Abstract
Background
Immune dysfunction has been associated with autism, yet whether maternal immune status during pregnancy plays a causal role remains to be clarified.
Methods
We conducted a population-based case-control study nested within the cohort of infants born July 2000-September 2001 to women who participated in the prenatal screening program in Orange County, California. Cases (AU; n = 84) were children receiving services for autism at the Regional Center of Orange County. Two control groups were included: children with mental retardation or developmental delay (MR; n = 49) receiving services at the same regional center; and children not receiving services for developmental disabilities, randomly sampled from the California birth certificate files (GP; n = 160). Maternal autoantibody reactivity to fetal brain protein was measured by Western blot in archived mid-pregnancy blood specimens drawn during routine prenatal screening. Presence of specific bands and band patterns were compared between the three study groups.
Results
The pattern of maternal mid-gestation antibody reactivity to human fetal brain protein varied by study group and by autism onset type, although most differences did not reach statistical significance. Reactivity to a band at 39 kDa was more common among mothers of children with autism (7%) compared with mothers of MR (0%; p = .09) and GP control subjects (2%; p = .07), and simultaneous reactivity to bands at 39 kDa and 73 kDa was found only in mothers of children with early onset autism (n = 3).
Conclusions
Our findings indicate that further studies of prenatal immune markers might be a productive area for etiologic and biologic marker discovery for autism.
Keywords: ASD, autism, autoimmune, biologic marker, neonatal, prenatal
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterized by impairments in social interaction, verbal and nonverbal communication, and stereo-typed behaviors and interests (1). Autism spectrum disorders encompass a broad range of phenotypes, and diagnosis is currently based solely on behavioral criteria (2). The etiology of ASD is not well understood, although it likely involves both genetic and environmental factors (3). Although the age of symptom onset would indicate a prenatal or early postnatal etiology, to date no human fetal or neonatal biological markers have been reliably identified for this spectrum of disorders. Such markers might help elucidate etiology and could allow for earlier identification and therapeutic intervention, improving the prognosis for ASD patients (4) and possibly contributing to the development of prevention strategies.
Immune system dysregulation has been reported in ASD in several studies (reviewed by Ashwood et al. 2006) (5). Systemic immunologic aberrations in individuals with autism have often been associated with autoimmunity. Of particular interest, auto-antibodies reactive against brain and central nervous system proteins have been found more frequently in children with autism compared with unaffected children (6-17).
In addition to the presence of autoantibodies in children with autism, a few studies suggest the presence of autoantibodies to brain proteins in the blood of mothers of children with autism. Dalton et al. (2003) (18) demonstrated maternal IgG antibody reactivity to adult rat cerebellar Purkinje cells in a mother of two children with neurodevelopmental disorders, one with ASD and the other with a severe language disorder. These authors also reported the presence of behavioral deficits in the pups of a mouse injected during gestation with the serum from this woman (18). Similarly, Zimmerman et al. (2007) (19) recently reported differing patterns of serum immunoreactivity to prenatal rat brain in specimens from mothers of children already diagnosed with autism compared with specimens from mothers of control children. This study also demonstrated that immunoreactivity persisted in maternal circulation for up to 18 years after delivery. Finally, we recently reported that autoantibodies to human fetal brain proteins at 37 kDa and 73 kDa were significantly more often present in the plasma of mothers of children with autism compared with control mothers (20). In that study, maternal plasma was collected on average 3.5 years after the birth of the study child. Because the overall circulating IgG profile of any individual changes over time as a result of subsequent exposures and immune responses, it is not clear that antibodies measured in mother’s blood several years after delivery of her child represent the antibody profile present during the prenatal period.
The transplacental passage of maternal IgG isotype antibodies has long been known as a mechanism for fetal immune instruction (21) and protection (22,23). However, autoantibodies that react to fetal “self”-proteins can also cross the placenta and potentially impact fetal development. In this study, we tested the hypothesis that the profile of autoantibody reactivity to fetal brain protein in mid-pregnancy serum collected from mothers who delivered a child subsequently diagnosed with autism is different from the profiles characterizing mothers of children subsequently diagnosed with other developmental disorders and mothers of children with apparent normal development.
Methods and Materials
Subjects
The study sample was drawn from the EMA (Early Markers for Autism) Study, a population-based case-control study designed to evaluate molecular biomarkers for autism in archived prenatal and neonatal blood specimens from the same mother-baby pairs. All study procedures were approved by the institutional review boards (IRBs) of the State of California and Kaiser Permanente Northern California.
Women were eligible for inclusion in the EMA Study if they participated in the prenatal expanded alphafetoprotein screening program (XAFP) in Orange County, California and delivered a live born infant from July 2000 to September 2001 in California. Three groups of children born to women in the cohort were identified: children with autism (AU), children with mental retardation or developmental delay but not autism (MR), and general population control subjects (GP). Children with AU or MR were ascertained from the California Department of Developmental Services (DDS), which operates a system of 21 Regional Centers (RC) that coordinate services for persons with autism, mental retardation, and other developmental disabilities. Referrals to the RCs come from pediatricians and other clinical providers, the education system, friends, and family members. The DDS and the RCs are mandated to provide services to individuals with autistic disorder and children with other Pervasive Developmental Disorders (PDDs) who have MR (IQ < 70) or are substantially handicapped. The GP control subjects were randomly sampled from the birth certificate files at a 2:1 ratio after excluding all past or current DDS/RC clients. Control subjects were frequency matched to autism cases by gender, birth month, and birth year. Only mother-child pairs for whom a maternal specimen was found in the Project Baby’s Breath (PBB) prenatal specimen archive (see following) were included in the present study. At the time of case and control ascertainment, children were 3-4 years old.
Diagnostic Verification
Following a protocol initially developed by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (24), trained medical record abstractors reviewed and abstracted detailed diagnostic and clinical data from RC records for all children receiving services for AU or MR. Final case status was determined by expert clinical review (RLH) of the abstracted information. The ASD status was based on DSM-IV-TR criteria. The ASD onset type was based on parental report or clinical observations recorded in the RC records and categorized as “early” (no statement of loss of social and/or language skills or statement of early and sustained delays or plateauing of skills without actual loss), “regressive” (statement reflecting clear loss of previously acquired language and/or social skills), or unknown. The MR determination was based on composite scores on standardized cognitive and functional tests recorded in RC records (MR: composite score < 70; no MR: all scores > 70 or some scores < 70 and others > 70; unknown: no standardized scores in chart). After expert review, the final analytic sample consisted of 84 children with AU with or without MR (early onset [n = 64]; regressive [n = 17]; unknown [n = 3]), 49 with MR without AU, and 160 GP control subjects.
Specimen Collection
Maternal mid-pregnancy blood specimens were retrieved from the PBB prenatal screening specimen archive maintained by the California Department of Public Health. The prenatal archive contains nearly 200,000 serum and blood cell pellet specimens collected for routine prenatal Expanded Alphafeto-protein (XAFP) screening at 15-19 weeks gestation from pregnant women living in three Southern California counties in 2000-2001. Specimens were collected in serum separator tubes by obstetrical care service providers and underwent XAFP testing at a central laboratory within 7 days of collection (median time = 3 days). After 1-2 days under refrigeration, leftover specimens were stored at -20°C. Consent forms for the XAFP screening program, completed at the time of the blood collection, stipulate that specimens and results from prenatal testing might be used for legitimate research purposes given appropriate IRB approval.
Determination of Reactivity to Fetal Brain Protein
Western blots were performed as described elsewhere (25). Briefly, 300-μg human fetal brain protein medley (Clontech, Mountain View, California), prepared from a pooled sample of 63 spontaneously aborted male and female fetuses 20-40 weeks gestation, was separated under reducing conditions on 4%-15% sodium dodecyl sulfate (SDS)-polyacrylamide prep gels (Bio-Rad, Hercules, California) and transferred electrophoretically to .2-μm-pore nitrocellulose membranes (Whatman, Florham Park, New Jersey) at 35 V for 14 hours. Magic mark XP molecular weight marker (Invitrogen, Carlsbad, California) was used in the single marker lane allowing chemiluminescent visualization of marker bands from 20 kDa to 220 kDa. After transfer, the blots were blocked in 10% Casein Block (Pierce Biotechnology, Rock-ford, Illinois) and then cut into strips including the MW marker and 24 fetal brain strips. Strips were placed in mini-incubation trays (Bio-Rad) on a rocking platform with 700 μL of 1:400 maternal plasma diluted in phosphate-buffered saline (PBS)/.05% Tween 20/.5% Casein Block (PBSTC) for 1.5 hours and then washed in PBS/.05% Tween 20 (PBST) 5 times for 5 min each. Zymax horseradish peroxidase conjugated Goat anti-Human IgG (Invitrogen) diluted 1:25,000 in PBST was added, and strips were incubated for 30 min with rocking. After secondary antibody incubation, strips were washed 5 times for 5 min with PBST and subsequently incubated with SuperSignal West Pico (Pierce Biotechnology) chemiluminescent substrate for 5 min. The strips were then removed from the incubation trays and arranged on a glass plate for imaging with a FluorChem 8900 with AlphaEaseFC software (Alpha Innotech, San Leandro, California) with a 1, 3, 5 min stacked movie acquisition.
Band presence and apparent molecular weight were determined with the image analysis capabilities of the AlphaEaseFC software. After defining the loading well position (protein migration start-point) and the dye front (end-point), relative migration (Rf) of each of the molecular weight markers was calculated. A point-by-point curve fit was applied to the Rf of the molecular weight markers and was used to determine the molecular weight of bands of maternal immunoreactivity to fetal brain. The presence of IgG heavy- and light-chain bands at approximately 25 kDa and 50 kDa, arising from reactivity of the secondary antibody to endogenous IgG present in the protein preparations, provided an internal reference for each sample strip and were used to verify uniform protein migration. Reference points from the IgG heavy- and light-chain bands were also used to determine the band Rf for those bands between 26 kDa and 56 kDa for increased accuracy. Blots were analyzed completely before revealing the diagnosis of the child. Bands were considered to be the same between samples when < 4% difference was observed in Rf. The threshold for assigning the presence of a band was a twofold-higher densitometry reading above background on the strip.
Statistical Analysis
Two-way comparisons between groups—AU versus GP, AU versus MR, and MR versus GP—were made with a Fisher exact test applied to all bands individually and in all possible band combinations to determine individual as well as grouped associations with diagnosis. Differences were considered significant at p < .05. Statistical significance was evaluated without correction for multiple comparisons, because we sought to identify all possible associations between individual bands and band patterns and autism.
We conducted, in addition to the key contrasts among AU, MR, and GP groups, secondary analyses to compare AU with regression versus AU early onset and to additionally compare these AU subgroups with GP control subjects.
Results
The characteristics of the study population are shown in Table 1. The distribution of maternal race was similar across the three study groups, although the proportion of mothers with Hispanic ethnicity and the proportion born in Mexico were lower in the AU group than the MR and GP groups. Maternal age was on average higher in the AU group. The prenatal blood specimen was collected at a similar point during pregnancy (17 weeks gestational age) in all three study groups, and maternal weight at the time of blood collection did not differ between groups.
Table 1.
Demographic Characteristics of Participants in the Early Markers for Autism Study
| AU (n = 84) |
MR (n = 49) |
GP (n = 160) |
AU vs. GP | AU vs. MR | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p | p | |
| Gender | ||||||||
| Male | 73 | 86.9 | 29 | 59.2 | 140 | 87.5 | .89 | .0003 |
| Female | 11 | 13.1 | 20 | 40.8 | 20 | 12.5 | ||
| Plurality | .42 | 1.0 | ||||||
| Singleton | 81 | 96.4 | 47 | 95.9 | 157 | 98.1 | ||
| Multiplea | 3 | 3.6 | 2 | 4.1 | 3 | 1.9 | ||
| Parityb | .23 | .05 | ||||||
| Primiparous | 42 | 50.0 | 16 | 32.7 | 67 | 41.9 | ||
| Multiparous | 42 | 50.0 | 33 | 67.3 | 93 | 58.1 | ||
| Mother’s Race | .06 | .63 | ||||||
| White | 57 | 67.9 | 37 | 75.5 | 127 | 79.4 | ||
| Asian | 19 | 22.6 | 9 | 18.4 | 28 | 17.5 | ||
| Other | 6 | 7.1 | 3 | 6.1 | 5 | 3.1 | ||
| Missing | 2 | 2.4 | 0 | 0 | ||||
| Mother’s Ethnicity | .0009 | .0001 | ||||||
| Hispanic | 20 | 23.8 | 28 | 57.1 | 73 | 45.6 | ||
| Non-Hispanic | 64 | 76.2 | 21 | 42.9 | 87 | 54.4 | ||
| Mother’s Birth Country | <.0001 | <.0001 | ||||||
| US | 45 | 53.6 | 16 | 32.7 | 72 | 45.0 | ||
| Mexico | 9 | 10.7 | 22 | 44.9 | 58 | 36.3 | ||
| Other | 30 | 35.7 | 11 | 22.4 | 30 | 18.8 | ||
| Maternal Age (yrs) mean (SD) | 30.9 | (5.2) | 28.3 | (5.2) | 28.2 | (5.5) | .0002 | .006 |
| Paternal Age (yrs) mean (SD) | 34.0 | (6.3) | 33.0 | (7.9) | 31.0 | (6.4) | .0009 | .41 |
| Maternal Weight at XAFP blood draw (lbs) mean (SD) | 145.1 | (26.7) | 149.1 | (38.7) | 146.9 | (33.7) | .64 | .53 |
| Gestational Age at XAFP blood draw (days) mean (SD) | 119.8 | (7.9) | 117.8 | (8.0) | 118.7 | (7.4) | .32 | .18 |
| Gestational Age at Birth (days) mean (SD) | 272.1 | (18.5) | 266.0 | (27.9) | 271.1 | (14.2) | .64 | .18 |
AU, children receiving services for autism at the Regional Center of Orange County; MR, children with mental retardation or developmental delay but not autism receiving services at the same regional center; GP, children not receiving services for developmental disabilities, randomly sampled from the California birth certificate files; XAFP, expanded alphafetoprotein screening program.
Multiple: twin or triplet.
Primiparous: no previous live births; multiparous: 1 or more previous live births.
Autoreactvity to a protein at approximately 32 kDa was observed significantly less frequently in serum of mothers of children with autism (3 of 84) compared with general population control subjects (18 of 152; p = .03) but was not significantly different compared with mothers of MR control subjects (2 of 48) (Table 2). Reactivity against proteins at both 39 kDa and 73 kDa was observed significantly more frequently in mothers of AU children (3 of 84) compared with GP control subjects (0 of 152; p = .04). Although not statistically significant in comparison with MR control subjects, the combination of reactivity at both 39 kDa and 73 kDa was seen exclusively among mothers of AU children (Table 2).
Table 2.
Reactivity to Specific Human Fetal Brain Proteins in Maternal Mid-Pregnancy Serum, the Early Markers for Autism Study
| AU (n = 84) | MR (n = 48) | GP (n = 152) | AU vs. GP | AU vs. MR | |
|---|---|---|---|---|---|
| Band | % | % | % | pa | pa |
| 32 kDa | 3.6 | 4.2 | 11.8 | .03 | 1.00 |
| 37 kDa | 7.1 | 4.2 | 7.9 | 1.00 | .71 |
| 39 kDa | 7.1 | 0 | 2.0 | .07 | .09 |
| 60 kDa | 19.0 | 25.0 | 23.7 | .51 | .51 |
| 73 kDa | 13.1 | 4.2 | 7.2 | .16 | .13 |
| 32 kDa:73 kDa | 2.4 | 0 | 1.3 | .62 | .53 |
| 37 kDa:73 kDa | 1.2 | 0 | 2.6 | .66 | 1.00 |
| 39 kDa:73 kDa | 3.6 | 0 | 0 | .04 | .55 |
| 60 kDa:73 kDa | 6.0 | 2.1 | 2.0 | .14 | .42 |
Abbreviations as in Table 1
Fisher exact test.
Of potential interest but not reaching statistical significance in these data, autoreactivity to a protein at approximately 39 kDa was observed in the serum of 6 of 84 mothers of AU children, compared with no mothers of MR control subjects (p = .09) and 3 of 152 mothers of GP children (p = .07). Reactivity to a band at 73 kDa was observed in 11 of 84 mothers of AU children but only 2 of 48 MR control mothers (p = .13) and 11 of 152 GP control mothers (p = .16). Simultaneous reactivity to proteins at both 60 kDa and 73 kDa also occurred somewhat more often in the mothers of AU children (5 of 84) than MR (1 of 48; p = .42) and GP control subjects (3 of 152; p = .14) (Table 2).
When examined by characteristics of AU onset, the patterns of reactivity to measured proteins were generally similar for mothers of children with regressive AU compared with mothers of GP control subjects. An exception is the combination of 60 kDa:73 kDa, which was observed somewhat more frequently in the mothers of children with regressive AU (2 of 17) than the mothers of GP control subjects (3 of 152; p = .08; Table 3). In contrast, the patterns of reactivity for mothers of children with early onset AU differed in some respects from mothers of GP control subjects: reactivity to proteins at 39 kDa and to the combination of 39 kDa:73 kDa were significantly more common among mothers of children with early onset AU (Table 3). The combination of reactivity to bands at both 39 kDa and 73 kDa was seen only in the serum of three mothers of children with early onset AU and in no other subjects (i.e., regressive AU, MR, or GP control subjects) (Figure 1). Reactivity at 32 kDa was less common among the mothers of children with early onset AU, but the difference did not reach statistical significance (p = .07; Table 3).
Table 3.
Reactivity to Specific Human Fetal Brain Proteins in Maternal Mid-Pregnancy Serum, by Autism Onset Type, the Early Markers for Autism Study
| Regression (n = 17) | Early Onset (n = 64) | GP (n = 152) | AU w/Regression vs. GP | AU Early Onset vs. GP | AU w/Regression vs. AU Early Onset | |
|---|---|---|---|---|---|---|
| Band | % | % | % | pa | pa | pa |
| 32 kDa | 5.9 | 3.1 | 11.8 | .70 | .07 | .51 |
| 37 kDa | 11.8 | 6.3 | 7.9 | .64 | .78 | .60 |
| 39 kDa | 0 | 9.4 | 2.0 | 1.00 | .02 | .33 |
| 60 kDa | 11.8 | 21.9 | 23.7 | .37 | .86 | .50 |
| 73 kDa | 17.6 | 12.5 | 7.2 | .15 | .29 | .69 |
| 32 kDa:73 kDa | 5.9 | 1.6 | 1.3 | .27 | 1.00 | .38 |
| 37 kDa:73 kDa | 5.9 | 0 | 2.6 | .42 | .32 | .21 |
| 39 kDa:73 kDa | 0 | 4.7 | 0 | .02 | 1.00 | |
| 60 kDa:73 kDa | 11.8 | 4.7 | 2.0 | .08 | .36 | .28 |
Abbreviations as in Table 1
Fisher exact test
Figure 1.
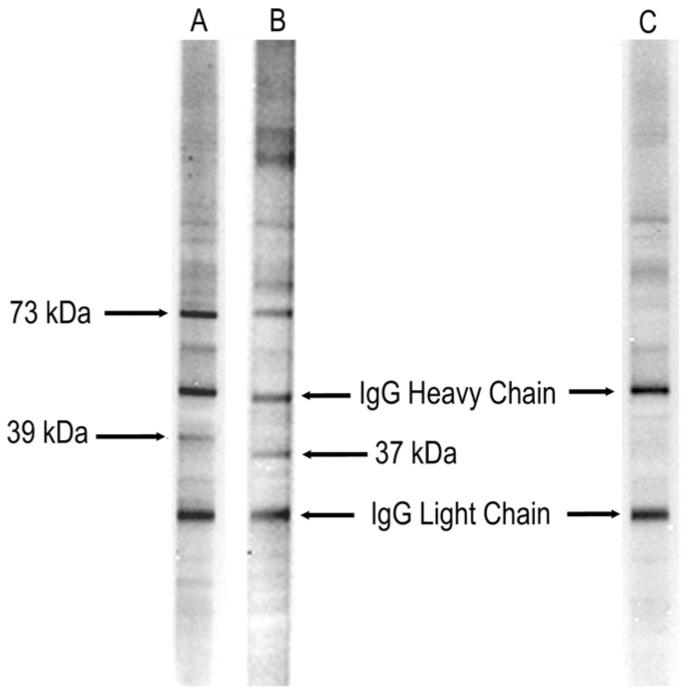
Representative lanes from western blot analysis of human fetal brain probed with serum from mothers of children with autism and those whose children are typically developing. Lane A represents a mother of a child with autism with the early onset phenotype with the 39-kDa:73-kDa band pattern. Lane B represents a mother of a child with regressive autism and the 37-kDa:73-kDa band pattern. Lane C represents a mother of a typically developing control child with no reactivity to fetal brain. The IgG heavy and light chains present in the fetal brain preparation are recognized by the secondary control and used as a reference marker.
The presence of specific bands or band patterns was not associated with maternal age, race, ethnicity, place of birth, parity, plurality, birth month, or child IQ (data not shown).
Discussion
Immune dysfunction in the mothers of children with autism during or around the time of pregnancy has been proposed as contributing to the abnormal brain development seen in affected children. Data supportive of some role for immune factors in the initiation or progression of atypical early brain development come from a variety of sources, but whether immune factors play a causal role or, rather, represent a downstream consequence of other, primary processes remains uncertain. A substantial obstacle to unraveling the complex biologic pathway leading to autism has been the lack of maternal biologic specimens obtained early in fetal brain development and linked to neurologic outcome data obtained after the children have reached the age at which autism can be reliably diagnosed. Prior studies of maternal immune biologic markers and autism have, of necessity, relied on maternal and child specimens obtained after a child has already received an autism diagnosis, several years after the process leading to autism was initiated.
To our knowledge, our study offers the first opportunity to evaluate immune markers in maternal specimens obtained during mid-gestation and in comparison with specimens from mothers whose children have mental retardation without autism or who are representative of the general population of children without either of these disorders. On the basis of results reported from evaluations of specimens obtained after the child’s diagnosis (18-20), we elected to begin the search for maternal biologic markers by focusing on autoantibodies to human fetal brain proteins measured in mid-pregnancy peripheral blood. With modest sample size for this first study, we found that the frequency of specific bands and band patterns differed some-what by study group and by autism onset type. Whereas between-group differences in reactivity to specific protein bands rarely achieved statistical significance in our study, our findings are similar to those reported with later-obtained specimens and indicate that further studies of maternal immune markers for autism might be a productive area for etiologic and biologic marker studies.
In the recently reported Braunschweig et al. (20) study, autoreactivity to human fetal brain proteins was evaluated in maternal specimens collected 2-5 years after delivery of the study child. In that study, we found that autoantibodies to proteins at 37 kDa and 73 kDa occurred significantly more often in plasma from mothers of children with autism when compared with mothers of children with other developmental delays or typical development. Reactivity to both the 37 kDa and 73 kDa protein bands was unique to the AU group; notably, 86% of the mothers who were positive for this doublet band pattern had children with regression. In contrast, in the current study, the 37 kDa:73 kDa doublet occurred in mothers of children in the GP control group as well as AU mothers and at a much lower frequency in AU mothers than in the Braunschweig et al. study. The one child with autism whose mother showed reactivity to this doublet was of the regressive phenotype, consistent with Braunschweig et al. We found, unlike the Braunschweig et al. study, that reactivity to the 39-kDa band was significantly more common among mothers whose children had early onset autism compared with control subjects, that reactivity to this band did not occur in any specimens from mothers of children with MR, and that reactivity to 39 kDa coupled with reactivity to 73 kDa was found only in the subgroup with early onset autism and not in either control population.
Although differences between the Braunschweig et al. study and the present one might be largely due to the timing of specimen collection, other factors that must be considered are the lower frequency of children with regression in the current study population compared with the Braunschweig et al. study (19% vs. 59%), differences in the control group (representative of the general population not enrolled with DDS in the current study, typically developing in the Braunschweig et al. study), differences in blood compartments analyzed (serum in current study, plasma in the Braunschweig et al. study), and sample processing. Similar laboratory platforms were used in both studies.
Zimmerman et al. (2007) (19) examined reactivity to fetal, postnatal, and adult rat brain in the serum of 11 mothers of children with autism and 10 mothers of control children. The serum was collected 2-18 years after the birth of the study child. They found that the pattern of antibody reactivity to prenatal but not postnatal or adult rat brain was significantly different in the serum of mothers of children with autism compared with control mothers. There were two distinct patterns observed for the mothers of children with autism, one with five prominent bands in the lower kDa range (< 37 kDa) and a second with a single prominent band at > 250 kDa. Further evidence of reactivity between maternal antibodies and rodent brain comes from a study reported by Dalton et al. (18), where the authors demonstrate immunohistochemical staining of neonatal rat brain by antibodies from a mother of multiple children with neurodevelopmental disabilities, one of which had autism.
These seemingly different results across studies might be due to the presence of differential autoantibody profiles among study cohorts or to experimental differences across studies, including the origin of the brain proteins used for antibody evaluation. It is striking, however, that maternal antibody reactivity against fetal brain protein was associated with autism in offspring in each of these studies. At present, we do not know the specific targets for any of the bands found to occur more frequently in mothers of children with autism in our study. We are now conducting further work to determine the protein targets of these antibodies. We anticipate that characterization of the protein targets will allow us to better understand the potential pathologic significance of our findings and guide us in creating specific screening assays.
Although several reports have suggested an increased rate of autoimmune diseases in the mothers of children with autism, limitations in study design hinder interpretation and findings regarding specific autoimmune diseases have been inconsistent (26-29). However, these data, together with the results from our current study, might suggest an overall alteration in the immune response in the mothers of children with autism during or around the time of pregnancy, not necessarily linked to a specific autoimmune condition or target organ. Detectable levels of maternal IgG are present in fetal circulation as early as 18 weeks gestation, and by 38 weeks gestation, fetal levels are comparable to maternal levels. Despite the beneficial nature of the majority of maternal IgG received by the fetus, a number of neonatal autoimmune diseases have been demonstrated to result from transmission of pathogenic maternal IgG, including neonatal lupus syndrome (30), neonatal anti-phospholipid syndrome (APS) (31), and abnormal thyroid function (32). Although these neonatal conditions are often transient, maternal antibody interference with developmental pathways can lead to permanent consequences, as in the case of congenital heart block associated with maternal systemic lupus erythematosus (SLE) autoantibodies. Furthermore, maternal IgG is observed in fetal brain and thus might have the potential to interfere with neurodevelopmental pathways.
Some limitations of the current study deserve mention. We were only able to measure autoreactivity in maternal serum at one point during pregnancy. Antibody production can vary over time and is influenced by exposure to infectious illness and injury (33). It is currently unknown whether the antibodies we measured were present earlier or later in the pregnancy or whether subsequent children born to those mothers with reactivity to fetal brain are at increased risk of autism. We were unable to measure patterns of reactivity in the serum of the study children, which could indicate whether a sustained autoimmune response was initiated by damage induced by maternal antibody exposure. A prospective pregnancy cohort study, now underway, with longitudinal follow-up of subsequent offspring born to mothers who already have one child with ASD will allow us to resolve some of these issues.
In addition, we did not look at reactivity to adult brain or other organs, as was done in previous studies (19,20), and thus do not know whether our findings are specific and limited to antigens expressed during fetal brain development or might also react to adult brain tissue. We did not have information on maternal history of autoimmune diseases or disease status during pregnancy, although no correlation between existing autoimmune conditions and the presence of antibodies to fetal brain proteins was noted in the Braunschweig et al. study (20). Although our study sample was larger than any reported to date and is representative of children born in a large and diverse region of southern California, our power to examine differences by phenotypic subtypes was limited. We were unable to validate the diagnostic status or type of autism onset by systematic clinical evaluation and, instead, relied on expert review of information recorded in service records. Finally, our general population control subjects were randomly sampled after excluding all past or current DDS/RC clients. This does not rule out the possibility that some of these children have ASD or another developmental delay for which they had not received services through DDS. To the extent that subject misclassification of this type might be present in these data, our ability to detect differences between subject groups would be diminished.
In this first study to examine prenatal antibody reactivity to human fetal brain, we have demonstrated variation in the pattern of maternal antibody reactivity during gestation to human fetal brain protein between mothers of children with autism and mothers of control children. Because of the complex and integrative nature of neurodevelopment, potentially pathogenic antibodies might yield diverse consequences. Further investigation in larger study populations is required to decipher the potential role of maternal antibodies in autism and other neurodevelopmental disorders.
Acknowledgments
Funding was provided by grants from the National Institute of Mental Health (R01- MH72565, LAC, principal investigator [PI]), the National Alliance for Autism Research (824/LC/01-201-004-00-00, LAC, PI), and the California Tobacco-Related Disease Research Program (8RT-0115, MK, PI). We thank Jack Collins, Roxana Odouli, and Tiffany Wong for project coordination; Julie Ruedaflores for record review and abstraction; Meredith Anderson and Daniel Najjar for assistance with data management and analysis; and Steve Graham and Debbie Hildebrandt for record linkage and specimen retrieval.
Footnotes
Drs. Croen, Grether, Kharrazi, Hansen, Ashwood, and Van de Water and Mr. Braunschweig, Ms. Haapanen, Ms. Yoshida, and Mr. Fireman reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 3.Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 4.Aman MG. Treatment planning for patients with autism spectrum disorders. J Clin Psychiatry. 2005;66(suppl 10):38–45. [PubMed] [Google Scholar]
- 5.Ashwood P, Wills S, Van de Water J. The immune response in autism: A new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 6.Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmun Rev. 2004;3:557–562. doi: 10.1016/j.autrev.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 7.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 8.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Ann NY Acad Sci. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- 9.Todd RD, Hickok JM, Anderson GM, Cohen DJ. Antibrain antibodies in infantile autism. Biol Psychiatry. 1988;23:644–647. doi: 10.1016/0006-3223(88)90012-1. [DOI] [PubMed] [Google Scholar]
- 10.Singh VK, Rivas WH. Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci Lett. 2004;355:53–56. doi: 10.1016/j.neulet.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Lin SX, Newell E, Nelson C. Abnormal measles-mumpsrubella antibodies and central nervous system autoimmunity in children with autism. J Biomed Sci. 2002;9:359–364. doi: 10.1007/BF02256592. [DOI] [PubMed] [Google Scholar]
- 12.Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997;17:88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 13.Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- 14.Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, et al. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59:354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1999;134:607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- 16.Kozlovskaia GV, Kliushnik TP, Goriunova AV, Turkova IL, Kalinina MA, Sergienko NS. Nerve growth factor auto-antibodies in children with various forms of mental dysontogenesis and in schizophrenia high risk group. Zh Nevrol Psikhiatr Im S S Korsakova. 2000;100:50–52. [PubMed] [Google Scholar]
- 17.Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: Maternally derived antibodies specific for fetal brain proteins. NeuroToxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, et al. Mechanisms of neonatal mucosal antibody protection. J Immunol. 2006;177:6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- 23.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- 24.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 26.Mouridsen SE, Rich B, Isager T, Nedergaard NJ. Autoimmune diseases in parents of children with infantile autism: A case-control study. Dev Med Child Neurol. 2007;49:429–432. doi: 10.1111/j.1469-8749.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 27.Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ. Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics. 2003;112:e420. doi: 10.1542/peds.112.5.e420. [DOI] [PubMed] [Google Scholar]
- 28.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 29.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 30.Tincani A, Danieli E, Nuzzo M, Scarsil M, Motta M, Cimaz R, et al. Impact of in utero environment on the offspring of lupus patients. Lupus. 2006;15:801–807. doi: 10.1177/0961203306071005. [DOI] [PubMed] [Google Scholar]
- 31.Soares Rolim AM, Castro M, Santiago MB. Neonatal antiphospholipid syndrome. Lupus. 2006;15:301–303. doi: 10.1191/0961203306lu2295cr. [DOI] [PubMed] [Google Scholar]
- 32.Fu J, Jiang Y, Liang L, Zhu H. Risk factors of primary thyroid dysfunction in early infants born to mothers with autoimmune thyroid disease. Acta Paediatr. 2005;94:1043–1048. doi: 10.1111/j.1651-2227.2005.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 33.Toptygina AP, Pukhalsky AL, Alioshkin VA. Immunoglobulin G subclass profile of antimeasles response in vaccinated children and in adults with measles history. Clin Diagn Lab Immunol. 2005;12:845–847. doi: 10.1128/CDLI.12.7.845-847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


