *p<0.05. One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
Temperature-Dependent transport
Carrier-mediated transport may be energy- dependent or independent (e.g., facilitated). Higher activation energy (>15 kcal/mol) is commonly associated with active transport, whereas lower activation energy (<10kal/mol) is associated with passive diffusion or facilitated transport. The results showed that both of the absorptive permeability (PAB) and secretory permeability (PBA) values of 20μM baohuoside I transport decreased significantly (p<0.05) when the temperature was lowered (Figure 4). At 37°C, the absorptive permeability was 5 times higher than that at 15°C, whereas the secretory permeability was approximately 12 times higher. At 4°C, the transport was so slow that the absorptive permeability and secretory permeability was not measurable. The apparent activation energies obtained using the Arrhenius equation were 16.4 and 19.6 kcal/mol for absorptive transport and secretory transport, respectively.
Fig. 4.
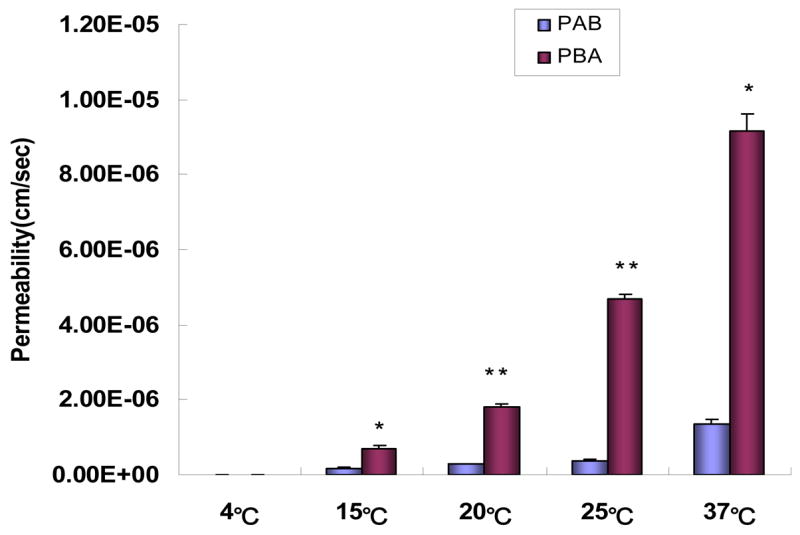
Permeabilities of baohuoside I (20μM) at different temperatures. The experiments were performed at 37°C. Permeabilities plotted here are similarly generated as described in Fig. 3. Each bar represents the average of three determinations and the error bars are the standard deviation of the means. The “*” symbol indicates that absorptive permeability (PAB) and secretory permeabilities (PBA) values at this concentration are significantly different from each other (p<0.01). One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
Inhibition Study
Two multidrug resistance-related protein (MRP) inhibitors leukotriene C4 (LTC4) and MK571, one breast cancer resistance protein (BCRP) inhibitor dipyridamole (24), and one p-glycoprotein inhibitor verapamil were used to determine which transporters might be involved in the transepithelial transport of baohuoside I. Additional studies were performed using icariin for the purposes of contrasting the results with that of baohuoside I. These inhibitors were chosen even though there is a possibility of cross talk (i.e., some inhibitors may inhibit more than one transporter). However, we recognized that there usually is some discrepancy as to the 50% effective concentrations (or IC50 value).
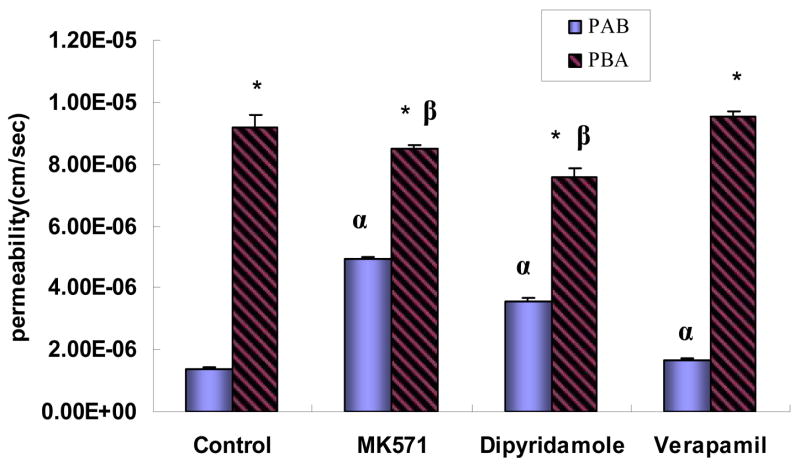
When an inhibitor is highly effective against an efflux transporter, the value of efflux ratio is expected to decrease significantly. The results (Fig. 5) indicated that in the presence of 50μM MK-571 the absorptive permeability of 20μM baohuoside I was 3.5 times higher than that of the control group, whereas the secretory permeability was reduced slightly (p>0.05), resulting in an efflux ratio reduction from 6.6 to 1.7. In the presence of 25μM dipyridamole, the absorptive permeability of 20μM baohuoside I was increased 2.5 times and the secretory permeability was reduced 20%, resulting in a 68% reduction in the efflux ratio. On the other hand, in the presence of 20μM verapamil, neither the absorptive permeability nor secretory permeability changed much (the efflux ratio only reduced 15%), indicating that this inhibitor was not effective.
Fig. 5.
Effects of various potential inhibitors on permeabilities of baohuoside I (20μM). The experiments were performed at 37°C. The inhibitors were present at the donor side of the cell monolayers at the stated concentration (50 μM for MK-571, 25 μM for dipyridamole, and 20 μM for verapamil). Permeabilities plotted here are similarly generated as described in Fig. 3. The “*” symbol indicates that PAB and PBA are significantly different (p<0.05) from each other. The symbol “α” indicates that the absorptive permeability (PAB) of baohuoside I in the presence of an inhibitor is significantly different (p<0.05) from that of the control, whereas the symbol “β” indicates the same for its secretory permeabilities (PBA). One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
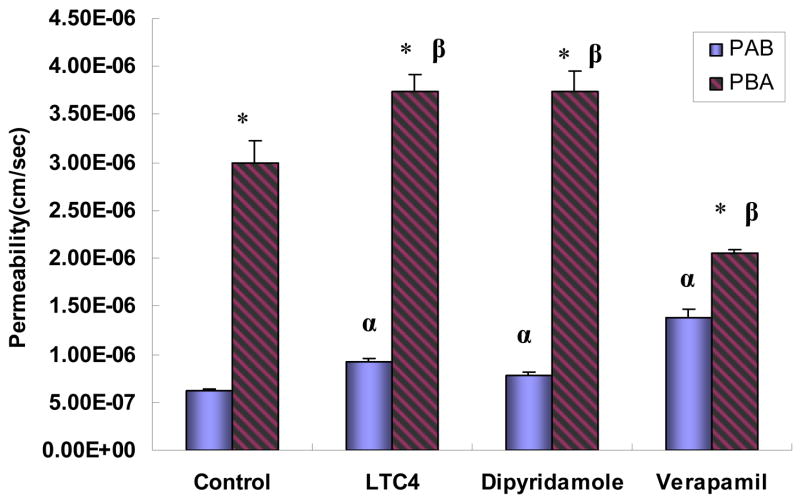
Parallel inhibition studies using 20μM icariin (Fig. 6) showed that both absorptive permeability and the secretory permeability increased and the efflux ratio remained about the same in the presence of MRP inhibitor LTC4 and BCRP inhibitor dipyridamole. However, in the presence of 20μM verapamil, the absorptive permeability significantly increased, whereas secretory permeability significantly decreased, which resulted in a 70% reduction in the efflux ratio (from 4.88 to 1.49).
Fig. 6.
Effects of various potential inhibitors on permeabilities of icariin (20μM). Permeabilities plotted here are similarly generated as described in Fig. 3. Absorptive permeability is expressed as PAB and secretory permeability is expressed as PBA. The experiments were performed at 37°C. The inhibitors were present at the donor side of the cell monolayers at the stated concentration (50 μM for MK-571, 25 μM for dipyridamole, and 20 μM for verapamil). The “*” symbol indicates that PAB and PBA are significantly different (p<0.01) from each other. The symbol “α” indicates that the absorptive permeability (PAB) of icariin in the presence of an inhibitor is significantly different (p<0.05) from that of the control, whereas the symbol “β” indicates the same for its secretory permeabilities PBA. One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
Effect of Concentrations on the Cellular Accumulation of Baohuoside I
Cellular accumulation of baohuoside I was assessed as a function of concentration (5μM~40μM). The results showed that both apical loading and basolateral loading of this flavonoid significantly raised its cellular levels and that the accumulation increased with the increasing concentration, suggesting no saturation of cellular accumulation (Fig. 7). The cellular accumulation was higher for basolateral loading than apical loading (Fig. 7), although the effects were not as pronounced as the difference in vectorial transport (Fig. 3).
Fig. 7.
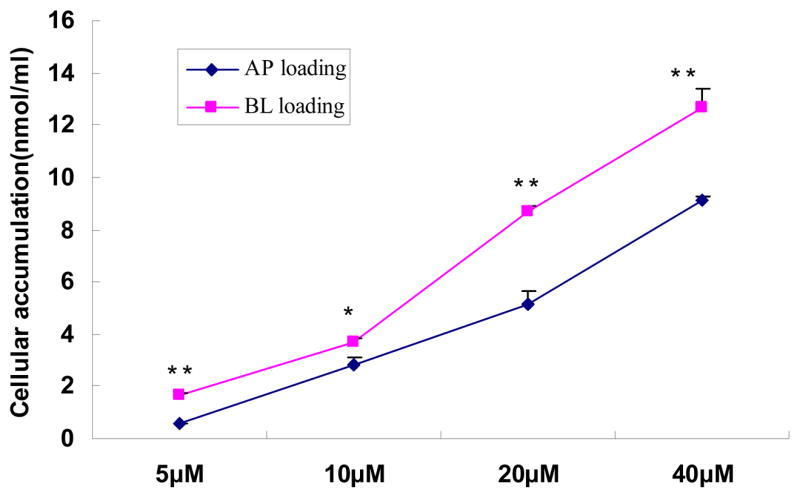
Effect of concentration on the cellular accumulation of baohuoside I at 37°C. The accumulation was measured after 5 hr incubation of cell monolayers with baohuoside I. Each data point is the average of three determinations, and the error bars represent the standard deviation of the mean. The “*” symbol indicated statistically significant difference between loading side. One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically.
Effect of Inhibitors on the Cellular Accumulation of Baohuoside I
We also determined intracellular accumulation of baohuoside I in the presence of verapamil, MK-571, and dipyridamole (Table 2). The result indicated that all three inhibitors significantly increased (at least 220%) the intracellular drug concentration when loaded from the apical side, but the effect of MK-571 was the most profound. Unlike apical loading, inhibitors had less effect on enhancing cellular accumulation from basolateral loading (at most 203%) and only MK-571 was effective, indicating that verapamil and dipyridamole was not interfering with the function of MRP2.
Table 2.
Effects of Transporter Inhibitors on the Cellular Accumulation of Baohuoside I
| Cellular accumulation(nmol/mg⋆)
|
|||
|---|---|---|---|
| Inhibitor | Concentration (μM) | Apical loading (mean ±SD) | Basolateral loading (mean ±SD) |
| None(control) | - | 5.167±0.453 | 8.698±0.212Δ |
| MK571 | 50 | 17.301±1.000* | 17.669±0.523* |
| Dipyridamole | 25 | 11.589±0.971* | 12.701±1.960* |
| Verapamil | 20 | 11.410±1.119* | 10.392±1.134 |
The cellular accumulation of baohuoside I (20μM) was measured after four hour incubation with prenylated flavonoids. The obtained values were expressed as nmol per mg since each cell monolayer has about 1 mg protein. Data are expressed as mean±SD (N=3). One-way ANOVA with Tamhane’s post hoc was used to analyze the data statistically. The “*” symbol indicates that the permeability PAB or PBA of baohuoside I in the presence of inhibitor is significantly different from that of the control. The “Δ” symbol indicates that PAB and PBA are significantly different in a particular experiment.
Effects of Processing Temperature on Herbal Prenylated Flavonoid Content
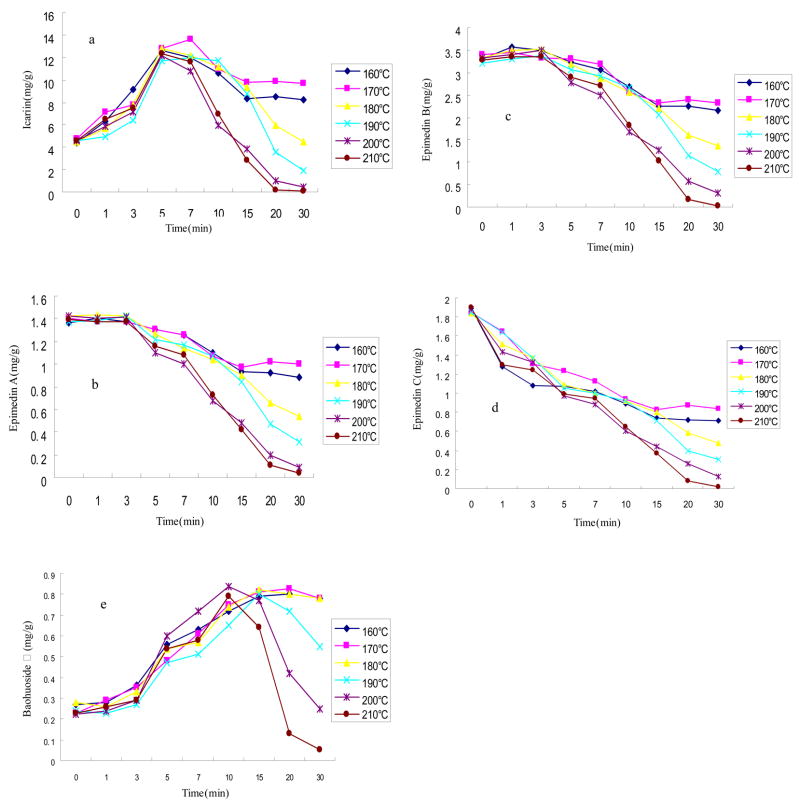
Since YYH is often used after heat-processing in Chinese traditional clinics to enhance efficacy, we determined here how heat processing affects the contents of different prenylated flavonoids. This is important in light of previous studies that showed substantial differences in permeabilities of different prenylated flavonoids. The results indicated that as the processing temperature was raised from 160°C to 210°C, the contents of icariin (Fig. 8a) and baohuoside I (Fig. 8e) increased first and then decreased with (heating) time, whereas the contents of epimedin A, epimedin B, and epimedin C appeared to decrease singularly with (heating) time (Fig. 8b, Fig. 8c and Fig. 8d). The icariin contents were the highest when the heating temperature was between 160°C ~170°C and the heating time was between 5~7min (Fig. 8a). The baohuoside I contents were the highest with temperature between 170–180°C, and heating time of 10–15 minutes (Fig. 8e). Because higher contents of baohuoside I are desired, which may be derived from either baohuoside I from the herb or from intestinal hydrolysis of icariin, heat processing procedures that will produce most of these two chemicals are considered better than others. Therefore, a heating temperature of 160–170 for 5–7 minutes appeared to be the best for maximizing the contents of icariin and baohuoside I in YYH.
Fig. 8.
Effects of processing temperature and time on the prenylated flavonoid content in Yin Yanghuo (Epimedium koreamum Nakai). The drug powder was heated at different temperature (from 160°C to 210°C)and/or for different length of time (from 0–30 min) and the contents of icariin (a), epimedin A (b), epimedin B (c), epimedin C (d), and baohuoside I (e) were analyzed using HPLC and plotted against the duration of the heating.
DISCUSSION
Our study provide strong evidence to show for the first time that prenylated flavonoids are likely to be poorly bioavailable because they have low absorptive permeability (PAB). The low PAB appears to be the result of apical efflux via BCRP and MRP2 (for baohuoside I) or the result of poor intrinsic permeability (for icariin, epimedin A, epimedin B and epimedin C), which is further limited by apical efflux transporters such as p-glycoprotein. Because heat processing affects the quantities of five main active prenylated flavonoids in YYH, an optimized method of heating can be developed to enrich more absorbable bioactive flavonoids such as icariin or baohuoside I.
This is the first in-depth investigation of transport of prenylated flavonoids, and the results clearly indicate that epimedin flavonoid di- or triglycosides, with sugars attached to two different phenolic groups on the flavonoid ring (Fig. 1), are poorly permeable (Table 1). Removal of a glucose molecule (from 7-O position of icariin) makes the resulting molecule (baohuoside I) more permeable (Table 1) but retention of the sugar at the 3-O group means that baohuoside I remains to be extensively effluxed (Fig. 5). Earlier investigation suggests that icaritin, which does not have the sugar moiety at the 3-OH group, is also more active than icariin (2), possibly because icaritin is more permeable than icariin. Therefore, our data support the hypothesis that prenylated flavonoid glycosides are poorly bioavailable because they have poor absorptive permeability. This is consistent with works of Walle and co-workers and our own earlier investigations which showed poor absorptive transport of other flavonoid glycosides (15,20,22,23). Whereas removal of all sugar moieties from the prenylated flavonoid could further improve their permeabilities and potentially their biological activities, attempts to conduct studies using an aglycone (i.e., no sugars attached to the ring) of icariin proved to be unfruitful because the aglycone is not soluble enough in water (<0.5 μg/ml). Therefore, one sugar moiety may be needed for this class of compound to remain soluble in water, a necessary prerequisite for absorption. Furthermore, the presence of this sugar moiety could make the flavonoid monoglycosides for glucose transporter as shown previously (14,21). In the present study, however, eliminating glucose from the apical buffer did not increase the transport of baohuoside I (not shown).
We believe the second reason for prenylated flavonoids to be poorly bioavailable is because they are extensively effluxed by the apically located efflux transporters. These efflux transporters are active since transport of baohuoside I was strongly dependent on temperature (Fig. 4). Inhibition studies indicate that baohuoside I was effluxed via BCRP and MRP2 (Fig. 5 and Table 2), two active transporters well known for their intestinal efflux functions.
Interestingly, the main transporter responsible icariin efflux was p-glycoprotein whereas the main transporters responsible baohuoside I efflux were MRP2 and BCRP (Fig. 5 and Fig. 6). It is apparent here that a deletion of 7-O-glucose from baohuoside I structure (Fig. 1) will change the transporter responsible for its efflux. Whereas it has been known for a while that MRP2 is responsible for glycoside efflux (23), it has not been reported in literatures that addition of an additional glucose to flavonoid will result in change in the efflux transporter. The later is not a total surprise, however, as p-glycoprotein is shown to be responsible for the transport of digoxin, a triglycoside, in Caco-2 cells (25,26). On the other hand, BCRP remains to be the transporter partially responsible for the efflux of baohuoside I but not icariin, suggesting that the functions of efflux transporter BCRP is also sensitive to glycosidic structures of its substrates. The fact that flavonoids are effluxed by BCRP, however, is consistent with earlier investigations by Morris and co-workers that showed BCRP is also responsible for efflux of several flavonoid aglycones (27,28).
Because of the differences in permeabilities of different prenylated flavonoids shown in this study and activities of different prenylated flavonoids shown elsewhere (2), we are confident that it is necessary to develop evidenced-based heat-processing method for Chinese herbs such as YYH. As a developing country, herbs remain a big if not dominant player in medical cares in China and accounts for approximately 50% of domestic pharmacy market. Vast majority of herbs need to be processed before they are used in clinics. A popular method of processing is heating, which is used to maintain or enhance its effectiveness and reduce the toxicity. Heating also can kill bacteria and other microorganisms and reduce significantly the aqueous contents of the herbs, making transportation and storage easier. Previously, heating of the raw herbs are accomplished by traditional cooking-like method such as stir-fried, fried with adjuvant material, or hot steam. Because heating is likely to cause chemical changes, many researches attempted to explain how heat processing affects pharmacological efficacy and correlated to their phytochemical changes (29–31). This is the first report that investigates how heat processing affects the intestinal absorption and metabolism of phytochemicals. Our results show that heat processing has significant impact on phytochemical composition in herbs and possibly other plant materials. Assuming icariin and baohuoside I are active components in YYH, the results show that it is possible to optimize processing parameter to obtain the highest amounts of more bioavailable or other desirable phytochemicals in an herb.
In conclusion, the present studies clearly demonstrate for the first time that poor bioavailability of prenylated flavonoids in glycoside form is the result of poor intrinsic permeability and efflux by apical efflux transporters such as MRP2, BCRP and p-glycoprotein. The presence of 3 and 7-OH sugar moiety was unexpectedly shown to determine which transporter (MRP2/BCRP or p-glycoprotein) will mediate the apical efflux of these prenylated flavonoids. Because presence of sugar moiety will affect solubility and pharmacological effect, heat processing parameters need to be carefully controlled so that more pharmacologically active components may be present in the treated herbs such as YYH, thereby increasing or maintaining the efficacy.
Acknowledgments
The work is supported by NIH GM 70737 to MH and National Natural Science Foundation of China (Grant No. 30572372) to YC, YHZ and XBJ. Y.C. was also supported by a training grant from Jiangsu Provincial Academy of Chinese Medicine, Nanjing, Jiangsu, China.
References
- 1.Branca F, Lorenzetti S. Health effects of phytoestrogens. Forum Nutr. 2005:100–111. doi: 10.1159/000083773. Medline. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:26–36. doi: 10.1016/j.ejphar.2007.02.039. Medline. [DOI] [PubMed] [Google Scholar]
- 3.Itoigawa M, Ito C, Ju-ichi M, Nobukuni T, Ichiishi E, Tokuda H, Nishino H, Furukawa H. Cancer chemopreventive activity of flavanones on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2002;176:25–29. doi: 10.1016/S0304-3835(01)00740-6. Medline. [DOI] [PubMed] [Google Scholar]
- 4.Maiti A, Cuendet M, Croy VL, Endringer DC, Pezzuto JM, Cushman M. Synthesis and biological evaluation of (+/−)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem. 2007;50:2799–2806. doi: 10.1021/jm070109i. Medline. [DOI] [PubMed] [Google Scholar]
- 5.Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, De Keukeleire D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction. 2002;123:235–242. doi: 10.1530/rep.0.1230235. Medline. [DOI] [PubMed] [Google Scholar]
- 6.Stevens JF, Page JE. Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. Medline. [DOI] [PubMed] [Google Scholar]
- 7.Lin CC, Ng LT, Hsu FF, Shieh DE, Chiang LC. Cytotoxic effects of Coptis chinensis and Epimedium sagittatum extracts and their major constituents (berberine, coptisine and icariin) on hepatoma and leukaemia cell growth. Clin Exp Pharmacol Physiol. 2004;31:65–69. doi: 10.1111/j.1440-1681.2004.03951.x. Medline. [DOI] [PubMed] [Google Scholar]
- 8.Liu TZ, Chen CY, Yiin SJ, Chen CH, Cheng JT, Shih MK, Wang YS, Chern CL. Molecular mechanism of cell cycle blockage of hepatoma SK-Hep-1 cells by Epimedin C through suppression of mitogen-activated protein kinase activation and increased expression of CDK inhibitors p21(Cip1) and p27(Kip1) Food Chem Toxicol. 2006;44:227–235. doi: 10.1016/j.fct.2005.07.003. Medline. [DOI] [PubMed] [Google Scholar]
- 9.Yap SP, Shen P, Butler MS, Gong Y, Loy CJ, Yong EL. New estrogenic prenylflavone from Epimedium brevicornum inhibits the growth of breast cancer cells. Planta Med. 2005;71:114–119. doi: 10.1016/j.fct.2005.07.003. Medline. [DOI] [PubMed] [Google Scholar]
- 10.Editorial Cmmittee of Pharmacopoeia of People’s Republic of China. Pharmacopoeia of the People’s Republic of China. Vol. 1. Beijing: Chemical Industry Press; 2005. p. 229. [Google Scholar]
- 11.Jeong EJ, Liu X, Jia X, Chen J, Hu M. Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab. 2005;6:455–468. doi: 10.2174/138920005774330657. Medline. [DOI] [PubMed] [Google Scholar]
- 12.Andlauer W, Kolb J, Furst P. Absorption and metabolism of genistin in the isolated rat small intestine. FEBS Lett. 2000;475:127–130. doi: 10.1016/S0014-5793(00)01642-2. Medline. [DOI] [PubMed] [Google Scholar]
- 13.Anonymous. Isoflavones from tofu are absorbed and metabolized in the isolated rat small intestine. J Nutr. 2000;130:3021–3027. doi: 10.1093/jn/130.12.3021. Medline. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. J Pharmacol Exp Ther. 2003;304:1228–1235. doi: 10.1124/jpet.102.046409. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Hu M. Absorption and metabolism of flavonoids in the caco-2 cell culture model and a perused rat intestinal model. Drug Metab Dispos. 2002;30:370–377. doi: 10.1124/dmd.30.4.370. Medline. [DOI] [PubMed] [Google Scholar]
- 16.Hu M, Li Y, Davitt CM, Huang SM, Thummel K, Penman BW, Crespi CL. Transport and metabolic characterization of Caco-2 cells expressing CYP3A4 and CYP3A4 plus oxidoreductase. Pharm Res. 1999;16:1352–1359. doi: 10.1023/A:1018986605929. Medline. [DOI] [PubMed] [Google Scholar]
- 17.Hu M, Chen J, Lin H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307:314–321. doi: 10.1124/jpet.103.053496. Medline. [DOI] [PubMed] [Google Scholar]
- 18.Hu M, Chen J, Zhu Y, Dantzig AH, Stratford RE, Jr, Kuhfeld MT. Mechanism and kinetics of transcellular transport of a new beta-lactam antibiotic loracarbef across an intestinal epithelial membrane model system (Caco-2) Pharm Res. 1994;11:1405–1413. doi: 10.1023/A:1018935704693. Medline. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Wang S, Jia X, Bajimaya S, Tam V, Hu M. Disposition of Flavonoids via Recycling: Comparison of Intestinal versus Hepatic Disposition. Drug Metab Dispos. 2005;33:1777–1784. doi: 10.1124/dmd.105.003673. Medline. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Liu Y, Dai Y, Xun L, Hu M. Enteric disposition and recycling of flavonoids and ginkgo flavonoids. J Altern Complement Med. 2003;9:631–640. doi: 10.1023/A:1018935704693. Medline. [DOI] [PubMed] [Google Scholar]
- 21.Walgren RA, Lin JT, Kinne RK, Walle T. Cellular uptake of dietary flavonoid quercetin 4′-beta-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther. 2000;294:837–843. Medline. [PubMed] [Google Scholar]
- 22.Walgren RA, Walle UK, Walle T. Transport of quercetin and its glucosides across human intestinal epithelial Caco-2 cells. Biochem Pharmacol. 1998;55:1721–1727. doi: 10.1016/S0006-2952(98)00048-3. Medline. [DOI] [PubMed] [Google Scholar]
- 23.Walle UK, French KL, Walgren RA, Walle T. Transport of genistein-7-glucoside by human intestinal CACO-2 cells: potential role for MRP2. Res Commun Mol Pathol Pharmacol. 1999;103:45–56. Medline. [PubMed] [Google Scholar]
- 24.Zhang Y, Gupta A, Wang H, Zhou L, Vethanayagam RR, Unadkat JD, Mao Q. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharm Res. 2005;22:2023–2034. doi: 10.1007/s11095-005-8384-4. Medline. [DOI] [PubMed] [Google Scholar]
- 25.Fan Y, Rodriguez-Proteau R. Ketoconazole and the modulation of multidrug resistance-mediated transport in Caco-2 and MDCKII-MDR1 drug transport models. Xenobiotica. 2008;38:107–129. doi: 10.1007/s11095-005-8384-4. Medline. [DOI] [PubMed] [Google Scholar]
- 26.Nishio N, Katsura T, Inui KI. Thyroid Hormone Regulates the Expression and Function of P-glycoprotein in Caco-2 Cells. Pharm Res. 2007 doi: 10.1007/s11095-007-9495-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Yang X, Morris ME. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharm Res. 2004;21:1263–1273. doi: 10.1023/B:PHAM.0000033015.84146.4c. Medline. [DOI] [PubMed] [Google Scholar]
- 28.Anonymous. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. Medline. [DOI] [PubMed] [Google Scholar]
- 29.Biavatti MW, Koerich CA, Henck CH, Zucatelli E, Martineli FH, Bresolin TB, Leite SN. Coumarin content and physicochemical profile of Mikania laevigata extracts. Z Naturforsch [C] 2004;59:197–200. doi: 10.1515/znc-2004-3-412. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Kayan B, Bozer N, Pate B, Baker C, Gizir AM. Terpene degradation and extraction from basil and oregano leaves using subcritical water. J Chromatogr A. 2007;1152:262–267. doi: 10.1016/j.chroma.2006.11.037. Medline. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DJ, Xiu ZL, Lin XH, Qi DJ. Effects of three different drying methods on extraction and separation of ginsenosides from fresh ginseng. Zhong Xi Yi Jie He Xue Bao. 2004;2:292–294. doi: 10.3736/jcim20040418. Medline. [DOI] [PubMed] [Google Scholar]