Abstract
Apolipoprotein A-IV (apo A-IV) is a satiation protein synthesized in the small intestine and hypothalamus. To further understand its anorectic mechanisms, we used immunohistochemical techniques to characterize the distribution of apo A-IV in brain areas involved in energy homeostasis. Dense apo A-IV staining was detected in the arcuate (ARC) and ventromedial hypothalamic nuclei with less staining in cells in the paraventricular and dorsomedial nuclei. In the brainstem, apo A-IV staining was found in the nucleus of the solitary tract. Double staining immunohistochemistry revealed co-existence of apo A-IV with Neuronal Nuclei (a neuronal marker), but less with glial fibrillary acidic protein (a glial marker), in ARC, suggesting that apo A-IV is largely present in neurons. In the ARC, apo A-IV was co-localized with pro-opiomelanocortin (POMC), and apo A-IV administration stimulated hypothalamic POMC gene expression, suggesting that the brain apo A-IV system suppresses food intake by stimulating the ARC POMC system. To ascertain whether the apo A-IV detected in the brain is derived from the circulation, 125I-labeled recombinant rat apo A-IV was intravenously injected into mice. No increase of radioactive apo A-IV was found in the brain, consistent with a lack of uptake of co-injected 99mTc-labeled albumin, indicating that circulating apo A-IV is unable to cross the blood brain barrier. These data collectively support the hypothesis that apo A-IV, produced by neuronal cells, may exert its anorectic action by interacting with catabolic regulatory neuropeptides.
Keywords: Apolipoproteins, immunohistochemistry, blood brain barrier, neuropeptides
1. Introduction
Apolipoprotein A-IV (apo A-IV) is a major protein component of intestinal triacylglycerol-rich lipoproteins such as chylomicrons and very low-density lipoproteins. In rat, apo A-IV is synthesized in the intestine and the liver, with the intestine accounting for the majority of the circulating apo A-IV [1]. Several studies have provided evidence that apoA-IV plays a role in cholesterol and lipoprotein metabolism [2]. Also, strong in vivo evidence supports roles for apo A-IV in the control of ingestive behavior [3] and gastric function [4] and in protection against lipoprotein oxidation [5] and atherosclerosis [6].
In 2001, we demonstrated that apo A-IV is also synthesized in the hypothalamus, and growing evidence has implicated brain apo A-IV as playing an important role in the control of food intake and body weight [7–11]. Intracerebroventricular (icv) administration of apo A-IV significantly inhibits food intake in a dose-dependent manner without eliciting signs of toxicity [3], and blocking the action of endogenous apo A-IV locally within the brain with its antibody increases meal size, implying that endogenous apo A-IV in the brain exerts an inhibitory tone on feeding [3]. We have also found that the hypothalamus expresses the apo A-IV gene and that the expression is reduced by food deprivation and restored by lipid re-feeding [7].
Although apo A-IV is expressed in hypothalamus [7], its distribution and cellular localization in discrete hypothalamic nuclei have not been fully explored. Furthermore, little information exists regarding the distribution of apo A-IV protein in the brainstem, which is also intimately involved in the control of food intake. One goal of these experiments, therefore, was to determine the distribution of apo A-IV protein in discrete brain areas using immunohistochemistry.
The hypothalamic melanocortin system is important in the regulation of food intake and body weight [12]. Our previous studies demonstrated that central apo A-IV reduces food intake by potentiating the anorectic effect of central melanocortin agonists [13]. Using double staining of immunohistochemistry, we determined that apo A-IV is co-localized with POMC in the ARC, providing morphological evidence for the interaction of apo A-IV with POMC in the regulation of food intake.
Because apo A-IV is present in the cerebrospinal fluid [3] as well as in the blood, the immunostaining of apo A-IV we have previously observed in the brain could have originated in the periphery; i.e., circulating apoA-IV may cross the blood-brain-barrier (BBB) and/or enter the brain from the CSF and be taken up by brain cells. A final goal of these experiments therefore was to administer 125I-labeled recombinant rat apo A-IV intravenously and assess its uptake into brain.
2. Materials and Methods
2.1. Animals and surgery
Adult male Sprague-Dawley rats (250–275 g, Harlan, Indianapolis, IN) were housed individually in a light- and temperature-controlled room (lights on 0600–1800 h, 21°C). Food (pelleted chow, Teklad Rodent Chow, Harlan) and water were available ad libitum. At least one week after arrival in the laboratory, rats were anesthetized with intraperitoneal (ip) ketamine (80 mg/kg)/xylazine (1.6 mg/kg) and implanted with 22-gauge stainless steel cannulas (Plastics One) aimed at the 3rd-cerebral ventricle as described previously [14]. Placement of cannulas was confirmed several days later by administration of 10 ng of angiotensin II in saline (2 μl) while the animals were water replete. Animals that did not drink at least 5 ml of water within 30 min after injection were considered to have failed cannula placement and were excluded from the experiments. All procedures were performed in accordance with institutional guidelines of the IACUC at the University of Cincinnati.
In order to investigate the interaction of apo A-IV with neuropeptides in the hypothalamus, we tested the effects of apo A-IV on the gene expression of POMC, neuropeptide Y (NPY) and agouti-related protein (AgRP). Rats were randomly divided into 4 groups. Groups 1 and 2 were ad libitum-fed, and Groups 3 and 4 were fasted for 46 h. Groups 2 and 4 received icv apo A-IV (8 μg), and Groups 1 and 3 received icv saline as control. No food was provided after icv administration. The rats were sacrificed 2 h after the injections, and hypothalamic gene expression was characterized using qPCR measurement of POMC, NPY and AgRP.
2.2. Antibodies
Antiserum against apo A-IV was raised from goat and tested previously [15]. Mouse monoclonal antibodies, including anti-neuronal nuclei (NeuN), anti-glial fibrillary acidic protein (GFAP), anti-rat CD11B, and anti-galactocerebroside were purchased from Chemicon International, Inc. (Temecula, CA). POMC antibody was obtained from Phoenix Peptides (Belmont, CA)
2.3. Immunoblotting
Samples of hypothalamus were homogenized at 4°C in a buffer containing 0.1 mg ml−1 PMSF, 1 μg ml−1 leupeptin, 1 μg ml−1 pepstatin, 1 μg ml−1 aprotinin and 1 mM EDTA; then centrifuged at 15,000 rpm for 20 min at 4°C. The supernatant of each sample (containing 20 μg protein) was separated by 12% polyacrylamide gel electrophoresis, transferred to nitrocellulose sheets and blotted with goat polyclonal antibody against rat apo A-IV (1:3000 dilution) overnight at 4°C. The amount of immune complexes was detected using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). The reacted membranes were exposed to X-ray film (Kodak Scientific imaging film, Rochester, NY). The optical density of the bands was analyzed by an imaging program from NIH (freeware, National Institutes of Health, Bethesda, MD, USA).
2.4. Immunohistochemistry
For apo A-IV staining, rats were anesthetized with sodium pentobarbital (50 mg/kg, ip) and then perfused via the ascending aorta with 100 ml of a 0.9% NaCl solution followed by 500 ml of ice cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed from the skulls and placed in perfusion buffer for 4 h at 4°C and then in buffered sucrose (30%) for at least 24 h, and 30 μm serial coronal sections were subsequently cut using a microtome. After washing, the sections were blocked with 5% normal donkey serum in PBS containing 0.3% Triton X-100 for 2 h. Then, sections were incubated with a goat anti-rat apo A-IV antiserum (1:400 dilutions) overnight at 4°C. Secondary antibody was 1:200 diluted donkey anti-goat immunoglobulin conjugated to fluorescein Alexa 594 (Molecular Probes, Inc.). Imaging was performed on a Zeiss 510 microscope system. Omission of the primary antibody as well as substituting the primary antibody with apo A-IV pre-absorbed serum, were used to determine the specificity of the antibodies.
2.5. Double-labeling Immunohistochemistry
In order to identify which cell types contain apo A-IV in the ARC, we dual-labeled apo A-IV immunoreactivity with monoclonal antibodies against different cell-type markers, including antineuronal nuclei (NeuN, for neurons), anti-glial fibrillary acidic protein (GFAP, for astrocytes), and anti-rat CD11b (for microglia cells). Using the same method, we also detected whether apo A-IV is co-localized with POMC in the ARC of the hypothalamus. Here, we use the double-staining of apo A-IV with POMC as an example. Free-floating sections (30 μm) were cut through the hypothalamic ARC from brains of perfusion-fixed rats. After washing, the sections were blocked with 5% normal donkey serum in PBS containing 0.3% Triton X-100 for 2 h. Then, sections were incubated with a goat anti-rat apo A-IV antiserum (1:400 dilutions) and rabbit anti-POMC antibody (1:800) overnight at 4°C. Secondary antibodies were used as appropriate, including 1:200 diluted donkey anti-goat (for apo A-IV) and donkey anti-rabbit (for POMC) immunoglobulin conjugated to fluorescein, Alexa 488 or Alexa 594 (Invitrogen Co., Carlsbad, CA), respectively. The dilutions used and the duration of incubation were selected based on our preliminary studies. Confocal imaging was performed on a Zeiss 510 microscope system. Omission of the primary antibodies as well as substituting the primary antibody with apo A-IV pre-absorbed antiserum or normal rabbit serum for POMC antibody were used to determine the specificity of these antibodies. Basically, the protocol is the same for determining the co-localization of apo A-IV with other cell-type markers, except for the different dilution of the first antibodies. The monoclonal antibody against neuronal nuclei (NeuN) was diluted at 1:1000, the anti-glial fibrillary acidic protein (GFAP) at 1:1500, and anti-rat CD11b at 1:1000. The second antibody for these cell-type antibodies was the same, which was 1:200 diluted donkey anti-mouse immunoglobulin conjugated to fluorescein, Alexa 488.
For quantitative assessments of the percentage of double-labeled cells, we analyzed five sections from the area of ARC that contained both apo A-IV and NeuN (an example of other tested proteins) cells. A cell was determined to be singly labeled when visible only under the fluorescence filter corresponding to the emission wavelength of one of the secondary antibody complexes used, e.g. 594 nm and not 488 nm in the case of NeuN. When the cell was visible at both 594- and 488-nm filters, it was deemed to be double labeled. Cell counts were obtained from each side in each section. For each animal, the total number of single-labeled apo A-IV, singled-labeled NeuN, and double-labeled apo A-IV/NeuN cells were counted in each area, and the percentage of apo A-IV cells that contained NeuN nuclei was calculated by 2 independent observers.
2.6. qPCR for apo A-IV mRNA measurement
Total hypothalamic RNA was isolated with Tri Reagent (Molecular Research Center, Inc.). Total RNA (100 ng) from hypothalamus was reverse-transcribed to first-strand complementary DNA (Amersham Pharmacia Biotech). qPCR was performed in a 25-μl final reaction volume with an iCycler iQ Detection System using iQ™ SYBR Green Supermix (Bio-Rad, Laboratories Inc). qPCR conditions were as follows: 95°C for 3 min for one cycle, followed by 38 cycles of 95°C for 30 sec and 58°C for 30 sec on an iCycler iQ real-time PCR detection system. No other products were amplified because melting curves revealed only one peak in each sample. Threshold cycle (Ct) readings for each of the unknown samples were then used, and the results were transferred and analyzed in Excel using the delta delta CT method (Perkin-Elmer Applied Biosystems) [9]. Cyclophilin mRNA levels from each sample were used as internal controls to normalize the mRNA levels. The same condition was also used for measuring mRNA levels of other neuropeptides, including POMC, NPY and AgRP. The sequences of the primers for rat apo A-IV, rat POMC, NPY, AgRP and cyclophilin were determined using primer design Software (Integrated DNA Technologies) and listed in Table 1.
Table 1.
Primer sequences and product size for qPCR
| Gene | Accession no. | Forward primer | Reverse primer | size (bp) |
|---|---|---|---|---|
| Rat apo A-IV | M00002 | 5′-aagctgaaaggcaacacgga-3′ | 5′-tgcctgaacttctccatctgc-3′ | 152 |
| POMC | NM_139326 | 5′-cgcccgtgtttcca-3′ | 5′-tgacccatgacgtacttcc-3′ | 69 |
| NPY | NM_012614 | 5′-ccttgcgacactacatcaa-3′ | 5′-ggggcattttctgtgcttt-3′ | 108 |
| AgRP | AF206017 | 5′-ttcccagagttctcaggtcta-3′ | 5′-atctagcacctctgccaaa-3′ | 98 |
| Cyclophilin | M19533 | 5′-attcatgtgccagggtggtgac-3′ | 5′-tcagtcttggcagtgcagat-3′ | 182 |
2.7 Blood brain barrier studies
Recombinant rat apo A-IV was synthesized according to the procedure described previously [11;16], and labeled with Iodo-Bead reagent (Pierce Chemicals, Rockford, IL) and purified on a column of Sephadex G-10 according to manufacturer’s instructions. Bovine serum albumin (BSA) was 99mTc-labeled following the protocol of Banks et al. [17;18]. BSA is a vascular marker of a functionally intact blood brain barrier (BBB) because it has no significant entry into the CNS when the BBB is intact.
For multiple-time regression analysis, anesthetized C57BL/6J mice were injected with 125I-labeled apo A-IV and 99mTc-labeled BSA (at 1 × 106 cpm) in 200 μl of lactated Ringer’s solution through the exposed left jugular vein. Arterial blood was collected via the right carotid artery at 1, 2.5, 5, 7.5, 15 and 30 min. Immediately following, the animals were decapitated and the whole brain was removed, rinsed with saline for removing excess blood, and weighed. The levels of radioactivity in the brain tissue and plasma were determined in a dual channel γcounter, which can recognize a difference between 125I-labeled apo A-IV and 99mTc-labeled BSA. The ratio of radioactivity of the brain tissue (cpm/g) to serum (cpm/μl) was calculated and plotted over time. This same experiment was repeated with 125I-labeled leptin (PerkinElmer Life and Analytical Sciences, Inc., Waltham, MA) and 99mTc-labeled BSA, and time points (at 1, 7.5, 15 and 30 min) were used to verify our experimental procedure because leptin has been reported to cross the BBB in normal mice [18]. The unidirectional influx constant (Ki, expressed in ml per g per min) was determined from the linear relationship between tissue/serum ratios and exposure time. The slope of this regression line represents the Ki [18].
2.8 Data presentation and analysis
All photographs were prepared by using Adobe Photoshop 7.0 for IBM by adjusting brightness/contrast and were labeled (including scale bars). The data content of the images was not altered in any way. Two-way ANOVA followed by Tukey’s test was utilized for analysis of gene mRNA levels. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Specificity of polyclonal antibody against rat apo A-IV
The specificity of purified goat polyclonal antibody against rat apo A-IV was examined by Western blot analysis. The antibody detected a single immunoreactive band in hypothalamic homogenate with 43 kDa of molecular weight (Fig. 1A), corresponding to the known molecular weight of rat apo A-IV (3). The abolition of immunoblotting (Fig. 1B) by pre-absorbing the antibody with purified rat apo A-IV demonstrates the specificity of the antibody. This band was not seen in hypothalamic extracts of apo A-IV knockout mice (Fig. 1C).
Figure 1.
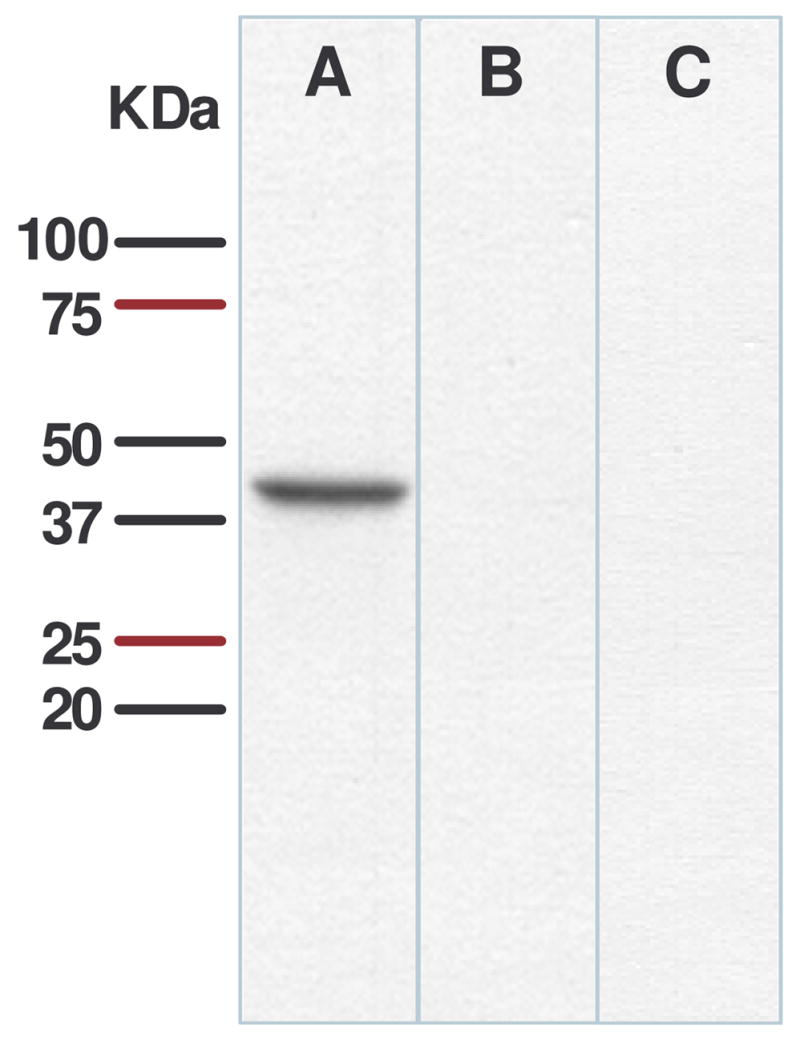
Immunoblots of rat hypothalamic proteins probed with antibody against apo A-IV. Anti-apo A-IV antibody recognized a band (A) of 43 kDa molecular weight, corresponding to the known molecular weight of rat apo A-IV. This staining was specifically inhibited by preabsorption with apo A-IV (B). No band was observed in hypothalamic protein extracted from apo A-IV knockout mice (C).
3.2. Distribution of apo A-IV in the hypothalamus and brainstem
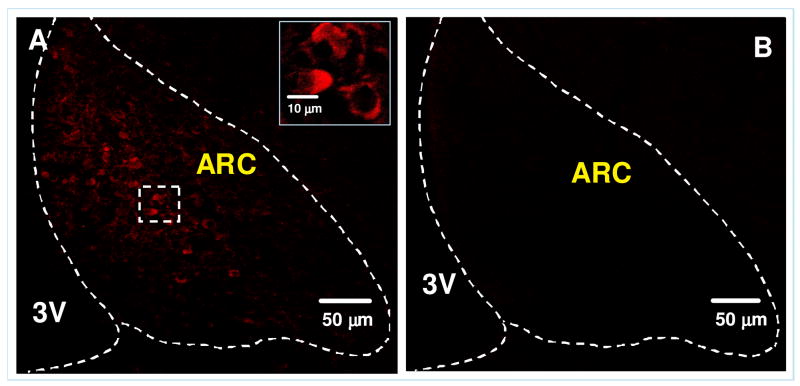
As summarized in Table 2, apo A-IV immunoreactivity was detected in several nuclei of the hypothalamus and brainstem. Immunofluorescence staining revealed apo A-IV-positive cell bodies localized in the ARC, with particularly dense staining in the medial-posterior part of the ARC and much less expression in the lateral ARC (Fig. 2A). Most of the reaction product was located in the perinuclear zone of cells and in proximal segments of the processes of labeled cells. Under the conditions used for immunohistochemistry, control sections of ARC treated with apo A-IV pre-absorbed antiserum had no apo A-IV immunoreactivity (Fig. 2B), indicating that the immunostaining is specific.
Table 2.
Qualitative summary of apo A-IV in the brain
| Brain structures | Apo A-IV staining |
|---|---|
| Arcuate nucleus (ARC) | +++ |
| Paraventricular nucleus (PVN) | ++ |
| Dorsomedial nucleus (DMH) | − |
| Ventromedial nucleus (VMH) | +++ |
| Lateral hypothalamic area (LHA) | − |
| Nucleus of the solitary tract (NTS) | ++ |
A 4-point scale was used to rate the data: +++, high density; ++, moderate density; +, low density; −, no nuclear signal.
Figure 2.
Immunofluorescence photomicrograph showing the presence of apo A-IV in the ARC of rat hypothalamus (A). No apo A-IV immunostaining was observed in control rat brain section treated with pre-absorbed apo A-IV antiserum. Solid boxes in each image are high-magnified image of the area dotted boxes. 3V: Third ventricle.
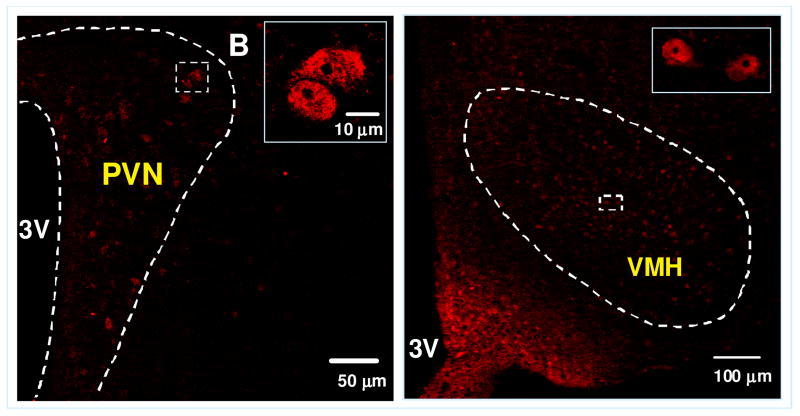
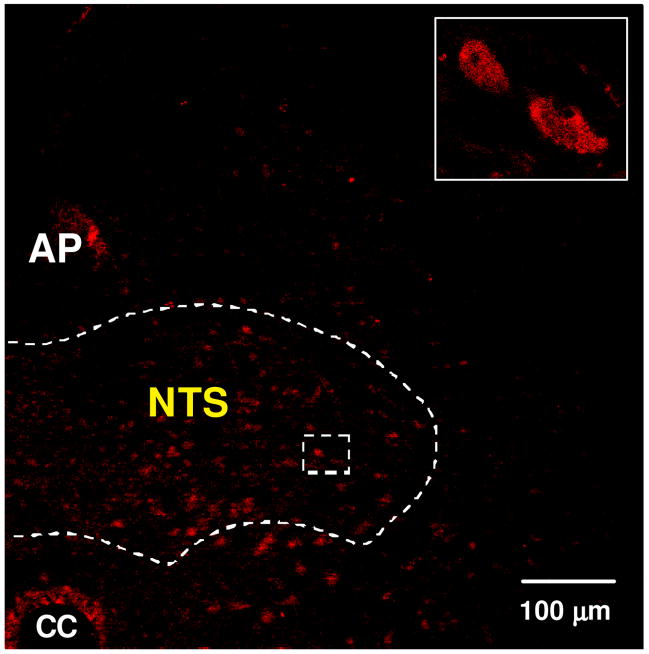
Within the PVN, apo A-IV was detected chiefly in ventral and periventricular parvocellular areas thought to be involved in food intake and autonomic regulation, with much less in the magnocellular PVN (Fig. 3A). While apo A-IV staining was detected in all three subnuclei (dorsomedial, central and ventrolateral) of the VMN, staining was concentrated primarily in the central portion of the VMN (Fig. 3B). By comparison, apo A-IV immunoreactivity was not detected in the lateral hypothalamic area (LHA) or dorsomedial nuclei (DMN) of the hypothalamus (Image not shown). In the NTS, apo A-IV staining was more prominent in the intermedial than medial and dorsomedial aspects (Fig. 4). Overall, the distribution of apo A-IV in the brain is consistent with a role in the regulation of energy homeostasis [19].
Figure 3.
Immunofluorescence photomicrograph showing the presence of apo A-IV in the PVN and VMH of the hypothalamus. Solid boxes in each image are high-magnified image of the area dotted boxes. 3V: Third ventricle.
Figure 4.
Immunofluorescence photomicrograph showing the presence of apo A-IV in the NTS of brainstem. Solid boxes in each image are high-magnified image of the area dotted boxes. 3V: Third ventricle, CC: central canal, AP: area postrema
3.3 Apo A-IV in the ARC is mainly localized in neurons
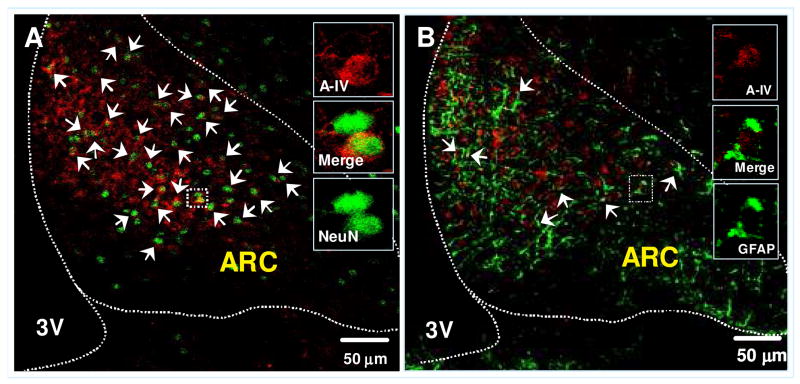
Our confocal microscopy examination of the double-labeled sections revealed that more than 78% of the apo A-IV-positive cells are neurons (Fig. 5A), 11% are astrocytes (Fig. 5B), 3% are microglial cells, and 8% are in all other cell types (pictures not shown).
Figure 5.
Confocal image of double-immunofluorescent staining for apo A-IV (red) and cell type maker (green): NeuN (for neuronal cells, Fig 6A) or GFAP (for astrocytes, Fig. 6B) in the ARC of the hypothalamus. The colocalizations of apo A-IV with cell type marker proteins are depicted in yellow color and indicated by arrows. Solid boxes in each image are high-magnified image of the area dotted boxes. Sections are representative of 4 animals in which staining was examined. 3V: Third ventricle.
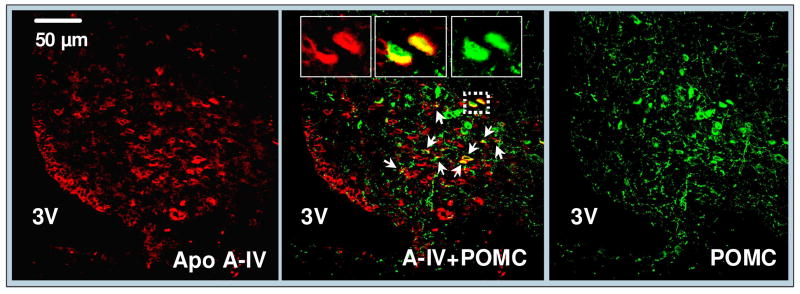
3.4 Apo A-IV is partially co-localized with POMC in the ARC
Confocal microscopy examination revealed that ~23% of POMC-containing neurons in the ARC contain apo A-IV (Fig. 6). This observation provided morphological evidence for a potential interaction of apo A-IV with POMC.
Figure 6.
Co-localization of apo A-IV (red) and POMC (green) in the ARC of the hypothalamus. The yellow color of apo A-IV and POMC, as indicated by arrows, depicted the colocalization of apo A-IV with POMC immunofluorescence. Solid boxes in each image are high-magnified image of the area dotted boxes. Sections are representative of 4 animals in which staining was examined. 3V: Third ventricle.
3.5 Apo A-IV reverses the reduction of POMC mRNA induced by fasting
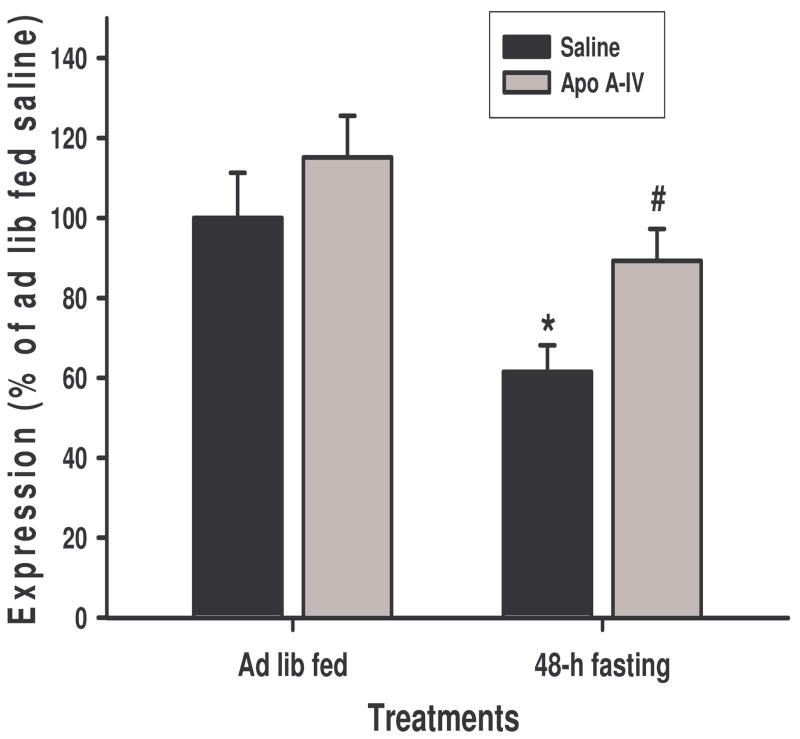
We further determined the effects of apo A-IV on the gene expression of POMC, NPY and AgRP in ad lib fed and 48-h fasted rats. As depicted in Figure 7, 48-h fasting significantly reduced POMC mRNA level (P < 0.05), and icv administration of apo A-IV reversed this reduction, demonstrating apo A-IV’s stimulatory effect on hypothalamic POMC gene expression. Consistent with a previous report [20], 48-h fasting also significantly increased NPY and AgRP mRNA levels. Following icv apo A-IV, there was a non-significant trend toward decreased NPY and AgRP gene expression (data not shown).
Figure 7.
Hypothalamic POMC mRNA levels measured by qPCR. 48-h fasting caused a significant reduction of POMC mRNA levels, which were reversed by icv administration of apo A-IV (8 μg). Mean ± SEM, n = 7. *P < 0.05, compared to ad libitum-fed saline group. #P < 0.05, compared to 48-h fasted saline controls.
3.6. Circulating apo A-IV does not cross the blood-brain barrier
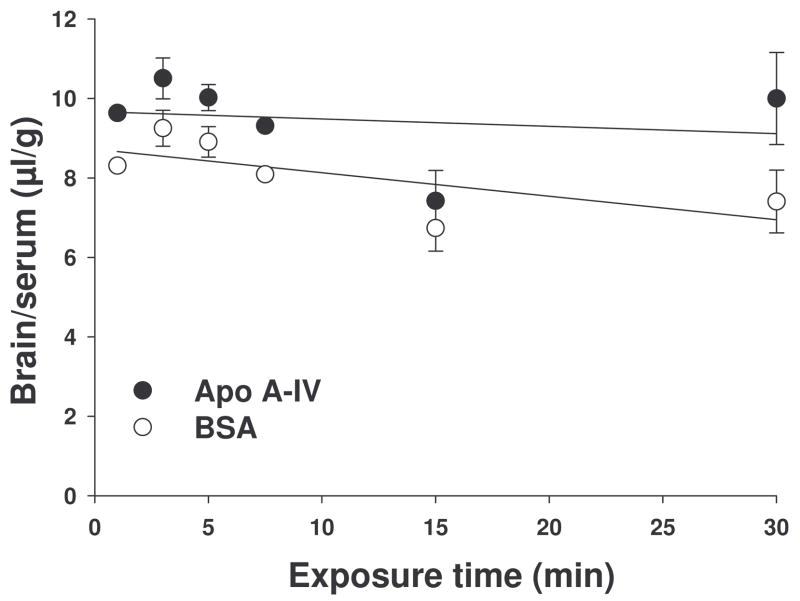
Multiple-time regression analysis was used for calculation of unidirection influx constant (Ki) from blood to brain [21]. As depicted in Fig. 8, the influx rates for 125I-labled apo A-IV and for 99mTc albumin are not significantly different from zero or from each other, implying that apo A-IV is not able to cross the BBB in these conditions. As a control, and to verify the experimental procedure, we performed identical studies using 125I-labeled leptin as described by Banks et al. [18]. Consistent with published data [18], there was a significant increase in the presence of leptin in the brain over time (Ki = 4.250 × 10−4 ml/g per min), indicating that our methodology was indeed capable of detecting leptin transit across the BBB.
Figure 8.
The ratio of brain (cpm) to serum (cpm) over time for both 125I-labeled recombinant apo A-IV and 99mTc albumin, where the slope of the linear portion of the relationship is Ki. The Ki of 125I-labled apo A-IV was −0.675 ± 0.151 × 10−4 ml/g per min. The Ki of the co-injected 99mTc albumin was −0.323 ± 0.138 × 10−4 ml/g per min. The rate of entry, reflected by the slope of the lines, is not significantly different for 125I-labeled apo A-IV from co-injected 99mTc-labeled albumin (BSA). n = 3 for each time point
4. Discussion
Using a specific polyclonal antibody against rat apo A-IV to perform immunohistochemistry, we established, for the first time, a detailed distribution of apo A-IV-containing cells in rat hypothalamus and brainstem, areas involved in the regulation of energy homeostasis. Apo A-IV immunoreactive cells were abundantly detected in the ARC and VMN, with less staining in the PVN and DMN. In the brainstem, a high density of stained cells and fibers was observed in the NTS.
The ARC has been identified as a prime regulatory area in the brain. Several neuropeptides, including AgRP, NPY and POMC, are synthesized in ARC neurons, and these cells are a primary target for leptin, a feeding-inhibitory hormone secreted predominantly by adipose tissue [22]. Our finding that apo A-IV protein is highly expressed in ARC suggests an important role of apo A-IV in this nucleus and its associated neural circuits. The observation is also consistent with previous studies [10;13], and we now extend that previous work by reporting that apo A-IV is primarily expressed in neurons. This is consistent with a previous study reporting apo A-IV expression in cultured neurons [14].
In the PVN, apo A-IV expression was localized primarily in the medial and periventricular parvocellular subnuclei, areas known to participate in the control of food intake and autonomic function [23]. Lesions of the PVN have profound effects on food intake and body weight regulation [24], and several neurotransmitters with orexigenic as well as anorectic properties exert their influence on feeding behavior most potently when injected directly into this nucleus. For example, NPY and galanin stimulate feeding when injected into the PVN [25;26], whereas glucagon-like peptide-1 (GLP-1) and corticotropin-releasing-factor (CRF) inhibit food intake when injected there [27]. Several peptidergic neurons regulated by leptin project to the PVN from the ARC [28;29], and several peptides that affect feeding and body weight, such as CRF and galanin, are localized within the PVN [30;31].
The VMN has long been implicated in the control of food intake [32]. Based on classic brain lesioning experiments, a “two-centers’ hypothesis in which the VMN signals satiety and the lateral hypothalamic area (LHA) signals hunger was prominent for years. Electrical stimulation of the VMN resulted in decreased feeding, whereas stimulation of the LHA increased appetite [33]. Recently, Dhillon et al. reported that targeted deletion of leptin receptors in VMN neurons resulted in increased food intake and body weight in mice [34]. Our discovery that apo A-IV is concentrated in the medial VMN is of interest in light of recent evidence that neurons in this brain area provide tonic excitatory input to POMC neurons in the ARC [35]. Whether these neurons are among those that contain apo A-IV and are important for energy homeostasis is therefore an interesting and as yet unanswered question.
In the present study, we also observed that apo A-IV is abundantly present in the NTS. Afferent input from the gastrointestinal tract generated by gastric distention or gut peptides is transmitted via vagal afferent fibers to the NTS, which in turn is activated in a leptin-sensitive manner to influence satiety [36]. Recently, it was reported that NTS lesions block the ability of both cholecystokinin (CCK) and glucagon to inhibit food intake [37]. Our detection of apo A-IV in the NTS neurons suggests a role of apo A-IV in processing visceral sensory information.
Compelling evidence suggests that food intake and body weight are controlled by neuropeptides acting within the CNS. CNS circuits involved in food intake can be conceptually divided into anabolic and catabolic systems, and both are well-represented in the ARC. NPY, AgRP and POMC are key peptides in these systems, and it is the balance between these systems that ultimately determines the animal’s ingestive behavior and energy expenditure [19]. We hypothesized that apo A-IV exerts its anorectic action by interacting with these ARC neuropeptides. To evaluate this, we assessed the effects of icv apo A-IV on hypothalamic POMC, NPY and AgRP gene expression in 48-h fasted rats. We found that apo A-IV significantly reversed the reduction of POMC mRNA in these fasted rats, but not ad lib fed rats. The observation of co-localization of apo A-IV with POMC in the ARC indicates that such effect may be direct.
The molecular mechanisms mediating apo A-IV’s anorectic action currently remain unclear. Our finding of co-localization of apo A-IV and POMC in the ARC may help to elucidate the mechanism of apo A-IV-induced anorexia. As previously reported, leptin stimulates apo A-IV synthesis and secretion from neurons in the hypothalamus [14]. The increased apo A-IV might therefore stimulate POMC gene expression and lead to increased α-MSH release. This hypothesis is supported by our previous observation that a subthreshold dose of apo A-IV enhances the ability of MT-II to suppress feeding and that a subthreshold dose of SHU9119 attenuates the anorectic effect of apo A-IV (14). All of these data indicate a synergistic interaction between the melanocortin system and apo A-IV in the brain to regulate the food intake [13].
Most apo A-IV is produced in the intestine of both humans and rodents [1], although some is also made in the liver [38]. Fujimoto et al. reported that the concentration of apo A-IV in cerebrospinal fluid increases following lipid intake [3], suggesting the possibility that apo A-IV found in the brain may have been synthesized in peripheral organs and transported into the brain from the circulation. Although several regulatory peptides and proteins are able to cross the BBB, whether this is also the case for apo A-IV has not been determined. In previous studies demonstrating apo A-IV mRNA in brain, we concluded that at least some of the apo A-IV protein present in brain was actually synthesized in the brain [7], but those findings did not address the possibility that circulating apo A-IV crosses the BBB. Therefore, examination of this possibility would help clarify the relation between central and peripheral pools of this protein as well as the relation between its central and peripheral effects.
Using multiple-time regression analysis, we found that the influx rates for 125I-labled apo A-IV and for the vascular marker 99mTc-albumin were not significantly different from zero or from each other, implying that circulating apo A-IV does not enter the brain in significant amounts after iv injection. In contrast and as reported by others [18], leptin did penetrate the BBB in parallel experiments. These observations lead us to conclude that apo A-IV identified in the brain is produced in the brain, and is not derived from peripheral tissues.
In conclusion, we have determined the apo A-IV is localized in neurons in multiple key brain areas important for energy homeostasis regulation. The co-localization of apo A-IV with POMC in the arcuate nucleus provides morphological evidence that apo A-IV’s anorectic action is associated with melanocortin signaling in the hypothalamus. These observations set the stage for functional and mechanistic studies to assess the role of apo A-IV signaling in the control of food intake and body weight.
Acknowledgments
The authors acknowledge the technical assistance of Drs. Ming-dian Zhang and Li-yun Ma. This work was supported by research grants from National Institutes of Health DK 63907, DK 70992, DK54890, DK17844, DK56863 and HL082734.
References
- 1.Apfelbaum TF, Davidson NO, Glickman RM. Apolipoprotein A-IV synthesis in rat intestine: regulation by dietary triglyceride. Am J Physiol. 1987;252:G662–G666. doi: 10.1152/ajpgi.1987.252.5.G662. [DOI] [PubMed] [Google Scholar]
- 2.Dvorin E, Gorder NL, Benson DM, Gotto AM., Jr Apolipoprotein A-IV. A determinant for binding and uptake of high density lipoproteins by rat hepatocytes. J Biol Chem. 1986;261:15714–15718. [PubMed] [Google Scholar]
- 3.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest. 1993;91:1830–1833. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Intracisternal injection of apolipoprotein A-IV inhibits gastric secretion in pylorus-ligated conscious rats. Gastroenterology. 1994;107:1861–1864. doi: 10.1016/0016-5085(94)90833-8. [DOI] [PubMed] [Google Scholar]
- 5.Qin X, Swertfeger DK, Zheng S, Hui DY, Tso P. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am J Physiol. 1998;274:H1836–H1840. doi: 10.1152/ajpheart.1998.274.5.H1836. [DOI] [PubMed] [Google Scholar]
- 6.Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, Steinmetz A, Denefle P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273:966–968. doi: 10.1126/science.273.5277.966. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, Jackman A, Tso P. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1382–R1387. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Shen L, Liu Y, Woods SC, Seeley RJ, D’Alessio D, Tso P. Obesity induced by a high-fat diet down-regulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab. 2004 doi: 10.1152/ajpendo.00448.2003. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Shen L, Liu Y, Tajima D, Sakai R, Woods SC, Tso P. Diurnal Rhythm of Apolipoprotein A-IV in Rat Hypothalamus and Its Relation to Food Intake and Corticosterone. Endocrinology. 2004;145:3232–3238. doi: 10.1210/en.2003-1554. [DOI] [PubMed] [Google Scholar]
- 10.Liu M, Shen L, Doi T, Woods SC, Seeley RJ, Tso P. Neuropeptide Y and lipid increase apolipoprotein AIV gene expression in rat hypothalamus. Brain Res. 2003;971:232–238. doi: 10.1016/s0006-8993(03)02402-8. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D’Alessio D, Seeley RJ, Tso P, Woods SC. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol. 2006;290:R202–R207. doi: 10.1152/ajpregu.00502.2005. [DOI] [PubMed] [Google Scholar]
- 12.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gotoh K, Liu M, Benoit SC, Clegg DJ, Davidson WS, D’Alessio D, Seeley RJ, Tso P, Woods SC. Apolipoprotein A-IV interacts synergistically with melanocortins to reduce food intake. Am J Physiol Regul Integr Comp Physiol. 2006;290:R202–R207. doi: 10.1152/ajpregu.00502.2005. [DOI] [PubMed] [Google Scholar]
- 14.Shen L, Tso P, Woods SC, Sakai RR, Davidson WS, Liu M. Hypothalamic Apolipoprotein A-IV Is Regulated by Leptin. Endocrinology. 2007;148:2681–2689. doi: 10.1210/en.2006-1596. [DOI] [PubMed] [Google Scholar]
- 15.Doi T, Liu M, Seeley RJ, Woods SC, Tso P. Effect of leptin on intestinal apolipoprotein AIV in response to lipid feeding. Am J Physiol Regul Integr Comp Physiol. 2001 Sep;281(3):R753–R759. doi: 10.1152/ajpregu.2001.281.3.R753. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, Woods SC, Seeley RJ, Davidson WS, Tso P. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav. 2003;78:149–155. doi: 10.1016/s0031-9384(02)00959-9. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Kastin AJ. Physiological consequences of the passage of peptides across the blood-brain barrier. Rev Neurosci. 1993;4:365–372. doi: 10.1515/revneuro.1993.4.4.365. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 19.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 20.Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. doi: 10.1016/s0006-8993(02)03674-0. [DOI] [PubMed] [Google Scholar]
- 21.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 22.Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–396. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- 23.Shor-Posner G, Azar AP, Insinga S, Leibowitz SF. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol Behav. 1985;35:883–890. doi: 10.1016/0031-9384(85)90255-0. [DOI] [PubMed] [Google Scholar]
- 24.Gold RM, Jones AP, Sawchenko PE. Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol Behav. 1977;18:1111–1119. doi: 10.1016/0031-9384(77)90019-1. [DOI] [PubMed] [Google Scholar]
- 25.Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide’s effects in the brain. Peptides. 1990;11:995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- 26.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–R29. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 28.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 29.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 30.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 31.Leibowitz SF, Akabayashi A, Wang J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci. 1998;18:2709–2719. doi: 10.1523/JNEUROSCI.18-07-02709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stellar E. The physiology of motivation. Psychol Rev. 1954;61:5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 33.Valenstein ES, Cox VC, Kakolewski JW. Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science. 1968;159:1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- 34.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 36.Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. [DOI] [PubMed] [Google Scholar]
- 37.Edwards GL, Ladenheim EE, Ritter RC. Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am J Physiol. 1986;251:R971–R977. doi: 10.1152/ajpregu.1986.251.5.R971. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro A, Mangeney M, Loriette C, Thomas G, Pepin D, Janvier B, Chambaz J, Bereziat G. Effect of simvastatin on the synthesis and secretion of lipoproteins in relation to the metabolism of cholesterol in cultured hepatocytes. Biochim Biophys Acta. 1991;1086:279–286. doi: 10.1016/0005-2760(91)90171-d. [DOI] [PubMed] [Google Scholar]