Abstract
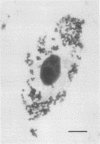
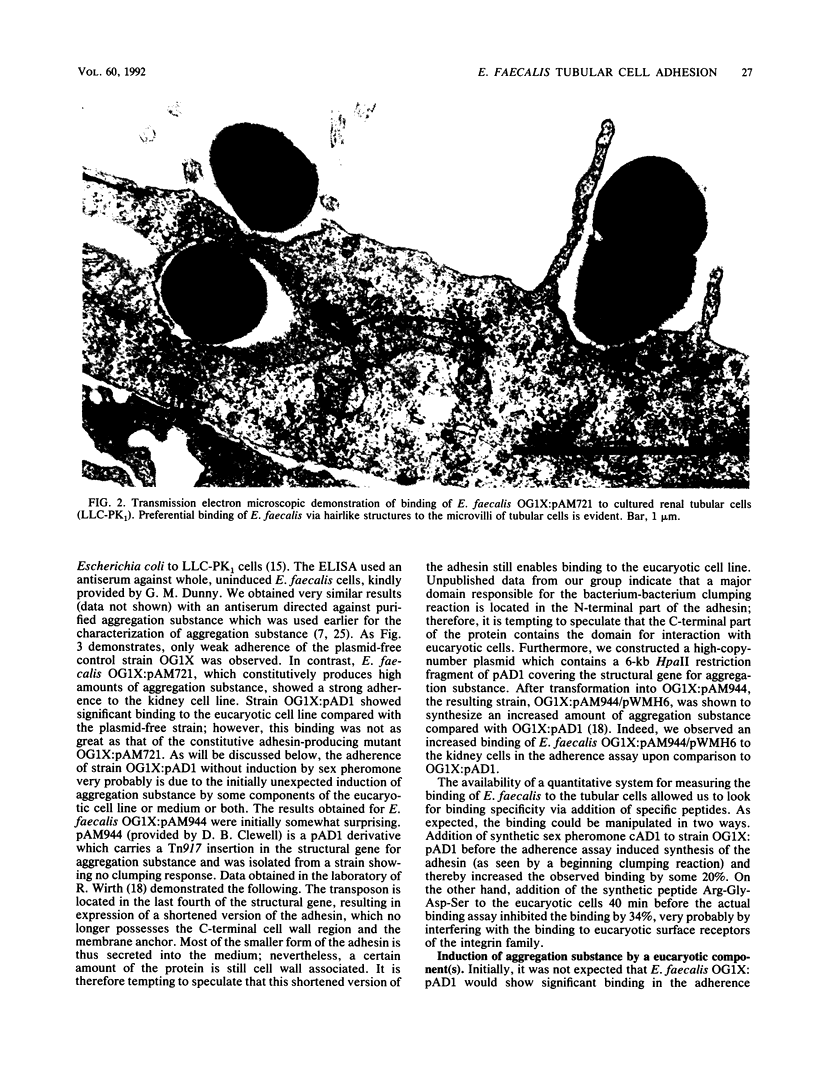
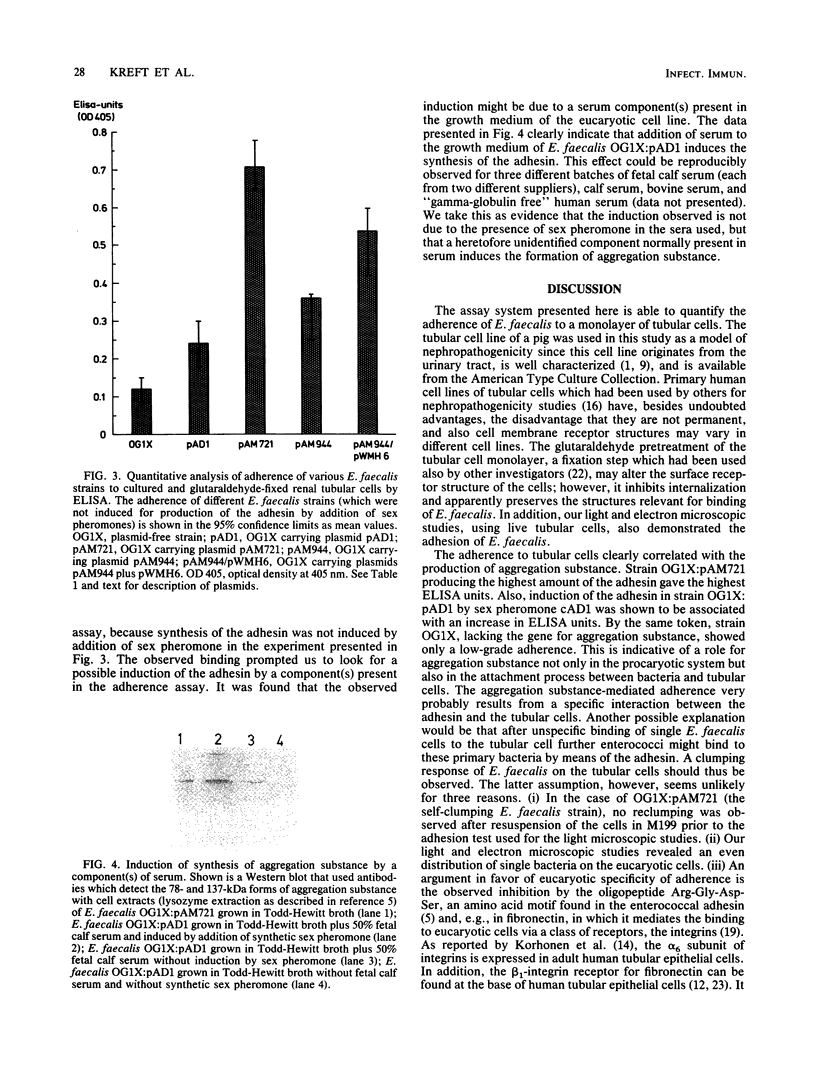
The sex pheromone system of Enterococcus faecalis is a unique, highly efficient plasmid collection mechanism for this species. A crucial role in this system is played by an adhesin called aggregation substance which enables the cell-cell contact between donor and recipient strains. The existence of the amino acid motif Arg-Gly-Asp-Ser in the adhesin prompted us to look for a possible binding of E. faecalis cells expressing aggregation substance to eucaryotic cells. We were able to show that the adhesin mediated binding to cultured renal tubular cells (porcine cell line LLC-PK1) via light microscopic, electron microscopic, and enzyme-linked immunosorbent assay-based studies. Synthesis of the adhesin was induced by some component(s) of serum. These data are interpreted to mean that aggregation substance is an adhesin mediating not only cell-cell contact between different E. faecalis strains but also binding of E. faecalis to eucaryotic cells, and therefore it might contribute to virulence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. C., Curthoys N. P., Lagenaur C. F., Puschett J. B. Characterization of primary cell cultures derived from rat renal proximal tubules. In Vitro Cell Dev Biol. 1989 Aug;25(8):714–722. doi: 10.1007/BF02623724. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Van der Schueren B., Jaspers M., Saison M., Spaepen M., Van Leuven F., Van den Berghe H., Cassiman J. J. Distribution of the beta 1 subgroup of the integrins in human cells and tissues. J Histochem Cytochem. 1989 Mar;37(3):299–307. doi: 10.1177/37.3.2645360. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. E., Clewell D. B. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J Bacteriol. 1987 Aug;169(8):3473–3481. doi: 10.1128/jb.169.8.3473-3481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli D., Lottspeich F., Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990 Jun;4(6):895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Galli D., Wirth R. Comparative analysis of Enterococcus faecalis sex pheromone plasmids identifies a single homologous DNA region which codes for aggregation substance. J Bacteriol. 1991 May;173(9):3029–3033. doi: 10.1128/jb.173.9.3029-3033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli D., Wirth R., Wanner G. Identification of aggregation substances of Enterococcus faecalis cells after induction by sex pheromones. An immunological and ultrastructural investigation. Arch Microbiol. 1989;151(6):486–490. doi: 10.1007/BF00454863. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Craig R. A., White B. A., Yagi Y., Clewell D. B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Ojha P. P., Susani M., Horvat R., Binder S., Hovorka A., Hillemanns P., Pytela R. A beta 1-integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol. 1989 Feb;134(2):481–489. [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., Yagi Y. Identification and partial characterization of a pheromone-induced adhesive surface antigen of Streptococcus faecalis. J Bacteriol. 1983 Aug;155(2):714–721. doi: 10.1128/jb.155.2.714-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M., Ylänne J., Laitinen L., Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990 Sep;111(3):1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre R., Kreft B., Hacker J. Genetically engineered S and F1C fimbriae differ in their contribution to adherence of Escherichia coli to cultured renal tubular cells. Infect Immun. 1990 Oct;58(10):3434–3437. doi: 10.1128/iai.58.10.3434-3437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Green D. M., Trifillis A. L., Johnson D. E., Chippendale G. R., Lockatell C. V., Jones B. D., Warren J. W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990 May;58(5):1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Tanaka H., Sakagami Y., Isogai A., Fujino M., Kitada C., White B. A., An F. Y., Clewell D. B., Suzuki A. Isolation and structure of the Streptococcus faecalis sex pheromone, cAM373. FEBS Lett. 1986 Sep 29;206(1):69–72. doi: 10.1016/0014-5793(86)81342-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sannomiya P., Craig R. A., Clewell D. B., Suzuki A., Fujino M., Till G. O., Marasco W. A. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci U S A. 1990 Jan;87(1):66–70. doi: 10.1073/pnas.87.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Mori M., Sakagami Y., Isogai A., Fujino M., Kitada C., Craig R. A., Clewell D. B. Isolation and structure of bacterial sex pheromone, cPD1. Science. 1984 Nov 16;226(4676):849–850. doi: 10.1126/science.6436978. [DOI] [PubMed] [Google Scholar]
- Wanner G., Formanek H., Galli D., Wirth R. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immuno labelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151(6):491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Kessler R. E., Shaw J. H., Lopatin D. E., An F., Clewell D. B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983 Apr;129(4):1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]