Abstract
Johansen, J.A., L.G. Clemens, and A.A. Nunez. Characterization of Copulatory behavior in female mice: evidence for paced mating PHYSIOL BEHAV 56(6) 000-000, 2008. –In this study we characterized female mouse sexual behavior using a pacing paradigm similar to that used to evaluate sexual behavior in female rats. A pacing chamber was designed for use with mice and we compared the sexual behavior of female mice that were tested in both pacing and nonpacing paradigms and under different hormone conditions. We found that, like rats, female mice do pace their copulatory behavior by altering the temporal sequence of copulatory events. Female mice take longer to return to the male after an ejaculation, compared to either a mount or intromission. However, it is still unclear if female paced mating serves the same functions as it does in female rats. More work is needed to confirm that paced mating induces hormonal changes needed for pregnancy as is the case in rats.
Keywords: sexual behavior, female, mating, receptivity, estrogen, progesterone
Introduction
Sexual behavior has been carefully characterized in both male and female rats. However, mouse reproductive behavior has only been fully described for males [1, 2]. Female rat sexual behavior has been well studied, but it is unclear that the rat model of sexual behavior is applicable to mice [3, 4]. Mouse mating strategies differ from those of the rat. Male mice defend a territory and mate with females in it, excluding other males [5]. But in rats, a single female is likely to mate concurrently with several males [6–8]. Male rats and mice also exhibit different copulation patterns. Copulation in the rat is much shorter, consisting of a series of relatively few intromissions, leading to ejaculation [9]. Whereas copulation in mice consists of numerous intromissions with sustained intravaginal thrusting [9]. The mouse ejaculatory reflex is also different from that of the rat. In male mice, ejaculation leads to a shudder while maintaining intromission. The male then clutches the female with all four limbs and usually falls to his side, frequently carrying the female with him; he remains like this, fully intromitted for 13–25 seconds [9, 10]. During mating, female mice may receive more vaginal/cervical stimulation than female rats, and as a result they are likely to differ from female rats in situations in which they control the pace of copulation in response to coital stimulation.
The goal of the present study was to characterize normative female mouse sexual behavior under several hormonal conditions and testing paradigms. Particularly, we were interested in determining the patterns of behavior female mice exhibit when they are able to control the pace of copulation. We also investigated how the testing paradigm interacted with the hormonal conditions that represent early proestrus (only estradiol) or late proestrus (estradiol plus progesterone). In rats mating can start just before the rise in progesterone, but females are most receptive when progesterone is high [4].
Materials and Methods
Animals
Female Swiss Webster mice (Charles River Laboratories, Raleigh, North Carolina) were group housed 4–5 per cage, and male C57BL/6 mice (Charles River Laboratories, Raleigh, North Carolina) were singly housed in clear Plexiglas cages (27.5 × 17 × 12 cm). Animals were provided with food (Harlan Teklad 22/5 Rodent Diet 8640) and water ad libitum, and maintained on a reverse light dark cycle with lights off from 1100 to 1900 hours. All experiments were performed in compliance with the Michigan State University All University Committee on Animal Use and Care, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize any discomfort experienced by the animals.
Hormone manipulations
All female mice were ovariectomized via bilateral incisions under ketamine/xylazine anesthesia (1ml/kg/bw of cocktail 44mg ketamine/10mg xylazine/ml). The females were divided into three hormone treatment groups. Injections were given at 0900 hours. Treatment 1 (EB+P), Estradiol Benzoate (EB, Sigma) 0.5ug/0.03ml in oil vehicle was given subcutaneously on days 1, 2, and 3. On the fourth day Progesterone (P, Sigma) was given at 0.5mg/0.03ml in sesame oil vehicle, four hours prior to behavior testing. Treatment 2 (EB ONLY), EB was given to females on days 1, 2 and 3 at the same concentration as treatment 1. Sesame oil was given on day 4 instead of P. Treatment 3 (OIL, Sigma), consisted of only sesame oil vehicle injections of 0.03ml subcutaneously on days 1, 2, 3 and 4. On the fourth day, all animals were tested for sexual behavior.
Behavior testing
After at least a week of recovery following ovariectomy, all animals were allowed to gain sexual experience prior to testing by pairing males and females overnight in the males’ homecage. For these pairings, all females were given hormone treatment 1, consisting of EB+P injections to induce sexual receptivity. After sexual experience the females were then randomly assigned to one of the three hormone treatments as described above and tested in both a nonpacing and pacing test, in a counter balanced order. For all tests, females were paired with a male that previously showed reliable sexual behavior and were tested with the same male in both pacing and nonpacing tests. Behavior tests were conducted under dim red illumination at 1300, two hours after lights out. Males were allowed to acclimate to the testing chambers for 5 min before introducing the female. All behavior tests were videotaped and analyzed with an event recording program (Observer version 2.0). For both pacing and nonpacing conditions, if no intromissions were observed in the initial 20 minutes the test was ended. Otherwise tests continued until an ejaculation was received in nonpacing tests, or after the female returned to the male following an ejaculation in pacing tests. Only females receiving ejaculations in either a pacing or nonpacing test were included in the statistical analyses.
Female Paced tests
Pacing chambers used for rats utilize the large sex difference in body size to allow females the opportunity to escape from the male. A large Plexiglas chamber is divided into a “male” chamber and “escape” chamber by a barrier with small holes that males are not able to fit through. Female rats can pass easily through the holes in the divider separating the two chambers, giving them the opportunity to escape the male rat. Male and female mice are approximately the same size, and because rat pacing chambers utilize the size difference between sexes to separate the two we could not use them. Therefore, we designed new chambers for these experiments. The testing chamber consisted of a Plexiglas arena (60cm × 45cm × 45cm ) with a 10cm tall Plexiglas barrier demarking the male side vs. the female side. The female could easily jump over this barrier to escape the male. The barrier was placed so that the female side was 20 × 45 × 45 cm vs the male side 40 × 45 × 45 cm. The males were tethered to the male side of the cage by placing a plastic collar around their neck, and attaching them to a ring affixed above the chamber that swiveled, allowing them access to all areas on the male side, but preventing them from crossing the barrier over to the female side. The females had free access to all areas of the testing chamber, including an area where they could escape and avoid the male.
The frequency of mounts, intromissions, and ejaculations were recorded, as were the latency to the first occurrence of each of these behaviors. Intromissions were further classified as male or female terminated. If the female pushed the male off it was deemed female terminated. If the male dismounted first, then it was deemed male terminated. The inter-intromission interval, or time between intromissions and the duration of each intromission were measured. We also measured how many times the female escaped to the female chamber (percent exits), and also the time for the female to return after each escape (return latency) after a mount, intromission and ejaculation. Lordosis was not reliably shown by the females, and males could intromit without the female displaying lordosis. Our females had limited sexual experience, and were tested only two times, which appears not enough sexual experience to show reliable lordosis in mice. Female mice typically show very low lordosis quotients, around 20% with only 2–3 sexual experiences [11, 12]. Instead of lordosis, rejections or acceptances of the male were used as an index of female receptivity. Rejections consisted of the biting and fighting by the female when the male came into contact with her, and were often accompanied by audible vocalizations. Acceptances resulted in mounts or mounts with intromissions.
Nonpacing tests
The testing chamber consisted of the same arena as in the pacing test, but without the barrier. Males remained tethered, but were allowed a greater range of movement and had access to all parts of the chamber. In this situation, the females had no opportunity to avoid contact with the male. Behavioral measures included the same measures as in the pacing paradigm, except for percent exits and return latencies.
We started with 10 animals per hormone group, however not all received ejaculations. In the EB+P group 7 females received an ejaculation in nonpacing tests, and 8 females received an ejaculation in pacing tests. In the EB Only group, 6 females received and ejaculation in nonpacing tests, and 4 females received an ejaculation in pacing tests. Oil treated animals never received an ejaculation, and only 7 females were tested in each testing paradigm.
Statistical analysis
Statview version 5.0.1 and SPSS version 13 were used to analyze the data. Two-way ANOVAs were run with hormone treatment and behavioral test as a between groups factors, for most measures of male and female behavior. A more conservative between group analysis was adopted to compare the two testing paradigms, as not all females received an ejaculation in both testing conditions. To analyze return latencies and percent exits within pacing tests, two-way ANOVAs with hormone treatment as a between groups factor, and behavioral measure as a repeated factor, (3 levels) were used. A Fisher’s PLSD post hoc test was run when there were significant main effects. A paired t-test was used to determine if there was a sex bias in the termination of intromissions. For all significant differences p was less than .05.
Results
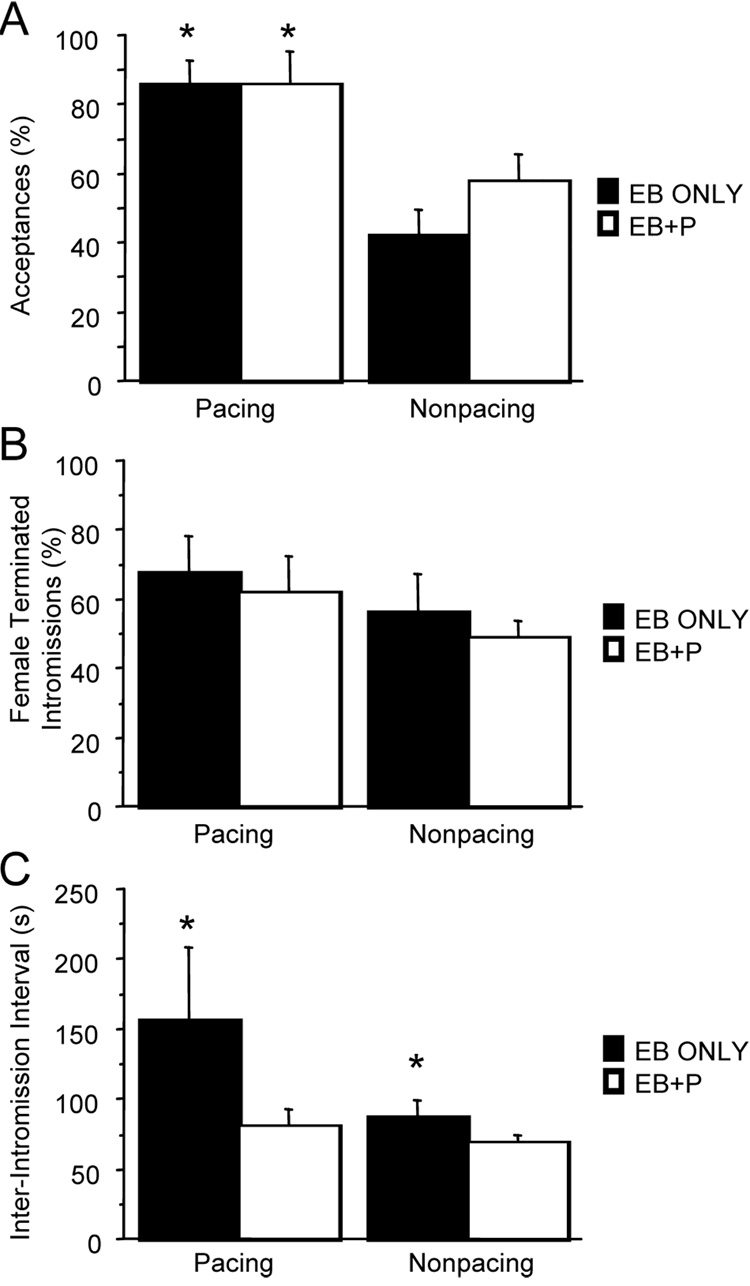
We utilized acceptances, as a measure of receptivity in female mice. Acceptances were the percentage of approaches by the male that resulted in a mount or intromission. All other approaches resulted in rejections by the female. For both hormonal conditions, females accepted the males’ approaches significantly more often in the pacing test (Fig. 1A). There was a main effect of test (p<.0005), with no significant effect of hormone treatment or significant interaction. Oil treated females did not receive any intromissions and only a few mounts, thus acceptance scores were very low and excluded from statistical analysis.
Figure 1.
A) The percentage of male approaches that resulted in acceptances (mounts or mounts with intromissions) by the female, were significantly higher in the pacing test compared to the nonpacing test (F(1,21)=16.589, p=.0005). There was no main effect of hormone treatment (F(1,21)=.766, p=.39) or significant interaction (F(1,21)=.794, p=.38). *Significantly different from nonpacing. B) The percent of intromissions terminated by the females did not differ between testing paradigms (F(1,21)=1.551, p=.2) or hormonal treatments (F(1,21)=.446, p=.5) and there was no significant interaction (F(1,21)=.009, p=.9). C) There was a main effect of testing paradigm (F(1,21)=4.4, p=.048), and main effect of hormone (F(1,21)=6.1, p=.02) but no interaction (F(1,21)=2.28, p=.146) on the inter-intromission interval (III). Post-hoc tests failed to detect a significant effect of testing paradigm, but found that the III was significantly longer in EB-only females (p = .05) in both testing paradigms. *Significantly longer than EB+P treated females within testing paradigm
Females were just as likely to terminate an intromission as were the males, (p=.60). There were no main effects of test or hormone treatment on the percent of intromissions terminated by the female (Fig. 1B). There was a main effect of hormone treatment on the interval between intromissions (III) (p<.02), and a main effect of test (p<.04), but no significant interaction. Under both testing conditions the III was longer for females receiving only EB (Fig. 1C). No significant effects of testing paradigm were found with post-hoc tests.
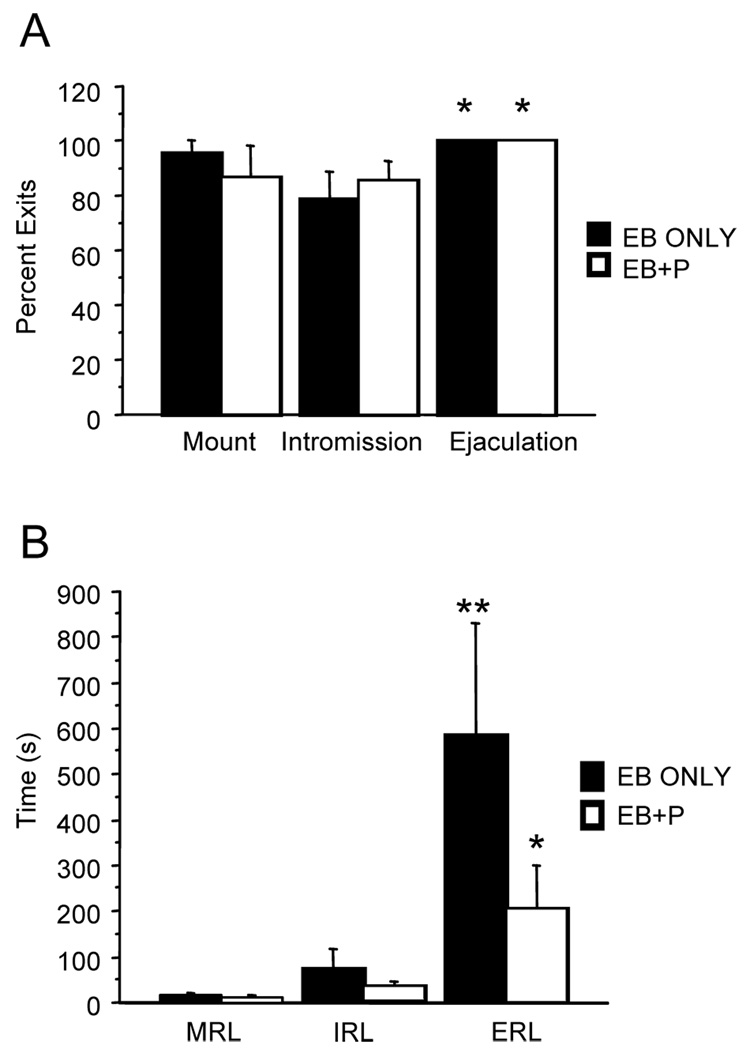
There was a main effect on percent exits (p<.04). In the pacing tests, females exited more often after an ejaculation (p<.01) compared to an intromission (Fig. 2A). There were no differences between the two hormonal conditions on this measure. There was a main effect on return latencies (p<.0007), and a significant interaction (p<.03) but no main effect of hormone treatment. EB+P treated females took longer to return after an ejaculation than a mount (p<.04, Fig. 2B), while females treated with EB took longer to return after an ejaculation compared to either a mount (p<.02, Fig 2B) or intromission (p<.03, Fig, 2B).
Figure 2.
A) Females were just as likely to exit the male chamber after either a mount or intromission, but were more likely to exit after an ejaculation (p<.01) under both hormone conditions. There was a main effect of percent exits (F(2,16)=3.9, p<.04) but no main effect of hormone (F(1,8)=.009, p=.9) and no significant interaction (F(2,16)=.784, p=.4). *Significantly different from exits after an intromission. B) Female mice took longer to return following greater amounts of stimulation (F(2,18)=11.1, p=.0007). There was no main effect of hormone treatment (F(1,9)=4.2, p=.06), but there was a significant interaction (F(2,18)=3.9, p=.03). EB only treated females returned longer after an ejaculation compared to either a mount (p=.02) or intromission (p=.03), while EB+P treated females returned longer after an ejaculation compared to a mount (p=.04) *Significantly different from MRL. **Significantly different from MRL and IRL
There was a main effect of test on mount frequency with significantly more mounts in a nonpacing test compared to a pacing test (p<.04, Table 1). There was no main effect of hormone and no significant interaction. There were no significant effects of test or female hormonal condition on all other measures of male sexual behavior shown in Table 1.
Table 1.
Male measures of sexual behavior, values expressed means ± SEM.
| Mount Frequency | Intromission Frequency | Mount Latency (s) | Intromission Latency (s) | Intromission duration (s) | Ejaculation Latency (s) | |
|---|---|---|---|---|---|---|
| EB+P | ||||||
| Nonpacing (7) | 9.4±2.9* | 27.2±8.0 | 135.7±68.6 | 291.7±44.6 | 20.5±3.1 | 1848.7±400 |
| Pacing (8) | 3.7±1.5 | 27.0±6.7 | 378.0±106.3 | 449.0±120.2 | 17.8±3.1 | 1752.5±303 |
| EB Only | ||||||
| Nonpacing (6) | 10.0±4.6* | 19.6±2.2 | 402.9±154.3 | 484.3±171.6 | 17.4±2.9 | 1789.0±283 |
| Pacing (4) | 2.5±1.2 | 10.50±2.5 | 378.1±89.1 | 589.8±110.2 | 22.7±4.2 | 1748.3±523 |
| Oil | ||||||
| Nonpacing (7) | 1.0±0.86 | 136.6±85.0 | ||||
| Pacing (7) | 0.86±0.86 | 27.0±27.0 |
Significantly different than respective pacing group
Discussion
The main finding of this study was that, similar to what has been described for female rats, female mice change their behavior when given the opportunity to escape from the male during copulation, and they display these patterns under hormonal conditions that emulate those of early or late matings on the night of proestrus. In the female-paced condition, female mice leave the male chamber following almost all mounts, intromissions and ejaculations. This results in a significant reduction in the frequency of male mounts and in the number of female rejections as compared to what is seen under testing conditions that do not permit an escape from the male.
Female mice take longer to return to the male as coital stimulation increases; they take longer to return after an ejaculation compared to either a mount or intromission. However, unlike rats, the time to return after an intromission did not differ from that after a mount suggesting that mice do not distinguish a mount from an intromission. Also different from rats [3], intromission intervals did not differ across testing conditions, particularly when the animals received optimal hormonal stimulation (EB+P).
In female rats, their pacing of copulation results in the temporal pattern of cervical-vaginal stimulation optimal to promote luteal function, by inducing prolactin surges and maintenance of the corpora lutea typical of pseudopregnancy and early pregnancy [3, 13]. In rats, pacing allows the female to increase the effectiveness of each intromission, requiring fewer intromissions to become pregnant or pseudopregnant [14]. However, in female mice it is unclear what role intromissions have in inducing female progestational changes, or if intromissions alone are sufficient to induce pseudopregnancy and a functional corpora lutea [10, 13, 15–17]. In mice, the ejaculatory reflex, rather than the pattern of intromissions, may be more important for inducing pro-gestational changes, thus differing from rats [10, 15–17].
Our results suggest that intromissions alone are not enough to affect return latencies, as female mice only delay returning to the male following ejaculations. The ejaculatory reflex in male mice would produce more stimulation than that of the rat, as male mice remain fully intromitted for much longer than male rats. Male mice undergo an anatomical change after the ejaculatory shudder, a penile cup is formed that lasts from 5–12 seconds after the shudder, and then recedes leaving a copulatory plug [10]. This added stimulation may be necessary to induce luteal activity in female mice.
Interestingly, ovariectomized female mice treated with ovarian hormones, show higher levels of rejection, and therefore less acceptances of the male, when they can not otherwise pace copulation by escaping from the male compartment. Females may be using rejections (turning, biting, vocalizations) as a strategy to space the intromissions when they can not avoid contact with the male by escaping. Female mice are similar in size to males, and therefore may use rejection as a very effective mean of controlling the pace of copulation. Although there was a trend for longer intervals between intromissions for EB only treated females, the intromission intervals did not differ significantly between testing paradigms, further suggesting that the female was able to control timing of copulation during a nonpacing test. Interestingly, mount frequency, but not intromission frequency, increased in nonpacing tests, compared to pacing tests. This may also reflect the female’s ability to control copulation as more mounts failed to result in an intromission during nonpacing tests.
It is not known if under natural conditions female mice use rejections or escapes (or both strategies) to control coital stimulation. Unlike rats in which several males mate concurrently with the same female [6–8], male mice are solitary and defend a territory by excluding other adult males [5]. Mating takes place in these individual territories [5] and it is not clear if the female can use escape as a strategy to pace copulation. However in a semi-natural testing paradigm, Garey et al. 2002, showed that female mice display darting (or escaping) behavior after mounts and ejaculations. Females tested in this semi-natural environment could utilize nest boxes to keep away from the male after coital stimulation, therefore timing copulation [18]. In that study, the investigators could not differentiate mounts from intromissions, but it is likely that either a mount or intromission resulted in darting, given that our data show escape occurring after both types of contact with the male is just as likely. Given these observations in a semi-natural environment, it is possible that female mice use escape as a means of controlling copulation. However rejections may also be used when escape is not an option. In addition, female mice may terminate intromissions as another way to control coital stimulation during mating, but our data indicates that this is used equally whether or not escaping the male is possible.
Mice are not the only species in which females may use alternatives to escaping to pace coital stimulation. In hamsters, another solitary species, females typically stay in a lordosis posture while mating. However, they too may actively control copulation, in spite of an apparent immobile posture [19]. Female hamsters make postural changes of the perineum during lordosis to facilitate successful intromission by the male [20, 21]. However, it is still unknown what pattern of intromissions is optimal for promoting progestational changes in this species [19].
In summary, female mice do pace copulation, perhaps by utilizing several strategies, including rejections when escape is not possible, but it is unclear if this pacing serves the same function as in female rats. More work is needed to determine the effects of paced mating on reproductive success and to elucidate the mechanisms involved in the induction of hormonal changes needed for pregnancy in female mice.
Acknowledgments
We wish to thank Jiming Fang and Justin Whitney for technical support. We also thank the anonymous reviewers for their very helpful suggestions. This work was supported by an NIEHS Superfund grant (P42ES04911) and an NSF grant (IBN972883) to L.G. Clemens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burns-Cusato M, Scordalakes EM, Rissman EF. Of mice and missing data: what we know (and need to learn) about male sexual behavior. Physiol Behav. 2004;83(2):217–232. doi: 10.1016/j.physbeh.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 2.McGill TE. Sexual behaviour in three inbred strains of mice. Behaviour. 1962;19:341–350. [Google Scholar]
- 3.Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23(4):473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein JD. Neuroendocrine Regulation of Feminine Sexual Behavior: Lessons from Rodent Models and Thoughts About Humans. Annual Review of Psychology. 2008;59(1) doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- 5.Bronson FH. The reproductive ecology of the house mouse. Q Rev Biol. 1979;54(3):265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- 6.McClintock MK. The Behavioral Endocrinology of Rodents: A Functional Analysis. BioScience. 1983;33(9):573–577. [Google Scholar]
- 7.McClintock MK, Anisko JJ. Group mating among Norway rats I. Sex differences in the pattern and neuroendocrine consequences of copulation. Animal Behaviour. 1982;30(2):398–409. [Google Scholar]
- 8.McClintock MK, Anisko JJ, Adler NT. Group mating among Norway rats II. The social dynamics of copulation: Competition, cooperation, and mate choice. Animal Behaviour. 1982;30(2):410–425. [Google Scholar]
- 9.Hull EM, Dominguez JM. Sexual behavior in male rodents. Hormones and Behavior. 2007;52(1):45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGill TE, Coughlin RC. Ejaculatory reflex and luteal activity induction in Mus musculus. J Reprod Fertil. 1970;21(2):215–220. doi: 10.1530/jrf.0.0210215. [DOI] [PubMed] [Google Scholar]
- 11.Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol. 2003;15(10):978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 12.Kudwa AE, et al. Roles of estrogen receptors [alpha] and [beta] in differentiation of mouse sexual behavior. Neuroscience Neuroactive Steroids: Old Players in a New Game. 2006;138(3):921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Erskine MS, et al. Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res. 2004;153(2):295–315. doi: 10.1016/j.bbr.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J Reprod Fertil. 1990;90(2):375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- 15.Diamond M. Intromission pattern and species vaginal code in relation to induction of pseudopregnancy. Science. 1970;169(949):995–997. doi: 10.1126/science.169.3949.995. [DOI] [PubMed] [Google Scholar]
- 16.Land RB, McGill TE. The effects of the mating pattern of the mouse on the formation of corpora lutea. J Reprod Fertil. 1967;13(1):121–125. doi: 10.1530/jrf.0.0130121. [DOI] [PubMed] [Google Scholar]
- 17.McGill TE. Preejaculatory stimulation does not induce luteal activity in the mouse Mus musculus. Horm Behav. 1972;3(2):83–85. doi: 10.1016/0018-506x(72)90010-4. [DOI] [PubMed] [Google Scholar]
- 18.Garey J, et al. Temporal and Spatial Quantitation of Nesting and Mating Behaviors among Mice Housed in a Semi-Natural Environment. Horm Behav. 2002;42(3):294–306. doi: 10.1006/hbeh.2002.1823. [DOI] [PubMed] [Google Scholar]
- 19.Bradley KC, Haas AR, Meisel RL. 6-Hydroxydopamine lesions in female hamsters (Mesocricetus auratus) abolish the sensitized effects of sexual experience on copulatory interactions with males. Behav Neurosci. 2005;119(1):224–232. doi: 10.1037/0735-7044.119.1.224. [DOI] [PubMed] [Google Scholar]
- 20.Noble RG. Limited coital stimulation facilitates sexual responses of the female hamster. Physiol Behav. 1979;23(6):1007–1010. doi: 10.1016/0031-9384(79)90289-0. [DOI] [PubMed] [Google Scholar]
- 21.Noble RG. Sex responses of the female hamster: effects on male performance. Physiol Behav. 1980;24(2):237–242. doi: 10.1016/0031-9384(80)90080-3. [DOI] [PubMed] [Google Scholar]