Abstract
Relapse to cocaine use after prolonged abstinence is an important clinical problem. This relapse is often induced by exposure to cues associated with cocaine use. To account for the persistent propensity for relapse, it has been suggested1 that cue-induced cocaine craving increases over the first several weeks of abstinence and remains high for extended periods. We and others identified an analogous phenomenon in rats that was termed ‘incubation of cocaine craving’: time-dependent increases in cue-induced cocaine-seeking over the first months after withdrawal from self-administered cocaine2–4. Cocaine-seeking requires the activation of glutamate projections that excite receptors for α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) in the nucleus accumbens5–7. Here we show that the number of synaptic AMPA receptors in the accumbens is increased after prolonged withdrawal from cocaine self-administration by the addition of new AMPA receptors lacking glutamate receptor 2 (GluR2). Furthermore, we show that these new receptors mediate the incubation of cocaine craving. Our results indicate that GluR2-lacking AMPA receptors could be a new target for drug development for the treatment of cocaine addiction. We propose that after prolonged withdrawal from cocaine, increased numbers of synaptic AMPA receptors combined with the higher conductance of GluR2-lacking AMPA receptors8,9 causes increased reactivity of accumbens neurons to cocaine-related cues, leading to an intensification of drug craving and relapse.
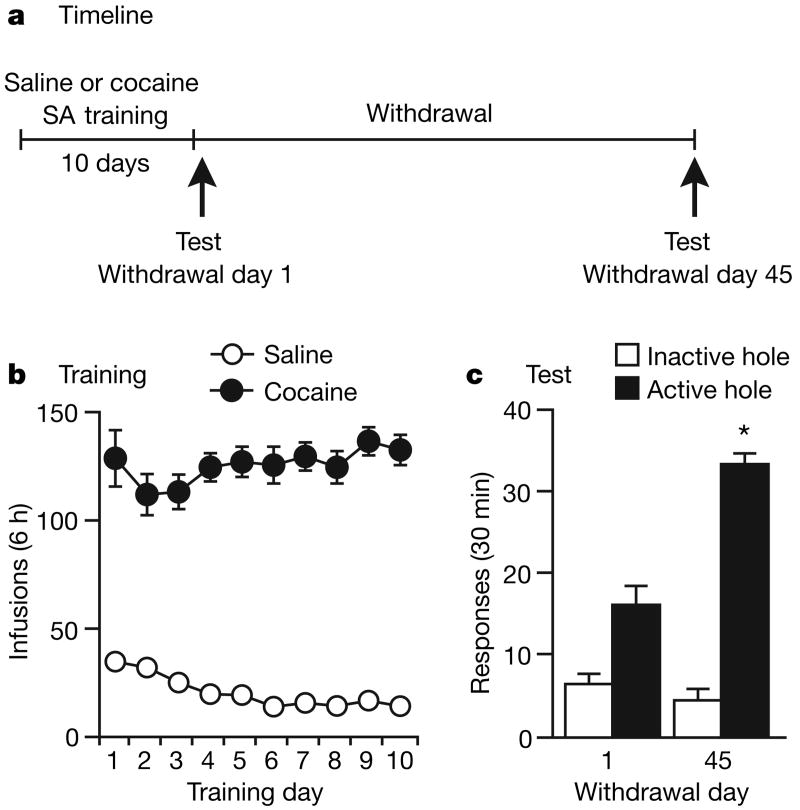
For 10 days we trained rats for 6 h a day to nose-poke to receive intravenous cocaine or saline infusions (Fig. 1a, b); these infusions were paired with a 5-s light cue. After 1 or 45 days of withdrawal from self-administration of cocaine or saline, we assessed cue-induced cocaine-seeking in a 30-min extinction test. In this test, rats were exposed to cues previously associated with cocaine availability, but nose-poke responding in the previously active hole (a measure of cocaine-seeking) did not result in cocaine infusions. Consistent with prior results2,3 was our observation that cue-induced cocaine-seeking was significantly greater on withdrawal day 45 than on withdrawal day 1 (Fig. 1c), confirming that cocaine craving incubates over time. On the basis of a critical role for glutamate-dependent plasticity in other addiction models10–12, and our previous work13,14, we proposed that time-dependent increases in accumbens AMPA receptor transmission underlie the incubation of cocaine craving. To test this hypothesis, we trained rats to self-administer cocaine or saline (as described above) for subsequent biochemical analysis after 1 or 45 days of withdrawal. The experimental groups were as follows: withdrawal day 1, saline (WD1-SAL); withdrawal day 1, cocaine (WD1-COC); withdrawal day 45, saline (WD45-SAL); and withdrawal day 45, cocaine (WD45-COC). We determined AMPA receptor distribution with a bis(sulphosuccinimidyl)suberate (BS3) protein crosslinking assay that enables the quantification of surface and intracellular receptor pools in tissue harvested after treatments in vivo13,14 (Supplementary Information).
Figure 1. Time-dependent increases in cue-induced cocaine-seeking (incubation of cocaine craving).
a, Experimental timeline. SA, self-administration. b, Training: the number of infusions (each paired with a 5-s light cue) during training are shown (means ± s.e.m.). Cocaine (0.5 mg kg−1 per infusion) supported self-administration, as indicated by high nose-poking in the active hole. Responding in the inactive hole was very low (not shown). c, Drug-seeking tests: shown are the number (means and s.e.m.) of nose-pokes in the previously active hole (a measure of cocaine-seeking) and inactive hole during a 30-min test performed under extinction conditions (nose-pokes deliver cue but not cocaine). Cocaine-seeking increased on withdrawal day 45 (withdrawal day × hole interaction: F1,12 = 14.9, P < 0.01) (n = 7 per group). Asterisk, significantly different (P < 0.05) from withdrawal day 1.
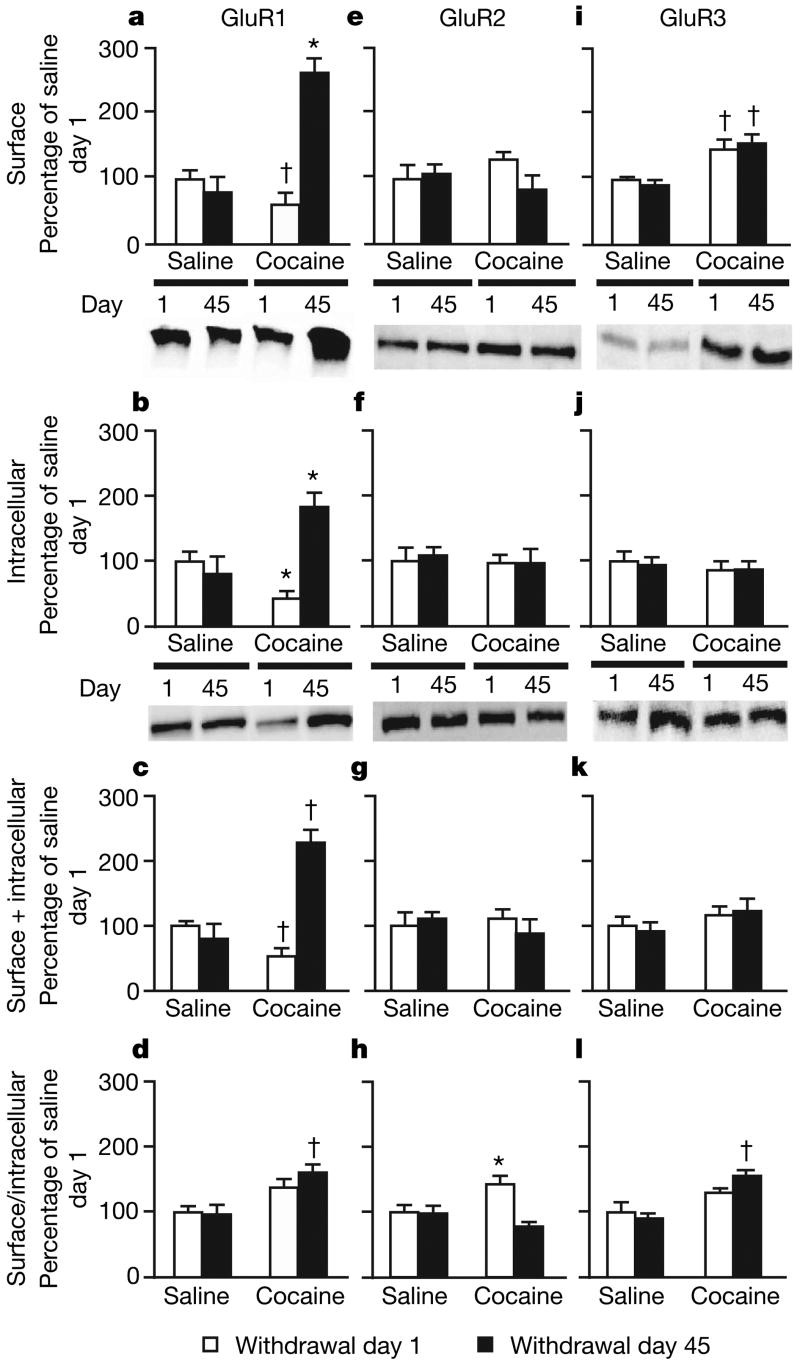
We found substantial (2–3-fold) increases in surface, intracellular and total GluR1 levels in the WD45-COC group compared with all other groups (Fig. 2a–c), as well as a more modest increase in the surface/intracellular ratio of GluR1 (Fig. 2d). Thus, the major effect of prolonged withdrawal from cocaine is increased expression of GluR1, rather than redistribution of pre-existing GluR1, suggesting either increased GluR1 synthesis or decreased GluR1 degradation. The magnitude of increased GluR1 expression indicates a locus in medium spiny neurons, which make up more than 90% of accumbens neurons15. We found opposite changes in GluR1 on withdrawal day 1: rats in the WD1-COC group had significantly lower surface, intracellular and total GluR1 levels (Fig. 2a–c). Intermediate effects were observed in an additional experiment in which we examined receptor expression on withdrawal days 3 and 21 (Supplementary Fig. 1), indicating that GluR1 levels increase gradually after withdrawal from cocaine. For GluR2 we found a small increase in the surface/intracellular ratio in cocaine-exposed rats on withdrawal day 1, but no changes on withdrawal day 45 (Fig. 2h). For GluR3 we found increased surface expression on both withdrawal days 1 and 45, indicating a time-independent effect (Fig. 2i).
Figure 2. Accumbens GluR1 and GluR3 expression increase after withdrawal from cocaine self-administration.
a–d, GluR1 increased markedly in cocaine-exposed rats on withdrawal day 45 (surface (a), intracellular (b) and total (c) GluR1; drug exposure × day interaction, F1,56 = 9.9, F1,56 = 9.2 and F1,56 = 12.1, respectively; P < 0.01). d, Surface/intracellular ratio. e–h, GluR2 was unchanged except for a small increase in the surface/intracellular ratio in cocaine-exposed rats on withdrawal day 1 (drug exposure × day interaction, F1,53 = 4.0; P < 0.01). i–l, Surface GluR3 and the surface/intracellular ratio increased after self-administration of cocaine (drug exposure, F1,48 = 4.4 and F1,48 = 3.9, respectively; P < 0.05). Data (means and s.e.m., n = 12–18 per group) are expressed as percentages of the values in the saline group on withdrawal day 1. Asterisk, significantly different (P < 0.05) from other conditions; dagger, significantly different (P < 0.05) from saline group on withdrawal day 1.
These results suggest that after prolonged withdrawal from cocaine, the normal complement of GluR2-containing AMPA receptors is supplemented by the addition of GluR2-lacking receptors (GluR1/3 and/or homomeric GluR1). We obtained additional support for this conclusion from a quantitative co-immunoprecipitation experiment (Supplementary Fig. 2). This effect is specific to accumbens AMPA receptors: we found no evidence for the formation of GluR2-lacking AMPA receptors in the ventral tegmental area or cingulate cortex after withdrawal from cocaine (Supplementary Figs 3, 4), nor did we find significant changes in accumbens N-methyl-d-aspartate receptor subunits (Supplementary Fig. 5).
The accumbens consists of two major subregions, termed core and shell, which can be distinguished on the basis of connectivity and morphology15. The core and shell have different roles in drug-related behaviours, with some evidence suggesting that the core is more significant in cue-induced cocaine-seeking16. To study potential core-shell differences, we assessed another cohort of cocaine self-administering rats after 1 or 45 days of withdrawal. We divided the accumbens into core and shell subregions, crosslinked with BS3, and analysed GluR1–3 (Supplementary Fig. 6). In the core we found robust time-dependent increases in GluR1 and modest increases in GluR3; in the shell we found that surface GluR1 was increased on withdrawal day 45. These results suggest that GluR2-lacking AMPA receptors form in both core and shell, but this effect may be more pronounced in the core.
Next we determined whether the time-dependent changes in AMPA receptor expression were influenced by performing a test for cue-induced cocaine-seeking (under extinction conditions). We trained rats to self-administer cocaine as described above. We assessed the brains of four groups of rats that were either tested (‘test’) or not tested (‘no-test’) for cue-induced cocaine-seeking after 1 or 45 days of withdrawal from cocaine; rats in the test condition were killed immediately after the 30-min cocaine-seeking test. We found increased surface and total GluR1 levels on withdrawal day 45 (Supplementary Fig. 7a–c) in both the test and no-test conditions, replicating results from our first experiment (Fig. 2a–c). No-test rats also showed a small decrease in the surface/intracellular ratio of GluR2 on withdrawal day 45 (Supplementary Fig. 7h). These data suggest that the test for cocaine-seeking had a minimal effect on AMPA receptor distribution in the accumbens.
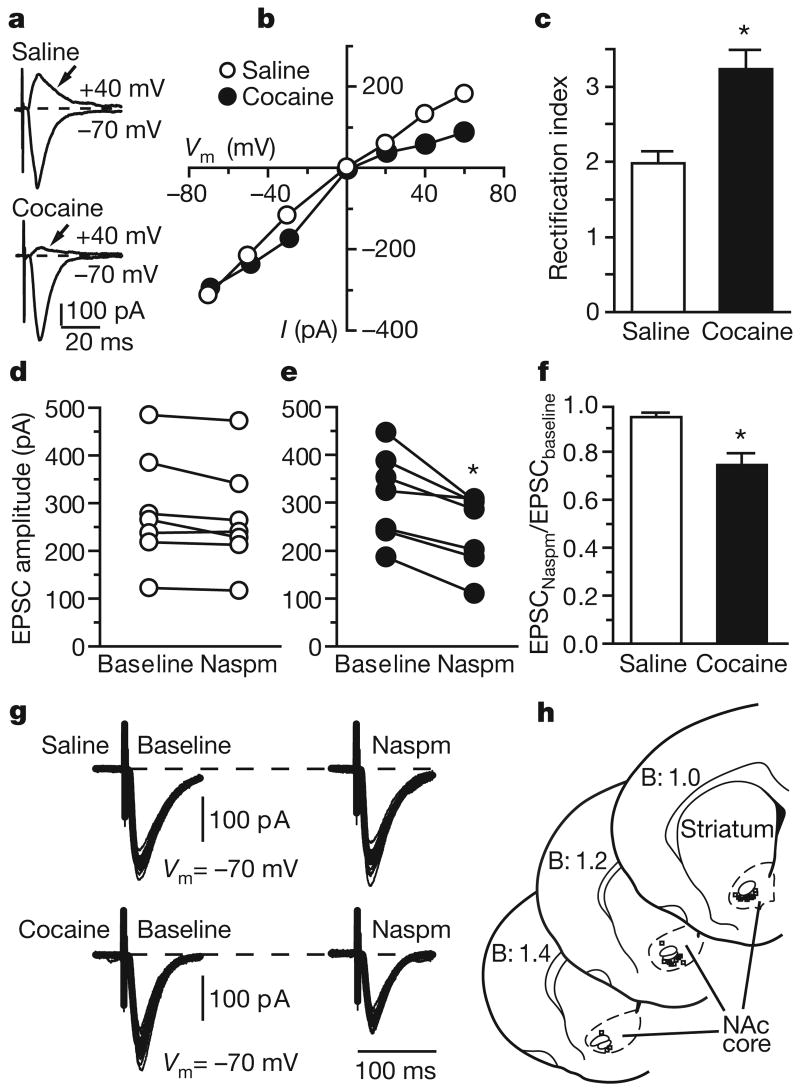
To confirm our biochemical results we performed whole-cell patch-clamp recordings of medium spiny neurons in the accumbens core after 42–47 days of withdrawal from saline or cocaine self-administration. GluR2-lacking AMPA receptors have unique properties: permeability to Ca2+, resulting in greater conductance, and inwardly rectifying currents due to voltage-dependent block by polyamines8,9. Current–voltage relationships of evoked excitatory postsynaptic currents (EPSCs; Supplementary Information) in accumbens neurons revealed significantly greater inward rectification in the cocaine-exposed group (Fig. 3a–c). Furthermore, bath application of 1-naphthylacetylsperimine (Naspm), a selective blocker of GluR2-lacking AMPA receptors, decreased evoked EPSC amplitude only in neurons recorded from the cocaine-exposed group (Fig. 3d–g). Thus, GluR2-lacking AMPA receptors contribute significantly to accumbens synaptic transmission only after prolonged withdrawal from cocaine. In addition, we found that neurons from cocaine-exposed rats showed a change in the distribution of spontaneous EPSC (sEPSC) amplitude as a result of an increased number of high-amplitude sEPSC (Supplementary Fig. 8b). Both the results with Naspm and the increased sEPSC amplitude predict enhanced responsiveness of accumbens neurons to excitatory inputs after prolonged withdrawal from cocaine. Neurons from cocaine-exposed rats also showed an increased frequency of AMPA-receptor-mediated sEPSCs (Supplementary Fig. 8a). This is unlikely to reflect increased probability of release, because the paired-pulse ratio did not differ between cocaine-exposed and saline-exposed rats (Supplementary Fig. 8c, d). Increased sEPSC frequency may be due to the formation of new synaptic contacts in the accumbens after withdrawal from cocaine17.
Figure 3. GluR2-lacking AMPA receptors are detected in accumbens neurons after prolonged withdrawal from self-administration of cocaine.
a, Evoked EPSC recorded after 42–47 days of withdrawal from saline or cocaine self-administration. b, Current–voltage relationships for neurons shown in a. c, Rectification index (EPSC−70 mV/EPSC+40 mV; Supplementary Information) from 13 and 8 neurons recorded from four cocaine-exposed and three saline-exposed rats (t19 = 3.47; asterisk, P < 0.01). Data are means and s.e.m. d, e, Naspm (200 μM, 5–10 min) decreased evoked EPSC amplitude in cocaine-exposed rats (e) (t6 = 4.72; asterisk, P < 0.01, baseline versus Naspm, seven cells per group) but not in saline-exposed rats (d). f, The effect of Naspm illustrated as the evoked EPSC amplitude normalized to baseline (t12 = 3.73; asterisk, P < 0.01). g, Representative traces illustrating the effect of Naspm after 10 min of bath application. h, Location of recordings. Numbers indicate distance rostral to Bregma (B) in mm.
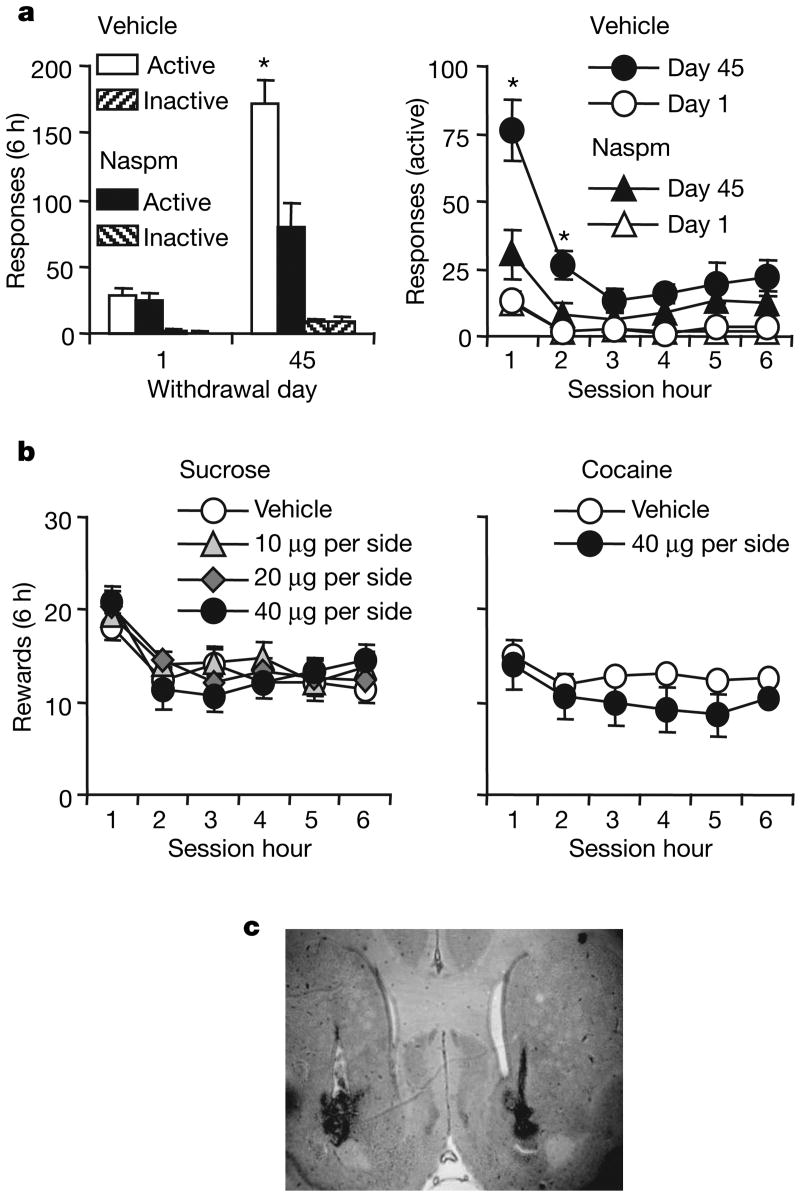
To test the functional role of new GluR2-lacking receptors, we injected Naspm (or vehicle) into the accumbens of cocaine-exposed rats before tests for cue-induced cocaine-seeking. Naspm decreased cue-induced cocaine-seeking on withdrawal day 45, demonstrating that GluR2-lacking AMPA receptors mediate the expression of incubation of cocaine craving (Fig. 4a). Naspm did not alter cue-induced cocaine-seeking on withdrawal day 1 (Fig. 4a). This finding is consistent with a lack of differences in AMPA receptor subunit expression and distribution on cocaine withdrawal day 1 compared with the drug-naive saline condition (Fig. 2), in which GluR2-lacking receptors are expressed at very low levels and contribute minimally to synaptic transmission in the accumbens (Fig. 3 and Supplementary Fig. 2). Naspm did not alter stable cocaine or sucrose self-administration (Fig. 4b).
Figure 4. Enhanced cue-induced cocaine-seeking after prolonged withdrawal from cocaine self-administration is inhibited by blockade of GluR2-lacking AMPA receptors.
a, Cue-induced cocaine-seeking tests. Left: responses (means and s.e.m.) on the previously active or inactive levers after injections of Naspm or vehicle into the accumbens 15 min before extinction tests on withdrawal days 1 or 45 (n = 10–14 per group). Right: responses on previously active lever at each hour of test (Naspm dose × withdrawal day × session hour × lever interaction, F1,45 = 4.6; asterisk, P < 0.05 compared with other groups). b, Injections of Naspm into the accumbens had no effect on stable self-administration of oral sucrose or intravenous cocaine. Results are numbers of oral sucrose deliveries (left; 0.75 ml per delivery, n = 10) or intravenous cocaine deliveries (right; 0.75 mg kg−1 per infusion, n = 5) (means ± s.e.m.). c, Placement of cannulae, showing injector tips.
We propose that the synaptic incorporation of GluR2-lacking AMPA receptors enhances the responsiveness of accumbens neurons to glutamate inputs from cortical and limbic regions, as a result of increases in the absolute number of surface AMPA receptors (Fig. 2) as well as the higher conductance of GluR2-lacking AMPA receptors8,9. Thus, when cocaine-associated cues are presented after prolonged withdrawal from cocaine and glutamate is released in the accumbens, neurons in this brain area respond more robustly, leading to enhanced cocaine-seeking.
Our results are consistent with a large body of literature implicating increased AMPA receptor transmission in the accumbens in cocaine-seeking5–7 and psychomotor sensitization13,14,18–21 after prolonged withdrawal from cocaine, and with the finding that increased neuronal activity in the accumbens correlates with the incubation of cocaine craving22. However, our results differ from the finding that cue-induced cocaine-seeking after prolonged withdrawal is not decreased in GluR1-knockout mice23. These latter results should be interpreted with caution in view of the potential for compensation during development and/or offsetting changes in other neuronal pathways. A previous study found that viral overexpression of GluR1 or GluR2 in the accumbens shell decreases cocaine-seeking during early withdrawal24. Many differences exist between our study and this previous study, including focus on core versus shell, long versus short withdrawal, and single versus multiple extinction tests. An important consideration is that our conclusions are based on measuring and manipulating endogenous surface AMPA receptors.
Recent work has highlighted the importance of GluR2-lacking AMPA receptors in long-term potentiation (LTP) and depression (LTD), experience-dependent plasticity, and synaptic scaling8,9. Synaptic scaling is a form of homeostatic plasticity in which prolonged activity blockade causes enhanced excitatory synaptic transmission. Synaptic scaling may have parallels to our model. After withdrawal from cocaine, cortical areas providing excitatory input to the accumbens show metabolic hypoactivity10,25, raising the possibility that accumbens GluR2-lacking AMPA receptors scale up as a homeostatic response to prolonged decreases in synaptic activation. Scaling-induced increases in GluR1 have been reported to occur through increased dendritic GluR1 synthesis26 as well as decreased GluR1 protein stability27.
Here we have demonstrated that GluR2-lacking AMPA receptors are produced in the accumbens during prolonged withdrawal from cocaine and mediate the incubation of cocaine craving. Our work adds to a growing consensus that perturbations in synaptic transmission during disease states cause compensatory changes in AMPA receptor subunit composition that alter the properties of neuronal networks8,9. For cocaine addiction, the production of GluR2-lacking AMPA receptors may exacerbate disease processes by increasing the reactivity of accumbens neurons to cocaine-associated cues that promote craving and relapse. A question for future research is whether GluR2-lacking receptors in the accumbens also contribute to drug-induced and stress-induced cocaine craving and relapse that also occur after prolonged withdrawal3,10. Finally, our results, and those of others on the formation of GluR2-lacking AMPA receptors in the ventral tegmental area after acute exposure to cocaine28,29, suggest that these receptors could be a new drug target for the treatment of addiction.
Methods Summary
All procedures are based on our previous work2,3,13,14,30.
Behavioural procedures
Male rats were trained to nose-poke (biochemical and electrophysiology experiments) or lever-press (Naspm behavioural experiment) for 6 h a day for 10–12 days; each cocaine infusion was paired with a tone–light or light cue. After self-administration training, the rats were tested for cue-induced cocaine-seeking after 1 or 45 days of withdrawal. During testing, responding with a lever-press or a nose-poke led to contingent presentations of the cue previously paired with cocaine infusions, but not cocaine. Responding on the previously active lever or hole was the operational measure of cocaine-seeking.
Biochemistry
After the appropriate withdrawal period (or immediately after the drug-seeking test in Supplementary Fig. 7), the rats were decapitated. The accumbens was rapidly dissected and brain slices (400 μm thick) prepared with a tissue chopper. Crosslinking of slices with BS3 (30 min), subsequent tissue processing, and quantification of surface and intracellular protein levels by SDS–PAGE and western blotting were performed as described in Methods. Values for surface, intracellular and total receptor subunit levels were normalized to total protein in the lane determined with Ponceau S.
Electrophysiology
Coronal slices (300 μm thick) containing the accumbens were obtained after 42–47 days of withdrawal from self-administration of saline or cocaine. Recordings were conducted in voltage-clamp configuration at 33–35 °C with patch electrodes filled with caesium gluconate, spermine (0.1 mM) and QX-314 (1 mM). Synaptic responses of medium spiny neurons were elicited by local stimulation of excitatory inputs with a bipolar electrode. Stimulation intensity (0.05–0.3 mA) was based on the minimum amount of current necessary to elicit a synaptic response with less than 15% variability in amplitude 10 min after obtaining the whole-cell configuration. Both spontaneous and evoked EPSCs were collected before and after bath application of Naspm (100–200 μM) for 10 min.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Acknowledgments
We thank R. J. Wenthold for help in establishing quantitative co-immunoprecipitation methods used in Supplementary Fig. 2. This work was supported by US Public Health Service grants DA09621, DA015835, DA00453 and NARSAD (M.E.W.), DA020654 (M.M.), predoctoral National Research Service Award DA021488 (K.L.C.), Rosalind Franklin University of Medicine and Science start-up funds (K.Y.T.) and the Intramural Research Program of the National Institute on Drug Abuse (Y.S.).
Footnotes
Author Contributions K.L.C., M.M. and M.E.W. were responsible for overall study design. K.L.C. conducted and analysed cocaine self-administration experiments (except the Naspm experiment) and protein crosslinking studies. M.M. trained K.L.C. in drug self-administration procedures and helped with these experiments. J.M.R. conducted and analysed co-immunoprecipitation experiments. K.Y.T. designed electrophysiological experiments, L.J.H. conducted them, and K.Y.T. analysed the data. Y.S. and K.L.C. designed the Naspm behavioural experiment, J.L.U. performed it, and Y.S. and J.L.U. analysed the data. K.L.C., Y.S. and M.E.W. wrote the paper with the help of the other authors.
References
- 1.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 2.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47 1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Neisewander JL, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-15-j0006.2000. RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suto N, et al. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- 8.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 11.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nature Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 12.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 13.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann NY Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- 16.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 1:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao WD, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 22.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mead AN, Zamanillo D, Becker N, Stephens DN. AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology. 2007;32:343–353. doi: 10.1038/sj.npp.1301045. [DOI] [PubMed] [Google Scholar]
- 24.Sutton MA, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 25.Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- 26.Ju W, et al. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nature Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien RJ, et al. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 28.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 29.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 30.Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.