Abstract
We used an overlay method to study the ability of human salivary glycoproteins to serve as receptors for several strains of streptococci that colonize the oral cavity. Parotid and submandibular-sublingual salivas were collected as ductal secretions, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. The resulting blots were overlaid with [35S]methionine-labeled bacteria, and salivary components to which the bacteria bound were detected by autoradiography. Potential glycoprotein receptors were identified for 8 of the 16 strains tested. In three cases (Streptococcus sanguis 72-40 and 804 and Streptococcus sobrinus OMZ176), highly specific interactions with a single salivary component were detected. Removal of sialic acid residues from the low-molecular-weight salivary mucin prevented adherence of one of these strains (S. sanguis 72-40), suggesting that this saccharide either mediates binding or is a critical component of the receptor site. In the remaining five strains (Streptococcus gordonii G9B and 10558, S. sanguis 10556, and Streptococcus oralis 10557 and 72-41), interactions with multiple salivary components, including the low-molecular-weight salivary mucin, highly glycosylated proline-rich glycoproteins, and alpha-amylase, were detected. These results suggest that some oral streptococci can bind specifically to certain of the salivary glycoproteins. The interactions identified may play an important role in governing bacterial adherence and clearance within the oral cavity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Hashimi I., Levine M. J. Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol. 1989;34(4):289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Minaguchi K., Latreille P., Kim H. S. Alleles at the PRB3 locus coding for a disulfide-bonded human salivary proline-rich glycoprotein (Gl 8) and a null in an Ashkenazi Jew. Am J Hum Genet. 1990 Oct;47(4):686–697. [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Hasty D. L., Simpson W. A. Isolation and characterization of a 60-kilodalton salivary glycoprotein with agglutinating activity against strains of Streptococcus mutans. Infect Immun. 1986 Feb;51(2):405–413. doi: 10.1128/iai.51.2.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Beachey E. H., Simpson W. A. Inhibition of the interaction of Streptococcus sanguis with hexadecane droplets by 55- and 60-kilodalton hydrophobic proteins of human saliva. Infect Immun. 1986 Aug;53(2):278–284. doi: 10.1128/iai.53.2.278-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bennick A., Chau G., Goodlin R., Abrams S., Tustian D., Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Arch Oral Biol. 1983;28(1):19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Leffler H. The specific glycosphingolipid composition of human ureteral epithelial cells. J Biochem. 1985 Nov;98(5):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135383. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Douglas C. W. The binding of human salivary alpha-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28(7):567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- Emilson C. G., Ciardi J. E., Olsson J., Bowen W. H. The influence of saliva on infection of the human mouth by mutans streptococci. Arch Oral Biol. 1989;34(5):335–340. doi: 10.1016/0003-9969(89)90106-4. [DOI] [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Fisher S. J., Prakobphol A., Kajisa L., Murray P. A. External radiolabelling of components of pellicle on human enamel and cementum. Arch Oral Biol. 1987;32(7):509–517. doi: 10.1016/s0003-9969(87)80013-4. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989 Sep;68(9):1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece-Castro B. L., Prakobphol A., Burlingame A. L., Leffler H., Fisher S. J. Structure and bacterial receptor activity of a human salivary proline-rich glycoprotein. J Biol Chem. 1991 Sep 15;266(26):17358–17368. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989 Dec;57(12):3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
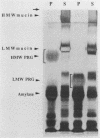
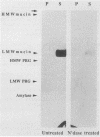
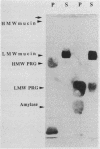
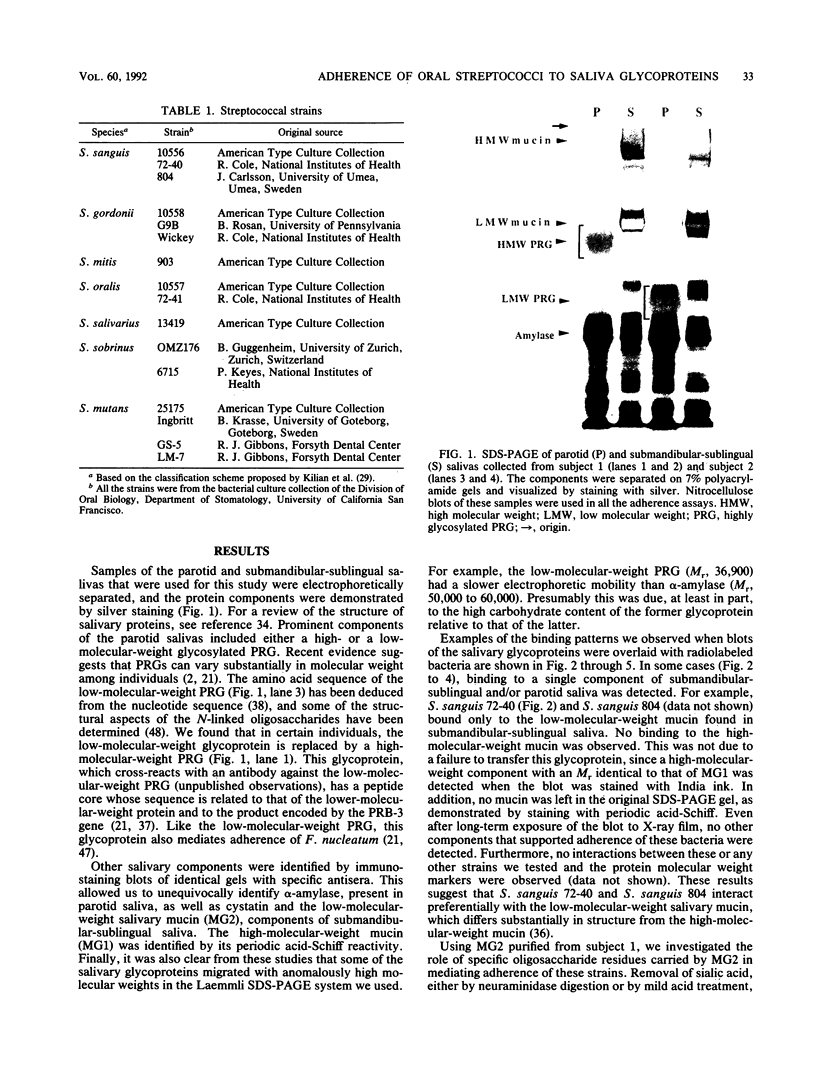
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis R. E., Prakobphol A., Levine M. J., Reddy M. S., Jones P. C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987 Nov 1;258(2):452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Azen E. A., Goodman P. A., Smithies O. Many protein products from a few loci: assignment of human salivary proline-rich proteins to specific loci. Genetics. 1988 Sep;120(1):255–265. doi: 10.1093/genetics/120.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Malamud D., Appelbaum B., Kline R., Golub E. E. Bacterial aggregating activity in human saliva: comparisons of bacterial species and strains. Infect Immun. 1981 Mar;31(3):1003–1006. doi: 10.1128/iai.31.3.1003-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D. The functions of saliva. J Dent Res. 1987 Feb;66(Spec No):623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Loveless R. W., Lawson A. M., Chai W., Lachmann P. J., Childs R. A., Thiel S., Feizi T. A library of oligosaccharide probes (neoglycolipids) from N-glycosylated proteins reveals that conglutinin binds to certain complex-type as well as high mannose-type oligosaccharide chains. J Biol Chem. 1989 Aug 15;264(23):13834–13839. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- Prakobphol A., Murray P. A., Fisher S. J. Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Jul;164(1):5–11. doi: 10.1016/0003-2697(87)90359-9. [DOI] [PubMed] [Google Scholar]
- Reddy M. S., Levine M. J., Tabak L. A. Structure of the carbohydrate chains of the proline-rich glycoprotein from human parotid saliva. Biochem Biophys Res Commun. 1982 Feb 11;104(3):882–888. doi: 10.1016/0006-291x(82)91331-6. [DOI] [PubMed] [Google Scholar]
- Rosan B., Appelbaum B., Golub E., Malamud D., Mandel I. D. Enhanced saliva-mediated bacterial aggregation and decreased bacterial adhesion in caries-resistant versus caries-susceptible individuals. Infect Immun. 1982 Dec;38(3):1056–1059. doi: 10.1128/iai.38.3.1056-1059.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Bergey E. J., Reddy M. S., Levine M. J. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989 Sep;57(9):2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Nagata K., Nakamura R., Tsunemitsu A., Misaki A. Interaction of parotid saliva basic glycoprotein with Streptococcus sanguis ATCC 10557. J Periodontol. 1980 Sep;51(9):499–504. doi: 10.1902/jop.1980.51.9.499. [DOI] [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990 Jul 5;265(19):11251–11258. [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Jain N. K., Bryan A. R., Cohen R. E., Monte L. D., Zawacki S., Nancollas G. H., Slomiany A., Slomiany B. L. Adsorption of human salivary mucins to hydroxyapatite. Arch Oral Biol. 1985;30(5):423–427. doi: 10.1016/0003-9969(85)90070-6. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Adherence of Streptococcus salivarius HB and HB-7 to oral surfaces and saliva-coated hydroxyapatite. Infect Immun. 1980 Oct;30(1):150–158. doi: 10.1128/iai.30.1.150-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M. K., Chassy B. M., Cisar J. O. Cloning and expression of a type 1 fimbrial subunit of Actinomyces viscosus T14V. J Bacteriol. 1987 Apr;169(4):1678–1683. doi: 10.1128/jb.169.4.1678-1683.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]