Abstract
Endemic Burkitt lymphoma (BL) is etiologically associated with Epstein-Barr virus (EBV) and ecologically linked to Plasmodium falciparum malaria. However, these infections imperfectly correlate with BL epidemiology. To obtain recent epidemiological data, we studied district- and county-specific BL incidence and standardized incidence ratios using data collected from 1997 through 2006 at Lacor Hospital in northern Uganda, where studies were last done more than 30 years ago. Among 500 patients, median age was 6 years (inter-quartile range 5-8) and male-to-female ratio was 1.8:1. Among those known, most presented with abdominal (56%, M: F 1.4:1) vs. only facial tumors (35%, M: F 3.0:1). Abdominal tumors occurred in older (mean age: 7.0 vs. 6.0 years; p<0.001) and more frequently in female children (68% vs. 50%; OR 2.2, 95% CI 1.5-3.5). The age-standardized incidence was 2.4 per 100,000, being 0.6 in 1-4 year olds, 4.1 in 5-9 year olds and 2.8 in 10-14 year olds and varied 3-4-fold across districts. The incidence was lower in districts that were far from Lacor and higher in districts that were close to Lacor. While districts close to Lacor were also more urbanized, the incidence was higher in the nearby perirural areas. We highlight high BL incidence and geographic variation in neighboring districts in northern Uganda. While distance from Lacor clearly influenced the patterns, the incidence was lower in municipal than in surrounding rural areas. Jaw tumors were characterized by young age and male gender, but presentation has shifted away from facial to mostly abdominal.
Keywords: Africa, cancer, malaria, Epstein-Barr virus, clustering, epidemiology
Introduction
Burkitt lymphoma (BL) is a highly proliferative B cell tumor first described in Ugandan children by Denis Burkitt fifty years ago 1. Subsequent population, hospital, and laboratory studies defined its epidemiology and substantially shaped our understanding of the role infections, the environment, and host-genetics in cancer development 2. BL is rare 3 but is 100-fold more common in tropical Africa and Papua New Guinea 4, which has led to the distinction between sporadic (low incidence) and endemic (high incidence) BL. Endemic BL often presents as facial (jaw or orbital) tumors, whereas sporadic BL presents as tumors involving abdominal organs or bone marrow. Compared to background rates in Western countries, the increased risk of BL in persons with AIDS (AIDS-related BL) has focused attention to immunosuppression as a co-factor. However, the relationship with immunosuppression is complex 5. Increased risk has not been demonstrated in persons with HIV/AIDS in endemic BL areas of Africa 6, 7 and immunosuppression does not seem to increase the risk of BL linearly in persons with AIDS 8, 9. All BL forms are histologically indistinguishable and harbor a molecular signature lesion: reciprocal chromosomal translocation of coding sequences of cellular-MYC on the long arm of chromosome 8 and promoter sequences of heavy chain immunoglobulin genes on long arms of chromosome 14 (80%) or light chain immunoglobulin genes on chromosomes 2 or 22 (20%) 10. The translocation disrupts the structure and function of c-MYC, causing it to be constitutively expressed when immunoglobulin genes are activated. Expression of c-MYC leads to hyper-proliferation of translocation-bearing B cells, increasing their risk for developing genetic errors, which ultimately increase the risk of BL 11. Translocation of c-MYC is considered essential for BL to develop, but it is apparently not sufficient because low level c-MYC rearrangements have been reported in healthy Caucasians [12]. The frequency and natural history of c-MYC translocation in healthy Africans is unknown.
BL is associated with Epstein-Barr virus (EBV) 12, but EBV is ubiquitous worldwide and alone cannot explain the uneven geographic distribution of BL [2, 13]. Plasmodium falciparum malaria was hypothesized as the geographic co-factor for endemic BL, based on ecologic studies 13. However, malaria transmission is imperfectly correlated with BL and epidemiological evidence for association is weak 14. Clusters of BL, akin to mini-epidemics, were reported in the West Nile 15, 16 and Bwamba 17 districts of Uganda in the 1960s and in Malawi in the 1990s 18, but they were not observed in Ghana 19 or Tanzania 20. Occurrence of clusters could suggest exposure to environmental factors which “move about” or are sporadic in some areas but constant in others20. In Uganda and Malawi, BL clusters reportedly coincided with epidemic activity of Chikungunya and Onyong-nyong viruses in the general population 18, but civil disturbances in Uganda prevented studies to test these associations. Carpenter et al., 21 recently reported significant association between endemic BL and high anti-malarial antibody titers, but the cases and controls came from dissimilar geographic areas and clustering was not evaluated. Recent improvements in technologies to diagnose 22 and map BL provide new opportunities to study to some of the old unanswered questions. To obtain recent data on the general epidemiology of endemic BL and refocus attention on this model disease23, we analyzed data collected from a BL registry in northern Uganda, an area last studied in the 1970s.
Methods
St. Mary’s Hospital, Lacor (http://www.lhospital.org), is a Catholic mission hospital established in 1959 in Gulu in northern Uganda about 350 km from Kampala, Uganda’s capital. With ~500 beds (108 for children) and treating ~280,000 patients annually, it is the 3rd largest hospital in the country, offering general and specialized services to people living within ~100 mile radius. BL treatment is given at no cost to patients. A BL registry was established in 1992 to keep track of patients. Cases are diagnosed clinically and confirmed using cytology or histology by a senior pathologist at Makerere University Medical School in Kampala. Data were available on age, sex, tribe, address (district, sub-county, parish or village), date of admission, duration of symptoms, diagnosis and tumor location. Analysis was restricted to cases from 10 neighboring districts (locator map in Figure 1) treated from 1997 through 2006, years for which registry data were considered reasonably complete. Northern Uganda lies in savannah woodland between 2000-4000 feet above sea level and receives ~1000-1500 mm of rainfall in two seasons from March through June (heavy rains) and from September through November (light rains). Average temperature is 60-80° F and humidity is ~30%, and malaria transmission is holoendemic year-around. Historically, BL incidence was higher in northern than in southern Uganda (~3-4-fold). The average population density is low compared to the country average (65 vs. 124/ km2) and people live in grass-thatched houses on small subsistence farms. The population is mostly Nilotic, with 80% belonging to the Luo tribes of Acholi or Langi. About 60% of the population live within 5 km of a health center or hospital and have relatively easy access to transport. We assumed that BL cases from this region would be referred to Lacor Hospital because it is the only hospital in the region with facilities to both diagnose and treat BL.
Figure 1.
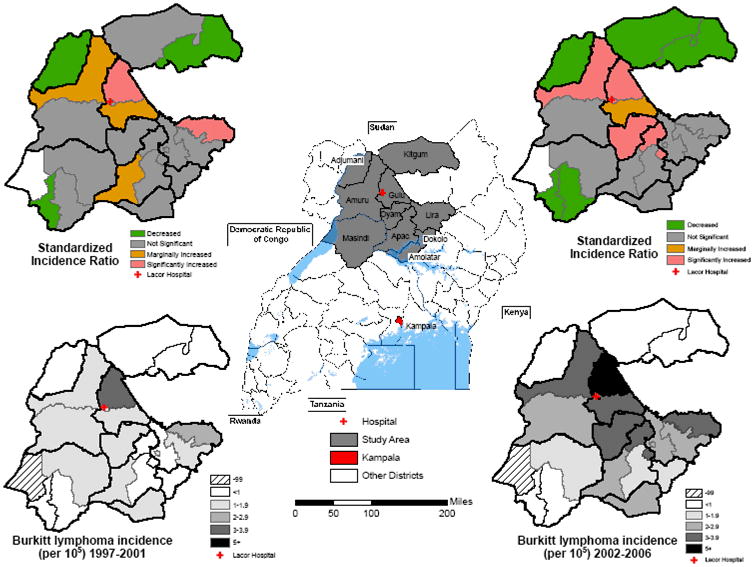
Map showing location of study area in northern Uganda and the names and district boundaries (centre map). Study maps show, on the lower panels the crude BL incidence and, on the upper panels, the standardized incidence ratio, by county for the periods 1997-2001(left) and 2002-2006 (right). Bold lines indicate district boundaries, thin lines indicate county boundaries. Hatch marked area indicates counties without any case counts.
We calculated BL incidence in children (ages 0-14 years) using annual (mid-year) age- and sex-specific-population projections obtained from the Uganda Bureau of Statistics. The population data included district-level population counts from the 1991 and 2002 censuses and the mid-year population estimates for the inter-censual years from 1992-2001 and extrapolations from 2003-2006, and age-, sex-, parish-level (Parish is the smallest administrative unit in a district for which population counts are obtained during census) population census data from the 2002 census. To impute county-level populations by year, we used the Parish census data for 2002 in combination with the district data for each year, assuming that the age-specific distributions in a given county remained the same across the study period. Overall, district-, county-, age-, sex-, calendar-period-specific BL incidence and standardized incidence ratios with 95% confidence intervals (CI) by county were calculated. The expected numbers of cases were calculated by applying age-, race-, sex-, calendar year-, and registry-specific incidence rates from the combined population to the person-time distribution in the district or county. We assumed that incidence was determined by Poisson distribution. District and county incidence were also age-standardized to the world standard population of Segi (1960) by the direct method. Odds ratios of association and 95% confidence intervals (95% CI) between categorical variables were determined using chi-square tables, while differences in the means of continuous variables were determined using the unpaired t-test. Two-sided p-value <0.05 was considered statistically significant.
Results
Of 515 cases registered in children from 1997 and 2006, 15 lacked a date of diagnosis and were excluded. In the 500 remaining cases, the number of cases registered increased from 132 in 1997-2001 to 368 in 2002-2006. The annual percentage of cases increased gradually in the first 5 years, but abruptly in 2004-2005 (Figure2A). More cases were diagnosed in July-December than in earlier months (283 vs. 217; Table), with the peak number diagnosed between July and September. Children aged <7 years were diagnosed more frequently from January to June compared to older children (OR 1.7, 95% CI 1.1-2.4). The median age was 6 years (inter-quartile range (IQR) 5-8 years) in both boys and girls, and the overall male: female ratio was 1.8:1. The majority of cases presented with abdominal (with or without jaw) than with only facial tumors (average 56% vs. 35%; Figure 2A). Children with abdominal tumors were older than those with only jaw tumors (mean age: 7.0 vs. 6.0 years; p<0.001). The proportion of children with jaw tumors peaked at ~3 years then decreased steadily (Figure 2B). Conversely, the proportion of children with abdominal tumors increased with progressively with age. The male to female ratio in children presenting with abdominal tumors was 1.4:1, but the ratio was 3.0:1 in children presenting with only jaw tumors. The median duration of symptoms was 4 weeks (IQR 2-8 weeks), but it was shorter in children with only jaw versus abdominal tumors (5.7 vs. 7.9 weeks, p=0.03). The five children who had central nervous system (CNS) involvement reported symptoms lasting <2 weeks, but the frequency of CNS disease is underrepresented because lumbar punctures were done only when CNS disease was suspected on clinical indications.
Figure 2.
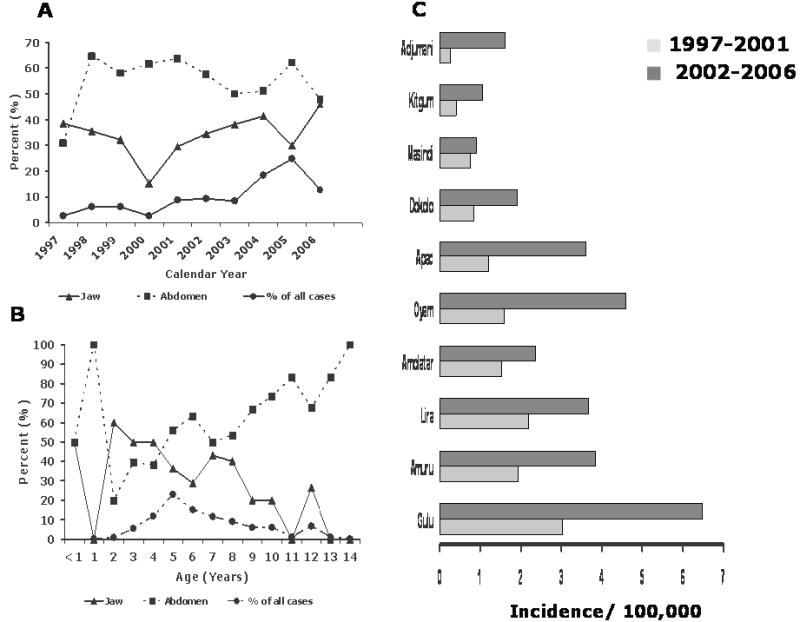
A: Line graph showing the percent of cases presenting as abdominal (with or without jaw) or only jaw tumors by calendar year and percent of cases by calendar year (1997-2006)
B: Line graph showing the percent of children with only jaw, abdominal (with or without jaw) and the percent of cases BL by age (0 through 14 years).
C: Bar graphs showing age-standardized (to the world population) incidence rates for BL for 10 districts in northern Uganda for calendar-year periods 1997-2001 and 2002-2006.
The crude annual incidence was 1.8 per 100,000 children (2.4 in males vs. 1.3 females). The age-standardized incidence was 2.4 per 100,000, and it doubled from 1.5 in 1997-2001 to 3.1 in 2002-2006. The age with the highest number of cases was 5 years. The incidence rose from 0.6 in 1-4 year olds to 4.1 in 5-9 year olds and then decreased to 2.8 in 10-14 year olds. Overall incidence was 3-4-fold higher in the districts with the highest incidence compared to those with the lowest incidence, but the relative incidence pattern remained the same in the first as in the last 5 years of the study (Figure 1 & 2C). Age-standardized incidence was lower in districts that were far from the hospital (e.g., 0.5 in Kitgum to the north-east and 0.7 in Masindi to the south-west) and higher in districts close to the hospital (3.4, 2.7, 2.6, and 2.3, respectively, for Gulu, Amuru, Oyam, and Lira districts; Figure 1). BL incidence was high in two districts (Gulu and Lira) that have the two largest urban centers and the two provincial hospitals in the region. However, within these two districts the incidence was higher in the nearby rural counties than in the municipality counties. Specifically, within Gulu District, the incidence was higher in rural Aswa County than in urban Gulu Municipality County (4.5 vs. 3.6). Similarly, in Lira District the incidence was higher in rural Otuke County than in urban Lira Municipality County (2.8 vs. 1.8). Five counties (Kilak, Aswa, Gulu Municipality, Otuke and Oyam; Figure 1) had significantly elevated BL SIRs compared to the average incidence for the whole region. However, only one of these five counties is urban (Gulu Municipality).
Discussion
Our study updates the general epidemiology of BL in northern Uganda, which was described more than thirty years ago 12, 13. As expected, we observed similarities with historical patterns, including the high incidence, peak incidence at ages 5-9 years, predominance in males, variation by geography, and short duration of symptoms 13. As previously reported 24, young children (<7 years) were more likely to be admitted in the months immediately following the rainy season (June to September), possibly because of increased risk associated with, albeit lagging, the peak malaria season. Our findings underscore previous observations that jaw tumors peak by age 3 years and are 3-fold more common in boys than girls. This unique pattern of jaw lymphomas in endemic BL is unexplained. Differences from historical patterns13 include a shift away from jaw to mostly abdominal disease presentation. A similar, fairly rapid shift was observed in Ghana in the 1970s 25, but its significance is unclear.
Compared with historical rates in northern Uganda (range: 1.9-6 per 100,000) from the 1960s 13, 26, BL incidence in our current study is broadly similar. However, comparing with historical rates is risky because inaccuracies in population measurement and secular changes for non-biological reasons can distort trends. In our study area, civil disturbance by a brigand group calling itself the Lord’s Resistance Army disrupted services and likely contributed to under-ascertainment of cases, especially in the 1990s. When studies in the 1960s were done, conditions were more stable, but the medical infrastructure and transport service were certainly much less developed and census data were probably less accurate than currently. In the old Mengo District of southern Uganda, BL incidence declined from 0.75 to 0.26 during the 1960s but in a more recent study in the 1990s, it was found to be increased (~3.4) 27, a finding attributed to better medical services and improved reporting in later years. There are indications in our data that underreporting was important. Examined at a county level, BL incidence was lower in counties that were further from Lacor than those that were closer, especially during 1997-2001, a time of civil unrest in northern Uganda. Theoretically, referral of cases to elsewhere in Uganda could also be a factor. The Uganda Cancer Institute (UCI) in Kampala, the capital of Uganda, is a well-known national cancer treatment center. However, it is located 350 km away. According to a recent report21, only 6 of 247 (2.4%) of BL patients treated at UCI from 1994 through 1998 came from our study region, a loss of cases that would not have disturbed our patterns. Non-diagnosis of BL could also contribute. During our study, the number of cases increased throughout the study period, and included abdominal cases, which are harder to diagnose, suggesting that increases are likely due to improvements in case ascertainment rather than absolute increase in incidence in the population.
Compared with incidence in Western Kenya in districts that abut Lake Victoria and also experience holoendemic malaria, BL incidence in northern Uganda is about 1.5-2-fold higher (0.6-1.2 per 100,000 in Kenya) 28. The median age at diagnosis of the lake-shore cases was 6 years (IQR 5.0–8.0) and males were predominant, but the cases presented mostly with jaw tumors. Ecologic analyses showed that BL incidence in Kenya was strongly correlated with the intensity of malaria transmission, being lowest in the low malaria risk upland areas (0.39) and highest in the malaria-endemic lake-shore region (1.23, with a relative risk of 3.5 for high vs. low malaria risk areas) 14, 28. Even so, statistically significant clustering was observed in some, but not all, high intensity malaria transmission areas, suggesting possible influences of co-factors other than malaria 14. Our county-level analyses showing variable BL incidence in neighboring counties and districts echo the results from Western Kenya. While distance from Lacor clearly influenced our patterns, the incidence was lower in municipal than in surrounding rural areas, suggesting distance and socioeconomic status in urban centers are not the sole determinants of BL variation. Similar patterns, that could not be explained as case-ascertainment artifacts, have been reported in some 15, 16, but not all studies 26 conducted in northern Uganda in the 1960s. Such geographic variation is likely to reflect factors that may be sporadic in some areas, but constant in others 20. Morrow suggested that the intensity and type of malarial infestation was responsible for variation in BL 13. Malaria transmission is influenced by mosquito density and by the frequency of mosquito bites at the household-level (estimated to range from 300 to 1500 mosquito bites per person per year in northern Uganda 29), which in turn is influenced by use of pesticides, bed-nets, socio-cultural practices, and physical factors including standing water, vegetation and land use practices. However, these factors are erroneously thought to be invariant for whole regions, and the debate often ignores other factors that co-exist with malaria, such as intestinal parasites, that could independently influence or modulate BL risk by influencing immune responses to EBV or malaria.
Proximity to a hospital is a plausible non-biologic explanation for geographic variation in BL incidence. However, we expect that most cases with BL come to medical attention because patients present with inexorably and rapidly progressive physical symptoms. Moreover, most people in northern Uganda live within 5 km of a health center and have relatively easy access to transport. The higher incidence of BL in the rural than the urban counties of the urban districts (Otuke in Lira, Aswa in Gulu, and Kilak in Amuru; Figure 1) supports this reasoning and is in accord with an earlier report that BL is more common in rural areas 19.
We are intrigued by data, noted by us and others, that jaw tumors peak at a very young age and occur disproportionately in boys 30. This pattern suggests that exposures that influence tumor tropism differ between young boys and girls and that the prevalence or influence of these exposures wanes with age. The peak age at 3 years for jaw tumors is similar to childhood leukemia, which has been postulated to develop from leukemogenic cells that arise prenatally 31. Whether jaw tumors develop from prenatally existing c-MYC translocations is unknown. Jaw tumors may arise because of insults to the oral mucosa or to dentition at a very young age, such as from local weaning practices or infant oral dental mutilation. For example, a practice called “ebinyo” involving extirpation of the primary canine tooth follicles of infants using crude instruments and application of saliva to the dental gum is widespread in northern Uganda 32. The preponderance of jaw tumors in males has been observed, but not emphasized, in other reports. Our study highlights this imbalance in jaw-male pattern, prompting us to wonder whether a similar pattern is observed in sporadic BL. If so, it might suggest genetic factors on the sex chromosomes. The Y-chromosome has been postulated to induce earlier and/or faster eruption of deciduous teeth 33, which may be biologically relevant.
Our study has limitations. Case ascertainment is likely incomplete; thus we focused our interpretation on patterns rather than on absolute risk. Histological diagnosis was done locally without confirmation from current-state-of-the art molecular methods 22. Our results are, nonetheless, comparable with results from other studies conducted in Africa 14, 30. The strengths of our study include its focus on a well-defined geographic region, relatively large size, and use of data from a BL hospital registry, which minimized errors. Our study highlights local capacity for BL research and the richness of epidemiologic opportunity in a hospital in a resource poor country grappling with challenges of BL treatment 34. International investigators should seek to strengthen this capacity for collaborative BL research to answer outstanding questions, including improved geographic and clinical characterization of BL, identifying co-factors, and determining the prevalence and natural history of translocations in B-cells in healthy populations. Such studies will provide access to populations and appropriate specimens to help further refine BL diagnosis, identify genetic risk factors 2, and bring treatment benefits to African patients with BL 23.
To conclude, we highlight high BL incidence and geographic variation in neighboring districts in northern Uganda. While distance from Lacor clearly influenced the patterns, the incidence was lower in municipal than in surrounding rural areas. The preponderance of jaw tumors in young boys and the shift from facial to abdominal presentation are unexplained.
Table 1.
Characteristics of cases in the Burkitt Lymphoma Registry at Lacor Hospital
| Characteristic | N | % |
|---|---|---|
| Sex | ||
| Female | 176 | 35% |
| Male | 324 | 65% |
| Age group, years | ||
| 0-4 | 96 | 19% |
| 5-9 | 327 | 65% |
| 10-14 | 77 | 16% |
| District | ||
| Adjumani | 17 | 3% |
| Amulatar | 18 | 4% |
| Amuru | 46 | 9% |
| Apac | 79 | 16% |
| Dokolo | 16 | 3% |
| Gulu | 101 | 20% |
| Kitgum | 13 | 3% |
| Lira | 115 | 23% |
| Masindi | 28 | 6% |
| Oyam | 67 | 13% |
| Calendar period | ||
| 1997-2001 | 132 | 26% |
| 2002-2006 | 368 | 74% |
| Tumor location* | ||
| Facial | 177 | 35% |
| Abdomen | 271 | 54% |
| Abdomen and face | 9 | 2% |
| Not recorded/other | 43 | 9% |
| Tumor stage † | ||
| A | 22 | 4% |
| B | 23 | 5% |
| C | 157 | 31% |
| D | 131 | 26% |
| Not recorded | 167 | 33% |
| Calendar month diagnosed | ||
| Jan-June | 217 | 43% |
| Jul-Dec | 283 | 57% |
Not recorded/other includes 5 cases recorded as having CNS involvement, but peripheral sites of disease was not recorded
BL staged according to a hierarchical four stage system: A-single extra-abdominal tumor (typically jaw or orbital); B-multiple extra-abdominal tumors; C-any intra-abdominal tumor; D-any involvement of the central nervous system or bone marrow.
Acknowledgments
We are grateful to the Uganda Bureau of Statistics (Kampala, Uganda) for giving us population census files, Mathew Airola at Westat Inc, (Rockville, MD) for illustrating geographic variation of BL, Ruth Parsons and Stella Munuo at Information Management Systems (Rockville, MD) for preparing analysis data files and performing analyses. This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (contracts N02-CP-31003 and N01-CO-12400). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–23. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3:182–7. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 3.Levine PH, Connelly RR, Berard CW, O’Connor GT, Dorfman RF, Easton JM, DeVita VT. The American Burkitt Lymphoma Registry: a progress report. Ann Intern Med. 1975;83:31–6. doi: 10.7326/0003-4819-83-1-31. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Sohier R, O’Conor GT. Geographic distribution of Burkitt’s lymphoma. IARC Sci Publ. 1985:155–64. [PubMed] [Google Scholar]
- 5.Mbulaiteye SM, Parkin DM, Rabkin CS. Epidemiology of AIDS-related malignancies an international perspective. Hematol Oncol Clin North Am. 2003;17:673–96. v. doi: 10.1016/s0889-8588(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Garcia-Giannoli H, Raphael M, Martin A, Katangole-Mbidde E, Wabinga H, Ziegler J. Non-Hodgkin lymphoma in Uganda: a case-control study. Aids. 2000;14:2929–36. doi: 10.1097/00002030-200012220-00015. [DOI] [PubMed] [Google Scholar]
- 7.Mbulaiteye SM, Katabira ET, Wabinga H, Parkin DM, Virgo P, Ochai R, Workneh M, Coutinho A, Engels EA. Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer. 2006;118:985–90. doi: 10.1002/ijc.21443. [DOI] [PubMed] [Google Scholar]
- 8.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003;32:527–33. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99:962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia K, Huppi K, Spangler G, Siwarski D, Iyer R, Magrath I. Point mutations in the c-Myc transactivation domain are common in Burkitt’s lymphoma and mouse plasmacytomas. Nat Genet. 1993;5:56–61. doi: 10.1038/ng0993-56. [DOI] [PubMed] [Google Scholar]
- 11.Kelly K, Siebenlist U. The role of c-myc in the proliferation of normal and neoplastic cells. J Clin Immunol. 1985;5:65–77. doi: 10.1007/BF00915003. [DOI] [PubMed] [Google Scholar]
- 12.de-The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt’s lymphoma from Ugandan prospective study. Nature. 1978;274:756–61. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 13.Morrow RH., Jr Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt’s lymphoma. IARC Sci Publ. 1985:177–86. [PubMed] [Google Scholar]
- 14.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt’s lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120:121–7. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 15.Williams EH, Smith PG, Day NE, Geser A, Ellice J, Tukei P. Space-time clustering of Burkitt’s lymphoma in the West Nile district of Uganda: 1961-1975. Br J Cancer. 1978;37:109–22. doi: 10.1038/bjc.1978.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams EH, Spit P, Pike MC. Further evidence of space-time clustering of Burkitt’s lymphoma patients in the West Nile District of Uganda. Br J Cancer. 1969;23:235–46. doi: 10.1038/bjc.1969.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow RH, Pike MC, Smith PG, Ziegler JL, Kisuule A. Burkitt’s lymphoma: a time-space cluster of cases in Bwamba County of Uganda. Br Med J. 1971;2:491–2. doi: 10.1136/bmj.2.5760.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Bosch CA. Is endemic Burkitt’s lymphoma an alliance between three infections and a tumour promoter? Lancet Oncol. 2004;5:738–46. doi: 10.1016/S1470-2045(04)01650-X. [DOI] [PubMed] [Google Scholar]
- 19.Biggar RJ, Nkrumah FK. Burkitt’s lymphoma in Ghana: urban-rural distribution, time-space clustering and seasonality. Int J Cancer. 1979;23:330–6. doi: 10.1002/ijc.2910230310. [DOI] [PubMed] [Google Scholar]
- 20.Siemiatycki J, Brubaker G, Geser A. Space-time clustering of Burkitt’s lymphoma in East Africa: analysis of recent data and a new look at old data. Int J Cancer. 1980;25:197–203. doi: 10.1002/ijc.2910250206. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, Wabinga H, Jaffe H, Beral V. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122:1319–23. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 22.Hummel M, Bentink S, Berger H, Klapper W, Wessendorf S, Barth TF, Bernd HW, Cogliatti SB, Dierlamm J, Feller AC, Hansmann ML, Haralambieva E, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–30. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro RC, Sandlund JT. Burkitt lymphoma in African children: A priority for the global health agenda? Pediatr Blood Cancer. 2008 doi: 10.1002/pbc.21528. [DOI] [PubMed] [Google Scholar]
- 24.Williams EH, Day NE, Geser AG. Seasonal variation in onset of Burkitt’s lymphoma in the West Nile District of Uganda. Lancet. 1974;2:19–22. doi: 10.1016/s0140-6736(74)91350-6. [DOI] [PubMed] [Google Scholar]
- 25.Nkrumah FK. Changes in the presentation of Burkitt’s lymphoma in Ghana over a 15- year period (1969-1982) IARC Sci Publ. 1984:665–74. [PubMed] [Google Scholar]
- 26.Morrow RH, Pike MC, Smith PG. Further studies of space-time clustering of Burkitt’s lymphoma in Uganda. Br J Cancer. 1977;35:668–73. doi: 10.1038/bjc.1977.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82:1585–92. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainey JJ, Mwanda WO, Wairiumu P, Moormann AM, Wilson ML, Rochford R. Spatial distribution of Burkitt’s lymphoma in Kenya and association with malaria risk. Trop Med Int Health. 2007;12:936–43. doi: 10.1111/j.1365-3156.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 29.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D’Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25. [PubMed] [Google Scholar]
- 30.Amusa YB, Adediran IA, Akinpelu VO, Famurewa OC, Olateju SO, Adegbehingbe BO, Komolafe EO, Faponle AF, Olasode BJ. Burkitt’s lymphoma of the head and neck region in a Nigerian tertiary hospital. West Afr J Med. 2005;24:139–42. doi: 10.4314/wajm.v24i2.28184. [DOI] [PubMed] [Google Scholar]
- 31.Greaves M. In utero origins of childhood leukaemia. Early Hum Dev. 2005;81:123–9. doi: 10.1016/j.earlhumdev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Bataringaya A, Ferguson M, Lalloo R. The impact of ebinyo, a form of dental mutilation, on the malocclusion status in Uganda. Community Dent Health. 2005;22:146–50. [PubMed] [Google Scholar]
- 33.Alvesalo L. Sex chromosomes and human growth. A dental approach. Hum Genet. 1997;101:1–5. doi: 10.1007/s004390050575. [DOI] [PubMed] [Google Scholar]
- 34.Wakabi W. Kenya and Uganda grapple with Burkitt lymphoma. Lancet Oncol. 2008;9:319. doi: 10.1016/s1470-2045(08)70088-3. [DOI] [PubMed] [Google Scholar]


